Published online Jun 28, 2022. doi: 10.13105/wjma.v10.i3.99
Peer-review started: February 16, 2022
First decision: April 13, 2022
Revised: April 27, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: June 28, 2022
Processing time: 139 Days and 5.9 Hours
Viral hepatitis continues to be a major health concern leading to hepatic decompensation ranging from acute hepatitis to cirrhosis and hepatocellular carcinoma. The hepatic and extrahepatic manifestations are not only debilitating but also associated with a significant economic burden. Over the last two decades, the field of virology has made significant breakthroughs leading to a better understanding of the pathophysiology of viral hepatitis, which in turn has led to new therapeutic options. The advent of direct-acting antiviral agents changed the landscape of hepatitis C virus (HCV) therapy, and new drugs are in the pipeline for chronic hepatitis B virus (HBV) treatment. There has also been a significant emphasis on screening and surveillance programs, widespread availability of vaccines, and linkage of care. Despite these efforts, significant gaps persist in care, and there is a pressing need for increased collaboration and teamwork across the globe to achieve a reduction of disease burden and elimination of HBV and HCV.
Core tip: Viral hepatitis is an important etiology for acute and chronic hepatic dysfunction with significant mortality and morbidity. This review aims to summarize the recent advances in the field and to focus on new novel therapeutic approaches as well as highlighting the barriers to achieving a complete cure. We also focus on preventive measures and strategies to optimize care.
- Citation: Ahmed Z, Shetty A, Victor DW, Kodali S. Viral hepatitis: A narrative review of hepatitis A–E. World J Meta-Anal 2022; 10(3): 99-121
- URL: https://www.wjgnet.com/2308-3840/full/v10/i3/99.htm
- DOI: https://dx.doi.org/10.13105/wjma.v10.i3.99
Epidemiology: Hepatitis A virus (HAV) belongs to the Picornaviridae family and is a single-stranded RNA virus that affects around 1.5 million people annually[1]. HAV is a resilient virus and is able to survive in most environments for months despite freezing temperatures, acidic environments, and exposure to chemical agents, thus making it an ideal agent for infection through exposure to contaminated water and food supplies[2]. Since the advent of the HAV vaccine in 1996, there has been a significant decline in HAV incidence rate worldwide[3]. Incidence and prevalence depend on socio
Prevalence of viral hepatitis in pediatric population: The prevalence in children of HAV varies based on region from where the data are reported, with higher exposure rates in Africa and South East Asia compared to Europe and the USA[6]. Global estimates for HBV prevalence are significantly lower among children, estimated at 1.3% in children under 5 years. There are limited data on hepatitis D prevalence in children[7]. Estimates show that HCV infection in the pediatric population to be approximately 5 million children and adolescents globally. Studies show that the prevalence rates are rising, ranging from 0.05%–0.36% in the USA and Europe to 1.8%–5.8% in developing countries, including Mexico[8-10]. A systematic review of hepatitis E infection in pediatric population projected a worldwide, seroprevalence of 10% with rising prevalence with age[11].
Disease phases: The incubation period of HAV is usually 14–28 d, and patients are contagious for 2 wk prior to, and up to 1–2 wk after symptom onset[6]. Most patients recover spontaneously without chronic consequences[12]. Clinical presentation is variable where children can be completely asymptomatic, while adults can present with jaundice, changes in stool and urine color. Relapsing hepatitis A is characterized by the reappearance of clinical features and laboratory abnormalities consistent with acute hepatitis A after initial resolution of symptoms. Relapse can occur during 6 mo after initial illness. The duration of clinical relapse is generally < 3 wk, however biochemical relapse can last as long as 12 mo. A minority of patients can progress and develop acute liver failure and may need a liver transplant[13,14]. Hepatitis A infection resolves completely in the majority (> 99%) of the cases[15]. HAV infection, unlike some other viruses, does not cause chronic liver disease[16].
Hepatitis A vaccine and future directions: The current recommendations by The Advisory Committee on Immunization Practices are two shots of HAV vaccine, 6 mo apart[17]. There has been a decline in HAV infection from 11.7 to 0.4 cases per 100 000 population, a reduction by 96.6% because of aggressive screening and vaccination protocols[18]. Despite intense public health measures, sporadic hepatitis A cases continue to occur, highlighting the need for ongoing efforts for screening, surveillance, immunization, and education programs.
Epidemiology: Hepatitis B has emerged as a global health problem with estimated 350 million cases worldwide of chronic hepatitis B infection[19]. WHO Western Pacific Region and the WHO African Region are estimated to have the highest burden of chronic hepatitis B infection. Countries with high prevalence include Ghana, Gabon, Somalia, China, Cambodia and Mongolia[20]. In the USA, 2.2 million have chronic hepatitis B (CHB) with a higher prevalence (3.45%) among first-generation immigrants[21]. Patients with CHB carry a 15%–40% lifetime risk of developing serious sequelae of infection with an increased risk of death from complications such as cirrhosis and[22] hepatocellular carcinoma (HCC)[23].
Disease transmission and phases: The route of hepatitis B virus spread is via contact of blood or bodily fluids of an infected person. The route of HBV transmission varies depending on the prevalence and geographic area. Vertical transmission at birth and close household contact among children are among the more common modes in Asia and Sub-Saharan Africa where HBV is endemic[24]. In areas with low prevalence, especially developed nations, transmission of HBV among adults usually occurs via sexual transmission, percutaneous inoculation through contaminated needles, blood transfusions, or healthcare-associated risk factors such as hemodialysis[24-29]. In the USA and Europe, prevalence rates are higher in areas with a larger ratio of immigrant population, who likely contracted HBV in their country of origin[30,31].
The natural history of HBV depends on the age of the patient at which infection is acquired. For example, in adults, it usually presents as an acute, self-resolving infection where patients who are immunocompetent develop hepatitis B surface antibody to hepatitis B surface antigen (HBsAg), while only 1%–5% progress to developing chronic infection[32]. In contrast, the majority of patients infected by vertical transmission or horizontal infection during early childhood are likely to develop CHB, with the risk of developing CHB rising to 90% if the infection was acquired at birth and 16%–30% if infected during childhood[33,34].
Chronic hepatitis B can be divided into five phases based on the patient’s viral load, elevation in liver enzymes, and hepatitis B serologies[35]. The early high replicative phase or immune tolerant phase is characterized by positive hepatitis B e antigen (HBeAg), high levels of HBV DNA and normal serum alanine transaminase (ALT). The next stage’s hallmark is immune activation, where HBeAg remains positive along with high levels of HBV DNA and elevated serum ALT with associated hepatic necroinflammation. Based on the immune activation, the disease may progress to loss of HBeAg and development of hepatitis B e antibody (anti-HBe). This stage is characterized by moderate to high levels of DNA with risk of progression to hepatic fibrosis and cirrhosis. In the nonreplicative phase (previously known as inactive carrier phase), in which HBV DNA is usually low or undetectable, HBeAg is absent and patients have normal serum ALT. Lastly, the HBsAg loss/occult phase is defined by loss of HBsAg but detectable HBV DNA in the liver and measurable HBV DNA in serum[36].
Extrahepatic manifestations: Both acute and chronic hepatitis B have extrahepatic manifestations. Polyarteritis nodosa is vasculitis of small and medium-sized vessels and manifests as a serious systemic complication of hepatitis B[37]. HBV-associated glomerulonephritis is commonly seen in children and is self-limited. In adults however HBV glomerulonephritis can slowly progress to renal failure[38]. Approximately one-third of the patients with hepatitis B can have the serum-sickness-like arthritis-dermatitis prodrome[39]. Many cutaneous disorders typically related to immune complex deposition are associated with hepatitis B. These include bullous pemphigoid, lichen planus and Gianotti–Crosti syndrome (papular acrodermatitis of childhood). Neurological manifestations include Guillain Barré syndrome, anxiety/depression and psychosis[40].
Definition of cure: Spontaneous seroconversion is the spontaneous loss of HBeAg and development of anti-HBe. This state is associated with low HBV-DNA levels and clinical remission of liver disease in many patients[41,42]. There is improvement in liver fibrosis when patients have HBeAg seroconversion[43]. Overall 0.5% and 0.8% of chronically infected patients will clear HBsAg per year[44]. This clearance of HbsAg is referred to as the recovery phase of hepatitis B.
Resolved CHB is characterized by sustained loss of HBsAg in a patient who was previously HBsAg positive, along with undetectable HBV-DNA levels and no clinical or histological evidence of active viral infection[45].
Functional cure is defined as loss of HbsAg with gain of anti-HbS. True cure is defined as elimination of HBsAg and closed covalent circular DNA (cccDNA)[45].
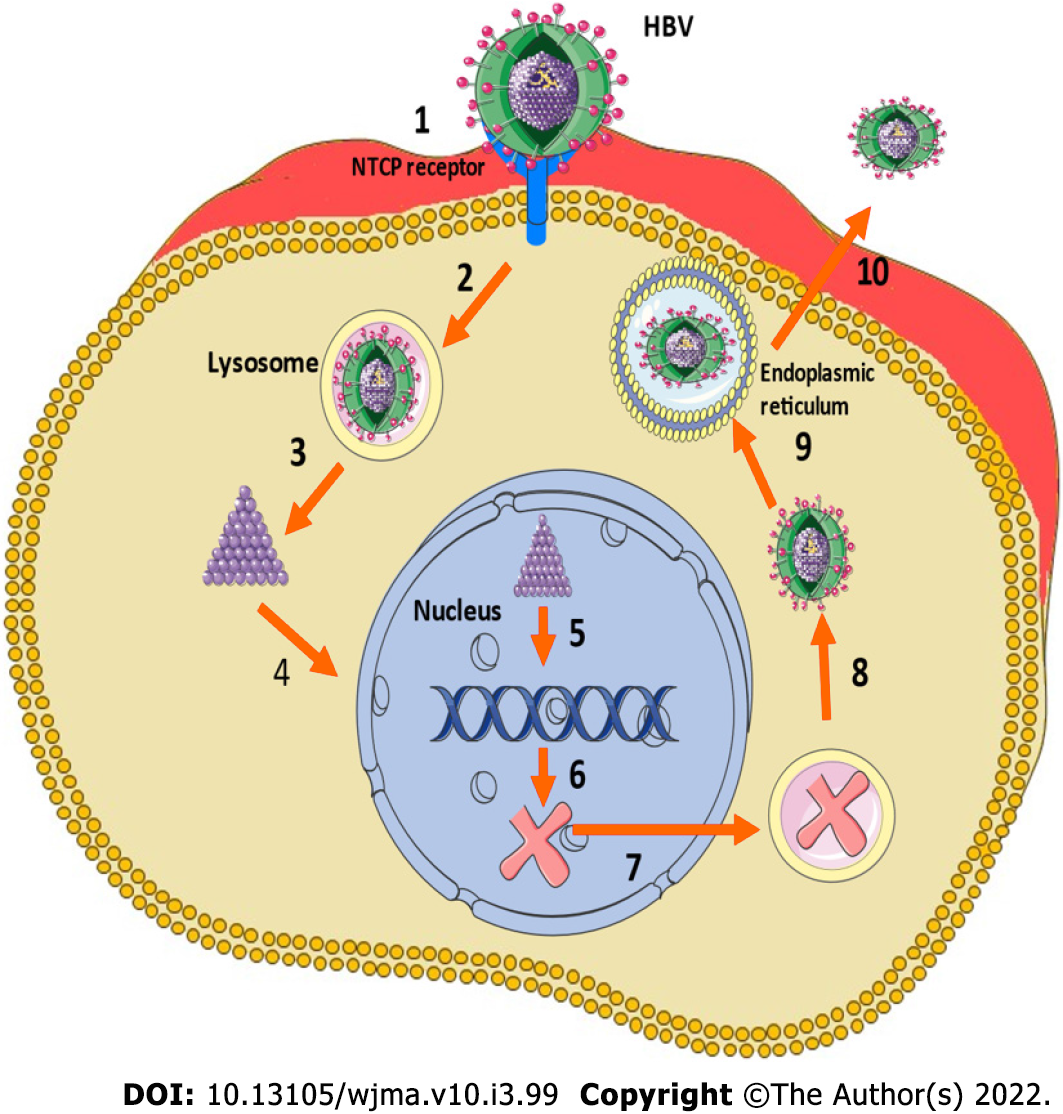
HBV is a DNA virus with a complex structure and categorized into 10 different genotypes (A–J) based on global distribution, and the severity of the disease, risk of HCC, and response to certain treatments[46]. HBV enters the hepatocytes as a consequence of an interaction between the surface antigen and the sodium taurocholate cotransporting polypeptide[47]. After entry into the hepatocyte, the cccDNA develops when the relaxed circular DNA integrates with host cell nucleus, and at the time of HBV replication, cccDNA can generate pregenomic RNA to function as the template for the fully double-stranded DNA[48]. Figure 1 shows the lifecycle of HBV virus.
A few copies of cccDNA can initiate a full-blown infection after active replication, especially when the host is immunosuppressed[49]. Persistent cccDNA has been detected in hepatocytes of patients with resolved HBV infections, and hence the ultimate goal of HBV eradication should aim to clear any remnant cccDNA[42-46]. Another important reason to aim for clearance of cccDNA is the risk of progression to cirrhosis and HCC in patients with low to no HBV DNA in serum, highlighting the important of the role of ccc DNA[50,51].
HBV is unique when compared to other hepatotropic viruses as there is a lack of innate response during HBV infection[52,53]. Chronic HBV affects the immune system by interfering with the function of T cells and in the synthesis of neutralizing antibodies, which are essential in mounting an appropriate immune response to the virus[54,55]. HBV exposure in utero induces a state of trained immunity against HBV[56], and HBV exposed neonates have variable levels of IL-10 and proinflammatory cytokines, and new pharmacotherapeutics exploring this pathway needs further research[56,57]. The goals of therapy have been to control viral replication so that inflammation, development of fibrosis and cirrhosis, and risk of HCC can be reduced, hence lowering the risk of decompensated liver disease and its sequelae and need for a liver transplant.
HBV vaccine, although available since 1982, was not widely available because of its high cost. The Global Alliance for Vaccines and Immunization was able to increase vaccine coverage in the early 2000s[58]. There still are major discrepancies in the availability and utilization of the vaccine, especially with regard to universal birth dose administration[59]. Different regions of the world have variable vaccine administration rates. In 2016, the rate of universal HBV single dose vaccine administration was 93% in the Western Pacific followed by 73% in Southeast Asia, 49% in America and Europe and only 19% in the African Region[60]. WHO recommends HBV vaccine at birth, followed by two or three doses at least 4 wk apart. Hepatitis B vaccine within 12 h is recommended for newborns born to mothers whose HBsAg status is unknown[61]. Adults who were not vaccinated as children can also receive HBV vaccine with first dose as soon as possible followed by 2 doses at 1 and 6 mo after the first dose[61]. Currently approved vaccines in the USA include single antigen hepatitis B vaccine and combined hepatitis A and hepatitis B vaccines. In adults aged 19–59 years they can either receive two doses 4 wk apart of single antigen hepatitis B vaccine or a three-dose series for the combination vaccine.
Multiple factors account for variation in vaccination and linkage of care for HBV across the globe. In China, the major limitation to access and care is secondary to the large population, high prevalence and the low coverage of diagnosis and treatment programs[62]. In resource-rich nations like Australia and New Zealand, despite subsidized screening, specialist management, and treatment for HBV, the barriers include lack of awareness about the implications of HBV infection in healthcare workers, and absence of consistent clinical guidelines regarding diagnosis and referral to a specialist[63,64]. In the USA, a recent systematic review highlighted the obstacles to care, which include access to medical care and lack of education and awareness amongst the patients, along with fear of stigma regarding the diagnosis as barriers to testing and care[65]. Implementation of effective vaccination policies worldwide, along with strategies to prevent vertical transmission and widely available testing and treatment, would be necessary to attain a reduction in HBV infections worldwide[66].
Current therapies and limitations: Current treatment options for CHB include interferons (IFNs) and nucleoside analogs but they suppress the viral replication and do not eliminate the virus, and aid in achieving a functional cure[67]. In HBeAg+ patients, the loss of serum HBeAg and appearance of HBeAb and loss of circulating HBV DNA is the major goal[22]. Current therapies lead to HBeAg seroconversion in only 20%–30% of treated patients and a mortality reduction by 50% over a 10-year period[22,68,69]. Table 1 summarizes the current antiviral therapies for adults and children.
| Drug | Adult dosing | Pediatric dosing | Potential side effects | Pregnancy category |
| Peg-IFN-a-2a (adult) IFN-a-2b (children) | 180 mcg wkly | > 1 yr dose: 6 million IU/m2 three times wkly | Flu-like symptoms, fatigue, mood disturbances, cytopenia, autoimmune disorders in adults, anorexia, and weight loss in children | C |
| Entecavir | 0.5 mg daily | > 2 yr dose: weight-based to 10-30 kg; above 30 kg: 0.5 mg daily | Lactic acidosis (decompensated cirrhosis only) | C |
| Tenofovir dipovoxil fumarate | 300 daily | > 12 yr | Fanconi syndrome, osteomalacia, lactic acidosis | B |
| Tenofovir alafenamide | 25 mg daily | - | Lactic acidosis | There are insufficient human data on use during pregnancy to inform a drug-associated risk of birth defects and miscarriage |
| Lamivudine | 100 mg daily | 2 yr dose: 3 mg/kg daily to max 100 mg | Pancreatitis lactic acidosis | C |
| Adefovir | 10 mg daily | 12 yr | Acute renal failure Fanconi syndrome lactic acidosis | C |
| Telbivudine | 600 mg daily | - | Creatine kinase elevation and myopathy peripheral neuropathy lactic acidosis | B |
Goal of new therapies: Eradication of cccDNA is the ultimate goal of ongoing research for novel HBV treatments. Hurdles to measurement of cccDNA include lack of sophisticated assays and challenges to biopsy. There is a need for surrogate markers for loss of cccDNA, HBV DNA, HBeAg[70]. Table 2 summarizes the newer therapies.
| drug class | Drug | Company | Phase |
| Core protein inhibitors | AB-506 | Arbutus Biopharma | 1 |
| ABI-H0731 | Assembly Biosciences | 1,2 | |
| ABI-H2158 | Assembly Biosciences | 1 | |
| EDP-514 | Enanta Pharmaceuticals | 1 | |
| JNJ-6379 | Johnson & Johnson | 1,2 | |
| JNJ-0440 | Johnson & Johnson | 1 | |
| RO7049389 | Roche | 1 | |
| siRNA, antisense RNA | AB-729 | Arbutus Biopharma | 1 |
| DCR-HBVS | Dicerna Pharmaceuticals | 1 | |
| GSK/IONIS-HBV-LRx | Ionis/GlaxoSmithKline | 1,2 | |
| IONIS-HBVRx | Ionis/GlaxoSmithKline | 1,2 | |
| JNJ-3989 (ARO-HBV) | Johnson & Johnson | 1,2 | |
| RO7062931 | Roche | 1 | |
| Vir-2218 (ALN-HBV02) | Vir Biotechnology/Alnylam | 1 | |
| pol/RT inhibitor | Tenofovir exalidex | ContraVir Pharmaceuticals | 1,2 |
| HBsAg secretion inhibitors | REP-2139 | Replicor | 1,2 |
| REP-2165 | Replicor | 1,2 | |
| HBV entry inhibitor | Bulevirtide | Hepatera Ltd | 1,2 |
| TLR-7 agonists | AL-034 | Johnson & Johnson/Alios | 1 |
| RG-7854 | Roche | 1 | |
| RO7020531 | Roche | 1 | |
| TLR-8 agonist | GS-9688 | Gilead Sciences | 1,2 |
| Therapeutic vaccines | AIC-649 | AiCuris | 1 |
| INO-1800 | Inovio Pharmaceuticals | 1 | |
| TG1050 | Transgene | 1 | |
| RIG-I and NOD2 agonist | Inarigivir | Spring Bank | 1,2 |
| Apoptosis inducer | APG-1387 | Ascentage Pharma | 1 |
| FXR agonist | EYP-001 | Enyo Pharma | 1,2 |
Gene editing – future direction: The major hurdle to eradication of HBV is that current antiviral therapies do not eradicate latently integrated or nonreplicating episomal viral genomes. Furthermore, HBV infection disseminates extensively beyond the liver and broad range of cell lines, including neurons, endothelial cells, macrophages, polymorphic nuclear leukocytes, peripheral blood mononuclear cells, and are permissive for HBV replication.
Gene editing provides the ability to alter an organism’s DNA. Targeted endonucleases are highly specific enzymes designed to introduce DNA double-strand breaks into desired target sequences .The major classes of DNA-cleaving enzymes include zinc finger nucleases, Tal-effector nucleases, RNA-guided engineered nucleases such as CRISPR/Cas9 and mega nucleases/homing endonucleases[71].
The features that make HBV amenable are small viral genome (3.2 kb) with four proteins (envelope, nucleocapsid, polymerase, and X protein). HBV also has low to intermediate mutability rate and the polymerase mutation rate rages from 1.4 × 105–3.2 × 105 mutations/site/year. These factors make it a good target for cleavage enzyme. CRISPR/Cas9 inhibits HBV replication and can be used to target HBV[72,73]. Recently, when Cas9 and guide RNAs were delivered using plasmids into mouse liver, cccDNA could be cleaved, disrupted and cleared[74]. Animal studies after gene editing of CRISPR-Cas9 gene showed improved survival with entacavir with reduced HBV DNA and cccDNA levels[75]. Recent findings of removal of full-length 3175-bp integrated HBV DNA fragment using CRISPR-Cas9 demonstrated that CRISP-Cas9 system may emerge as powerful tool capable of promoting a radical or “sterile” HBV cure[76,77].
Globally, hepatitis C virus (HCV) infection prevalence is 1%, and there are about 2.3 million cases in the USA[78]. The highest prevalence is in the Eastern Mediterranean and European Regions, followed by South East Asian and Western Pacific Regions. Countries with high prevalence are Russia, Gabon, Egypt and Syria[78,79]. HCV is an RNA virus, and similar to HBV, it is transmitted via contacting blood or body fluids of infected individuals, with most common routes of transmission being intravenous drug use, blood product transfusion, solid organ transplantation, or unintentional cross-contamination in hospitals and other medical facilities[80]. Intranasal cocaine use and tattoos administered in unclean parlors are other risk factors[81,82]. Perinatal transmission, though very rare, has been reported in 2%–8% of infected mothers[83].
Hepatitis C in pediatric populations: The estimated prevalence of HCV in 2018 in the pediatric population aged 0–18 years was 0.13% corresponding to 3.26 million children[84]. Direct-acting antivirals (DAAs) are the treatment option for HCV infection in children and adolescents aged ≥ 3 years. Presence of extrahepatic manifestations like rash, advanced fibrosis, cryoglobulinemia, and glomerulonephritis is an indication for early antiviral therapy. Table 3 summarizes the treatment options in pediatric populations.
| Regimen | Patient population | Duration (wk) |
| Genotype 1 | ||
| Ledipasvir/sofosbuvir | Prior exposure to DAA and IFN (± ribavirin) , no cirrhosis | 12 |
| Prior exposure to DAA and IFN (± ribavirin) , compensated cirrhosis | 24 | |
| Glecaprevir/pibrentasvi | Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an NS5A inhibitor but no NS3/4A protease inhibitor exposure, no cirrhosis, compensated cirrhosis | 16 |
| Age ≥ 12 yr or weighing ≥ 45 kg with prior exposure to NS3/4A protease inhibitors but no NS5A inhibitor exposure, no cirrhosis, compensated cirrhosis | 12 | |
| Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, with compensated cirrhosis | 12 | |
| Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, without cirrhosis | 8 | |
| Genotype 2 | ||
| Glecaprevir /pibrentasvir | Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, without cirrhosis | 8 |
| Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, compensated cirrhosis | 12 | |
| Genotype 3 | ||
| Glecaprevir/pibrentasvir | Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, no cirrhosis or compensated cirrhosis | 16 |
| Genotype 4 | ||
| Glecaprevir/pibrentasvir | Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, compensated cirrhosis | 12 |
| Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, no cirrhosis | 8 | |
| Ledipasvir/sofosbuvir | Age ≥ 3 yr with prior exposure to an IFN (± ribavirin) plus an HCV protease inhibitor regimen, no cirrhosis or compensated cirrhosis | 12 |
| Genotype 5 | ||
| Ledipasvir/sofosbuvir | Age ≥ 3 yr with prior exposure to an IFN (± ribavirin) plus an HCV protease inhibitor regimen, no cirrhosis or compensated cirrhosis | 12 |
| Glecaprevir/pibrentasvir | Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, no cirrhosis | 8 |
| Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, with compensated cirrhosis | 12 | |
| Genotype 6 | ||
| Glecaprevir/pibrentasvir | Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, no cirrhosis | 8 |
| Age ≥ 12 yr or weight ≥ 45 kg with prior exposure to an IFN-based regimen (± ribavirin) and/or sofosbuvir but no exposure to NS3/4A or NS5A protease inhibitors, with compensated cirrhosis | 12 | |
| Ledipasvir/sofosbuvir | Age ≥ 3 yr with prior exposure to an IFN (± ribavirin) plus an HCV protease inhibitor regimen, no cirrhosis, compensated cirrhosis | 12 |
Extrahepatic manifestations: Chronic HCV, which is untreated can cause chronic inflammation, followed by progressive liver fibrosis leading to the development of cirrhosis and HCC[85,86]. Various extrahepatic manifestations are reported in chronic HCV infection like mixed cryoglobulinemia, vasculitis, glomerulonephritis, and B-cell non-Hodgkin’s lymphoma, along with increased rates of insulin resistance, diabetes, atherosclerosis, and cognitive impairment[87-89]. A meta-analysis of 102 studies looking at prevalence, quality of life, and economic burden of extrahepatic manifestations of HCV showed diabetes (15%) and depression (25%) were the most common extrahepatic manifestations[90].
Barriers to elimination: The goal of WHO has been to develop and work on strategies to reduce new infections while treating patients who are infected with HCV[91].
Since the advent of oral DAAs in 2014, there has been a dramatic change in the landscape of HCV therapy[92,93]. DAA therapies are not only well-tolerated and safe but offer cure rates of > 95%[94,95]. In comparison to IFNs, treatment with DAAs is short term[96]. With the new agents and multiple options, treatment can be tailored based on presence and absence of cirrhosis, decompensated disease, coinfection with human immunodeficiency virus (HIV) and renal function and need for dialysis. Table 4 summarizes the available DAAs, their target population, and genotype coverage. DAAs are promising and have changed the landscape of chronic hepatitis C infection, but there are several barriers to care and cure and below mentioned are a few.
| Regimen | Patient population | Duration (wk) |
| Genotype 1 | ||
| Daclatasvir + sofosbuvir | Decompensated cirrhosis regardless of subtype | 12 |
| HIV/HCV coinfection when antiretroviral regimen cannot be made to accommodate recommended regimens | 12 | |
| Elbasvir/grazoprevir | Treatment naive or Peg/RBV experienced regardless of cirrhosis | 12 |
| Severe renal impairment (CKD stage 4/5) | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Glecaprevir/pibrentasvir | Treatment naive or Peg/RBV experienced without cirrhosis | 8 |
| Treatment naive or Peg/RBV experienced with cirrhosis, and non-NS5A failures (including NS3) regardless of cirrhosis | 12 | |
| Post liver transplant without cirrhosis | 12 | |
| Severe renal impairment (CKD stage 4 or 5) | 8–12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Ledipasvir/sofosbuvir | Treatment naive regardless of cirrhosis | 12 |
| Treatment naive, no cirrhosis, non-black, HIV negative, and HCV RNA <106 IU/mL | 8 | |
| Peg/RBV (± NS3 protease inhibitor) experienced without cirrhosis | 12 | |
| Decompensated cirrhosis, treatment naive or Peg/RBV (± NS3 protease inhibitor) experienced | 12 | |
| Decompensated cirrhosis, prior sofosbuvir failure only | 24 | |
| Post liver transplant regardless of cirrhosis or decompensation | 12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Sofosbuvir/velpatasvir | Treatment naive or Peg/RBV ± NS3 protease inhibitor experienced regardless of cirrhosis | 12 |
| GT1b, non-NS5A DAA experienced regardless of cirrhosis | 12 | |
| Decompensated cirrhosis, treatment naive or Peg/RBV (± NS3 protease inhibitor) experienced | 12 | |
| Decompensated cirrhosis, DAA failure (including NS5A)b | 24 | |
| Sofosbuvir/velpatasvir/voxilaprevir | NS5A failures (including NS3 protease inhibitor) regardless of cirrhosis | 12 |
| GT1a, non-NS5A failures (including NS3 protease inhibitors) regardless of cirrhosis | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Genotype 2 | ||
| Daclatasvir + sofosbuvir | Decompensated cirrhosis | 12 |
| Post liver transplant regardless of cirrhosis or decompensation | 12 | |
| Glecaprevir/pibrentasvir | Treatment naive or Peg/RBV experienced without cirrhosis | 8 |
| Treatment naive or Peg/RBV experienced with cirrhosis, and sofosbuvir failures regardless of cirrhosis | 12 | |
| Post liver transplant without cirrhosis | 12 | |
| Severe renal impairment (CKD stage 4 or 5) | 8–12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Sofosbuvir/velpatasvir | Treatment naive, or Peg/RBV or non-NS5A experienced regardless of cirrhosis | 12 |
| Decompensated cirrhosis, treatment naive or Peg/RBV or non-NS5A experienced | 12 | |
| Decompensated cirrhosis, DAA failure (including sofosbuvir ± NS5A)b | 24 | |
| Post liver transplant with decompensated cirrhosis | 12 | |
| Sofosbuvir/velpatasvir/voxilaprevir | NS5A failures | 12 |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Genotype 3 | ||
| Daclatasvir + sofosbuvir | Decompensated cirrhosis | 12 |
| Post liver transplant regardless of cirrhosis or decompensation | 12 | |
| Glecaprevir/pibrentasvir | Treatment naive without cirrhosis | 8 |
| Treatment naive with compensated cirrhosis | 12 | |
| Post liver transplant without cirrhosis | 12 | |
| Severe renal impairment (CKD stage 4 or 5) | 8–12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Sofosbuvir + elbasvir/grazoprevir | Peg/RBV experienced with compensated cirrhosis | 12 |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Sofosbuvir/velpatasvir | Treatment naive without cirrhosis | 12 |
| Treatment naive with cirrhosis or Peg/RBV experienced without cirrhosis | 12 | |
| Decompensated cirrhosis, treatment naive or Peg/ RBV experienced | 12 | |
| Decompensated cirrhosis, previously exposed to DAA (including sofosbuvir ± NS5A)b | 24 | |
| Post liver transplant with decompensated cirrhosis | 12 | |
| Sofosbuvir/velpatasvir/voxilaprevir | Peg/RBV experienced with cirrhosis, or DAA failure (including NS5A inhibitors) regardless of cirrhosis | 12 |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Genotype 4 | ||
| Daclatasvir + sofosbuvir | Decompensated cirrhosis | 12 |
| HIV/HCV coinfection when antiretroviral regimen cannot be made to accommodate recommended regimens | 12 | |
| Elbasvir/grazoprevir | Treatment naive or Peg/RBV experienced with prior relapse, regardless of cirrhosis | 12 |
| Severe renal impairment (CKD stage 4/5) | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Glecaprevir/pibrentasvir | Treatment naive or Peg/RBV experienced without cirrhosis | 8 |
| Treatment naive or Peg/RBV experienced with cirrhosis | 12 | |
| Post liver transplant without cirrhosis | 12 | |
| Severe renal impairment (CKD stage 4 or 5) | 8–12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Treatment naive regardless of cirrhosis or Peg/RBV experienced without cirrhosis | 12 | |
| Decompensated cirrhosis, treatment naive or Peg/ RBV experienced | 12 | |
| Decompensated cirrhosis, sofosbuvir failure | 24 | |
| Post liver transplant regardless of cirrhosis or decompensation | 12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Sofosbuvir/velpatasvir | Treatment naive or Peg/RBV experienced regardless of cirrhosis | 12 |
| Decompensated cirrhosis, treatment naive or Peg/ RBV (± NS3 protease inhibitor) experienced | 12 | |
| Decompensated cirrhosis, DAA failure (including NS5A) | 24 | |
| Sofosbuvir/velpatasvir/voxilaprevir | NS5A failures (including NS3 protease inhibitors) regardless of cirrhosis | 12 |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Sofusbuvir/ledipasvir | Treatment naive, compensated cirrhosis – not for decompensated cirrhosis | 12 |
| Genotype 5 or 6 | ||
| Glecaprevir/pibrentasvir | Treatment naive or Peg/RBV experienced without cirrhosis | 8 |
| Treatment naive or Peg/RBV experienced with cirrhosis | 12 | |
| Post liver transplant without cirrhosis | 12 | |
| Severe renal impairment (CKD stage 4 or 5) | 8–12 | |
| Post kidney transplant regardless of cirrhosis | 12 | |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis | ||
| Ledipasvir/sofosbuvir | Treatment naive or Peg/RBV experienced regardless of cirrhosis | 12 |
| Decompensated cirrhosis, treatment naive or Peg/ RBV experienced | 12 | |
| Decompensated cirrhosis, sofosbuvir failure | 24 | |
| Post liver transplant regardless of cirrhosis or decompensation | 12 | |
| Sofosbuvir/velpatasvir | Treatment naive or Peg/RBV experienced regardless of cirrhosis | 12 |
| Decompensated cirrhosis, treatment naive or Peg/RBV (± NS3 protease inhibitor) experienced | 12 | |
| Decompensated cirrhosis, DAA failure (including NS5A) | 24 | |
| Sofosbuvir/velpatasvir/voxilaprevir | NS5A failures (including NS3 protease inhibitors) regardless of cirrhosis | 12 |
| Not for decompensated cirrhosis or post liver transplant with cirrhosis |
There remains a general misunderstanding and also lack of awareness regarding HCV in the general population worldwide, as evident by a 2017 WHO Global Hepatitis Report, where only 20% of 71 million people with HCV worldwide, were aware of the infection at the time of confirmation[91]. A large population-based study in the USA from 2001 to 2008 showed only 49.7% of patients with HCV infection were aware of their status[97]. Another National Health and Nutrition Examination Survey study showed that cirrhosis was equally common in patients who were unaware of their diagnosis compared to those who knew about their infection[98]. At a patient level, fear of the stigma associated with the diagnosis and at the provider level, lack of time, knowledge, and discomfort in asking about high-risk behaviors are barriers to screening, testing, and cure[99]. The provider perceptions have changed over the years and now most providers believe that they play an active role in their patient’s treatment and their decisions to start treatment are not influenced by high risk behaviors amongst patients[100].
In developing countries, the absence of screening programs and limited resources have resulted in the vast majority of patients being undiagnosed[101-103]. A systematic review and meta-analysis of studies published after 1995 showed that in Africa, only 19% of blood transfusions are screened for HCV due to cost constraints[104].
The screening strategies have to be tailored according to the population and the country to make these cost effective[105]. As shown in a systematic review of 67 screening programs, in low HCV-prevalence populations, prescreening can increase efficiency, whereas in high prevalence countries widespread screening programs are cost effective[106-108].
High risk groups: Intravenous drug users (IVDUs) are a well-known high-risk population and the global burden of HCV in injecting drug users is approximately 67%[109]. In Europe the prevalence of HCV is estimated to be 50 times higher in individuals who inject drugs compared with the general population[110]. In the past, this subset of HCV patients was not regarded as eligible for treatment due to concern for poor adherence, reinfection and psychiatric ailments[111]. Current guidelines recommend that people who inject drugs (PWIDs) should not be excluded from HCV treatment[112] and multiple recent studies have shown that there is direct relationship between influences of IV drugs on the efficacy of DAA therapy among adherent patients[113-115], and the SIMPLIFY trial demonstrated that PWIDs should be offered HCV treatment regardless of ongoing drug use[116]. In this high-risk group testing, access to care, prescription of DAA therapy, along with the elimination of stigma associated with the infection have been proposed as effective strategies for this specific population[117-119].
Prevention of HBV/HCV infection: Both HBV and HCV can be transmitted perinatally, via needle stick injury and via household contacts. Perinatal transmission for HBV can be prevented by providing hepatitis B immunoglobulins and vaccines within 12 h of birth to infants of HbsAg-positive mothers[120]. Unfortunately for HCV infection, there are no interventions or prophylactic measures that have been proven to prevent perinatal transmission. Management of needle stick injury for HBV depends on the vaccination status of exposed individual and HBV status of patient. For individuals who suffer needle stick injury and are unvaccinated, vaccination series should be initiated. For vaccinated individuals with documented vaccine response no treatment is required. If the vaccination status is unknown, then its recommended to check anti-Hbs titers and if negative, initiating vaccine series is recommended[121]. Recommendations for prevention of HCV after needle stick injury include testing for HCV RNA, HCV antibodies and ALT immediately after the event, repeat laboratory analysis in 2–8 wk, and referral to specialist if infection occurs[122]. Household contacts should be extensively counselled and education includes measures to avoid sharing razors or toothbrushes etc. that predisposes one to contact with body fluids, HCV/HBV positive individuals should refrain from donating blood, organ and tissue[123].
HBV/HCV coinfection: The worldwide incidence of HBV/HCV coinfection is reported to range from 5% to 15%[124,125]. The incidence varies significantly depending on geographic location, with higher incidence in endemic areas[126]. HBV/HCV coinfection leads to higher rate of cirrhosis, HCC and decompensated liver disease compared to monoinfection[124,127]. Four serological profiles are seen in coinfection–codominant, HCV dominant, HBV dominant, and neither replicative, and these can evolve over period of time[128]. The aim in these scenarios would be to identify and eradicate the dominant virus and then monitor for reactivation of the other virus. Close monitoring of HBV DNA and HCV RNA is essential before determining viral dominance[129]. HBV monoinfection is treated with a nucleo(s)tide analog (e.g., entacavir or tenofovir, lamivudine) and/or pegylated IFN (Peg-IFN). Currently, DAAs are the mainstay of treatment for HCV monoinfection although Peg-IFN plus ribavirin is effective, but is rarely used[126].
HBV/HCV infection after liver transplantation: HBV recurrence after liver transplantation is a major causes of graft failure, graft cirrhosis and allograft dysfunction. Patients can be categorized into high and low risk for recurrent HBV based on pretransplant viral load, HbeAg positivity and history of antiviral drug resistance[130]. Combination of potent nucleos(t)ide analog and hepatitis B immunoglobulin (HBIG) is recommended after liver transplantation for the prevention of HBV recurrence in patients with CHB. Recent data suggest that patients with low risk of recurrence need to be on continued monoprophylaxis with nucleos(t)ide analogs; however, HBIG can be discontinued[77]. HCV recurrence after liver transplantation is universal in patients with HCV viremia at the time of transplantation. The viral levels are shown to rebound and reach pretransplant level with 72 h and DAA therapy should be started within this timeframe to prevent graft reinfection and loss[131].
Hepatitis delta virus (HDV) is a defective virus that encodes its own genome but needs HBsAg and hence HBV for replication, propagation and transmission[132]. The two high-risk groups at risk of infection include IVDUs and patients with high-risk sexual behaviors[133]. In the USA, HDV infection was considered to be a rare, but data over the last decade estimating seroprevalence of HDV have shown higher rates, especially in Asians and immigrants[45].
Transmission: HDV is transmitted parenterally and sexually, while vertical transmission is thought to be rare[134,135]. In low-endemicity regions and developed nations, IVD use is the main route of transmission[133].
Clinical presentation: HDV infection is always associated with HBV infection as HBV is integral to the assembly of the hepatitis D virion and release. Two major patterns of infection can occur: superinfection and coinfection.
Coinfection is concurrent infection with both HDV and HBV. Clinically, the presentation is difficult to differentiate from other causes of hepatitis and especially acute HBV[136]. Patients who are coinfected can present with symptoms that can be mild to severe fulminant hepatitis[137]. Coinfection is usually self-limited, but it is important to highlight the fact that coinfection can cause severe fulminant hepatitis compared to superinfection[137].
Superinfection occurs when HDV infects an individual with CHB, in whom pre-existing HBsAg provides an ideal environment for HDV expression. Patients progress from acute hepatitis to chronic infection in up to 90% of cases, whereas the rest either resolve or progress to fulminant disease[136]. Chronic HDV infection, in comparison to HBV monoinfection, is more severe and, up to 70% percent of patients rapidly progress to cirrhosis within 5–10 years[138].
Diagnosis and management: In patients suspected to have HDV, the first-line screening test is ELISA for anti-HDV. The acute phase of HDV infection is characterized by positive IgM anti-HDV in serum. IgG anti-HDV antibodies are representative of chronic HDV infection or past exposure[139]. HDV screening for all HBV-infected individuals is recommended by the European guidelines whereas in the USA screening is limited to patients with high-risk factors such as HIV or HCV infections and patients with low or undetectable HBV DNA presenting with elevated aminotransferases[45]. Screening of all HBsAg-positive patients should be considered given concerns regarding underestimation of prevalence of HDV[140,141]. This approach would lead to more accurate determination of HDV prevalence and would also lead to earlier intervention and treatment[142].
The current treatment option for chronic HDV infection is Peg-IFN-α for 12 mo based on guidelines from the American Association for the Study of Liver Diseases, Asian Pacific Association for the Study of the Liver, and the European Association for the Study of the Liver[45,77,143]. Overall, the response rate to therapy is low with only a 10%–20% rate of sustained HDV clearance and 10% rate of HbsAg clearance with 1 year of standard IFN-α[144,145]. Studies revealed that 1 year of therapy with Peg-IFN proved to have a better response rate than standard IFN therapy however it hardly exceeded 25% of sustained HDV clearance[146,147].
Combination therapy involving standard IFN-α with ribavirin[148] or lamivudine[149,150] is not more efficacious than monotherapy with IFN for chronic hepatitis D. Similar results are obtained when Peg-IFN-α is used in combination with ribavirin[151] or adefovir[147].
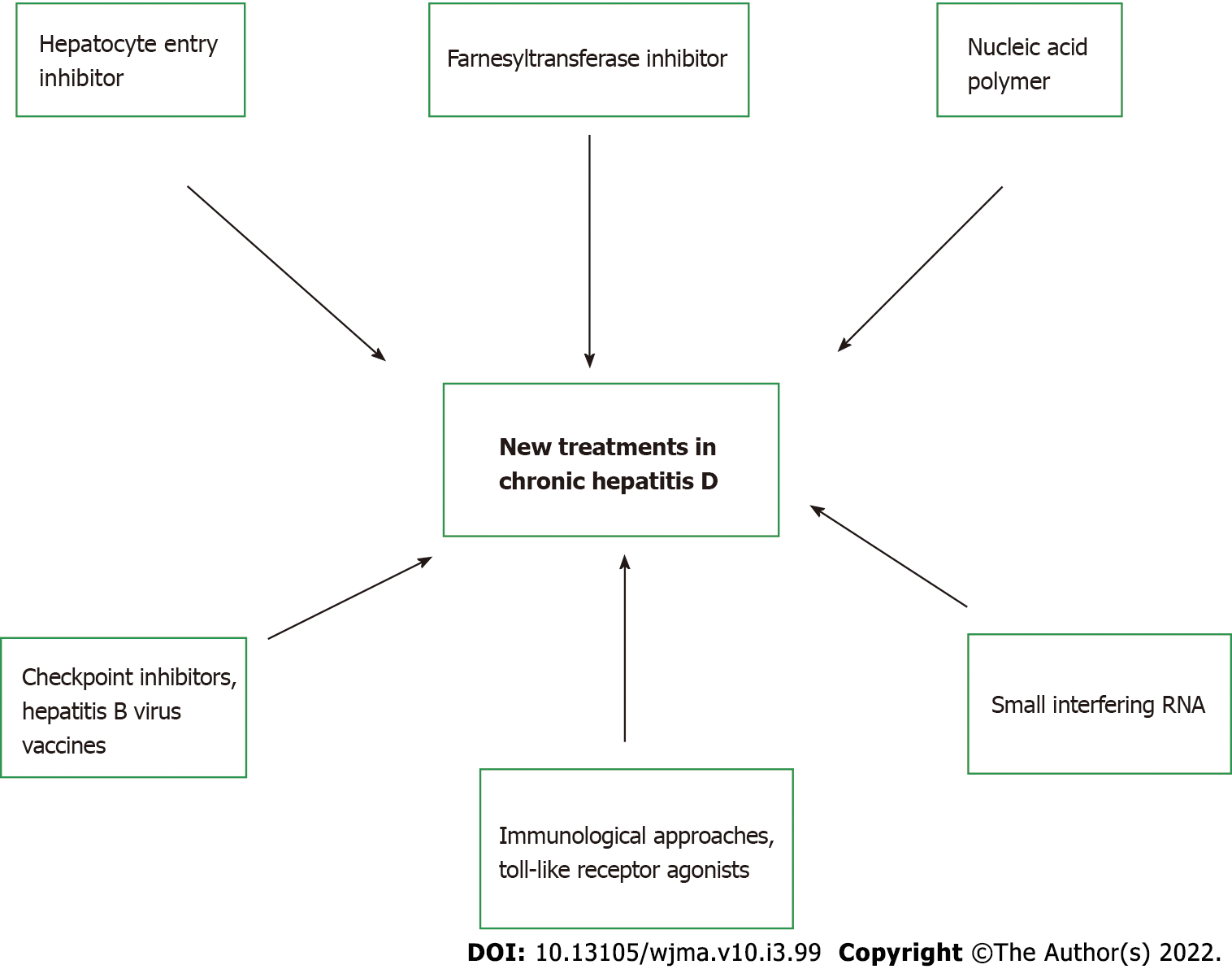
Novel therapeutics: Given the low overall virological response rate and high rate of relapse, there is an increasing need for therapeutic strategies aimed at improving efficacy and offering it to patients for whom IFN is contraindicated due to advanced liver disease. Currently, three new medications that affect HDV life cycle are being studied in clinical trials, with varying mechanism of actions: hepatocyte entry inhibitors, farnesyltransferase inhibitors, and nucleic acid polymers. Table 5 summarizes these novel therapies with the associated adverse effects. Figure 2 highlights the different targeted approaches for treatment of chronic hepatitis D.
| Drug | Mode of action | Administration route | Phase of study | Adverse effect |
| Myrcludex B | Interferes with hepatitis D virus entry into hepatocyte | Subcutaneous, daily for 6 mo | Ib, IIa | Lipase and amylase elevation in phase I but not in phase II study |
| Through sodium taurocholate cotransporting | ± pegylated interferon (Peg-IFN) | Elevation of taurine- and glycine-conjugated bile acids without apparent clinical consequences | ||
| polypeptide inhibition | Thrombocytopenia, neutropenia, lymphopenia, and eosinophilia: Generally mild, transient | |||
| Lonafarnib | Farnesyltransferase inhibitor, inhibits virion assembly | Oral, 2 to 12 mo, ± ritonavir | II | Gastrointestinal toxicity (anorexia, nausea with or without vomiting, diarrhea, weight loss): Dose dependent and in lower dose cohorts generally mild and well tolerated |
| ± peg-IFN | ||||
| Rep-2139-Ca | Nucleic acid polymer, binds with high affinity to | Intravenous infusion, once wkly | II | Hair loss, dysphagia, anorexia, dysgeusia, in hepatitis B study: Related to heavy metal exposure at the trial site |
| Amphipathic proteins, which are required at various | for 4-6 mo ± peg-IFN | Administration route-related side effects: peripheral grade 1 hyperemia, fever, chills, and headache | ||
| Stages of the viral life cycle |
Additional approaches: siRNAs have shown early promise in this field. In a phase IIa clinical trial that showed that a single injection of siRNA ARC-520d decreased HbsAg levels in HbeAg negative CHB patients in a dose-dependent fashion[152]. A multi-dose extension study of up to 12 doses, with once monthly dosing, demonstrated an increased decline of HbAg level, especially in HbeAg-positive rather than HbeAg-negative patients[153]. The study was stopped because of adverse effects of the carrier molecule but demonstrated the effect and highlighted the scope of siRNA as a potential treatment option.
Currently, for the management of HDV, new approaches such as DNA vaccines[154], anti-HB immune complexes[155]https://www-ncbi-nlm-nih-gov.ezproxy3.lhl.uab.edu/pmc/articles/PMC5580405/-ref-75, and immunologically active adjuvants such as β-glucosylceramide are being explored. Targeting the HBV and immune system interaction is another area that has garnered significant interest. Preclinical studies have shown that the Toll-like receptors (TLRs) play a key role in sensing pathogen-associated molecule patterns and activating intracellular antiviral pathways as well as the production of proinflammatory cytokines and antiviral effectors like IFN[156]. In a study assessing the safety, pharmacokinetics, and pharmacodynamics of oral TLR-7 agonist, GS-920 led to induction of peripheral mRNA expression of IFN-stimulated gene 15 production in CHB patients; however, there was no effect on HBV DNA[157]. Immune checkpoint inhibitors have also been studies in chronic viral hepatitis, and a phase Ib clinical study of nivolumab in CHB patients highlighted its tolerance and association with significant decline in HbsAg after a single dose over a 24-wk period[158].
HEV is a small, nonenveloped virus and belongs to the Hepeviridae family and is further classified into genotypes 1–4 and 7[159]. Globally an estimated 2.2 billion people are infected by HEV with 70 000 deaths attributed to HEV annually[160,161].
HEV is mainly transmitted via contaminated water and consumption of undercooked pork or wild boar and other foods, while reports of blood transfusion related transmission has been recently recognized[162,163]. Outbreaks of HEV1 and HEV2 genotypes are documented in areas with inadequate sanitary conditions and lack of access to clean water[164]. The prevalence of anti-HEV IgG in Africa ranges from 4.6% to 10.7%, and 34% to 94% in Asia[165-169]. HEV prevalence is probably underestimated as seen in a large German cohort study, as the majority of practitioners do not regularly test for HEV in the presence of acute hepatitis symptoms; in part, due to lack of high clinical suspicion but also absence of standardized testing, leading to increased morbidity and mortality among susceptible individuals[170].
The diagnosis of HEV infection is often challenging given lack of standardized testing and need for HEV PCR for definitive diagnosis[171-173]. Paradoxically, in immunocompromised hosts who do not mount an adequate antibody response, PCR testing should be the cornerstone of diagnosis[174,175]. There is no US FDA-approved diagnostic test and available serological assays have variable sensitivities and specificities making accurate diagnosis often challenging[176-178].
Clinical presentation: Clinical presentation in HEV is variable, ranging from asymptomatic carriers to fulminant hepatitis. In acute HEV, the incubation period is typically 3–8 wk. followed by a short prodrome leading to a symptomatic phase that can last for several days to weeks (mean 4–6 wk)[179]. HEV can also infect patients with chronic liver disease and can cause decompensation, and lead to high mortality[180-182]. Extrahepatic manifestations of HEV include rash, arthralgias, Guillain–Barré syndrome palsies, and pseudotumor cerebri[183].
Based on the patients’ immune response to acute HEV, some may progress to chronic HEV infection, which is defined by persistent elevated aminotransferase levels for at least 3 mo combined with positive serum HEV RNA and consistent histological findings on liver biopsy[182]. Chronic HEV primarily occurs in immunosuppressed patients such as organ transplantation recipients or those with HIV infection, and hemodialysis[184-189].
Infection with HEV, specifically genotype 1, during pregnancy leads to increased risk of adverse outcomes to the fetus such as spontaneous abortion, in utero fetal demise, and premature delivery, while placing the mother at risk of severe hepatitis and complications[190]. HEV in pregnancy is associated with eclampsia, hemorrhage, and acute liver failure, and carries a high mortality rate of 15%–25%, especially in the third trimester[182,191].
Treatment: Acute HEV in immunocompetent hosts is self-limiting illness followed by spontaneous clearance and usually does not require treatment[192]. Monotherapy with ribavirin is the current treatment of choice for patients with chronic HEV infection[193]. Three months of ribavirin monotherapy for chronic HEV has been associated with around 78% sustained virological response[194]. No established treatment for HEV is available for pregnant women as ribavirin is contraindicated in pregnancy, hence supportive care is recommended[195,196]. Peg-IFN, as an alternative to ribavirin has shown limited success[197,198]. There is a need for direct-acting novel therapies as HEV remains a serious public health concern particularly among pregnant women and immunocompromised patients. The current efforts for these drug developments are focusing either on the inhibition and manipulation of host components or developing DAA therapies that can target viral enzymes without affecting host components[199].
Hepatitis E vaccine and surveillance programs: The need for hepatitis E vaccine was recognized secondary to its worldwide prevalence and severe complications in high risk populations. Early studies of recombinant vaccines in healthy adults have shown promising results, but study populations have unfortunately not included the high-risk groups who are most susceptible to severe and chronic HEV. A Nepalese randomized, placebo controlled, double blinded , phase 2 clinical trial of a recombinant HEV vaccine given at 0, 1 and 6 mo to 898 patients (vs 896 placebo) revealed vaccine efficacy of 88.5% in intention to treat analysis[200]. In a different phase 3 clinical trial, Hecolin (Xiamen Innovax Biotech, China) had 112 604 participants, of which 56302 in the study arm and 56 302 in the placebo arm, received three doses of rHEV and hepatitis B vaccine at 0, 1 and 6 mo respectively. The vaccine efficacy of 95.5%was reported in an intention to treat analysis[201]. This vaccine is only approved and commercially available in China[202].
An HEV vaccine that is available worldwide would reduce the incidence of the infection in endemic areas and also confer protection to travelers[203]. Areas and countries with high prevalence should also focus on improved sanitation, access to clean water with a specific focus on high-risk groups, especially pregnant women and patients with chronic medical conditions[167,204].
With the recent advancements in the area of molecular virology, the landscape for the management of viral hepatitis has evolved dramatically. We have a better understanding of the molecular structure of these pathogens and their interplay with our immune system, which has paved the way for novel drugs and therapeutics. While the success of the decade is focused on DAAs as the cure for HCV, the burden of chronic HBV and HDV infections persists as research is ongoing for both a cure for HBV and treatment options for HDV. Drugs that hold promise regarding complete eradication of HBV cccDNA from hepatocytes are under investigation and may be pivotal in complete eradication of the infection in the future. Despite the advancement in the field of serological and PCR testing for HBV, HCV, and HDV, there is a continued need for improvements in screening protocols for these infections. Standardized testing along with options for treatment and vaccination remain areas of interest for HEV. Work continues on implementation of universal vaccination for HAV and HBV, while clinical trials are ongoing for HEV vaccination. There remains a pressing need for increased collaborative efforts to help combat these illnesses, as we continue to learn about the viral hepatitis to fill the gaps in our knowledge.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balaban YH, Turkey; Mogahed EA, Egypt A-Editor: Zhu JQ, China S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87:261-76. [PubMed] |
| 2. | Siegl G, Weitz M, Kronauer G. Stability of hepatitis A virus. Intervirology. 1984;22:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Rein DB, Stevens G, Flaxman A, Wittenborn JS, Timothy N, Wiktor SZ, Wiersma ST. The global burden of hepatitis a virus in 1990 and 2005. Journal of Hepatol. 2014;1:S303. |
| 4. | Aggarwal R, Goel A. Hepatitis A: epidemiology in resource-poor countries. Curr Opin Infect Dis. 2015;28:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 5. | Ikobah JM, Okpara HC, Ekanem EE, Udo JJ. Seroprevalence and predictors of hepatitis A infection in Nigerian children. Pan Afr Med J. 2015;20:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | WHO. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review. 2010. Available from: https://apps.who.int/iris/bitstream/handle/10665/70180/WHO_?sequence=1. |
| 7. | WHO. World health statistics 2018: monitoring health for the SDGs, sustainable development goals: World Health Organization; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/324835/9789241565707-eng.pdf. |
| 8. | El-Shabrawi MH, Kamal NM. Burden of pediatric hepatitis C. World J Gastroenterol. 2013;19:7880-7888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1360] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 10. | Escobedo-Meléndez G, Fierro NA, Roman S, Maldonado-González M, Zepeda-Carrillo E, Panduro A. Prevalence of hepatitis A, B and C serological markers in children from western Mexico. Ann Hepatol. 2012;11:194-201. [PubMed] |
| 11. | Verghese VP, Robinson JL. A systematic review of hepatitis E virus infection in children. Clin Infect Dis. 2014;59:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 12. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1210] [Article Influence: 172.9] [Reference Citation Analysis (1)] |
| 13. | Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 207] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Ciocca M. Clinical course and consequences of hepatitis A infection. Vaccine. 2000;18 Suppl 1:S71-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D. Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine (Baltimore). 1992;71:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Manka P, Verheyen J, Gerken G, Canbay A. Liver Failure due to Acute Viral Hepatitis (A-E). Visc Med. 2016;32:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | American Academy of Pediatrics Committee on Infectious Diseases. Hepatitis A vaccine recommendations. Pediatrics. 2007;120:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Murphy TV, Denniston MM, Hill HA, McDonald M, Klevens MR, Elam-Evans LD, Nelson NP, Iskander J, Ward JD. Progress Toward Eliminating Hepatitis A Disease in the United States. MMWR Suppl. 2016;65:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1989] [Article Influence: 198.9] [Reference Citation Analysis (3)] |
| 20. | WHO. Hepatitis B. World Health Organization Fact Sheet No. 204. 2017. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. |
| 21. | Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 314] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 22. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2169] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 23. | Vittal A, Ghany MG. WHO Guidelines for Prevention, Care and Treatment of Individuals Infected with HBV: A US Perspective. Clin Liver Dis. 2019;23:417-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Hou J, Liu Z, Gu F. Epidemiology and Prevention of Hepatitis B Virus Infection. Int J Med Sci. 2005;2:50-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Abbas Z, Jafri W, Shah SH, Khokhar N, Zuberi SJ; Pakistan Society of Gastroenterology and G. I. Endoscopy. PGS consensus statement on management of hepatitis B virus infection--2003. J Pak Med Assoc. 2004;54:150-158. [PubMed] |
| 26. | Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 27. | Francis DP, Favero MS, Maynard JE. Transmission of hepatitis B virus. Semin Liver Dis. 1981;1:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Davis LG, Weber DJ, Lemon SM. Horizontal transmission of hepatitis B virus. Lancet. 1989;1:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, Larney S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192-e1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1009] [Cited by in RCA: 1049] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 30. | Chu JJ, Wörmann T, Popp J, Pätzelt G, Akmatov MK, Krämer A, Reintjes R. Changing epidemiology of hepatitis B and migration--a comparison of six Northern and North-Western European countries. Eur J Public Health. 2013;23:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Koc ÖM, Kremer C, Bielen R, Buscchots D, Hens N, Nevens F, Robaeys G. Prevalence and risk factors of hepatitis B virus infection in Middle-Limburg Belgium, year 2017: Importance of migration. J Med Virol. 2019;91:1479-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Mysore KR, Leung DH. Hepatitis B and C. Clin Liver Dis. 2018;22:703-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, Chen CL. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 526] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 34. | McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, Schluep T. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 36. | Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 385] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 37. | Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E, Cohen P; French Vasculitis Study Group. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine (Baltimore). 2005;84:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Gupta A, Quigg RJ. Glomerular Diseases Associated With Hepatitis B and C. Adv Chronic Kidney Dis. 2015;22:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Han SH. Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis. 2004;8:403-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Kappus MR, Sterling RK. Extrahepatic manifestations of acute hepatitis B virus infection. Gastroenterol Hepatol (N Y). 2013;9:123-126. [PubMed] |
| 41. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 42. | Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Int. 2009;3:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, Lai ST, Wong WM, Lai LS, Poon RT, Lo CM, Fan ST, Lau GK; Hong Kong Liver Fibrosis Study Group. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 556] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 45. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2815] [Article Influence: 402.1] [Reference Citation Analysis (0)] |
| 46. | Kramvis A, Kew M, François G. Hepatitis B virus genotypes. Vaccine. 2005;23:2409-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1593] [Article Influence: 122.5] [Reference Citation Analysis (1)] |
| 48. | Revill PA, Locarnini SA. New perspectives on the hepatitis B virus life cycle in the human liver. J Clin Invest. 2016;126:833-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 689] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 50. | Pollicino T, Saitta C. Occult hepatitis B virus and hepatocellular carcinoma. World J Gastroenterol. 2014;20:5951-5961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Raimondo G, Pollicino T. Occult HBV infection. Hepatitis B Virus in Human Diseases: Springer; 2016; 277-301. Available from: https://link.springer.com/chapter/10.1007/978-3-319-22330-8_13. |
| 52. | Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669-6674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 549] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 53. | Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79:9369-9380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 346] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 54. | Boeijen LL, Hoogeveen RC, Boonstra A, Lauer GM. Hepatitis B virus infection and the immune response: The big questions. Best Pract Res Clin Gastroenterol. 2017;31:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Ferrari C. HBV and the immune response. Liver Int. 2015;35 Suppl 1:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 56. | Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, Urbani S, Chong YS, Guccione E, Bertoletti A. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun. 2015;6:6588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 57. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 439] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 58. | Muraskin W. The last years of the CVI and the birth of the GAVI. Public-private partnerships for public health. 2002: 115-168. Available from: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/480/2012/09/Partnerships_book.pdf#page=126. |
| 59. | Aslam A, Ishtiaq R, Lau DTY. Timely Administration of Birth Dose Hepatitis B Virus Vaccine May Break the Chain of Perinatal Transmission. Hepatology. 2019;69:2284-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Implementation of hepatitis B birth dose vaccination – worldwide, 2016. Wkly Epidemiol Rec. 2018;93:61-72. [PubMed] |
| 61. | Robinson CL, Bernstein H, Poehling K, Romero JR, Szilagyi P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:130-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Liu J, Liu M. [Progress and challenges in achieving the WHO goal on 'Elimination of Hepatitis B by 2030' in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 63. | van Gemert C, Wang J, Simmons J, Cowie B, Boyle D, Stoove M, Enright C, Hellard M. Improving the identification of priority populations to increase hepatitis B testing rates, 2012. BMC Public Health. 2016;16:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Howell J, Pedrana A, Cowie BC, Doyle J, Getahun A, Ward J, Gane E, Cunningham C, Wallace J, Lee A, Malani J, Thompson A, Hellard ME. Aiming for the elimination of viral hepatitis in Australia, New Zealand, and the Pacific Islands and Territories: Where are we now and barriers to meeting World Health Organization targets by 2030. J Gastroenterol Hepatol. 2019;34:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Yeo YH, Nguyen MH. Review article: current gaps and opportunities in HBV prevention, testing and linkage to care in the United States-a call for action. Aliment Pharmacol Ther. 2021;53:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D, Hallett TB. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 67. | Block TM, Gish R, Guo H, Mehta A, Cuconati A, Thomas London W, Guo JT. Chronic hepatitis B: what should be the goal for new therapies? Antiviral Res. 2013;98:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Evans AA, London WT, Gish RG, Cohen C, Block TM. Chronic HBV infection outside treatment guidelines: is treatment needed? Antivir Ther. 2013;18:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M; Asian-Pacific consensus update working party on chronic hepatitis B. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | Viganò M, Lampertico P. Clinical implications of HBsAg quantification in patients with chronic hepatitis B. Saudi J Gastroenterol. 2012;18:81-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Stone D, Niyonzima N, Jerome KR. Genome editing and the next generation of antiviral therapy. Hum Genet. 2016;135:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Kennedy EM, Kornepati AV, Cullen BR. Targeting hepatitis B virus cccDNA using CRISPR/Cas9. Antiviral Res. 2015;123:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, Scott DA, Zhang F, Rice CM, Bhatia SN. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep. 2015;5:10833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 74. | Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res. 2015;118:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 75. | Stone D, Long KR, Loprieno MA, De Silva Feelixge HS, Kenkel EJ, Liley RM, Rapp S, Roychoudhury P, Nguyen T, Stensland L, Colón-Thillet R, Klouser LM, Weber ND, Le C, Wagoner J, Goecker EA, Li AZ, Eichholz K, Corey L, Tyrrell DL, Greninger AL, Huang ML, Polyak SJ, Aubert M, Sagartz JE, Jerome KR. CRISPR-Cas9 gene editing of hepatitis B virus in chronically infected humanized mice. Mol Ther Methods Clin Dev. 2021;20:258-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 76. | Li H, Sheng C, Wang S, Yang L, Liang Y, Huang Y, Liu H, Li P, Yang C, Yang X, Jia L, Xie J, Wang L, Hao R, Du X, Xu D, Zhou J, Li M, Sun Y, Tong Y, Li Q, Qiu S, Song H. Removal of Integrated Hepatitis B Virus DNA Using CRISPR-Cas9. Front Cell Infect Microbiol. 2017;7:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 77. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3768] [Article Influence: 471.0] [Reference Citation Analysis (1)] |
| 78. | Roudot-Thoraval F. Epidemiology of hepatitis C virus infection. Clin Res Hepatol Gastroenterol. 2021;45:101596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 79. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1468] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 80. | Preciado MV, Valva P, Escobar-Gutierrez A, Rahal P, Ruiz-Tovar K, Yamasaki L, Vazquez-Chacon C, Martinez-Guarneros A, Carpio-Pedroza JC, Fonseca-Coronado S, Cruz-Rivera M. Hepatitis C virus molecular evolution: transmission, disease progression and antiviral therapy. World J Gastroenterol. 2014;20:15992-16013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (5)] |
| 81. | Yang S, Wang D, Zhang Y, Yu C, Ren J, Xu K, Deng M, Tian G, Ding C, Cao Q, Li Y, Chen P, Xie T, Wang C, Wang B, Yao J, Threapleton D, Mao C, Ruan B, Li L. Transmission of Hepatitis B and C Virus Infection Through Body Piercing: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Fernandez N, Towers CV, Wolfe L, Hennessy MD, Weitz B, Porter S. Sharing of Snorting Straws and Hepatitis C Virus Infection in Pregnant Women. Obstet Gynecol. 2016;128:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Prasad MR, Honegger JR. Hepatitis C virus in pregnancy. Am J Perinatol. 2013;30:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Schmelzer J, Dugan E, Blach S, Coleman S, Cai Z, DePaola M, Estes C, Gamkrelidze I, Jerabek K, Ma S, Montoya S, Razavi-Shearer D, Razavi-Shearer K, Robbins-Scott S, Razavi H, El Sayed MH. Global prevalence of hepatitis C virus in children in 2018: a modelling study. Lancet Gastroenterol Hepatol. 2020;5:374-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 85. | He Q, He Q, Qin X, Li S, Li T, Xie L, Deng Y, He Y, Chen Y, Wei Z. The Relationship between Inflammatory Marker Levels and Hepatitis C Virus Severity. Gastroenterol Res Pract. 2016;2016:2978479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Ringelhan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philos Trans R Soc Lond B Biol Sci. 2017;372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 87. | Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 88. | Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 89. | Cacoub P, Comarmond C, Domont F, Savey L, Desbois AC, Saadoun D. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis. 2016;3:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 90. | Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology. 2016;150:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 91. | WHO. World Health Organization. Global Health Sector Strategies on Viral Hepatitis 2016-2021 2016. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_32-en.pdf?ua=1. |
| 92. | Park H, Wang W, Henry L, Nelson DR. Impact of All-Oral Direct-Acting Antivirals on Clinical and Economic Outcomes in Patients With Chronic Hepatitis C in the United States. Hepatology. 2019;69:1032-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 93. | Das D, Pandya M. Recent Advancement of Direct-acting Antiviral Agents (DAAs) in Hepatitis C Therapy. Mini Rev Med Chem. 2018;18:584-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 94. | Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of "perfectovir". Clin Infect Dis. 2015;60:1829-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 95. | Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 547] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 96. | Mohamed AA, El-Toukhy NER, Said EM, Gabal HMR, AbdelAziz H, Doss W, El-Hanafi H, El Deeb HH, Mahmoud S, Elkadeem M, Shalby HS, Abd-Elsalam S. Hepatitis C Virus: Efficacy of New DAAs Regimens. Infect Disord Drug Targets. 2020;20:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 98. | Udompap P, Mannalithara A, Heo NY, Kim D, Kim WR. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J Hepatol. 2016;64:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 99. | Shehata N, Austin T, Ha S, Timmerman K. Barriers to and facilitators of hepatitis C virus screening and testing: A scoping review. Can Commun Dis Rep. 2018;44:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Zhang G, Patel K, Moghe A, Reid A, Serper M, Calgaro L, Gibson S, Zickmund S, Shaikh O, Rogal S. Provider Perceptions of Hepatitis C Treatment Adherence and Initiation. Dig Dis Sci. 2020;65:1324-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | El Kassas M, Elbaz T, Elsharkawy A, Omar H, Esmat G. HCV in Egypt, prevention, treatment and key barriers to elimination. Expert Rev Anti Infect Ther. 2018;16:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Blach S, Sanai FM. HCV Burden and Barriers to Elimination in the Middle East. Clin Liver Dis (Hoboken). 2019;14:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | Ishizaki A, Bouscaillou J, Luhmann N, Liu S, Chua R, Walsh N, Hess S, Ivanova E, Roberts T, Easterbrook P. Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries. BMC Infect Dis. 2017;17:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 104. | Karoney MJ, Siika AM. Hepatitis C virus (HCV) infection in Africa: a review. Pan Afr Med J. 2013;14:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 105. | Friis RH, Sellers T. Epidemiology for public health practice: Jones & Bartlett Learning; 2020. |
| 106. | Zuure FR, Urbanus AT, Langendam MW, Helsper CW, van den Berg CH, Davidovich U, Prins M. Outcomes of hepatitis C screening programs targeted at risk groups hidden in the general population: a systematic review. BMC Public Health. 2014;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Kim DD, Hutton DW, Raouf AA, Salama M, Hablas A, Seifeldin IA, Soliman AS. Cost-effectiveness model for hepatitis C screening and treatment: Implications for Egypt and other countries with high prevalence. Glob Public Health. 2015;10:296-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 108. | Deuffic-Burban S, Huneau A, Verleene A, Brouard C, Pillonel J, Le Strat Y, Cossais S, Roudot-Thoraval F, Canva V, Mathurin P, Dhumeaux D, Yazdanpanah Y. Assessing the cost-effectiveness of hepatitis C screening strategies in France. J Hepatol. 2018;69:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9553] [Article Influence: 734.8] [Reference Citation Analysis (0)] |
| 110. | Hickman M, Martin NK, Giraudon I, Wiessing L, Hedrich D, Kalamara E, et al. Hepatitis C among drug users in Europe: epidemiology, treatment and prevention. Publication Office of the European Union; 2016. |
| 111. | Grebely J, Tyndall MW. Management of HCV and HIV infections among people who inject drugs. Curr Opin HIV AIDS. 2011;6:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 113. | Bruggmann P, Falcato L, Dober S, Helbling B, Keiser O, Negro F, Meili D; Swiss Hepatitis C Cohort Study. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Grassi A, Ballardini G. Hepatitis C in injection drug users: It is time to treat. World J Gastroenterol. 2017;23:3569-3571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, Goldberg DJ, Hellard ME. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57 Suppl 2:S80-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 116. | Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, Amin J, Bruneau J, Hellard M, Litwin AH, Marks P, Quiene S, Siriragavan S, Applegate TL, Swan T, Byrne J, Lacalamita M, Dunlop A, Matthews GV, Powis J, Shaw D, Thurnheer MC, Weltman M, Kronborg I, Cooper C, Feld JJ, Fraser C, Dillon JF, Read P, Gane E, Dore GJ; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 117. | Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57 Suppl 2:S32-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 118. | Wiessing L, Ferri M, Grady B, Kantzanou M, Sperle I, Cullen KJ; EMCDDA DRID group, Hatzakis A, Prins M, Vickerman P, Lazarus JV, Hope VD, Matheï C. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One. 2014;9:e103345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 119. | Grebely J, Dore GJ, Morin S, Rockstroh JK, Klein MB. Elimination of HCV as a public health concern among people who inject drugs by 2030 - What will it take to get there? J Int AIDS Soc. 2017;20:22146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 120. | Nelson NP, Jamieson DJ, Murphy TV. Prevention of Perinatal Hepatitis B Virus Transmission. J Pediatric Infect Dis Soc. 2014;3 Suppl 1:S7-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 121. | Coppola N, De Pascalis S, Onorato L, Calò F, Sagnelli C, Sagnelli E. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World J Hepatol. 2016;8:273-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (5)] |
| 122. | Moorman AC, de Perio MA, Goldschmidt R, Chu C, Kuhar D, Henderson DK, Naggie S, Kamili S, Spradling PR, Gordon SC, Russi MB, Teshale EH. Testing and Clinical Management of Health Care Personnel Potentially Exposed to Hepatitis C Virus - CDC Guidance, United States, 2020. MMWR Recomm Rep. 2020;69:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 123. | Zarski JP, Leroy V. Counselling patients with hepatitis C. J Hepatol. 1999;31 Suppl 1:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 124. | Pol S, Haour G, Fontaine H, Dorival C, Petrov-Sanchez V, Bourliere M, Capeau J, Carrieri P, Larrey D, Larsen C, Marcellin P, Pawlostky JM, Nahon P, Zoulim F, Cacoub P, de Ledinghen V, Mathurin P, Negro F, Pageaux GP, Yazdanpanah Y, Wittkop L, Zarski JP, Carrat F; French Anrs Co22 Hepather Cohort. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment Pharmacol Ther. 2017;46:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 125. | Senturk H, Tahan V, Canbakan B, Uraz S, Ulger Y, Ozaras R, Tabak F, Mert A, Ozbay G. Chronic hepatitis C responds poorly to combination therapy in chronic hepatis B carriers. Neth J Med. 2008;66:191-195. [PubMed] |
| 126. | Sagnelli E, Sagnelli C, Macera M, Pisaturo M, Coppola N. An update on the treatment options for HBV/HCV coinfection. Expert Opin Pharmacother. 2017;18:1691-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 127. | Cho LY, Yang JJ, Ko KP, Park B, Shin A, Lim MK, Oh JK, Park S, Kim YJ, Shin HR, Yoo KY, Park SK. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 128. | Mavilia MG, Wu GY. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J Clin Transl Hepatol. 2018;6:296-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 129. | Raimondo G, Brunetto MR, Pontisso P, Smedile A, Maina AM, Saitta C, Squadrito G, Tono N; Associazione Italiana Studio Fegato Cooperative Group. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology. 2006;43:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 130. | Maiwall R, Kumar M. Prevention and Treatment of Recurrent Hepatitis B after Liver Transplantation. J Clin Transl Hepatol. 2016;4:54-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 132. | Mentha N, Clément S, Negro F, Alfaiate D. A review on hepatitis D: From virology to new therapies. J Adv Res. 2019;17:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 133. | Chen HY, Shen DT, Ji DZ, Han PC, Zhang WM, Ma JF, Chen WS, Goyal H, Pan S, Xu HG. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 134. | Liaw YF, Chiu KW, Chu CM, Sheen IS, Huang MJ. Heterosexual transmission of hepatitis delta virus in the general population of an area endemic for hepatitis B virus infection: a prospective study. J Infect Dis. 1990;162:1170-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 135. | Weisfuse IB, Hadler SC, Fields HA, Alter MJ, O'Malley PM, Judson FN, Ostrow DG, Altman NL. Delta hepatitis in homosexual men in the United States. Hepatology. 1989;9:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | Smedile A, Farci P, Verme G, Caredda F, Cargnel A, Caporaso N, Dentico P, Trepo C, Opolon P, Gimson A, Vergani D, Williams R, Rizzetto M. Influence of delta infection on severity of hepatitis B. Lancet. 1982;2:945-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 277] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 137. | Farci P. Delta hepatitis: an update. J Hepatol. 2003;39 Suppl 1:S212-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 138. | Smedile A, Dentico P, Zanetti A, Sagnelli E, Nordenfelt E, Actis GC, Rizzetto M. Infection with the delta agent in chronic HBsAg carriers. Gastroenterology. 1981;81:992-997. [PubMed] |
| 139. | Shah PA, Choudhry S, Reyes KJC, Lau DTY. An update on the management of chronic hepatitis D. Gastroenterol Rep (Oxf). 2019;7:396-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 140. | Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of Hepatitis B and Hepatitis D Virus Infections in the United States, 2011-2016. Clin Infect Dis. 2019;69:709-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 141. | Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol. 2015;63:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 142. | Ahn J, Gish RG. Hepatitis D Virus: A Call to Screening. Gastroenterol Hepatol (N Y). 2014;10:647-686. [PubMed] |
| 143. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1945] [Article Influence: 216.1] [Reference Citation Analysis (0)] |
| 144. | Yurdaydin C. Treatment of chronic delta hepatitis. Semin Liver Dis. 2012;32:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 145. | Niro GA, Rosina F, Rizzetto M. Treatment of hepatitis D. J Viral Hepat. 2005;12:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 146. | Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, Pham BN, Maylin S, Bedossa P, Dény P, Marcellin P, Gault E. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology. 2006;44:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 147. | Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H, Koch A, Bock T, Dienes HP, Manns MP; HIDIT Study Group. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 358] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 148. | Kaymakoglu S, Karaca C, Demir K, Poturoglu S, Danalioglu A, Badur S, Bozaci M, Besisik F, Cakaloglu Y, Okten A. Alpha interferon and ribavirin combination therapy of chronic hepatitis D. Antimicrob Agents Chemother. 2005;49:1135-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 149. | Yurdaydin C, Bozkaya H, Onder FO, Sentürk H, Karaaslan H, Akdoğan M, Cetinkaya H, Erden E, Erkan-Esin O, Yalçin K, Bozdayi AM, Schinazi RF, Gerin JL, Uzunalimoğlu O, Ozden A. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat. 2008;15:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 150. | Canbakan B, Senturk H, Tabak F, Akdogan M, Tahan V, Mert A, Sut N, Ozaras R, Midilli K, Ozbay G. Efficacy of interferon alpha-2b and lamivudine combination treatment in comparison to interferon alpha-2b alone in chronic delta hepatitis: a randomized trial. J Gastroenterol Hepatol. 2006;21:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Niro GA, Ciancio A, Gaeta GB, Smedile A, Marrone A, Olivero A, Stanzione M, David E, Brancaccio G, Fontana R, Perri F, Andriulli A, Rizzetto M. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology. 2006;44:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 152. | Yuen M, Chan H, Given B, Hamilton J, Schluep T, Lewis DL. Phase II, dose-ranging study of ARC-520, a siRNA-based therapeutic, in patients with chronic hepatitis B virus. In American Association for the Study of Liver Diseases (AASLD) Liver Meeting, Boston, abstract LB-21. 2014. |
| 153. | Yuen MF, Liu K, Chan HL, Given BD, Schluep T, Hamilton J, Lai CL, Locarnini SA, Lau JY, Ferrari C, Gish RG. Prolonged RNA interference therapy with ARC-520 Injection in treatment naïve, HBeAg positive and negative patients with chronic HBV results in significant reductions of HBs antigen. J Hepatol. 2017;1:p.S27. |
| 154. | Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Bréchot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology. 2004;40:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 155. | Fontaine H, Kahi S, Chazallon C, Bourgine M, Varaut A, Buffet C, Godon O, Meritet JF, Saïdi Y, Michel ML, Scott-Algara D, Aboulker JP, Pol S; ANRS HB02 study group. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial--ANRS HB02 VAC-ADN. Gut. 2015;64:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 156. | Zhang E, Lu M. Toll-like receptor (TLR)-mediated innate immune responses in the control of hepatitis B virus (HBV) infection. Med Microbiol Immunol. 2015;204:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 157. | Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye Z, Pflanz S, Garrison KL, Gaggar A, Mani Subramanian G, McHutchison JG, Kottilil S, Freilich B, Coffin CS, Cheng W, Kim YJ. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015;63:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 158. | Gane E, Gaggar A, Nguyen AH, Subramanian GM, McHutchison JG, Schwabe C, Dunbar R. A phase1 study evaluating anti-PD-1 treatment with or without GS-4774 in HBeAg negative chronic hepatitis B patients. J Hepatol. 2017;1:S26-S27. |
| 159. | Meng XJ. Expanding Host Range and Cross-Species Infection of Hepatitis E Virus. PLoS Pathog. 2016;12:e1005695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 160. | Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 161. | Mirazo S, Ramos N, Mainardi V, Gerona S, Arbiza J. Transmission, diagnosis, and management of hepatitis E: an update. Hepat Med. 2014;6:45-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 162. | Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, Russell K, Tettmar KI, Tossell J, Ushiro-Lumb I, Tedder RS. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 398] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 163. | Colson P, Coze C, Gallian P, Henry M, De Micco P, Tamalet C. Transfusion-associated hepatitis E, France. Emerg Infect Dis. 2007;13:648-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 164. | Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol. 2011;3:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 165. | Goumba CM, Yandoko-Nakouné ER, Komas NP. A fatal case of acute hepatitis E among pregnant women, Central African Republic. BMC Res Notes. 2010;3:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 166. | You S, Rong Y, Zhu B, Zhang A, Zang H, Liu H, Li D, Wan Z, Xin S. Changing etiology of liver failure in 3,916 patients from northern China: a 10-year survey. Hepatol Int. 2013;7:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 167. | Ren F, Zhao C, Wang L, Wang Z, Gong X, Song M, Zhuang H, Huang Y, Shan H, Wang J, Liu Q, Ness P, Nelson KE, Wang Y. Hepatitis E virus seroprevalence and molecular study among blood donors in China. Transfusion. 2014;54:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 168. | Izopet J, Labrique AB, Basnyat B, Dalton HR, Kmush B, Heaney CD, Nelson KE, Ahmed ZB, Zaman K, Mansuy JM, Bendall R, Sauné K, Kamar N, Arjyal A, Karkey A, Dongol S, Prajapati KG, Adhikary D. Hepatitis E virus seroprevalence in three hyperendemic areas: Nepal, Bangladesh and southwest France. J Clin Virol. 2015;70:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 169. | Kang YH, Cong W, Zhang XY, Wang CF, Shan XF, Qian AD. Hepatitis E virus seroprevalence among farmers, veterinarians and control subjects in Jilin province, Shandong province and Inner Mongolia Autonomous Region, China. J Med Virol. 2017;89:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 170. | Anastasiou OE, Thodou V, Berger A, Wedemeyer H, Ciesek S. Comprehensive Evaluation of Hepatitis E Serology and Molecular Testing in a Large Cohort. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 171. | Herremans M, Bakker J, Duizer E, Vennema H, Koopmans MP. Use of serological assays for diagnosis of hepatitis E virus genotype 1 and 3 infections in a setting of low endemicity. Clin Vaccine Immunol. 2007;14:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 172. | Takahashi M, Kusakai S, Mizuo H, Suzuki K, Fujimura K, Masuko K, Sugai Y, Aikawa T, Nishizawa T, Okamoto H. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) Is highly specific for diagnosis of acute HEV infection. J Clin Microbiol. 2005;43:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 173. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 437] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 174. | Khudyakov Y, Kamili S. Serological diagnostics of hepatitis E virus infection. Virus Res. 2011;161:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 175. | Khuroo MS, Khuroo MS. Hepatitis E: an emerging global disease - from discovery towards control and cure. J Viral Hepat. 2016;23:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 176. | Vollmer T, Diekmann J, Eberhardt M, Knabbe C, Dreier J. Monitoring of Anti-Hepatitis E Virus Antibody Seroconversion in Asymptomatically Infected Blood Donors: Systematic Comparison of Nine Commercial Anti-HEV IgM and IgG Assays. Viruses. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 177. | Sue PK, Pisanic N, Heaney CD, Mixson-Hayden T, Kamili S, Nelson K, Schwarz KB, Forman M, Valsamakis A, Ticehurst J, Karnsakul W. Variability of hepatitis E serologic assays in a pediatric liver transplant recipient: challenges to diagnosing hepatitis E virus infection in the United States. Transpl Infect Dis. 2015;17:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 178. | Yoo N, Bernstein J, Caldwell C, Dong C, Drobeniuc J, Kamili S, Landry ML. Hepatitis E virus infection in a liver transplant recipient: delayed diagnosis due to variable performance of serologic assays. Transpl Infect Dis. 2013;15:E166-E168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 179. | Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 180. | Haim-Boukobza S, Coilly A, Sebagh M, Bouamoud M, Antonini T, Roche B, Yordanova O, Savary J, Saliba F, Duclos-Vallee JC, Samuel D, Ichai P, Roque-Afonso AM. Hepatitis E infection in patients with severe acute alcoholic hepatitis. Liver Int. 2015;35:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 181. | Pérez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect Genet Evol. 2014;22:40-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 182. | Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 458] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 183. | Fousekis FS, Mitselos IV, Christodoulou DK. Extrahepatic manifestations of hepatitis E virus: An overview. Clin Mol Hepatol. 2020;26:16-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 184. | Tavitian S, Péron JM, Huynh A, Mansuy JM, Ysebaert L, Huguet F, Vinel JP, Attal M, Izopet J, Récher C. Hepatitis E virus excretion can be prolonged in patients with hematological malignancies. J Clin Virol. 2010;49:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 185. | Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150:430-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 186. | Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 187. | Giordani MT, Fabris P, Brunetti E, Goblirsch S, Romanò L. Hepatitis E and lymphocytic leukemia in Man, Italy. Emerg Infect Dis. 2013;19:2054-2056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 188. | Wang Y, Metselaar HJ, Peppelenbosch MP, Pan Q. Chronic hepatitis E in solid-organ transplantation: the key implications of immunosuppressants. Curr Opin Infect Dis. 2014;27:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 189. | Kamar N, Izopet J. Does chronic hepatitis E virus infection exist in immunocompetent patients? Hepatology. 2014;60:427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 190. | Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 191. | Tsega E, Krawczynski K, Hansson BG, Nordenfelt E. Hepatitis E virus infection in pregnancy in Ethiopia. Ethiop Med J. 1993;31:173-181. [PubMed] |
| 192. | Kamar N, Rostaing L, Izopet J. Hepatitis E virus infection in immunosuppressed patients: natural history and therapy. Semin Liver Dis. 2013;33:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 193. | Shrestha A, P Gupta B, K Lama T. Current Treatment of Acute and Chronic Hepatitis E Virus Infection: Role of Antivirals. Euroasian J Hepatogastroenterol. 2017;7:73-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 194. | Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 195. | Debing Y, Gisa A, Dallmeier K, Pischke S, Bremer B, Manns M, Wedemeyer H, Suneetha PV, Neyts J. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology. 2014;147:1008-11.e7; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 196. | Debing Y, Ramière C, Dallmeier K, Piorkowski G, Trabaud MA, Lebossé F, Scholtès C, Roche M, Legras-Lachuer C, de Lamballerie X, André P, Neyts J. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol. 2016;65:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 197. | Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Ann Intern Med. 2010;153:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 198. | Kamar N, Abravanel F, Garrouste C, Cardeau-Desangles I, Mansuy JM, Weclawiak H, Izopet J, Rostaing L. Three-monthweeks pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant. 2010;25:2792-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 199. | Kinast V, Burkard TL, Todt D, Steinmann E. Hepatitis E Virus Drug Development. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 200. | Shrestha MP, Scott RM, Joshi DM, Mammen MP Jr, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, David MP, Seriwatana J, Vaughn DW, Safary A, Endy TP, Innis BL. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 201. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 548] [Article Influence: 36.5] [Reference Citation Analysis (1)] |
| 202. | Park SB. Hepatitis E vaccine debuts. Nature. 2012;491:21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 203. | Pagliusi S, Dennehy M, Kim H; DCVMN AGM Organizing Committee. Vaccines, inspiring innovation in health. Vaccine. 2018;36:7430-7437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 204. | Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O'Riordan J, Boland F, Harritshøj L, Nascimento MSJ, Ciccaglione AR, Politis C, Adlhoch C, Flan B, Oualikene-Gonin W, Rautmann G, Strengers P, Hewitt P. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |