Published online Jan 12, 2023. doi: 10.13105/wjma.v11.i1.5
Peer-review started: September 9, 2022
First decision: November 17, 2022
Revised: November 25, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 12, 2023
Processing time: 123 Days and 21.5 Hours
The coronavirus 2019 disease (COVID-19) is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2. This disease was designated by the World Health Organization as a pandemic on March 11, 2020, which is not seen before. There are no classical features among the cases of the disease owing to the involvement of nearly all body tissues by the virus. Hepatic involvement is one of the characteristics of the COVID-19 course. There are six possible mechanisms of such involvement: Direct virus injury, drug-induced effect, inflammatory cytokine storm, hypoxia-ischemic destruction, abnormalities in liver function tests, and pre-existing chronic liver diseases. Liver abnormalities are seen commonly in the severe or critical stage of COVID-19. Therefore, these abnor
Core Tip: There is a diversity of clinical manifestations of the coronavirus 2019 disease (COVID-19), ranging from classical presentations like fever, cough, and dyspnea to non-classical presentations like liver involvement. Direct injury, drug-induced hypoxia, abnormal liver function tests, cytokine storm, and a history of chronic hepatic diseases are the proposed mechanisms of liver involvement during the COVID-19 course. Liver involvement can determine the severity of the disease. Old age and a history of chronic diseases like diabetes mellitus are recognized risk factors for this involvement. Autoimmune hepatitis is an example of liver involvement following COVID-19 vaccination. However, complete vaccination with 3rd and 4th booster doses is required in patients with chronic liver diseases. We aim to summarize the various aspects of hepatic involvement during the COVID-19 course or following its vaccination.
- Citation: Al-Rawi TSS, Al-Ani RM. Liver dysfunction-related COVID-19: A narrative review. World J Meta-Anal 2023; 11(1): 5-17
- URL: https://www.wjgnet.com/2308-3840/full/v11/i1/5.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i1.5
The liver plays an essential role in the body. It deserves several physiological processes such as metabolism of the macronutrient, regulation of the blood volume, endocrine control of growth signaling pathways, support of body immunity, metabolism of cholesterol and lipid, and destruction of xenobiotic materials like certain drugs[1].
Among various causes of liver dysfunction, many viruses might attack the liver directly or indirectly. These include, but are not limited to, hepatitis A virus, hepatitis B virus (HBV), and hepatitis C virus (HCV). There is approximately 60% of patients in the previous pandemic in 2003, which was caused by the severe acute respiratory syndrome coronavirus (SARS-CoV), affected by different involvements of the liver[2]. Hence, from the beginning of the current coronavirus disease 2019 (COVID-19) pandemic, scientists have paid great attention to liver involvement due to the novel coronavirus (SARS-CoV-2). As such, a prior investigation from China reported that around 50% of the individuals with COVID-19 had dysfunction of the liver at a certain point in their disease course[3].
Liver abnormalities associated with COVID-19 might be due to direct liver damage by the SARS-CoV-2, drugs used for the disease, unrecognized previous liver abnormality, and cytokine storm, and as an indirect effect to the liver due to involvement of other body systems by the virus like the cardiopulmonary system[4].
Owing to the enormous research belonging to liver dysfunction-related COVID-19[4-8], we design this narrative review to update and summarize the epidemiological features, predisposing factors, pathophysiological mechanisms, hepatic manifestations due to COVID-19 or following vaccination, role of liver function tests in the assessment of COVID-19 severity, adverse effects of the therapeutic agents for the disease, and prognosis.
The source of SARS-CoV-2 is unknown and spreads quickly throughout the world. The WHO declared that COVID-19 is a pandemic on March 11, 2020[9]. COVID-19 could be transmitted by two major routes: One is direct contact (close contact) from individual to individual through aerosol and respiratory droplets produced by talking, sneezing, and coughing, and the other is indirect noncontact through contaminated objects and surfaces. The incubation period ranges from 1 to 14 d, with a median of 5.5 d[10,11]. Based on the WHO dashboard on August 10, 2022, there were 584065952 confirmed cases of COVID-19 globally, with the vast majority from Europe at 243772549, the Americas at 172407904, and the Western Pacific at 76247604. The total number of cases of deaths across the globe was 6418958, with the vast majority of deaths happening in the Americas (2797327), followed by Europe (2058965) and South-East Asia (793446) [World Health Organization. WHO coronavirus disease (COVID-19) situation dashboard. 2022; cited August 10, 2022. Available from: https://www.who.int/]. The number of COVID-19 cases is still sharply increasing, with over three million cases weekly.
A prior study has illustrated that males are more likely to have abnormal liver biochemical tests related to higher concentrations of C-reactive protein (CRP) and procalcitonin and a longer period time of hospitalization, about 20 d during severe COVID-19 compared to the control group with the normal biochemical test (16 d)[12]. A meta-analysis by Xu et al[13] has documented that males were more potential to have severe pneumonia than females. In addition, obesity, older age, and comorbidities were dangerous factors for death among hospitalized SARS-CoV-2 patients[14].
COVID-19 is characterized by rapid transmission through the lack of herd immunity with increased mortality, and the infection is increased in elderly individuals and becomes a greater danger to those who have hypertension, diabetes mellitus, and cardiorespiratory diseases[15-17].
COVID-19 is not occurring in the elderly only but also occurs in the pediatric population with a range of ages between 0-18 years with only 3% involvement. The infection has a slight predominance of males (51%). In the same study, it has been found that the infected adolescents were mainly aged 15-18 years, and that the occurrence of COVID-19 gradually decreased with younger ages[18].
SARS-CoV-2 is a positive sense single-stranded RNA virus. SARS-CoV and MERS-CoV are the original viruses that lead to the SARS-CoV-2 pandemic. Other subgenres of Sarbecovirus have caused the infection combined with acute respiratory symptoms in human beings, such as 229E, NL63, OC43, and HKU1. They lead to mild to severe diseases in the infected people[10,19]. The sequence of SARS-CoV-2 spike glycoproteins is significantly similar to that of SARS-CoV spike glycoproteins[20].
The receptor angiotensin-converting enzyme 2 (ACE-2) has been identified as the major viral receptor for SARS-CoV and SARS-CoV-2, and it facilitates these viruses to enter into target cells via the spike protein of the viruses. The mechanism includes the attachment of the virus to the surface of the host cell by linking to the ACE-2 receptor. SARS-CoV-2 gains access to the host via the ACE-2 receptor[21,22]. The expression of the ACE-2 receptor is widely shown on the surfaces of various types of human cells, systems, and organs. These include the muscular and nervous systems, alveolar epithelial cells in the lungs, nasal and oral mucosa, bronchial epithelial cells, nasopharynx, enterocytes of the small intestine, pancreas, liver, brain, heart, kidney, etc.[10,23-25].
The ACE-2 receptor is mainly expressed on cholangiocytes (bile duct) (60%) and has less expression (3%) on hepatocytes in the liver, while there is no expression of ACE-2 in Kupffer cells[26]. COVID-19-related hepatic injury could be defined as any impairment in infected individuals to the liver which occurs during the infection course and treatment phase of COVID-19 with or without the presence of liver disease.
The pathophysiology of liver injury induced by COVID-19 is complex and multifactorial. Other liver diseases should be considered, such as chronic hepatic disease due to autoimmune or viral disease, metabolic dysfunction-related fatty liver disease, cirrhosis, or liver transplant. An autopsy study on tissue from the liver of a COVID-19 subject revealed a relatively low viral titer owing to the absence of a viral inclusion body in the hepatic tissue. However, the pathological evaluation reported two findings: Mild active inflammation and moderate microvascular steatosis of the lobular portal part of the liver[27].
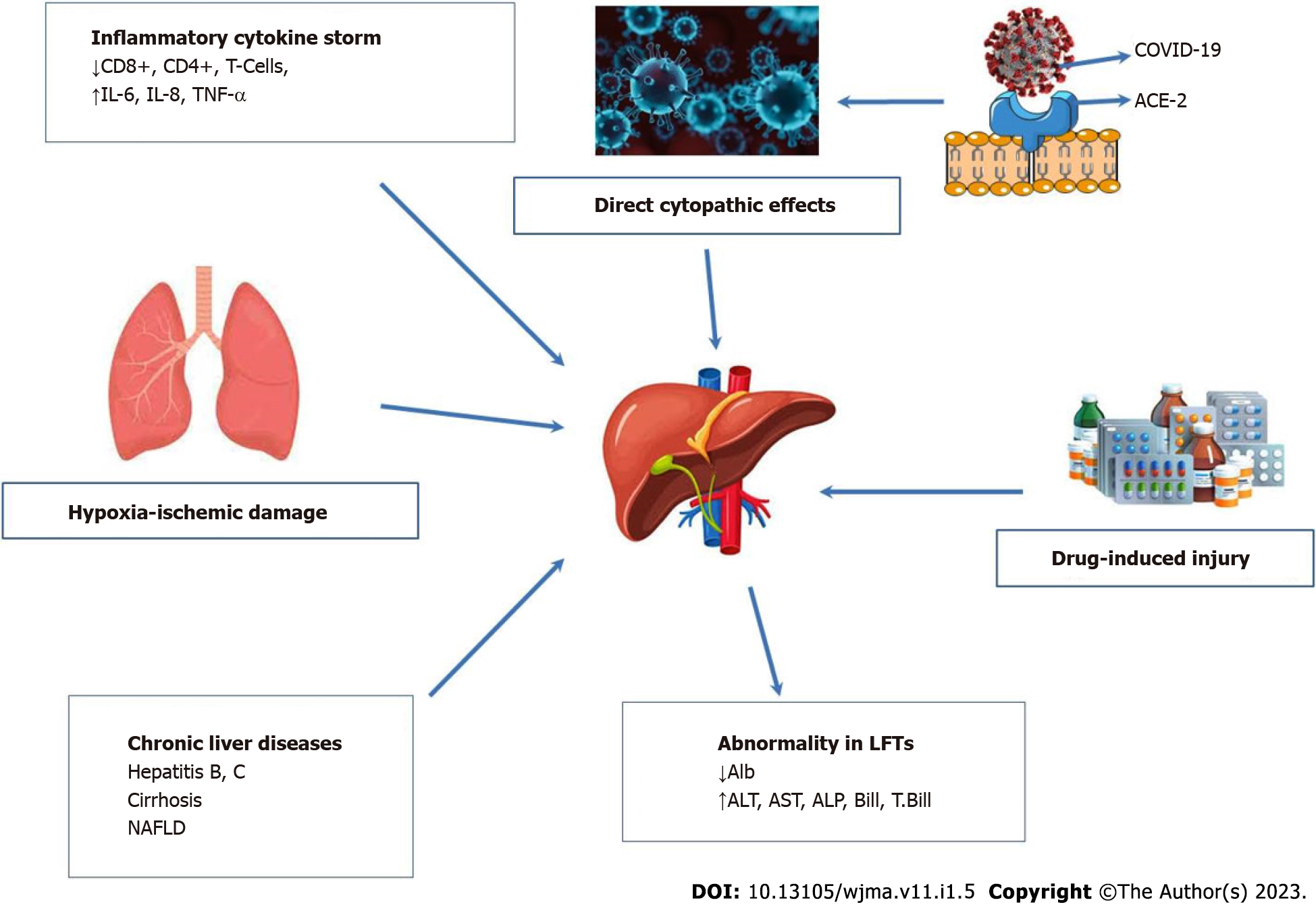
The mechanisms of liver injury related to COVID-19 are varied. Six probable mechanisms are proposed to clarify COVID-19 with liver disease, as shown in Figure 1.
The first mechanism is hypoxic-ischemic liver injury. A high level of aspartate transaminase (AST) in hepatitis could characterize ischemic hepatitis. The common outcome of COVID-19 is cardiomyopathy which happened in 33% of infected individuals in a series of critically ill United States (US) patients[28]. The hepatic ischemia, hypoxia, as well as impaired tissue perfusion in the course of COVID-19 could develop as a result of circulatory failure, multiple organ failure, pneumonia-correlated hypoxemia, and respiratory distress syndrome[29]. In mechanically-ventilated patients, high positive end-respiratory pressure and hepatic congestion can also increase the degree of hypoxic damage in hepatocytes[30,31].
Direct viral injury is also a possible mechanism of liver injury. It has been assumed that COVID-19 might cause cytopathic effects. The expression of the ACE-2 receptor occurs during the pathogenesis of liver injury associated with COVID-19. The reason is that when SARS-CoV-2 enters the liver on cholangiocytes, the spike proteins of SARS-CoV-2 bind to the ACE-2 receptor, and the viral replication will occur via interaction between the virus and ACE-2[32]. The expression of ACE-2 in cholangiocytes is considerably higher (about 60%) than that in hepatocytes (about 3%)[31,33]. The direct viral injury to bile duct epithelial cells could result from COVID-19-caused liver injury, which is recognized to significantly diminish the immune response and liver regeneration[34]. Moreover, it could be clarified by the fact that cholangiocytes have a crucial role in inflammation, liver regeneration capacity, and immune response. The loss of cholangiocytes leads to hepatocellular damage. However, the cytopathic effect of COVID-19 might not be the major reason for liver damage[34,35].
In liver biopsies from two infected individuals with COVID-19 who died, the particles of typical coronavirus were recognized in the cytoplasm of the hepatocytes; therefore, the cytopathic damage could be distinguished through endoplasmic reticulum dilatation, glycogen granule, and mitochondrial swelling[36].
Cholangiopathy is another mechanism to describe COVID-19-related liver injury. There is a broad domain of hepatic-biliary symptoms with COVID-19, including cholangiopathy's chronic and infrequent symptoms. It has been illustrated that the bile duct structure mimics secondary sclerosing cholangitis. It is ambiguous at this phase if these hepatic-biliary symptoms were an outcome of direct infection of the biliary tract and liver or if these demonstrated alterations of biliary tree ischemia. The complete recovery in COVID-19 patients was not reported with the increased concentrations of serum alkaline phosphatase (ALP) and bilirubin[37,38].
Drug-induced liver injury is also probable. COVID-19 requires drugs such as antiviral and antibody agents (protease inhibitors, azithromycin, receptor antagonist, and anti-interleukin IL-6 monoclonal antibody); such agents could cause hepatic injury. For example, remdesivir is a drug confirmed by the US Food and Drug Administration as a cause of liver injury[39]. The COVID-19-associated liver injury might also occur secondary to the potentially hepatoxic effects of different drugs, such as antivirals, acetaminophen, corticosteroids, immune modulators, and antibiotics, among others. The presence of liver inflammation and microvesicular steatosis characterized by small intracytoplasmic fat vacuoles (liposomes) which accumulate within hepatocytes in the liver biopsies of individuals with COVID-19 might also be drug-associated[27].
The interaction between drugs and cytochrome P-450 can demonstrate a few hepatic toxicities secondary to such medicines as acetaminophen, lopinavir/ritonavir, azithromycin, and hydroxychloroquine[26]. In the systematic review by Kulkarni et al[40], which included 20874 patients (107 articles), about a quarter of COVID-19 patients suffered drug-induced liver injury.
The histopathological analysis for liver biopsy samples from COVID-19 patients recorded nominal lobular and portal activity, simple micro-vesicular steatosis, mitosis, as well as hepatocellular necrosis in the liver tissue, and no viral inclusion bodies. The abnormality of histopathological results may be due to COVID-19-caused liver damage or drug-induced liver injury[41].
Hyper-inflammatory cytokine storm may also cause hepatic injury. The concentrations of inflammatory cytokines, including tumor necrosis factor (TNF), IL-1, and IL-6, were observed to be increased in COVID-19 patients by around 20%, resulting in a cytokine storm. Hepatocytes could be oversensitive to hypoxic hepatic injury during severe COVID-19; the further deterioration of hepatocytes occurs due to immune overreaction resulting in significantly abnormal liver biochemical tests[42]. COVID-19 patients with multiorgan failure in the intensive care unit (ICU) might be associated with severe liver dysfunction[43]. In addition, patients infected with SARS-CoV-2 with raised AST also have increased ferritin, IL-6, C-reactive protein, and lactate dehydrogenase compared to subjects with normal AST[44].
The over-activation of the immune system, which is correlated with COVID-19, might induce liver injury. A significant increase in the serum concentrations of inflammatory cytokines, including interferon-γ, IL-1β, IL-10, IL-6, TNF, and soluble IL-2 receptor, exists in subjects with SARS-CoV-2, particularly in those patients with severe pneumonia[45,46]. The result of that is liver injury mediated by the immune system through the stimulation of intrahepatic CD4+ and CD8+ cells, Kupffer cells, and T cells leading to dysregulated innate immune response[30,47]. This manifestation has also been characterized in infections caused by other viruses such as SARS-CoV and herpes simplex virus, Epstein-Barr virus, cytomegalovirus, adenovirus, and parvovirus. These viruses target the upper respiratory tract[47].
Patients infected with SARS-CoV-2 might have chronic liver diseases (CLD), for example, non-alcoholic fatty liver disease (NAFLD), HBV or HCV infection, and cirrhosis. In COVID-19 patients with a previous history of HBV or HCV infection and liver cirrhosis, there might be a synergistic effect between the drugs used for these diseases with the drugs used for the COVID-19 treatment. As a consequence, acute hepatitis happens[48].
All previous findings contribute to the hypothesis of COVID-19-associated liver damage. Another study has reported from post-mortem liver histopathology that microvesicular steatosis could occur with the overactivation of T cells, assuming that the liver injury is mediated through the immune system[49]. Endothelitites could be generated due to COVID-19, and damage the liver[50]. The involvement of endothelial cells in hepatic ischemia-reperfusion damage leads to the stimulation of oxidative stress via the reaction between the derivatives of nitric oxide and oxygen species[51].
SARS-CoV-2 RNA has been discovered in feces. It appears sensible that the inflammatory mediators and virus are present in the gut lumen, reaching the liver via portal circulation. The viral particles could be removed by Kupffer cells, thus resulting in a rising inflammatory response[26,50]. The cholangiocyte-related enzymes are gamma-glutamyl transferase (GGT) and ALP. However, the abnormal concentration of GGT might contribute to acute inflammatory stress since it is known as a biomarker for raised inflammation and oxidative stress[52].
In the case of chronic hepatitis B or C related to COVID-19, the counts of the white blood cells and monocytes significantly diminished compared to those in patients with COVID-19 alone, while the level of CD8+ T cells greatly increased, and HBV-infected patients with COVID-19 had a greater danger of thrombocytopenia[53]. In addition, the HCV and active infection of HCV have a weak relationship with COVID-19. Mangia and his colleagues have reported that HCV-infected patients have a lower risk of being infected with COVID-19. They suggested that antibodies to HCV could protect against COVID-19[54].
The metabolic syndrome NAFLD, which is the most frequent CLD, carries a highly raised risk for severe COVID-19. It was estimated in a meta-analysis of epidemiological studies that NAFLD was associated with a 5.2-fold increased risk of severe COVID-19[55]. A recent study by Jiuling and his colleagues has reported that a significant association was recorded between NAFLD and severe COVID-19; however, this association disappeared when the demographic (age and gender) and comorbid factors like obesity were adjusted, while the other metabolic perturbations (diabetes mellitus and hypertension) does not have an association with severe COVID-19[56]. CRP, D-dimer, and ferritin levels as well as lymphocyte and neutrophil counts are similar for both NAFLD and non-NAFLD patients. The liver parameters such as serum albumin, ALP, and serum bilirubin levels are comparable across both groups. In contrast, increased concentrations of alanine transaminase (ALT), AST, and GGT have been observed in NAFLD patients compared to non-NAFLD patients. The mortality and hospitalization stays have not increased in COVID-19 patients with NAFLD based on increased liver parameters[57].
A study by Pan et al[58] has illustrated liver injury for COVID-19 patients with NAFLD; it has found that liver injury happened in 50% and 75% of infected persons upon admission and during staying in the hospital, respectively. These findings are due to the increased expression of the ACE-2 receptor as well as chronic inflammation of the liver in NAFLD, which leads to liver injury. In addition, the degree of liver fibrosis in NAFLD may affect the consequence of SARS-CoV-2 infection, and the high or intermediate score of FIB4 has been associated with severe SARS-CoV-2 illness among patients with MAFLD[59].
Liver injury often cause the changes in liver function tests above normal ranges; AST > 40 U/L, ALT > 40 U/L (higher than 3 times the upper limit unit of normal (ULN), ALP > 130 U/L (2 × ULN), bilirubin > 1.1 mg/dL, and GGT > 48 U/L (2 × ULN) were monitored in patients with asymptomatic-to-severe/critical COVID-19. Despite that the accurate impact of SARS-CoV-2 on the liver is unclear, abnormal liver enzymes are present in around 15%-65% of COVID-19 patients. Liver function is normally impaired in patients with COVID-19 due to abnormal liver biochemical markers, which lead to an increase in the danger of progressing to severe disease during staying in the hospital with cholestasis hepatocellular injury[60,61]. A retrospective study by Lei and his colleagues documented the liver function tests regardless of COVID-19 severity; AST was elevated, followed by an elevation in ALT with a variant concentration in bilirubin. The mortality risk was significantly related to the levels of AST[62].
In the Singhai study, among 600 COVID-19 patients, 416 had mild COVID-19, 23 had moderate COVID-19, and 161 had severe COVID-19. The severity of COVID-19 could be classified as asymptomatic, mild, moderate, and severe/critical. Mild COVID-19 patients have no pneumonia and minor symptoms; moderate COVID-19 patients have respiratory tract symptoms, and fever, and show pneumonia without respiratory distress on imaging; the average hospitalization is 6.98 d. Severe COVID-19 patients have an arterial blood partial pressure/O2 concentration of less than 300 mmHg, and more than 50% have lung involvement on radiological imaging, hypoxia (oxygen saturation < 93%), or respiratory distress. Critical COVID-19 involved respiratory failure, shock, and multiorgan failure (5%) or death (2.3%); the average hospitalization is 11.41 d. The levels of AST and ALT are highest in moderate COVID-19, ALP is highest in mild COVID-19, and there are no different values in bilirubin between these groups[7,61,63].
The biomarker to diagnose the injury of cholangiocytes is GGT, but it is not raised in most patients. ALP is still at the normal level. The indices of albumin and total protein are diminished at admission, indicating that COVID-19 may directly damage the liver. At the same time, the indices of total bilirubin, direct bilirubin, indirect bilirubin, ALT, and AST levels are increased during admission, during treatment, as well as during hospitalization. Previous observations recorded that the aggravated liver dysfunction ( increased levels of AST and ALT) during the COVID-19 course, was significantly associated with COVID-19 severity[12,64,65]. CRP level is greatly increased during admission in COVID-19 patients and returned to the normal range before discharge[64].
Liver injury in severe cases was more severe than that in patients with mild and non-severe COVID-19. Severe infection was more likely to cause severe hepatic injury compared to a mild infection. Patients with hypertension or diabetes generally have an increase in liver enzymes, bilirubin, and ALP and a decrease in albumin (2.6–3 g/dL). It could be detected for early severe COVID-19 through the abnormality of the liver test[66-68]. Liver injury with COVID-19 was more frequently found among severe patients compared to non-severe patients and mild COVID-19 (about 45% for severe patients, 15% for mild COVID-19, and 10% for non-severe COVID-19)[69,70]. Pneumonia developed during COVID-19 is associated with a high level of CRP, mildly elevated levels of bilirubin and AST, and a low level of serum albumin, which leads to COVID-19-induced liver dysfunction[64]. Liver abnormalities might occur due to tissue hypoxemia and sepsis. The concentration of CRP is elevated in severe patients[71].
A significant correlation was observed between the elevation of AST, ALT, and bilirubin and the critical illness of COVID-19, and their concentrations are higher in critical COVID-19 compared to severe or mild COVID-19. Serum albumin decreased in the critical illness of COVID-19, and it is lower than that in severe COVID-19[72].
Several therapeutic agents are utilized to treat patients with COVID-19 and associated manifestations. There is no particular medication for COVID-19 at present, and antiviral drugs account for the significant treatment. These medications consist of antivirals (ritonavir, remdesivir, favipiravir, and lopinavir), antimalarials (chloroquine and hydroxychloroquine), some monoclonal antibody products, acetaminophen, steroids, antipyretics, immune-modulators, and corticosteroids. Since the liver metabolizes these drugs, they can lead to hepatotoxicity[73]. Paracetamol and acetaminophen are medicines used to block some complications of COVID-19[74]. The use of acetaminophen used as an antipyretic drug causes sudden hepatic failure at high doses, and the treatment doses utilized to heal SARS-CoV-2 may cause abnormal levels of ALT and AST and lead to mild liver injury[75].
The safety and effectiveness of ritonavir and lopinavir medicines were examined to treat COVID-19. They are accounted as human immunodeficiency virus protease inhibitors to inhibit viral replication via inhibiting the proteolytic cleavage of the polyprotein of virus polymerase[76]. Another study has demonstrated that ritonavir and lopinavir treatment caused increased concentrations of AST, ALT, and total bilirubin in a few infected persons[77].
Remdesivir inhibits viral replication through intracellular transformation to inhibit viral RNA polymerase[78]. The antiviral drug remdesivir antagonizes RNA polymerase. It has been utilized to treat patients with Marburg virus infection, Ebola virus disease, and hepatitis. Remdesivir has reported in vitro efficacy against COVID-19 and is partially metabolized through the cytochrome P450 enzymes[79]. A study by Lee et al[80] reported that remdesivir has safety and efficacy properties in about 80 COVID-19 patients with severe disease; the clinical effectiveness has been reported on hospitalized patients with a mean duration of oxygen therapy of about 10 d, and a time of staying in hospital of 10 d. A study by Van Laar and colleagues has demonstrated that remdesivir therapy causes hepatotoxic effects. In about 100 SARS-CoV-2 patients, 25 individuals had elevated ALT, and 35 had increased AST concentrations[81].
These agents have anti-inflammatory and antimalarial properties, and with the appearance of the SARS-CoV-2 pandemic, they have a potential therapeutic indicator for patients with COVID-19[82]. The appropriate mechanism of impact of hydroxychloroquine on the resistance of COVID-19 is by inhibiting the attachment of the spike protein of COVID-19 to the receptor of ACE-2, thus blocking the viral elements and the fusion of the cell membranes of the target cells. This might reduce the key processes which result from COVID-19, including proteolytic activity, lysosome activity, and autophagy in the host cells; hydroxychloroquine has an immunomodulatory effect by diminishing cytokine production[83]. A systematic review and randomized, parallel and clinical trial by Hernandez et al[84] consisting of about 80 patients with COVID-19 demonstrated no relationship between abnormality of hepatic function test and hydroxychloroquine therapy.
The therapeutic agent tocilizumab (IL-6 receptor monoclonal antibody) prevents the signal transduction of the cytokines pathway and blocks the pro-inflammatory actions[85]. Tocilizumab has many adverse effects, such as dizziness, sore throats, fungal infection, hypertension, and headache[86]. In the case of utilizing tocilizumab to treat severe COVID-19 patients, IL-6 is significantly elevated due to cytokine storm which worsens the COVID-19 course[87]. The inflammatory markers, for example, D-dimer and CRP, have also diminished when utilizing tocilizumab in around 50 patients with severe COVID-19, although the reduction in these markers has not greatly influenced the outcome[88]. Tocilizumab administration damaged the liver after 2 wk via the development of liver injury induced by the drug in around 90 patients with COVID-19. However, close monitoring should be done during and after giving tocilizumab to COVID-19 patients[89].
The antimicrobial therapeutic medicine azithromycin is utilized to heal bacterial infections, which has the ability to reduce severe lower respiratory tract infections[90]. Azithromycin binds to the ACE-2 receptor-COVD-19 spike protein complex, leading to a reduction in the downstream signaling. As a result, the effect of the virus is inhibited[91]. In COVID-19, azithromycin is used to prevent the first step of virus replication. The outcomes of clinical trials suggest that it should be given alone or with hydroxychloroquine[92]. The transaminase concentrations have significantly increased more than five times when using azithromycin in combination with ritonavir, hydroxychloroquine, and lopinavir to treat COVID-19 in patients with no prior history of hepatic disease[93].
SARS-CoV-2-infected patients could recover without specific medicines. So far, the impact of COVID-19 vaccination on CLD is still unknown. The early vaccination for COVID-19 is valuable for the proliferative responses of T lymphocytes and antibody production, resulting in diminished danger of COVID-19 severity. COVID-19 vaccination is necessary for those with liver diseases such as liver cirrhosis and those with liver transplant (LT). The acquisition of immunity following COVID-19 vaccination in patients with liver transplant is low in comparison with normal individuals. The neutralizing antibodies can be observed in approximately 48% of LT patients[94]. A study by Ruether et al[95] illustrated that the rates of T-cell response and serum conversion in the second COVID-19 vaccination were 36.6% and 63%, respectively. The percentage of serum conversion for patients with hepatic cirrhosis could reach 100% after the second vaccination.
A study demonstrated that SARS-CoV-19 infection was diagnosed after a single dose of vaccine in 62% and after a couple of doses in 38%. It is reported that COVID-19 vaccination reduced the infection by SARS-CoV-2, and as a result, the consequences of infection with CLD were improved (e.g., respiratory symptoms, hospitalization, invasive ventilation, ICU admission, and death)[96]. In patients with prior LT as well as cirrhosis, it is recommended to fully vaccinate to reduce the cases of severe infection. The immunity against COVID-19 begins after 2 wk of the first dose of the vaccine and elevates extra after the second dose[97].
To increase immunity and decrease COVID-19 cases, it is interesting to provide a booster dose of COVID-19 vaccination (3rd and 4th doses). The antibody titers were elevated after the third dose of COVID-19 vaccination in LT recipients who had negative antibody titers[98,99].
It is well-known that COVID-19 vaccines have local (like local injection site pain) or systemic adverse effects (like smell and taste abnormalities). Local side effects are more common in occurrence than systemic ones. Autoimmune hepatitis and HCV reactivation are examples of liver involvement following COVID-19 vaccination[100-102]. These conditions were reported on rare occasions as case reports. Even though they are identified as rare complications, one should consider them in determining the future safety of these vaccines.
Despite COVID-19 principally causing respiratory manifestations, it also could lead to extrapulmonary diseases as comorbidities, such as hyperglycemia and ketosis, thrombotic complications, cerebro
COVID-19 patients with CLD, particularly those with cirrhosis, have various forms of immune dysfunction which result in an increased risk of infection and abnormal inflammatory response during infection. Cirrhosis-associated immune dysfunction consists of decreased macrophage activation, combinations of the complement system, upregulation of Toll-like receptors, intestinal dysbiosis, and impaired neutrophil and lymphocyte function[104]. Individuals with pre-exciting CLD and cirrhosis are more likely to be infected by SARS-CoV-2. The etiology of hepatic disease could impact clinical outcomes in SARS-CoV-2 infection. In general, advanced age, diabetes, and obesity are risk factors for SARS-CoV-2 mortality and morbidity[105]. Nevertheless, such patients are not diagnosed with NAFLD because liver steatosis was not reported or alcohol use was not determined. Many contradictions throughout the literature have been illustrated in the case of the impact of NAFLD on the SARS-CoV-2 course. The contradiction might be correlated to difficulty in distinguishing the influence of NAFLD from different metabolic comorbidities; this could be due to the effect of virus-induced steatosis or different diagnostic criteria. A retrospective study of 202 patients with COVID-19 recognized NAFLD as a dangerous aspect for longer viral shedding times, abnormal concentrations of liver enzymes, and progressive COVID-19[49]. However, a study of 70 subjects with SARS-CoV-2 infection and autoimmune hepatitis revealed that there is an equivalent result to subjects with other causes of CLD and propensity score-matched controls despite the use of baseline immunosuppression in 86% of patients[106]. The major reason for death is CLD liver-correlated mortality preceded by SARS-CoV-2-induced pulmonary disease[107].
Of note, if individuals are infected with COVID-19 and have preexisting CLD, the increase in mortality and morbidity has occurred with the rising severity of cirrhosis. An increase in mortality was found for individuals who required intensive care, and only 10% of patients who underwent mechanical ventilation survived. However, a significant relationship has been illustrated between SARS-CoV-2-related mortality and preexisting severe liver cirrhosis, which results in a rise in the mortality percentage[107]. SARS-CoV-2, similar to influenza, could lead to acute-on-chronic liver failure (ACLF); ACLF could be caused by viral illness or bacterial infection, and ACLF is noticed through the increasing severity of the disease and liver decompensation[108].
Gut microbiota composition has the function of regulating the severity of COVID-19 by modulating the immune responses of the host; alterations to the gut microbiota composition are caused by cirrhosis and intestinal permeability. The changes in the gut-liver axis may participate in the course of severe COVID-19 noticed in the patient group[109].
It is worth mentioning that the main reason for deaths in individuals with COVID-19 and cirrhosis is respiratory failure, despite that the accurate path of this observation is still unclear. It is reasonable that the hallmark of severe SARS-CoV-2 infection, pulmonary thromboembolic disease, has a participatory role in the hypercoagulable case related to cirrhosis. Thromboprophylaxis is recommended during the period that COVID-19 patients stay in the hospital[110]. Given together, the relationship and coexistence of coagulopathy with both COVID-19 and cirrhosis are leading to a cumulative danger of thrombotic complications[111]. Moreover, research has reported with 40 patients that the use of thromboprophylaxis in individuals with COVID-19 and cirrhosis yielded no risk of hemorrhagic complications[112].
Abnormal liver function tests are common at the presentation and increased during the COVID-19 course. There are six proposed pathophysiological mechanisms of liver involvement: Hypoxia, direct viral effect, drug-induced liver injury, cytokine storm, elevated hepatic chemistry tests, and preexisting CLD. Various liver involvements occur, which include, but are not exclusive to, elevated AST and ALT, hyperbilirubinemia, prolonged prothrombin time, elevated ALP, GGT elevation, and low serum albumin level. Hepatic involvements determine the severity of COVID-19. Abnormal liver function tests are more in non-survivors than in survivors. Great care is highly recommended to avoid liver injury in COVID-19 patients by modulation of therapeutic agents and regular measurement of the liver function tests, particularly in patients with a history of CLD. COVID-19 vaccines have adverse effects on the liver, for example, resulting in autoimmune hepatitis. However, complete COVID-19 vaccination for patients with a history of CLD or those who were subjected to LT is highly recommended to avoid the occurrence of the disease and further hepatic destruction.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Iraq
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Duan Z, China; Ren S, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147-R1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 902] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 2. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 3. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 4. | Shousha HI, Ramadan A, Lithy R, El-Kassas M. Patterns of liver profile disturbance in patients with COVID-19. World J Clin Cases. 2022;10:2063-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 5. | Wang Y, Gao D, Li X, Xu P, Zhou Q, Yin J, Xu J. Early changes in laboratory tests predict liver function damage in patients with moderate coronavirus disease 2019: a retrospective multicenter study. BMC Gastroenterol. 2022;22:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Amin M. COVID-19 and the liver: overview. Eur J Gastroenterol Hepatol. 2021;33:309-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Przekop D, Gruszewska E, Chrostek L. Liver function in COVID-19 infection. World J Hepatol. 2021;13:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 8. | Teschke R, Méndez-Sánchez N, Eickhoff A. Liver Injury in COVID-19 Patients with Drugs as Causatives: A Systematic Review of 996 DILI Cases Published 2020/2021 Based on RUCAM as Causality Assessment Method. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 522] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 10. | Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1854] [Cited by in RCA: 2018] [Article Influence: 403.6] [Reference Citation Analysis (0)] |
| 11. | Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020;41:1100-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 713] [Cited by in RCA: 752] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 12. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 13. | Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging (Albany NY). 2020;12:12410-12421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Noor FM, Islam MM. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Community Health. 2020;45:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 15. | Parczewski M, Ciechanowicz A. Molecular epidemiology of SARS-CoV-2: a review of current data on genetic variability of the virus. Pol Arch Intern Med. 2020;131:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33:373-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 17. | Bradley SA, Banach M, Alvarado N, Smokovski I, Bhaskar SMM. Prevalence and impact of diabetes in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Diabetes. 2022;14:144-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Tlacuilo-Parra A, Calderón-Vega E, López-Jiménez JJ, Soto-Sumuano L, Olivera-Guerrero F, Guevara-Gutiérrez E. COVID-19 in the pediatric population of the state of Jalisco: spatiotemporal analysis of 1,515 cases. Bol Med Hosp Infant Mex. 2022;79:91-99. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1725] [Cited by in RCA: 1877] [Article Influence: 208.6] [Reference Citation Analysis (0)] |
| 20. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281-292.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6160] [Article Influence: 1232.0] [Reference Citation Analysis (0)] |
| 21. | Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, Muslin C, Jaramillo AMG, Barba-Ostria C, Cevallos-Robalino D, Sanches-SanMiguel H, Unigarro L, Zalakeviciute R, Gadian N, López-Cortés A. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;98:115094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 22. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14274] [Article Influence: 2854.8] [Reference Citation Analysis (0)] |
| 23. | Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126:1456-1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1325] [Cited by in RCA: 1377] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 24. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 25. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1245] [Cited by in RCA: 1313] [Article Influence: 262.6] [Reference Citation Analysis (0)] |
| 26. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 27. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5786] [Article Influence: 1157.2] [Reference Citation Analysis (2)] |
| 28. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1624] [Article Influence: 324.8] [Reference Citation Analysis (0)] |
| 29. | Ali N, Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 31. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (2)] |
| 32. | Lozano-Sepulveda SA, Galan-Huerta K, Martínez-Acuña N, Arellanos-Soto D, Rivas-Estilla AM. SARS-CoV-2 another kind of liver aggressor, how does it do that? Ann Hepatol. 2020;19:592-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. biorxiv. 2020;. [DOI] [Full Text] |
| 34. | Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 35. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 37. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 38. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 39. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1884] [Article Influence: 376.8] [Reference Citation Analysis (0)] |
| 40. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 41. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 42. | Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 44. | Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 45. | Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428-e436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 46. | Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383:2255-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1324] [Cited by in RCA: 2104] [Article Influence: 420.8] [Reference Citation Analysis (0)] |
| 47. | Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (2)] |
| 49. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 50. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 51. | Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 52. | Corti A, Belcastro E, Dominici S, Maellaro E, Pompella A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an 'antioxidant' enzyme. Free Radic Biol Med. 2020;160:807-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | Liu R, Zhao L, Cheng X, Han H, Li C, Li D, Liu A, Gao G, Zhou F, Liu F, Jiang Y, Zhu C, Xia Y. Clinical characteristics of COVID-19 patients with hepatitis B virus infection - a retrospective study. Liver Int. 2021;41:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Mangia A, Cenderello G, Verucchi G, Ciancio A, Fontana A, Piazzolla V, Minerva N, Squillante MM, Copetti M. Is positivity for hepatitis C virus antibody predictive of lower risk of death in COVID-19 patients with cirrhosis? World J Clin Cases. 2020;8:5831-5834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 55. | Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, Erőss B, Szakács Z, Hegyi P, Pár G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front Med (Lausanne). 2021;8:626425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 56. | Li J, Tian A, Zhu H, Chen L, Wen J, Liu W, Chen P. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin Gastroenterol Hepatol. 2022;20:1553-1560.e78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 57. | Nath P, Kumar R, Mallick B, Das S, Anand A, Panigrahi SC, Duseja A, Acharya SK, Chawla YK, Praharaj DL. Effect of Nonalcoholic Fatty Liver Disease (NAFLD) on COVID-19: A Single-Center Study of 3983 Patients With Review of Literature. Cureus. 2022;14:e26683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 58. | Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: A meta-analysis. Dig Liver Dis. 2021;53:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 59. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 60. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 61. | Kamal AM, Dumitrescu F, Mită A, Săbiescu DM, Alexandru DO, Gheorghe CE, Filip MM, Ionescu-Ciocâlteu A, Maria DT, Kamal D, Kamal CK. Liver Function Tests and FIB-4 Score as Predictors of Severity in COVID-19 Patients from the South-West of Romania. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 63. | Singhai A, Pavan GS, Panda S. Evaluation of liver function in symptomatic COVID-19 patients. J Family Med Prim Care. 2021;10:3252-3256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Nie XB, Shi BS, Zhang L, Niu WL, Xue T, Li LQ, Wei XY, Wang YD, Chen WD, Hou RF. Epidemiological features and dynamic changes in blood biochemical indices for COVID-19 patients in Hebi. World J Clin Cases. 2022;10:2404-2419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Ibrahim N, Hosri J, Bteich Y, Dib A, Abou Rached A. COVID-19 and Liver Dysfunction. Cureus. 2022;14:e21302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 66. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 67. | Yang Z, Xu M, Yi JQ, Jia WD. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60-63. [PubMed] |
| 68. | Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 69. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 70. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 71. | Chaibi S, Boussier J, Hajj WE, Abitbol Y, Taieb S, Horaist C, Jouannaud V, Wang P, Piquet J, Maurer C, Lahmek P, Nahon S. Liver function test abnormalities are associated with a poorer prognosis in Covid-19 patients: Results of a French cohort. Clin Res Hepatol Gastroenterol. 2021;45:101556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Kovalic AJ, Huang G, Thuluvath PJ, Satapathy SK. Elevated Liver Biochemistries in Hospitalized Chinese Patients With Severe COVID-19: Systematic Review and Meta-analysis. Hepatology. 2021;73:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 74. | Dar-Odeh N, Elsayed S, Babkair H, Abu-Hammad S, Althagafi N, Bahabri R, Eldeen YS, Aljohani W, Abu-Hammad O. What the dental practitioner needs to know about pharmaco-therapeutic modalities of COVID-19 treatment: A review. J Dent Sci. 2021;16:806-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Hoofnagle JH, Serrano J, Knoben JE, Navarro VJ. LiverTox: a website on drug-induced liver injury. Hepatology. 2013;57:873-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 76. | Osborne V, Davies M, Lane S, Evans A, Denyer J, Dhanda S, Roy D, Shakir S. Lopinavir-Ritonavir in the Treatment of COVID-19: A Dynamic Systematic Benefit-Risk Assessment. Drug Saf. 2020;43:809-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 77. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3627] [Article Influence: 725.4] [Reference Citation Analysis (0)] |
| 78. | Kindler E, Thiel V, Weber F. Interaction of SARS and MERS Coronaviruses with the Antiviral Interferon Response. Adv Virus Res. 2016;96:219-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 79. | Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295:6785-6797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 705] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 80. | Lee S, Santarelli A, Caine K, Schritter S, Dietrich T, Ashurst J. Remdesivir for the Treatment of Severe COVID-19: A Community Hospital's Experience. J Am Osteopath Assoc. 2020;120:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | van Laar SA, de Boer MGJ, Gombert-Handoko KB, Guchelaar HJ, Zwaveling J; LUMC-Covid-19 research group. Liver and kidney function in patients with Covid-19 treated with remdesivir. Br J Clin Pharmacol. 2021;87:4450-4454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Infante M, Ricordi C, Alejandro R, Caprio M, Fabbri A. Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert Rev Anti Infect Ther. 2021;19:5-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1336] [Cited by in RCA: 1341] [Article Influence: 268.2] [Reference Citation Analysis (1)] |
| 84. | Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19: A Living Systematic Review. Ann Intern Med. 2020;173:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 85. | Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008;151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 86. | Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13:1972-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 87. | Yang Y, Zhu XF, Huang J, Chen C, Zheng Y, He W, Zhao LH, Gao Q, Huang XX, Fu LJ, Zhang Y, Chang YQ, Zhang HJ, Lu ZJ. Nomogram for prediction of fatal outcome in patients with severe COVID-19: a multicenter study. Mil Med Res. 2021;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Amin S, Rahim F, Bahadur S, Noor M, Mahmood A, Gul H. The Effect of Tocilizumab on Inflammatory Markers in Survivors and Non-survivors of Severe COVID-19. J Coll Physicians Surg Pak. 2021;31:S7-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Gatti M, Fusaroli M, Caraceni P, Poluzzi E, De Ponti F, Raschi E. Serious adverse events with tocilizumab: Pharmacovigilance as an aid to prioritize monitoring in COVID-19. Br J Clin Pharmacol. 2021;87:1533-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, Jackson DJ, Baxi SN, Benson M, Burnham CD, Cabana M, Castro M, Chmiel JF, Covar R, Daines M, Gaffin JM, Gentile DA, Holguin F, Israel E, Kelly HW, Lazarus SC, Lemanske RF Jr, Ly N, Meade K, Morgan W, Moy J, Olin T, Peters SP, Phipatanakul W, Pongracic JA, Raissy HH, Ross K, Sheehan WJ, Sorkness C, Szefler SJ, Teague WG, Thyne S, Martinez FD. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314:2034-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 91. | Sandeep S, McGregor K. Energetics based modeling of hydroxychloroquine and azithromycin binding to the SARS-CoV-2 spike (S) protein-ACE2 complex. 2020. [DOI] [Full Text] |
| 92. | Wahab S, Ahmad MF, Hussain A, Usmani S, Shoaib A, Ahmad W. Effectiveness of Azithromycin as Add-on Therapy in COVID-19 Management. Mini Rev Med Chem. 2021;21:2860-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O, Katchman H. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 273] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 95. | Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, Wehmeyer M, Jahnke-Triankowski J, Mayer L, Hoffmann A, Fischer L, Addo MM, Lütgehetmann M, Lohse AW, Schulze Zur Wiesch J, Sterneck M. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol. 2022;20:162-172.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 96. | Moon AM, Webb GJ, García-Juárez I, Kulkarni AV, Adali G, Wong DK, Lusina B, Dalekos GN, Masson S, Shore BM, Barnes E, Barritt AS 4th, Marjot T. SARS-CoV-2 Infections Among Patients With Liver Disease and Liver Transplantation Who Received COVID-19 Vaccination. Hepatol Commun. 2022;6:889-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 97. | Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383:2439-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1902] [Article Influence: 380.4] [Reference Citation Analysis (0)] |
| 98. | Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, Segev DL. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Intern Med. 2021;174:1330-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 99. | Harberts A, Schaub GM, Ruether DF, Duengelhoef PM, Brehm TT, Karsten H, Fathi A, Jahnke-Triankowski J, Fischer L, Addo MM, Haag F, Luetgehetmann M, Lohse AW, Schulze Zur Wiesch J, Sterneck M. Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 mRNA Vaccination in Liver Transplant Recipients. Clin Gastroenterol Hepatol. 2022;20:2558-2566.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 100. | Kang SH, Kim MY, Cho MY, Baik SK. Autoimmune Hepatitis Following Vaccination for SARS-CoV-2 in Korea: Coincidence or Autoimmunity? J Korean Med Sci. 2022;37:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 101. | Lensen R, Netea MG, Rosendaal FR. Hepatitis C Virus Reactivation Following COVID-19 Vaccination - A Case Report. Int Med Case Rep J. 2021;14:573-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 102. | Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J Autoimmun. 2021;125:102745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 103. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2049] [Article Influence: 409.8] [Reference Citation Analysis (2)] |
| 104. | Noor MT, Manoria P. Immune Dysfunction in Cirrhosis. J Clin Transl Hepatol. 2017;5:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 105. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4209] [Article Influence: 841.8] [Reference Citation Analysis (0)] |
| 106. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 107. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 108. | Schütte A, Ciesek S, Wedemeyer H, Lange CM. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 109. | Bajaj JS. Altered Microbiota in Cirrhosis and Its Relationship to the Development of Infection. Clin Liver Dis (Hoboken). 2019;14:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Manolis AS, Manolis TA, Manolis AA, Papatheou D, Melita H. COVID-19 Infection: Viral Macro- and Micro-Vascular Coagulopathy and Thromboembolism/Prophylactic and Therapeutic Management. J Cardiovasc Pharmacol Ther. 2021;26:12-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 111. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 112. | Lemos ACB, do Espírito Santo DA, Salvetti MC, Gilio RN, Agra LB, Pazin-Filho A, Miranda CH. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |