Published online Jun 28, 2022. doi: 10.13105/wjma.v10.i3.186
Peer-review started: April 13, 2022
First decision: May 31, 2022
Revised: June 14, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: June 28, 2022
Processing time: 83 Days and 3.6 Hours
It is generally accepted that the incidence of hepatocellular carcinoma (HCC) in hepatitis C virus (HCV)-associated patients is higher than that in hepatitis B virus (HBV)-associated patients. The reason why this difference in the incidence of HCC occurs in patients with HBV and HCV infections remains unclear. We report the possibility that the contributing power of inflammation, which is the main risk factor for developing HCC, may be different with HBV and HCV infections.
To investigate this, we surveyed the hazard ratio of inflammation for HCC development which was identified by serum alanine aminotransferase (ALT) levels between patients with HBV and HCV infections.
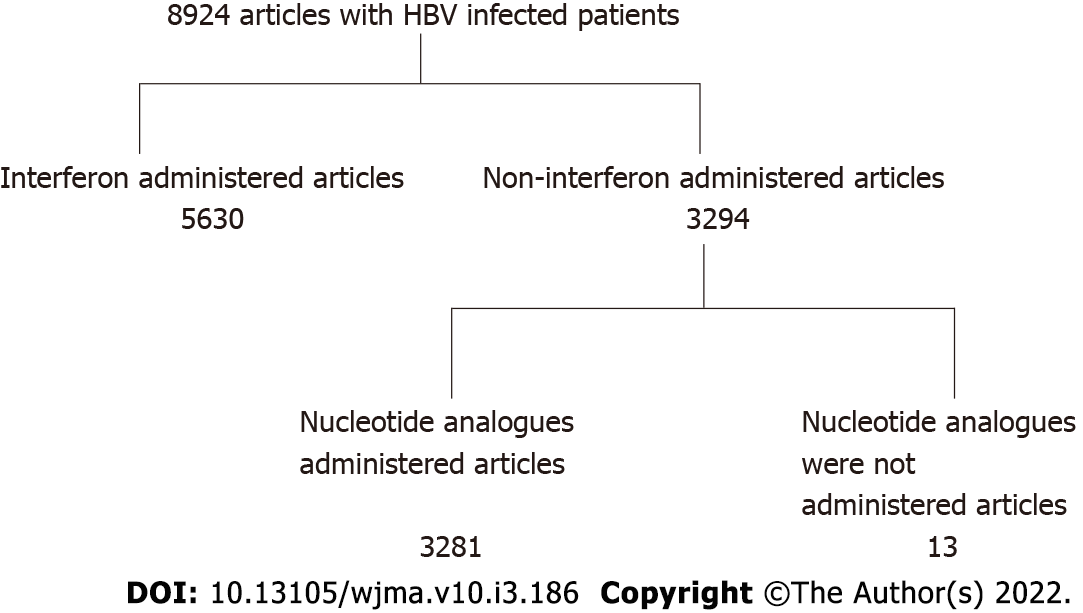
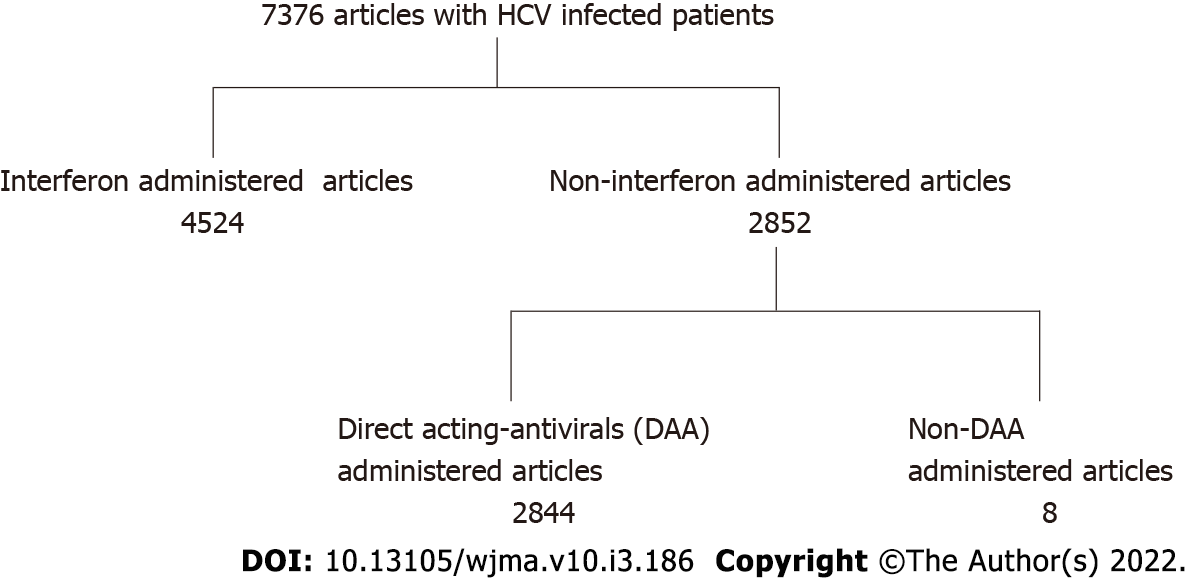
The PubMed database was searched (2001-2021) for studies published in English regarding the incidence of HCC identifying 8924 HBV-and 7376 HCV- infected patients. From these studies, interferon-treated patients with both HBV and HCV infections were excluded. Furthermore, in HBV patients, those administered nucleos(t)ide analogues were excluded, and in HCV patients, those administered direct acting antivirals were also excluded. Studies citing hazard ratios of HCC regarding inflammation (serum elevated alanine aminotransferase levels) were selected. Finally, there were 14 studies of HBV- infected patients and 8 studies of HCV-infected patients. We calculated the hazard ratio in patients in an inflammatory state (serum ALT levels were above the normal range).
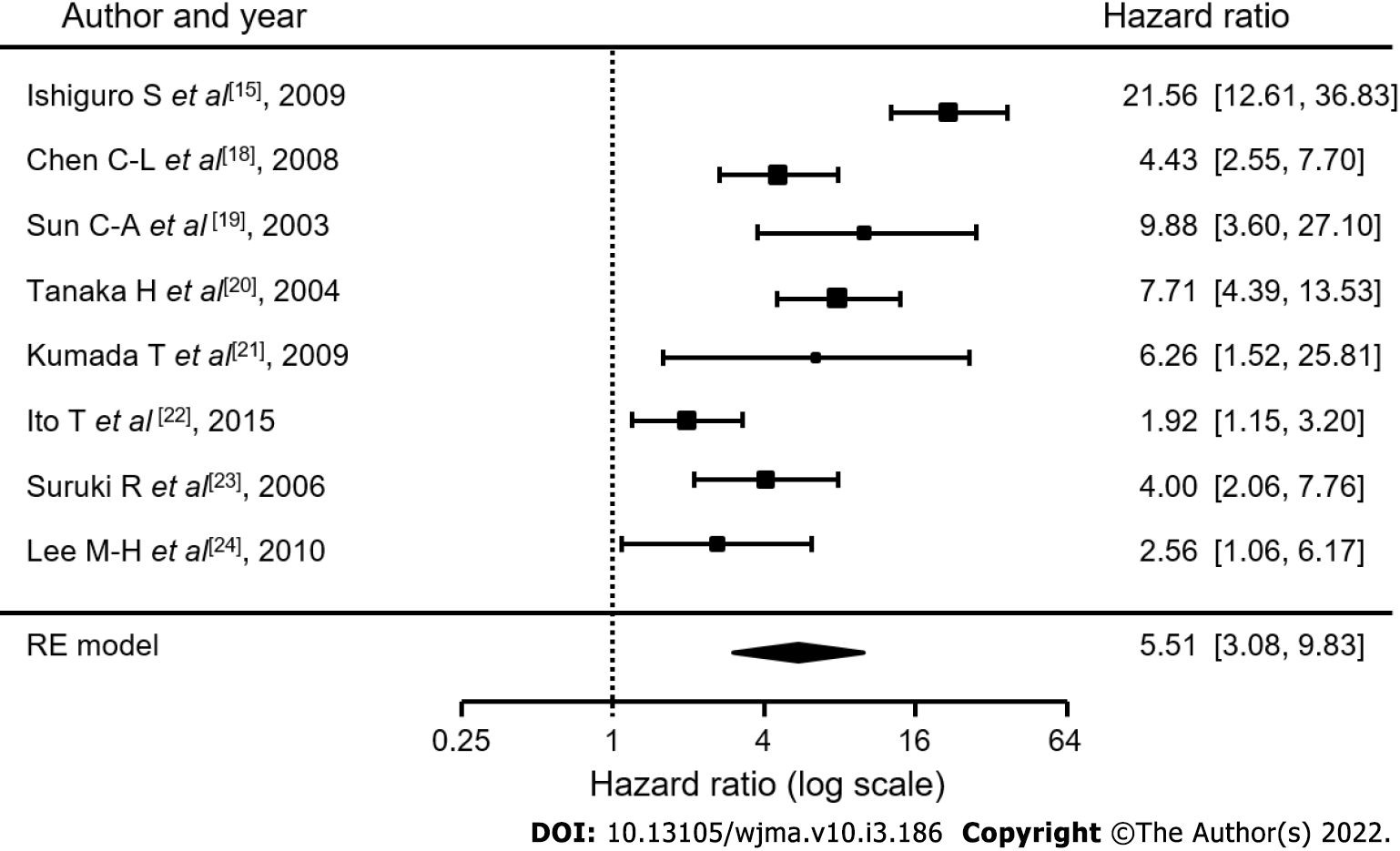
In the 14 studies of HBV patients, the average hazard ratio (HR) of elevated ALT for developing HCC was 2.74 [1.98-3.77] and that in the 8 studies of HCV-infected patients was 5.51 [3.08-9.83]. The HR of inflammation for HCC development in HCV-associated liver diseases is about twice that in HBV-associated liver diseases. HR in HCV-infected patients was significantly (P = 0.0391) higher than that in HBV-infected patients. In hepatitis B patients, the abnormal range adopted was 28-45 IU/L, and in hepatitis C patients, it was 20-50 IU/L. It was demonstrated that the abnormal ALT levels adopted in hepatitis B and C patients were very similar in this series.
The difference in the incidence of HCC development between HBV and HCV patients may depend on the difference in the hazard risk of ALT between HBV and HCV infections.
Core Tip: It is generally accepted that the incidence of hepatocellular carcinoma (HCC) in hepatitis C virus (HCV)-associated of patients is higher than that in hepatitis B virus (HBV)-associated patients. We demonstrated that the incidence of HCC in HCV-associated cirrhotic patients was 4.81%/year as compared with 3.23% in HBV-associated patients based on analytic assessment of already published papers. In HBV infection, alanine aminotransferase (ALT) is the second highest risk factor, and in HCV infection, ALT is the highest risk factor, for HCC development. The hazard ratio (HR) for developing HCC in the inflammatory state (serum ALT levels exceeded the normal range) was compared between HBV and HCV patients. In the 14 studies of HBV patients, the average HR was 2.74 as compared with 5.51 in the 8 studies of HCV patients (P = 0.0391). The difference in the incidence of HCC development between HBV and HCV patients may depend on the difference in the hazard risk of ALT for HCC development between HBV and HCV infections.
- Citation: Tarao K, Nozaki A, Komatsu H, Ideno N, Komatsu T, Ikeda T, Taguri M, Maeda S. Difference in incidence of developing hepatocellular carcinoma between hepatitis B virus-and hepatitis C virus-infected patients. World J Meta-Anal 2022; 10(3): 186-194
- URL: https://www.wjgnet.com/2308-3840/full/v10/i3/186.htm
- DOI: https://dx.doi.org/10.13105/wjma.v10.i3.186
It is generally accepted that the incidence of hepatocellular carcinoma (HCC) in hepatitis C virus (HCV)-associated of patients is higher than that in hepatitis B virus (HBV)-associated patients. We demonstrated that the incidence of HCC in HCV-associated cirrhotic patients was 4.81%/year as compared with 3.23% in HBV-associated patients based on analytic assessment of already published papers[1].
However, the reason why this difference in incidence of HCC occurs in patients with HBV and HCV infections remains unclear. We have been considering this for many years, and finally arrived at the possibility that the contributing power of inflammation, which is the main risk factor for developing HCC, may be different with HBV and HCV infections.
To investigate this, we surveyed the hazard ratio (HR) of inflammation which was identified by serum alanine aminotransferase (ALT) levels between patients with HBV and HCV infections.
Why ALT, not AST was adopted in this study was as follows: We previously demonstrated[2] the strong association between sustained high serum ALT levels (≥ 80 international units (INU) annual average) and the development of HCC in patients with HCV-LC (Child Stage A) by long-term observation lasting about 7 years, (Cancer 1999; 86: 589-595). In this series of the study, we also investigated the association between sustained high serum AST levels (≥ 80 INU) and development, but the association was not so strong as ALT. Moreover, many studies have demonstrated a close association between severe inflammation as estimated by higher serum ALT level and initiation of HCC development (Veldt et al[3]; Miyakawa et al[4]).
The PubMed database was searched (2001-2021) for studies published in English regarding the incidence of HCC in HBV or HCV infected patients. There were 8924 studies involving HBV patients, and 7376 studies of HCV patients. From these studies, interferon-treated patients with both HBV and HCV infections were excluded. Furthermore, in HBV patients, those who were administered nucleos(t)ide analogues were excluded, and HCV patients administered direct acting antivirals were also excluded. We also excluded articles which include co-existing liver disease such as alcoholic liver diseases and/or fatty liver diseases. Then, studies which dealt with the HR of HCC regarding inflammation (serum elevated ALT levels) were selected. Finally, there were 13 studies of HBV-infected patients[5-17], and 8 studies of HCV-infected patients[13,18-24] (Figures 1 and 2). In these selected papers, the HR of patients in a non-inflammatory state (serum ALT levels within normal range) was set as 1. We then calculated the HR in patients in an inflammatory state (serum ALT levels were above normal range).
Furthermore, for the purpose of comparing elevated ALT levels between hepatitis B and C patients, we examined the actual ALT levels cited in patients with chronic hepatitis B and hepatitis C included in this series (Tables 1 and 2) .
| Ref. | Actual elevated ALT levels |
| Kim et al[5] | Above normal levels |
| Du et al[6] | Above normal levels |
| Choi et al[7] | Above normal levels |
| Wen et al[8] | ≥ 25 IU/L |
| Hann et al[11] | Elevated |
| Chen et al[12] | ≥ 45 IU/L |
| Kumada et al[13] | Absence of persistently normal ALT levels |
| Chen et al[14] | Above normal levels |
| Ishiguro et al[15] | ≥ 30 IU/L |
| Ando et al[16] | ≥ 23 IU/L |
| Yamada et al[17] | ≥ 40 IU/L |
To compare HR of ALT for HCC between HBV and HCV patients, we calculated the weighted mean of HR for each type using the random effect model (Ref.: Dersimonian R, Laird N. Meta-analysis in Clinical trials. Controlled Clinic Trials 1986; 7: 177-188). To assess whether the mean HR among HBV patients was lower than that among HCV patients, we calculated the P value using a Z test. All reported p- values correspond to two-sided tests, and those P < 0.05 were considered significant. All analyses were performed using R (version 4.1.2) and R Studio (version 1.4) software.
In the 14 studies of HBV patients[5-17], the average HR of elevated ALT for developing HCC was 2.74 [1.98-3.77] (Figure 3), and that in 8 studies of HCV-infected patients[12,15-21] was 5.51 [3.08-9.83] (Figure 4). It was demonstrated that the HR of inflammation for HCC development in HCV-associated liver diseases is about twice that in HBV-associated liver diseases. The HR in HCV-infected patients was significantly (P = 0.0391) higher than that in HBV-infected patients.
In hepatitis B patients, the abnormal range adopted was 28-45 IU/L (Table 1), and in hepatitis C patients, it was 20-50 IU/L (Table 2). It was demonstrated that the abnormal ALT levels adopted in hepatitis B and C patients were very similar in this series.
There are many risk factors for developing HCC: Sex, age, ALT, α-fetoprotein, presence of cirrhosis, habitual alcohol consumption, tabaco, and diabetes mellitus are typically cited, and HBV-DNA[1,3,6-8,10] and the HBV genotype[9] are added for chronic HBV infection. The HCV genotype is also cited for HCV infection[21]. To study the impact of ALT on HCC development in chronic hepatitis B and chronic hepatitis C virus infections, we initially surveyed risk factors for HCC that are strongly associated with its development.
As shown in Table 3, the HR for developing HCC for each item in patients with chronic hepatitis B virus infection was 2.52 for sex, 3.15 for age, 2.212 for HBV-DNA, 3.37 for ALT, and 6.42 for presence of cirrhosis. Except for the presence of cirrhosis, ALT shows the highest risk ratio for HCC development.
| Ref. | Sex | Age | HBV-DNA | ALT | AFP | Presence of cirrhosis | HBV genotype | Alcohol use | Tabaco | DM |
| Kim et al[5] | 2.782 | 1.080 | 0.986 | 2.641 | 2.955 | 2.105 | 2.00 | |||
| Du et al[6] | 2.94 | 3.30 | 2.55 | 2.45 | ||||||
| Choi et al[7] | 1.67 | 1.05 | 1.02 | 1.54 | 1.21 | 1.54 | ||||
| Wen et al[10] | 1.93 | 5.34 | 1.93 | |||||||
| Hann et al[11] | 1.21 | 2.60 | ||||||||
| Chen et al[12] | 3.12 | 5.75 | 7.961 | 2.05 (Type C) | ||||||
| Kumada et al[13] | 6.011 | 5.125 | 3.939 | 6.779 | 18.033 | |||||
| Chen et al[14] | 1.2 | 2.0 | 1.6 | 1.7 | 2.3 | 1.9 | ||||
| Ishiguro et al[15] | 10.5 | 2.183 | ||||||||
| Ando et al[16] | 2.200 | 3.395 | 1.442 | 1.914 | 1.967 | |||||
| Yamada et al[17] | 1.44 | 5.867 | 5.59 | |||||||
| Average | 2.52 | 3.15 | 2.212 | 3.37 | 6.42 |
As shown in Table 4, in patients with chronic hepatitis C virus infection, it was 5.486 for age and 5.877 for ALT. The value for ALT was higher than that for age. In HBV infection, ALT is the second-highest risk factor, and in HCV infection, ALT is the higher risk factor.
In support of our findings, Benvegnù et al[25] demonstrated that patients with HCV infection with persistently elevated or fluctuating ALT levels during the observation period demonstrated a significantly higher rate of HCC development compared with patients in whom ALT remained or became normal during follow-up. This observation confirms that the activity of liver disease, which is characterized by inflammation, necrosis, and regeneration, plays an important role in promoting HCC development and suggests that medical interventions that limit disease activity may prevent or delay neoplastic transformation and tumor growth.
Furthermore, we demonstrated that the average HR of ALT for HCC development in HCV patients is about twice that in HBV patients (P < 0.05).
In conclusion, the difference in the incidence of HCC development between HBV and HCV patients may depend on the difference in the HR of ALT between HBV and HCV infections.
It is generally accepted that the incidence of hepatocellular carcinoma (HCC) in hepatitis C virus (HCV)-associated patients is higher than that in hepatitis B virus (HBV)-associated patients. We demonstrated that the incidence of HCC in HCV-associated cirrhotic patients was 4.81%/year compared with 3.23% in HBV-associated patients based on analytic assessment of already published papers.
The reason why this difference in incidence of HCC occurs in patients with HBV and HCV infections remains unknown. We considered the possibility that the contributing power of inflammation, which is the main risk factor for developing HCC, may be different with HBV and HCV infections.
To investigate this, we surveyed the hazard ratio of inflammation for HCC development, which was identified by serum alanine aminotransferase levels between patients with HBV and HCV infections.
The PubMed database was searched (2001-2021) for studies published in English regarding the incidence of HCC, identifying 8924 HBV-and7376 HCV-infected patients. From these studies, interferon-treated patients with both HBV and HCV infections were excluded. Furthermore, in HBV patients, those administered nucleos(t)ide analogues were excluded, and in HCV patients, those administered direct acting antivirals were also excluded. Studies citing hazard ratios of HCC regarding inflammation (serum elevated alanine aminotransferase levels) were selected. Finally, there were 14 studies of HBV- infected patients and 8 studies of HCV-infected patients. We calculated the hazard ratio in patients in an inflammatory state (serum ALT levels were above the normal range).
In the 14 studies of HBV patients, the average hazard ratio (HR) of elevated ALT for developing HCC was 2.74 [1.98-3.77], and that in the 8 studies on HCV-infected patients was 5.51 [3.08-9.83]. HR in HCV-infected patients was about twice that in HBV-infected patient, and was significantly (P = 0.0391) higher than that in HBV-infected patients. In hepatitis B patients, the abnormal range adopted was 28-45 IU/L, and in hepatitis C patients, it was 20-50 IU/L. It was demonstrated that the abnormal ALT levels adopted in hepatitis B and C patients were very similar in this series.
The difference in the incidence of HCC development between HBV and HCV patients may depend on the difference in the HR of ALT between HBV and HCV infections.
In this study, it was demonstrated that the HR of inflammation for HCC development in HCV-associated liver diseases is about twice that in HBV-associated liver diseases. So, we must optimally suppress inflammation in patients with HCV-associated liver diseases to prevent HCC development.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ciotti M, Italy; Talal A, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T, Taguri M, Tanaka K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019;8:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N, Totsuka S. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999;86:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Veldt BJ, Hansen BE, Ikeda K, Verhey E, Suzuki H, Schalm SW. Long-term clinical outcome and effect of glycyrrhizin in 1093 chronic hepatitis C patients with non-response or relapse to interferon. Scand J Gastroenterol. 2006;41:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Miyakawa K, Tarao K, Ohshige K, Morinaga S, Ohkawa S, Okamoto N, Shibuya A, Adachi S, Miura Y, Fujiyama S, Miyase S, Tomita K. High serum alanine aminotransferase levels for the first three successive years can predict very high incidence of hepatocellular carcinoma in patients with Child Stage A HCV-associated liver cirrhosis. Scand J Gastroenterol. 2009;44:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kim S, Lee Y, Bang SM, Bak H, Yim SY, Lee YS, Yoo YJ, Jung YK, Kim JH, Seo YS, Yim HJ, Um SH, Byun KS, Yeon JE. Early Normalization of Alanine Aminotransferase during Antiviral Therapy Reduces Risk of Hepatocellular Carcinoma in HBV Patients. J Clin Med. 2021;10:1840-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Du Y, Du B, Fang X, Shu M, Zhang Y, Chung H, Sun Y, Teng J, Visalath P, Qiu H, Cai W. ALT Flare Predicts Hepatocellular Carcinoma Among Antiviral Treated Patients With Chronic Hepatitis B: A Cross-Country Cohort Study. Front Oncol. 2021;10:615203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Choi J, Kim GA, Han S, Lim YS. Earlier Alanine Aminotransferase Normalization During Antiviral Treatment Is Independently Associated With Lower Risk of Hepatocellular Carcinoma in Chronic Hepatitis B. Am J Gastroenterol. 2020;115:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Wong GL, Chan HL, Tse YK, Yip TC, Lam KL, Lui GC, Wong VW. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Lee J, Sinn DH, Kim JH, Gwak GY, Kim HS, Jung SH, Paik YH, Choi MS, Lee JH, Koh KC, Yoo BC, Paik SW. Hepatocellular Carcinoma Risk of Compensated Cirrhosis Patients with Elevated HBV DNA Levels according to Serum Aminotransferase Levels. J Korean Med Sci. 2015;30:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Wen CP, Lin J, Yang YC, Tsai MK, Tsao CK, Etzel C, Huang M, Hsu CY, Ye Y, Mishra L, Hawk E, Wu X. Hepatocellular carcinoma risk prediction model for the general population: the predictive power of transaminases. J Natl Cancer Inst. 2012;104:1599-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Hann HW, Wan S, Myers RE, Hann RS, Xing J, Chen B, Yang H. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS One. 2012;7:e47687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, Lu SN, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL–HBV) Study Group. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240-1248, 1248.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kanamori A, Atsumi H, Takagi M, Arakawa T, Fujimori M. Incidence of hepatocellular carcinoma in patients with chronic hepatitis B virus infection who have normal alanine aminotransferase values. J Med Virol. 2010;82:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Ishiguro S, Inoue M, Tanaka Y, Mizokami M, Iwasaki M, Tsugane S; JPHC Study Group. Serum aminotransferase level and the risk of hepatocellular carcinoma: a population-based cohort study in Japan. Eur J Cancer Prev. 2009;18:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ando Y, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Ishikawa T, Nakano I, Hirooka Y, Goto H. Cumulative incidence and risk factors for the development of hepatocellular carcinoma in patients with chronic hepatitis B who achieved sustained disappearance of viremia by nucleos(t)ide analog treatment. Hepatol Res. 2018;48:E240-E251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Yamada R, Hiramatsu N, Oze T, Morishita N, Harada N, Yakushijin T, Iio S, Doi Y, Yamada A, Kaneko A, Hagiwara H, Mita E, Oshita M, Itoh T, Fukui H, Hijioka T, Katayama K, Tamura S, Yoshihara H, Imai Y, Kato M, Miyagi T, Yoshida Y, Tatsumi T, Kasahara A, Hamasaki T, Hayashi N, Takehara T; Osaka Liver Forum. Impact of alpha-fetoprotein on hepatocellular carcinoma development during entecavir treatment of chronic hepatitis B virus infection. J Gastroenterol. 2015;50:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 19. | Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, Wu MH, Chen CJ. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Tanaka H, Tsukuma H, Yamano H, Oshima A, Shibata H. Prospective study on the risk of hepatocellular carcinoma among hepatitis C virus-positive blood donors focusing on demographic factors, alanine aminotransferase level at donation and interaction with hepatitis B virus. Int J Cancer. 2004;112:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kanamori A, Atsumi H, Takagi M, Nakano S, Arakawa T, Fujimori M. Incidence of hepatocellular carcinoma in hepatitis C carriers with normal alanine aminotransferase levels. J Hepatol. 2009;50:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Ito T, Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S. Utility of the FIB-4 Index for hepatocarcinogenesis in hepatitis C virus carriers with normal alanine aminotransferase levels. J Viral Hepat. 2015;22:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Suruki R, Hayashi K, Kusumoto K, Uto H, Ido A, Tsubouchi H, Stuver SO. Alanine aminotransferase level as a predictor of hepatitis C virus-associated hepatocellular carcinoma incidence in a community-based population in Japan. Int J Cancer. 2006;119:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ, Chen CJ. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587-4593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Benvegnù L, Alberti A. Risk factors and prevention of hepatocellular carcinoma in HCV infection. Dig Dis Sci. 1996;41:49S-55S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |