Published online Jun 28, 2022. doi: 10.13105/wjma.v10.i3.162
Peer-review started: February 4, 2022
First decision: April 19, 2022
Revised: May 2, 2022
Accepted: June 22, 2022
Article in press: June 22, 2022
Published online: June 28, 2022
Processing time: 151 Days and 1 Hours
Mesenchymal stromal cell (MSC)-based cellular therapy promotes type I collagen production, enhance mechanical strength of tissues, and enhance biology at the bone-tendon interface, which primarily explains their potential clinical utility in rotator cuff (RC) tears.
To analyze the efficacy and safety of cellular therapy utilizing MSCs in the management of RC tears from clinical studies available in the literature.
We conducted independent and duplicate electronic database searches including PubMed, Embase, Reference Citation Anallysis, Web of Science, and Cochrane Library in August 2021 for studies analyzing the efficacy and safety of cellular therapy (CT) utilizing MSCs in the management of RC tears. Visual Analog Score (VAS) score for pain, American Shoulder and Elbow Surgeons (ASES) score, Disability of the Arm, Shoulder, and Hand score, Constant score, radiological assessment of healing, and complications such as retear rate and adverse events were the outcomes analyzed. Analysis was performed in R-platform using OpenMeta [Analyst] software.
Six studies involving 238 patients were included for analysis. We noted a significant reduction in VAS score for pain at 3 mo (weighed mean difference [WMD] = -2.234, P < 0.001) and 6 mo (WMD = -3.078, P < 0.001) with the use of CT, which was not maintained at long-term follow-up (WMD = -0.749, P = 0.544). Concerning functional outcomes, utilization of CT produced a significant short-term improvement in the ASES score (WMD = 17.090, P < 0.001) and significant benefit in functional scores such as Constant score (WMD = 0.833, P = 0.760) at long-term follow-up. Moreover, we also observed significantly improved radiological tendon healing during the long-term follow-up (odds ratio [OR] = 3.252, P = 0.059). We also noted a significant reduction in the retear rate upon utilization of CT in RC tears both at short- (OR = 0.079, P = 0.032) and long-term (OR = 0.434, P = 0.027) follow-ups. We did not observe any significant increase in the adverse events directly related to cellular therapy, as compared with the control group (OR = 0.876, P = 0.869).
Based on our comprehensive and critical review, we could observe that the utilization of CT in RC tear significantly reduced pain severity at 3 and 6 mo, improved short-term functional outcome, enhanced radiological tendon healing, and mitigated retear rates at both short- and long-term follow-ups. The literature also confirmed the relative safety of using MSC therapy in patients presenting with RC tears.
Core Tip: Biological augmentation of rotator cuff tears with mesenchymal stromal cell-based cellular therapy significantly reduces the pain severity and improves the functional outcome at 3 and 6 mo based on our critical review of clinical studies on the subject. Moreover, we noted enhanced radiological tendon healing, and mitigated retear rates at both short- and long-term follow-ups. We have also established the safety of using cellular therapy in patients presenting with rotator cuff tears.
- Citation: Muthu S, Mogulesh C, Viswanathan VK, Jeyaraman N, Pai SN, Jeyaraman M, Khanna M. Is cellular therapy beneficial in management of rotator cuff tears? Meta-analysis of comparative clinical studies. World J Meta-Anal 2022; 10(3): 162-176
- URL: https://www.wjgnet.com/2308-3840/full/v10/i3/162.htm
- DOI: https://dx.doi.org/10.13105/wjma.v10.i3.162
Rotator cuff (RC) tear is a common shoulder pathology, whose prevalence ranges from 4% in asymptomatic individuals younger than 40 years to 54% in patients aged over 60 years[1]. The etiology of these tears is multifactorial, and has been variously attributed to traumatic, mechanical, and inflammatory processes[2]. It has been well-demonstrated that the natural course of non-operatively managed RC tears in the majority of patients is a progressive deterioration of the anatomical tear without spontaneous regression of symptoms[3]. On the other hand, although surgical repair of a torn RC potentially aids in restoring the shoulder function as well as arresting the tear progression[4], the failure rates range between 0 and 78%, thereby giving room for improvement[5].
In view of obtaining predictable and consistent results in the management of RC tears, biological adjuvants like platelet-rich plasma (PRP) and stem cells (SC) have been tried to augment the regeneration of damaged RCs and improve the outcome following surgical repair[6]. Recently, mesenchymal stromal cells (MSCs) have been successfully employed in diverse animal and human models in the repair of various musculoskeletal structures like cartilages, bones, muscles, and tendons[7]. These cells (usually extracted from bone marrow or adipose tissue) possess a unique attribute described as “multipotency”, which denotes their ability to differentiate into other tissues of mesenchymal origin[2,8]. When delivered using appropriate scaffolds, these modalities of cellular therapy (CT) have shown great promise in enhancing the outcome following RC tears too[2,8-13]. MSCs are thought to promote type I collagen production, enhance mechanical strength of tissues, and ameliorate biology at the bone-tendon interface, which primarily explains their potential clinical utility in RC tears[8-13]. The concentrates of these cells may be delivered into the region of tendon injury, either via image-guided injections or through arthroscopic approach (intra-operatively)[8-13]. However, the major barriers to regular use of MSCs include lack of standardized techniques for preparation, inadequate clinical evidence, and potentially high cost:benefit ratios.
With this backdrop, in order to further enhance the understanding of their utility with clinical evidence on utilization of MSC-based CT in the management of RC tears, we performed a meta-analysis of clinical studies available in the literature to systematically analyze the efficacy and safety of CT utilizing MSCs in the management of RC tears.
We performed this meta-analysis following the guidelines made out by the Back Review Group of Cochrane Collaboration[14] and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[15].
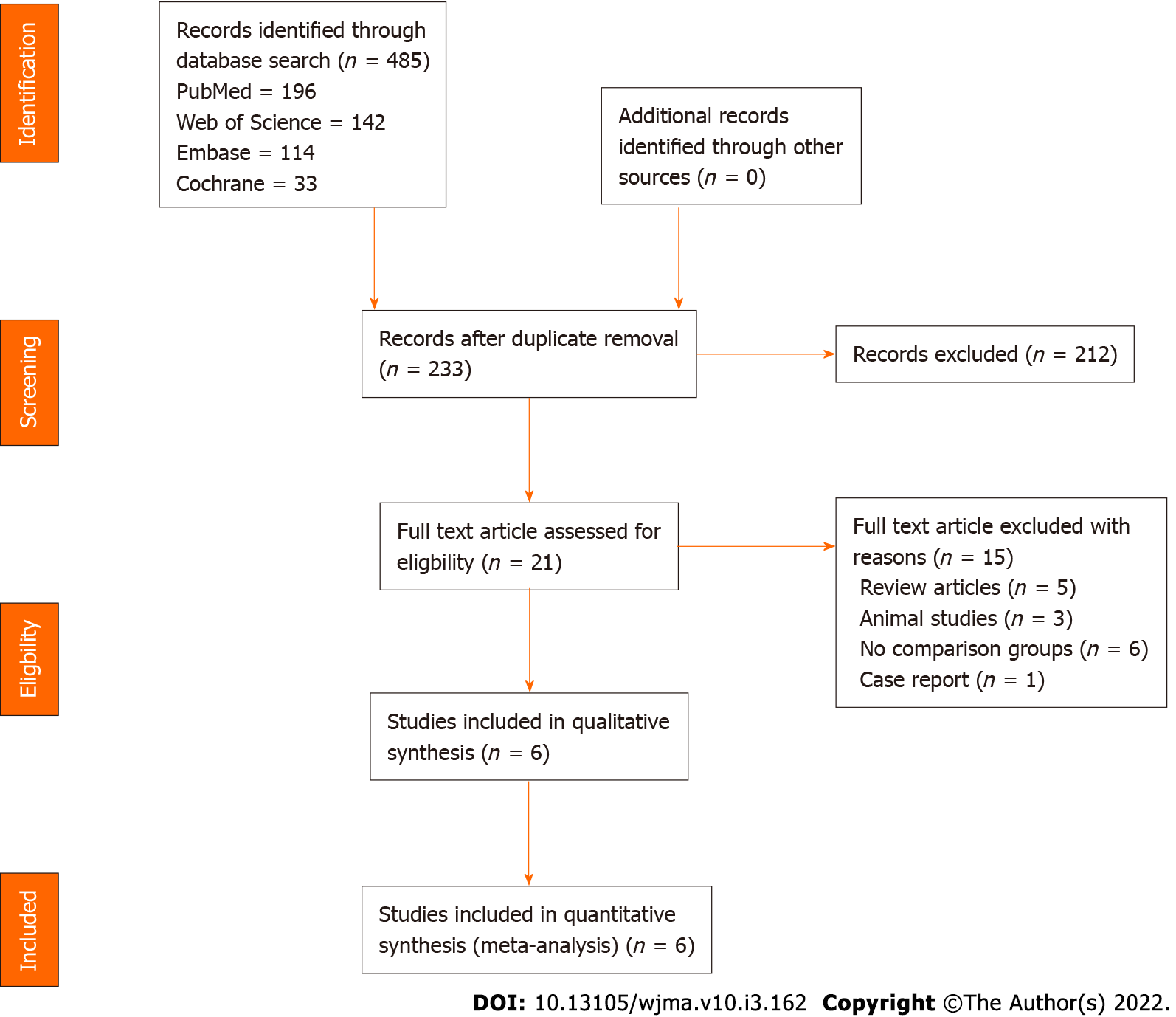
Two reviewers performed an independent electronic literature search for studies evaluating the efficacy and safety of MSC-based CT in the management of RC tears. We searched the following databases: PubMed, Embase, Reference Citation Analysis, Web of Science, and the Cochrane Library up to August 2021. No language or date restrictions were applied. Keywords used for the search were as follows: “Cellular therapy”, “Mesenchymal Stromal Cells”, “Stem Cell Therapy”, “Mesenchymal Stromal Cells”, “Bone marrow”, “Adipose”, “Rotator cuff tear”, and “Supraspinatus tear”. We have presented the search strategy used in one of the included databases in Supplementary Material 1. We also looked into the references of the included studies to identify additional studies that were not identified in the primary search. Based on the specified inclusion and exclusion criteria set as a priori, studies were analysed for inclusion into the analysis. In case of discrepancy between the authors upon selection of studies into the analysis, discussion was made until a consensus was obtained. PRISMA flow diagram of inclusion of studies in the analysis is given in Figure 1.
The PICOS criteria to include the studies into the review were as follows:
Population: Patients with RC tears.
Intervention: MSC-based biological therapy.
Comparator: Placebo.
Outcomes: Visual Analog Score (VAS) score for pain, American Shoulder and Elbow Surgeons (ASES) score, Disability of the Arm, Shoulder and Hand (DASH) score, Constant score, ultrasonogram (USG) or magnetic resonance imaging (MRI) based assessment of healing, and complications such as retear rate and adverse events reported.
Study design: Comparative clinical studies (CCSs).
Studies that had the following characteristics were excluded from the inclusion into the review: In vitro studies on stem cell therapy for tendon injury; Observational studies and non-comparative interventional studies on RC tears; Animal model studies of tendon injury investigating stem cell therapy; and Review articles on CT for RC tears.
The following relevant data from the included studies were retrieved by two reviewers for analysis.
Study characteristics: Year of publication, authors, country, nature of the study, level of evidence, and number of enrolled patients.
Baseline characteristics: Age, gender proportions, nature of RC tear, intervention for both the groups, source of MSC utilized, delivery method of MSCs, follow-up duration, and assessment parameters utilized.
Efficacy outcomes: VAS score for pain, ASES score, DASH score, Constant score, and USG/MRI based assessment of healing.
Safety outcomes: Complications such as retear rate and adverse events reported in the included studies.
In case of any disagreement between the authors in data collection, it was resolved by discussion until a consensus was achieved.
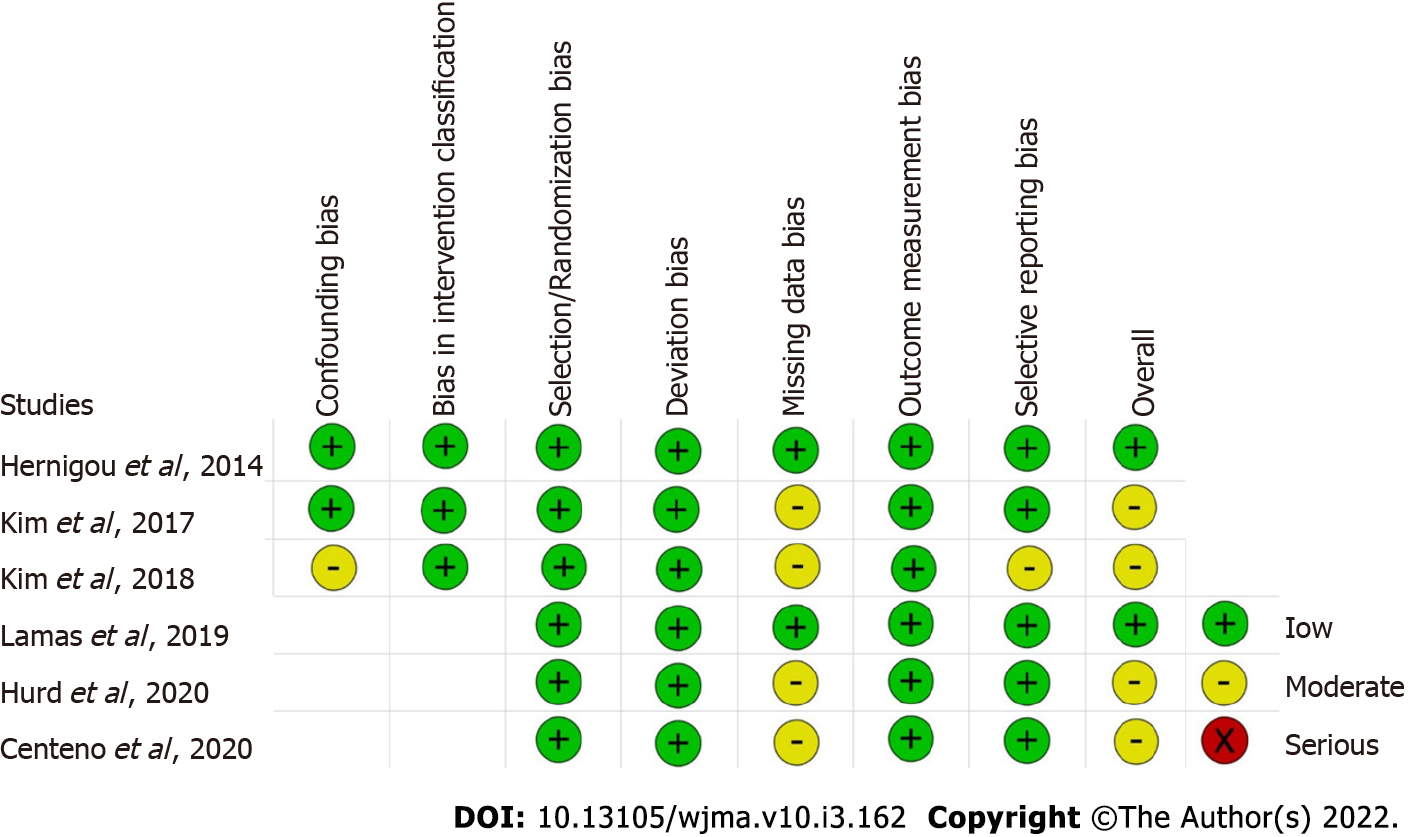
Two reviewers independently assessed the methodological quality of the included studies based on the ROB2 tool of Cochrane Collaboration for randomized studies. It consists of five domains of bias assessment including bias in randomization process, bias due to deviation from intended intervention, bias due to missing outcome data, bias in measurement of the outcome, and bias in selective reporting of results[16]. Similarly, the methodological quality of the non-randomized comparative studies were assessed using ROBINS-1 tool of Cochrane Collaboration which have seven domains of bias assessment including confounding bias and bias in intervention classification apart from the five domains described previously for randomized studies[17].
We conducted the meta-analysis in the R-platform with OpenMeta [Analyst][18]. We utilized odds ratio (OR) with 95% confidence interval (CI) for dichotomous outcomes and weighted mean difference (WMD) with 95%CI for continuous variable outcomes. We analyzed the heterogeneity in the included studies using the I2 test[19]. In case of I2 value < 50% and P value > 0.1, a fixed-effects model was used for evaluation. Otherwise, a random-effects model was used. We considered a P value < 0.05 to be significant. We also performed sensitivity analysis and subgroup analysis to analyse the source of heterogeneity when it existed. Funnel plot and normal quantile plot were used for the publication bias assessment of the outcomes in the included studies along with Egger’s regression test.
Electronic database search resulted in 485 articles which, after initial screening for duplicate removal, gave a total of 233 articles. Title and abstract screening were done in those 233 articles and 212 of them were excluded. Twenty-one articles were qualified for full-text review, of which 15 were excluded. A list of articles excluded from full-text review with reasons is presented in Supplementary Material 2. We included six studies[2,8-12] (3 randomized controlled trials[2,9,12], 1 prospective controlled study[8], and 2 retrospective comparative studies[10,11]) with 238 patients for meta-analysis. PRISMA flow diagram of study selection is given in Figure 1. We excluded most of the studies since they did not have a comparator group in their study design, which resulted in a low number of included studies for analysis. Considering the specificity of the research question, we considered it would be useful if a meaningful result could be arrived with the analysis of the comparative studies identified based on the predefined screening protocol. Four of the included six studies[2,8,11,12] utilized MSCs from bone marrow, while the remaining two studies[9,10] used adipose tissue as their source of MSCs. Only two of the included six studies[8,12] utilized platelet-rich plasma as an adjuvant to the cellular therapy intervention being used in them. We noted wide variability in the cellular dosage utilized in the included studies with a mean dosage of 141.15 ± 327.75 × 106 cells. While three studies[2,10,11] compared the intervention against the surgical repair of RC tears, two studies[8,12] compared it against exercise therapy, and one study[9] against steroid injection. There was also no uniformity among the included studies for the outcome measures utilized to analyze the efficacy of the intervention. The general characteristics of the studies included are given in Table 1. The protocols of intervention used in the case and control groups along with the measures of outcome assessment are given in Table 2.
| No. | First author | Year | Country | Study design | Sample size | Cases/controls | MSC source | PRP | Cellular dosage (106 cells) | Comparator intervention | Case age (SD) | Control age (SD) | Case male: Female | Control male: Female | Follow-up timeline (mo) |
| 1 | P Hernigou | 2014 | France | RCS | 90 | 45/45 | BM-MSC | No | 0.051 | Surgery | 61 ± 4.5 | 61 ± 4.5 | 21:24 | 20:25 | 6, 120 |
| 2 | YS Kim | 2017 | Korea | RCS | 70 | 35/35 | AD-MSC | No | 4.46 | Surgery | 59.2 ± 3.4 | 57.6 ± 2.9 | 15:20 | 13:22 | 28.3 |
| 3 | SJ Kim | 2018 | Korea | PCS | 24 | 12/12 | BM-MSC | Yes | 1 | Exercise therapy | 54.9 ± 7.6 | 59.6 ± 7.2 | 05:07 | 08:04 | 0.7, 3 |
| 4 | JR Lamas | 2019 | Spain | RCT | 13 | 8/5 | BM-MSC | No | 20 | Surgery | 57.8 ± 6.5 | 61.8 ± 3.8 | 06:02 | 02:03 | 12 |
| 5 | C Centeno | 2020 | USA | RCT | 25 | 14/11 | BM-MSC | Yes | 810 | Exercise therapy | 46 ± 11 | 49 ± 11 | 09:05 | 05:06 | 1, 12 |
| 6 | JL Hurd | 2020 | USA | RCT | 16 | 11/5 | AD-MSC | No | 11.4 | Steroid | 64.6 ± 9.6 | 57.6 ± 6.2 | 08:03 | 05:00 | 6, 12 |
| Ref. | MSC type | MSC source | Rotator cuff tear nature | Preparation method | Cellular dosage (106 cells) | Treatment group intervention | Control group intervention | Outcome measures |
| Hernigou et al[11] | BM-MSC | Auto | Full thickness | 150 mL of bone marrow aspirate was serially centrifuged to result in 12 mL of BMAC | 0.051 | Arthroscopic rotator cuff repair followed by injection of BMAC of 4 mL in bone-tendon junction, and 8 mL in the bone at the site of the footprint | Arthroscopic rotator cuff repair only | USG and MRI guided tear size assessment |
| Kim et al[4] | AD-MSC | Auto | Full thickness | The raw lipoaspirates were processed to obtain SVF using enzymatic digestion method using 0.075% collagenase | 4.46 | Arthroscopic rotator cuff repair followed by injection of AD-MSCs in SVF loaded in 2 mL of fibrin glue scaffold into the tendon-bone approximation and over the repaired tendon | Arthroscopic rotator cuff repair only | VAS, Constant score, UCLA scores, MRI guided tear size assessment |
| Kim et al[8] | BM-MSC | Auto | Partial thickness | BMAC from BIOMET MarrowStim Mini kit and PRP from BIOMET GPS III kit | 1 | USG guided injection of 2 mL BMAC with 1 mL of PRP | Rotator cuff exercises taught by experienced physical therapist done daily on their own for 3 mo | VAS, MMT scores, ASES, USG guided tear size assessment |
| Lamas et al[2] | CE-BM-MSC | Auto | Full thickness | The BM-MSCs isolated from aspirates were expanded during a 2-wk period | 20 | Open rotator cuff repair with autologous bone marrow in combination with a type I collagen membrane (OrthADAPT) | Open rotator cuff repair with a type I collagen membrane (OrthADAPT™) | VAS, Constant score, MRI guided tear size assessment |
| Centeno et al[12] | BM-MSC | Auto | Both partial and full thickness | 90 mL of bone marrow aspirate was serially centrifuged to a resultant 1-3 mL of buffy coat that is collected. In addition, 60mL of intravenous blood was drawn to isolate PRP and platelet lysate | 810 | USG guided injection of 1-2 mL of injectate with 60% by volume of BMAC, 20% of PRP, and 20% of platelet lysate | Stretches in all planes along with non-weighted exercises involving scapular stabilizing muscles, triceps, and the rotator cuff muscles for 3 mo | DASH score, SANE, MRI guided tear size assessment |
| Hurd et al[9] | AD-MSC | Auto | Partial thickness | Transpose RT/Matrase system | 11.4 | USG guided injection of 5 mL of AD-MSCs | Single injection of 80 mg of methylprednisolone in 2 mL plus 3 mL of 0.25% bupivacaine | Adverse events, ASES, SF-36 score, VAS, MRI guided tear size assessment |
The methodological quality of the included studies evaluated as per the RoB2 tool and ROBINS tool is presented in Figure 2. None of the included studies had a high risk of bias to be excluded from the analysis.
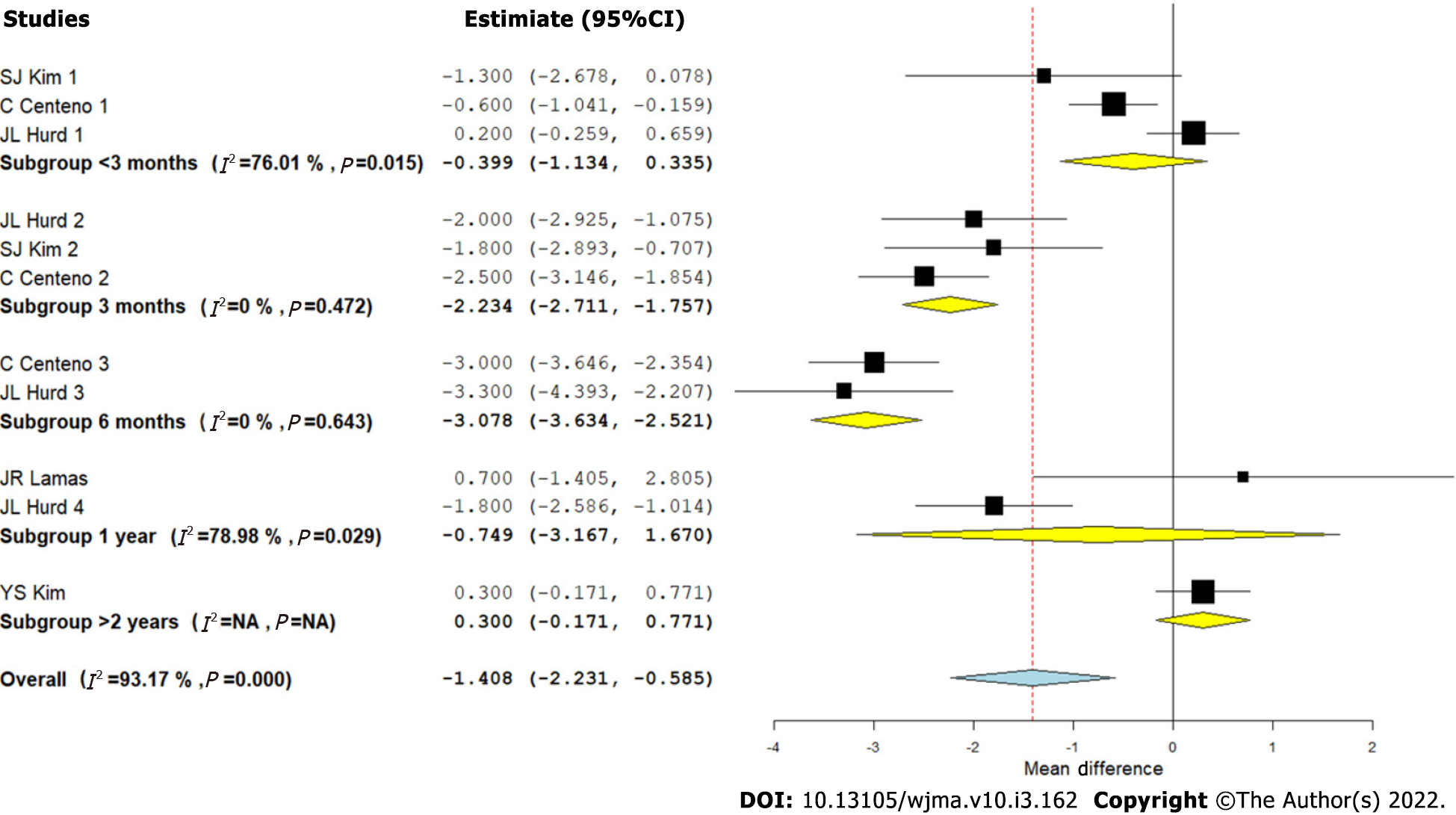
Visual Analog Scale score for pain: We analyzed five studies[2,8-10,12] comparing the VAS outcome upon using CT for RC tears against controls at varied time points. There was a significant heterogeneity observed between the included studies (I2 > 80%, P < 0.001). Hence, a random-effects model was used for analysis. We stratified the analysis based on the duration of follow-up in the included studies and found that upon utilizing CT in the management of RC tears, an overall significant reduction in VAS score for pain was noted compared to the controls (WMD = -1.408, 95%CI [-2.231, -0.585], P < 0.001). However, upon stratification of the studies based on the duration of their follow-up, it was noted that the pain reduction was not significant at < 3 mo (WMD = -0.399, 95%CI [-1.134, 0.335], P = 0.287), which improved significantly at 3 mo (WMD = -2.234, 95%CI [-2.711, -1.757], P < 0.001) and 6 mo (WMD = -3.078, 95%CI [-3.634, -2.521], P < 0.001). Upon analyzing the VAS scores at 1 year (WMD = -0.749, 95%CI [-3.167, 1.670], P = 0.544), and > 2 years (WMD = 0.3, 95%CI [-0.171, 0.771], P = 0.256), it was noted that the significance of the VAS reduction was lost at long-term follow-up as shown in Figure 3. Hence, utilization of CT produced a significant reduction of pain in the initial periods of inflammation and healing cascade caused by the injury and surgical repair procedure while in the long term, since the lesion heals in the surgical comparator groups, we did not note any significant difference (Figure 3).
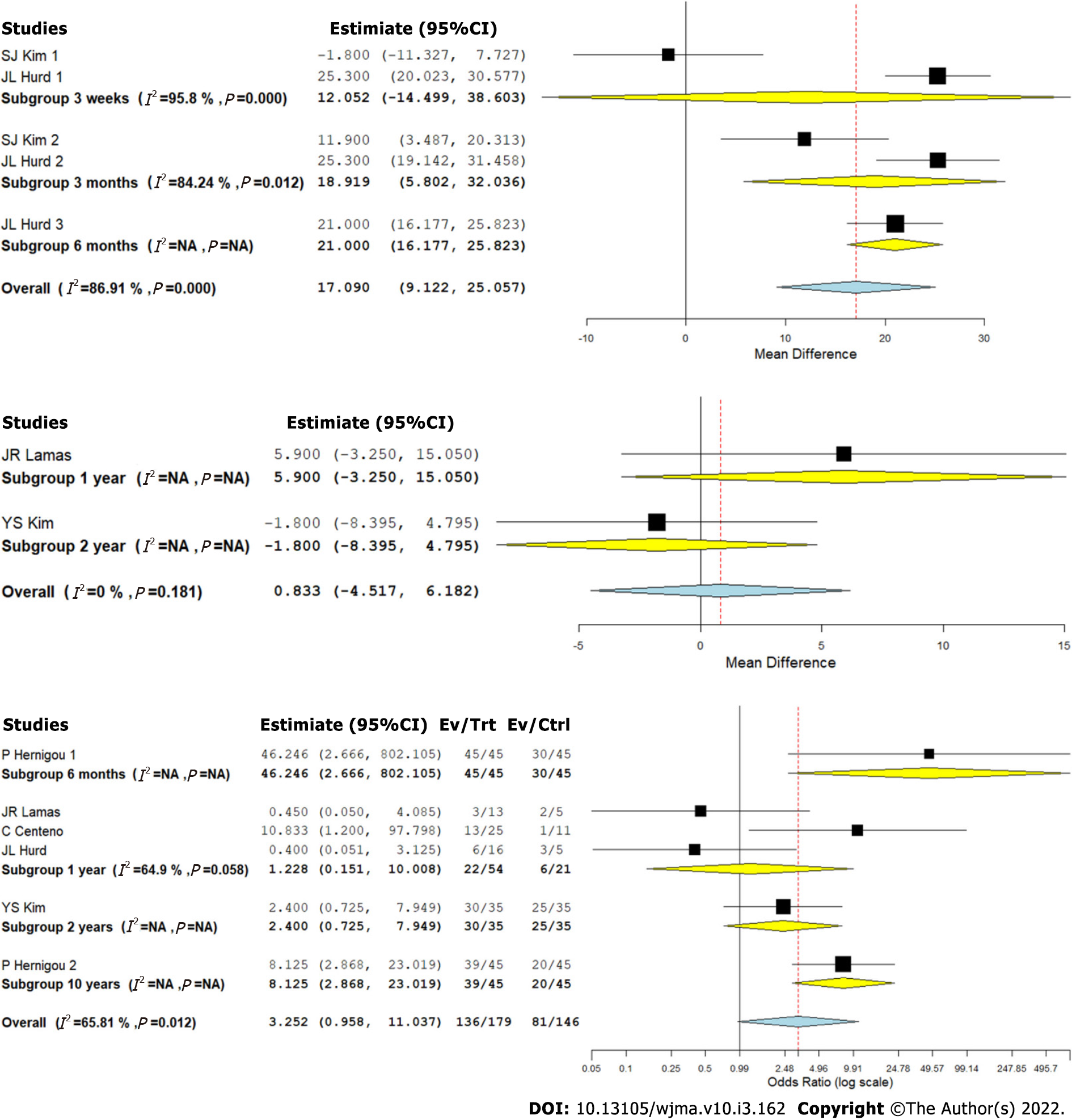
We analyzed two studies[8,9] comparing the ASES outcome upon using CT for RC tears against controls at varied time points. There was a significant heterogeneity observed between the included studies (I2 > 80%, P < 0.001). Hence, a random-effects model was used for analysis. We stratified the analysis based on the duration of follow-up in the included studies and found that upon utilizing CT in the management of RC tears, an overall significant improvement in ASES score was noted compared to the controls (WMD = 17.090, 95%CI [9.122, 25.057], P < 0.001). However, upon stratification of the studies based on the duration of their follow-up, it was noted that the functional improvement based on ASES score improvement was not significant at 3 wk (WMD = 12.052, 95%CI [-14.499, 38.603], P = 0.374), which improved significantly at 3 mo (WMD = 18.919, 95%CI [5.802, 32.036], P = 0.005) and 6 mo (WMD = 21.000, 95%CI [16.177, 25.823], P < 0.001) as shown in Figure 4A.
We analyzed two studies[2,8] comparing the Constant scores upon using CT for RC tears against controls at varied time points. There was no significant heterogeneity observed between the included studies (I2 < 50%, P = 0.181). Hence, a fixed-effects model was used for analysis. We stratified the analysis based on the duration of follow-up in the included studies and found that upon utilizing CT in the management of RC tears, we did not find any significant improvement in the Constant score compared to the controls (WMD = 0.833, 95%CI [-4.517, 6.182], P = 0.760). Both the studies included in the analysis compared the outcomes at 1 and 2 years (Figure 4B).
We analyzed five studies[2,9-12] reporting the MRI-based healing of RC tears upon using CT for RC tears against controls at varied time points. There was a significant heterogeneity observed between the included studies (I2 > 80%, P < 0.001). Hence, a random-effects model was used for analysis. We stratified the analysis based on the duration of follow-up in the included studies and found that upon utilizing CT in the management of RC tears, we did not note an overall significant difference in the healing of RC tears based on repeat MRI compared to the controls (OR = 3.252, 95%CI [0.958, 11.037], P = 0.059). However, upon stratification of the studies based on the duration of their follow-up, it was noted that the MRI based healing of the RC tears was significantly better at long-term follow-up compared to the controls (OR = 8.125, 95%CI [2.868, 23.019], P < 0.001) as shown in Figure 4C.
Hence, utilization of CT produced significant improvement in functional outcomes in short term based on the ASES score. Although similar consistent significant long-term benefit in function scores such as Constant score was not found in long term, significant radiological improvement in the tendon healing was noted in long term.
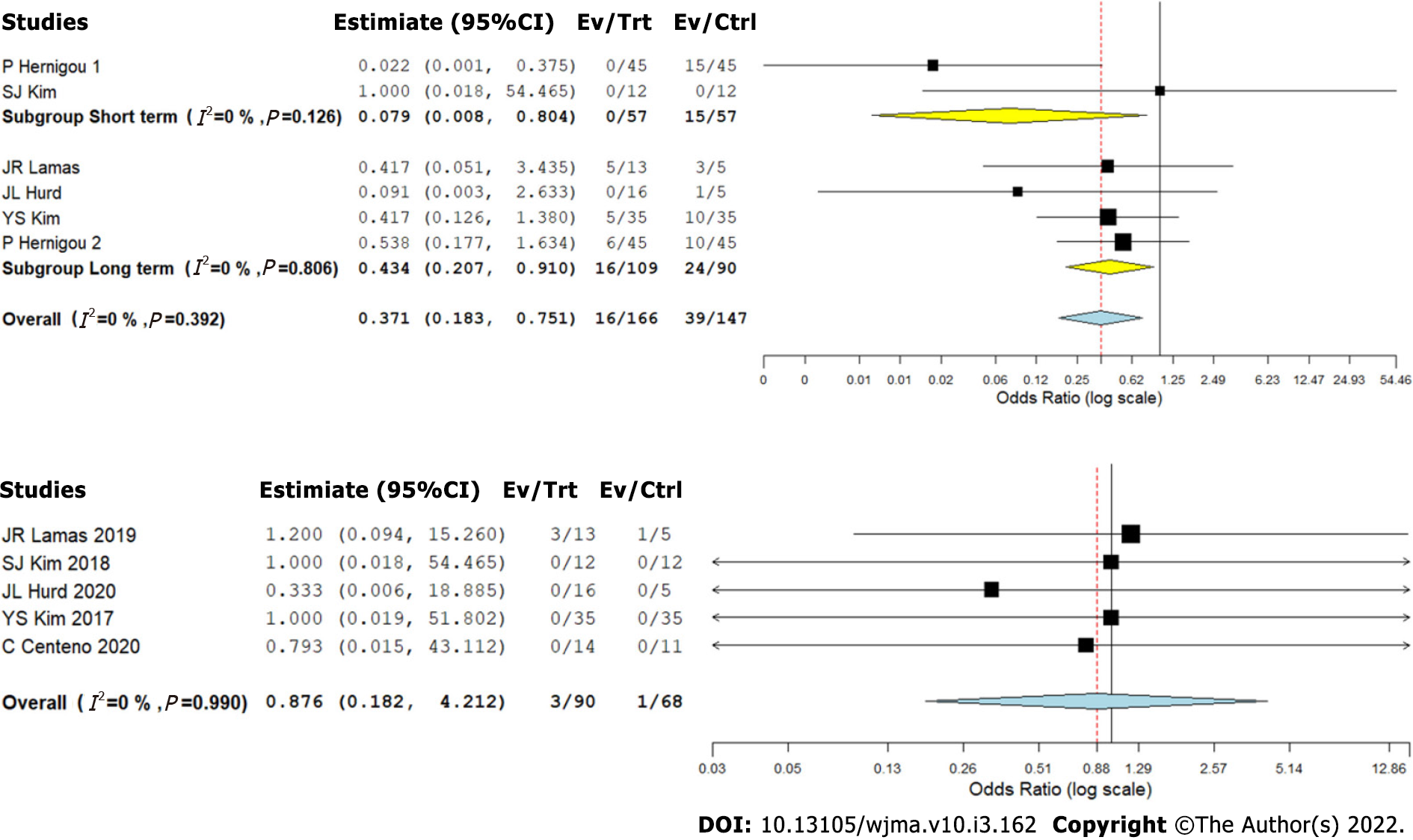
We analyzed five studies[2,8-11] reporting retear of RC tendons following RC tear management using CT against controls at varied time points. We did not note a significant heterogeneity among the included studies (I2 < 50%, P = 0.392). Hence, a fixed-effects model was used for analysis. We stratified the analysis based on the duration of follow-up in the included studies and found that upon utilizing CT in the management of RC tears, we noted an overall significant reduction in the retear rate compared to the controls (OR = 0.371, 95%CI [0.183, 0.751], P = 0.006). Upon stratification of the studies based on the duration of their follow-up, it was noted that utilization of CT for RC tears resulted in a significant reduction of retear rate both at short- (OR = 0.079, 95%CI [0.008, 0.804], P = 0.032) and long-term (OR = 0.434, 95%CI [0.207, 0.910], P = 0.027) follow-ups compared to the controls as shown in Figure 5A.
Five studies[2,8-10,12] reported adverse effects with a low heterogeneity among the included studies upon utilization of CT in RC tear management (I2 = 0.0%, P = 0.990). Hence, a fixed-effects model was used for analysis. On analysis, we did not find any significant increase in the adverse events compared to the controls (OR = 0.876, 95%CI [0.182, 4.212], P = 0.869) as shown in Figure 5B. No major serious adverse events with permanent effects such as death, tumor, or immune reaction to the intervention were noted during follow-up.
A sensitivity analysis was performed in each analysis. All the results (VAS score for pain, ASES score, Constant score, radiological healing, and complications such as retear rate and adverse events) maintained their consistency in significance even upon sequentially omitting each study in the meta-analysis. Similar consistency was noted upon reanalysis of the results by changing to a random-effects model. We performed subgroup analysis of outcomes with significant heterogeneity based on the duration of their follow-up and presented accordingly (Figures 3-5). We explored into the heterogeneity of the results based on the source, dosage of MSCs, and nature of RC tear (complete/partial) but we did not find any significant change in the summary of results obtained as shown in Supplementary Materials.
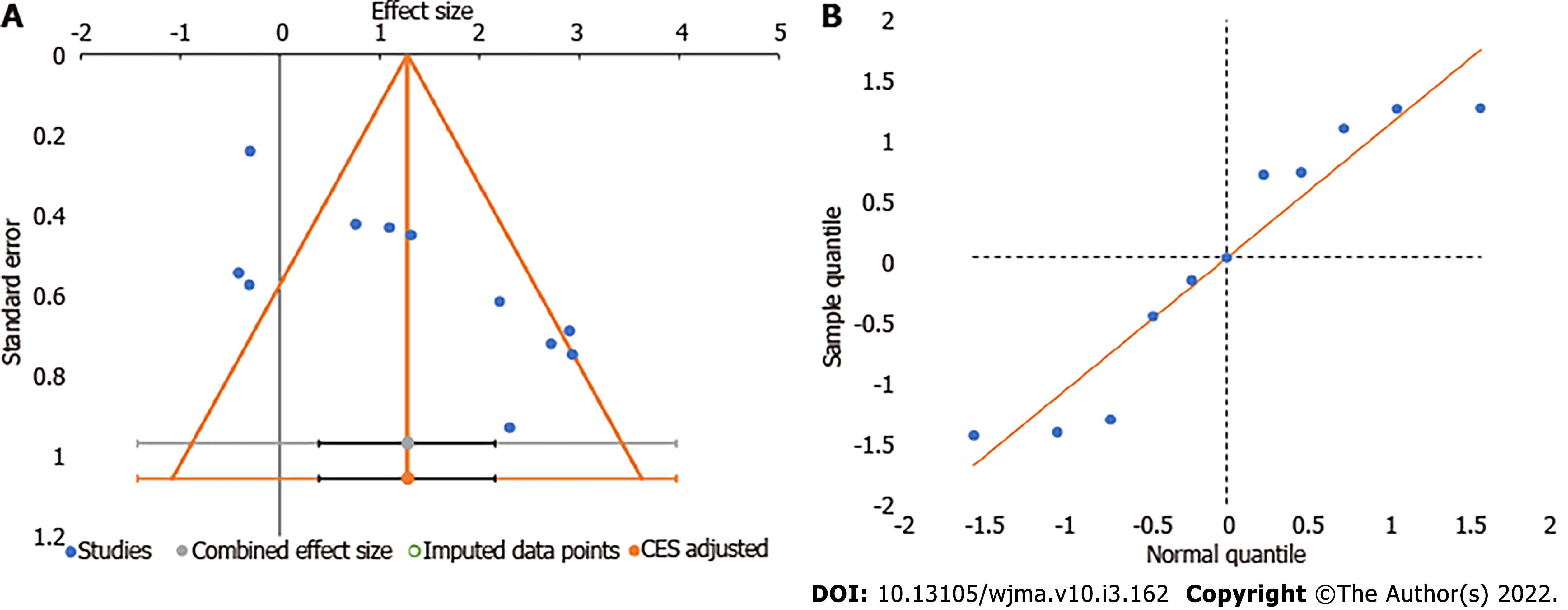
We utilized the funnel plot, normal quantile plot, and Egger’s regression test to analyze the publication bias in the reporting of studies on the subject analyzed. We did not find any significant publication bias by funnel plot and normal quantile plot as shown in Figure 6 or by Egger’s regression test (P = 0.019). We noted that all the studies were close to the 95%CI without significant heterogeneity in their distribution about the axes, implying minimal publication bias.
The lifetime chance of sustaining an RC tear has been reported to range between 25% and 40%[20], and the rate continues to rise with increasing age. Given the global increase in the elderly population, it is estimated that RC pathologies will continue to place immense demands on the overall healthcare system worldwide[21]. Among the non-surgical options, although local corticosteroid infiltration is highly popular, it has been associated with a high incidence of RC rupture resulting from an enhanced induction of non-tenocyte differentiation of human tendon stem cells[9]. With progressive advancements in arthroscopic procedures, surgical implantation for RC pathologies has increased tremendously over the past decade[21]. Consequently, the rate of retear has also worsened, with an overall reported incidence as high as 15% to 40%, irrespective of the surgical technique employed[22]. To overcome these aforementioned reasons, research efforts in the recent past have focused on enhancing the outcomes following management of RC tears, through advancements in surgical indications and decision making, novel surgical and non-surgical interventions, as well as improvements in rehabilitative strategies[2,8-12]. Over the past decade, one promising augmentative approach is the incorporation of biological agents into surgical and non-surgical strategies. In this context, the potential role of MSCs as biological adjuncts in RC injuries has been increasingly acknowledged[2,8-12].
Following an injury to a tendon, various physiological healing cascades are initiated. The earliest response is acute inflammation, which includes the recruitment of cells like leukocytes and thrombocytes to the site of injury[23]. These inflammatory cells further recruit various growth factors like transforming growth factor-beta (TGF-beta), platelet-derived growth, insulin-like growth factor, and fibroblast growth factor[24]. During the next proliferative stage of healing, these recruited cells stimulate the production of type-3 collagen and temporary extracellular matrix[24]. Finally, the remodeling phase occurs at around 1 to 2 mo following the injury where the type-3 collage gradually gets replaced by type-1 collagen. Biological augmentation techniques utilize these body's natural healing processes especially during the inflammatory and proliferative phases to facilitate the enhancement of tendon healing. PRP is a component of blood with a high concentration of platelets, which releases growth factors essential for tissue healing[25]. Hypocellularity is postulated as a major reason underlying the relatively unsatisfactory outcome following PRP injection[9]. In other words, there is a mismatch between the growth factors released by PRP and the insufficient number of stem cells at the site of RC tear[9,22]. Recent reports, therefore, seem to suggest that biological augmentative therapy involving MSCs circumvents this problem and offers greater benefits, as compared to PRP-alone administration[9].
In the current meta-analysis, we included six CCSs (3 RCTs – 238 patients), which discussed the influence of MSCs in RC tears[2,8-12]. While the effect of adjuvant MSCs was compared to the surgically-treated control RC injury population in 3 studies[2,10,11], non-surgically managed control population was included in the remaining trials[8,9,12].
Among the 3 studies involving conservatively-managed RC tear patients, Hurd et al[9] discussed the role of MSCs in partial injuries. Partial RC tendon ruptures are broadly classified based on location (articular, bursal, and interstitial), depth (grade 1 < 3 mm, grade 2 3-6 mm, grade 3 ≥ 6 mm), and tear area[9]. It has been reported that articular-sided RC tears are more common and have relatively lower healing rates, given the poorer vascularity[9,12,24]. Hurd et al[9] demonstrated that injection of adipose-derived into pathological RC tissues resulted in a reduced recruitment of inflammatory cells, enhanced tendon regeneration with mitigated scarring, improved collagen fiber arrangement, greater load-to-failure, and enhanced tensile strength of the injured tendons. They recommended that stem cell delivery can be a promising non-surgical option in these types of partial injuries with a relatively poor prognosis for healing.
The two main clinical parameters assessed in the reviewed studies were pain severity by VAS score[2,8-10,12] and functional outcome measures by ASES[8,9] and Constant scores[2,8]. We could observe significant heterogeneity in the reporting of both these parameters.
Based on our analysis, we could observe a significant improvement in the VAS score for pain at 3 and 6 mo in all patients who underwent CT. However, beyond this initial period, MSC therapy did not result in any significant difference compared to the control group. This observation was consistently reported by all the included studies[2,8-10,12]. A probable explanation for the observed effect could be due to the augmented healing in the treated group of patients demonstrating superior pain control during the early post-operative period.
The reviewed studies used ASES and Constant scores to report the functional outcome during follow-up. In the two studies[8,9] which reported the ASES scores, the outcome was significantly better at 3 and 6 mo following CT. However, the studies did not reveal any significant difference in ASES scores, before and after this time point (i.e., at 3 wk, 1 year, and 2 years). Both the studies that evaluated the Constant score compared the outcome at 1 and 2 years[2,8]. The above findings were in concordance with the results of the pain scores in the included studies showing early augmented healing and functional benefit in the intervention group compared to the controls.
The radiological healing was assessed in 5 studies[2,9-12] based on MRI. In contrast to our findings regarding the pain score and functional outcome, there was a statistically significant improvement in the radiological healing of the lesions at long-term follow-up (1 and 2 years). It has been well-acknowledged that the use of appropriate scaffolds is necessary to preserve the optimal survival, as well as reparative and differentiation capacities of MSCs[25]. BMAC-PRP complexes have previously been shown to enhance healing in diabetic ulcers, osteochondral deficiencies, and spinal injuries. Additi
Thus, our review suggests that the use of BMAC-PRP complexes in RC tears can be a potentially rewarding treatment option. Kim et al[8] reported that the proliferation of tenocytes and tendon stem cells, followed by synthesis of collagen type 3 by tenocytes at 6 wk post-injury, was enhanced by BMAC-PRP complexes. Therefore, in their study, they indicated that biological augmentation with these complexes may potentially result in more anatomical healing of the tears. In the study by Hernigou et al[11], augmentation with MSCs significantly improved the healing, quality, and the structural integrity of arthroscopically-repaired RC tears, as assessed by ultrasound and MRI performed at 6 mo and 10-year follow-up time points.
Based on the evidence from five studies[2,8-11], we could observe that the use of CT resulted in a significant reduction in the retear rates, at both the short-term as well as long-term follow-up. This corroborated our finding that CT resulted in better radiological healing of the lesions. The studies by Hernigou et al[11] and Kim et al[10], which included a total of 80 patients treated with MSCs in addition to arthroscopic RC repair, revealed a statistically significant improvement in the rate of tendon healing, structural integrity of healed tendon, and mitigated number of retears at both short-term and long-term follow-up. In the study by Hernigou et al[11], 87% of patients in the MSC group had intact tendon integrity at 10-year follow-up, as against 44% in the control population.
In one of the included studies[2], high failure rates secondary to detrimental inflammatory processes activated by a xenograft scaffold were reported similarly in both MSC and control groups. Apart from this issue that was unrelated to MSCs, none of the reviewed studies reported any significant adverse events directly related to the use of CT in patients with RC tears.
Our analysis had some limitations. We could not find blinding to be established in most of the studies included in the analysis which might invite room for bias from patients or observers with regard to the treatment given. We also noted heterogeneity across many reported outcomes in the included studies, which might be due to the variability in the follow-up time and the treatment protocols followed in the individual studies as shown in Table 2. However, we tried to address the impact of follow-up period through our stratified analysis of results at different time points to arrive at a meaningful conclusion. Moreover, patients in various stages of the disease process were included in the studies, which could have also contributed to the heterogeneity of their results.
Based on our comprehensive and critical review of the available literature analyzing the efficacy and safety of CT utilizing MSCs in the management of RC tears, we could observe that the utilization of CT significantly reduced pain severity at 3 and 6 mo, improved short-term functional outcome, enhanced radiological tendon healing, and mitigated retear rates at short- and long-term follow-up. The literature did not reveal any major adverse events directly related to MSC therapy in patients presenting with RC tears.
We recommend a large-scale, multicentric trial analyzing autologous and allogeneic sources of MSCs with standardized dosage and intervention protocol, evaluated with established outcome measures both at short- and long-term follow-up to further confirm the results of our analysis.
Rotator cuff (RC) tear is a common shoulder pathology, whose prevalence ranges from 4% in asymptomatic individuals younger than 40 years to 54% in patients aged over 60 years. The etiology of these tears is multifactorial, and has been variously attributed to traumatic, mechanical, and inflammatory processes. It has been well-demonstrated that the natural course of non-operatively managed RC tears in the majority of patients is a progressive deterioration of the anatomical tear without spontaneous regression of symptoms. On the other hand, although surgical repair of a torn RC potentially aids in restoring the shoulder function as well as arresting the tear progression, the failure rates range between 0 and 78%, thereby giving room for improvement.
Recently, mesenchymal stromal cells (MSCs) have been successfully employed in diverse animal and human models in the repair of various musculoskeletal structures like cartilages, bones, muscles, and tendons. These cells (usually extracted from bone marrow or adipose tissue) possess a unique attribute described as “multipotency”, which denotes their ability to differentiate into other tissues of mesenchymal origin. When delivered using appropriate scaffolds, these modalities of cellular therapy (CT) have shown great promise in enhancing the outcome following RC tears, too. MSCs are thought to promote type I collagen production, enhance mechanical strength of tissues, and ameliorate biology at the bone-tendon interface, which primarily explains their potential clinical utility in RC tears. The concentrates of these cells may be delivered into the region of tendon injury, either via image-guided injections or through arthroscopic approach (intra-operatively). However, the major barriers to regular use of MSCs include lack of standardized techniques for preparation, inadequate clinical evidence, and potentially high cost:benefit ratios.
To analyze the efficacy and safety of CT utilizing MSCs in the management of RC tears from clinical studies available in the literature.
We conducted independent and duplicate electronic database searches including PubMed, Embase, Web of Science, and Cochrane Library on August 2021 for studies analyzing the efficacy and safety of CT utilizing MSCs in the management of RC tears. Visual Analog Score (VAS) score for pain, American Shoulder and Elbow Surgeons (ASES) score, Disability of the Arm, Shoulder and Hand score, Constant score, radiological assessment of healing, and complications such as retear rate and adverse events were the outcomes analyzed. Analysis was performed in R-platform using OpenMeta [Analyst] software.
Six studies involving 238 patients were included for analysis. We noted a significant reduction in VAS score for pain at 3 mo (WMD = -2.234, P < 0.001) and 6 mo (WMD = -3.078, P < 0.001) with the use of CT, which was not maintained at long-term follow-up (WMD = -0.749, P = 0.544). Concerning functional outcomes, utilization of CT produced a significant short-term improvement in the ASES score (WMD = 17.090, P < 0.001) and significant benefit in functional scores such as Constant score (WMD = 0.833, P = 0.760) at long-term follow-up. Moreover, we also observed a significantly improved radiological tendon healing during the long-term follow-up (OR = 3.252, P = 0.059). We also noted a significant reduction in the retear rate upon utilization of CT in RC tears both at short- (OR = 0.079, P = 0.032) and long-term (OR = 0.434, P = 0.027) follow-up. We did not observe any significant increase in the adverse events directly related to CT, as compared with the control group (OR = 0.876, P = 0.869).
Based on our comprehensive and critical review of the available literature analyzing the efficacy and safety of CT utilizing MSCs in the management of RC tears, we could observe that the utilization of CT significantly reduced pain severity at 3 and 6 mo, improved short-term functional outcome, enhanced radiological tendon healing, and mitigated retear rates at short- and long-term follow-up. The literature did not reveal any major adverse events directly related to MSC therapy in patients presenting with RC tears.
We recommend a large-scale, multicentric trial analyzing autologous and allogeneic sources of MSCs with standardized dosage and intervention protocol, evaluated with established outcome measures both at short- and long-term follow-up to further confirm the results of our analysis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Chrcanovic BR, Sweden; Jing F, China; Tsang HW, China; Yan T, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Clement ND, Nie YX, McBirnie JM. Management of degenerative rotator cuff tears: a review and treatment strategy. Sports Med Arthrosc Rehabil Ther Technol. 2012;4:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Lamas JR, García-Fernández C, Tornero-Esteban P, Lópiz Y, Rodriguez-Rodriguez L, Ortega L, Fernández-Gutiérrez B, Marco F. Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials. 2019;20:387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Longo UG, Franceschi F, Berton A, Maffulli N, Droena V. Conservative treatment and rotator cuff tear progression. Med Sport Sci. 2012;57:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kim YS, Kim SE, Bae SH, Lee HJ, Jee WH, Park CK. Tear progression of symptomatic full-thickness and partial-thickness rotator cuff tears as measured by repeated MRI. Knee Surg Sports Traumatol Arthrosc. 2017;25:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Moosmayer S, Gärtner AV, Tariq R. The natural course of nonoperatively treated rotator cuff tears: an 8.8-year follow-up of tear anatomy and clinical outcome in 49 patients. J Shoulder Elbow Surg. 2017;26:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Gumucio JP, Flood MD, Roche SM, Sugg KB, Momoh AO, Kosnik PE, Bedi A, Mendias CL. Stromal vascular stem cell treatment decreases muscle fibrosis following chronic rotator cuff tear. Int Orthop. 2016;40:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 8. | Kim SJ, Kim EK, Kim SJ, Song DH. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res. 2018;13:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 9. | Hurd JL, Facile TR, Weiss J, Hayes M, Furia JP, Maffulli N, Winnier GE, Alt C, Schmitz C, Alt EU, Lundeen M. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res. 2020;15:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? Am J Sports Med. 2017;45:2010-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, Rouard H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38:1811-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 12. | Centeno C, Fausel Z, Stemper I, Azuike U, Dodson E. A Randomized Controlled Trial of the Treatment of Rotator Cuff Tears with Bone Marrow Concentrate and Platelet Products Compared to Exercise Therapy: A Midterm Analysis. Stem Cells Int. 2020;2020:5962354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Cho WS, Chung SG, Kim W, Jo CH, Lee SU, Lee SY. Mesenchymal Stem Cells Use in the Treatment of Tendon Disorders: A Systematic Review and Meta-Analysis of Prospective Clinical Studies. Ann Rehabil Med. 2021;45:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW; Editorial Board of the Cochrane Back, Neck Group. 2015 Updated Method Guideline for Systematic Reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976). 2015;40:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 531] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 15. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40536] [Article Influence: 10134.0] [Reference Citation Analysis (2)] |
| 16. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15259] [Article Influence: 2543.2] [Reference Citation Analysis (0)] |
| 17. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10862] [Article Influence: 1206.9] [Reference Citation Analysis (2)] |
| 18. | Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Stat Softw. 2012;49:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 19. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46536] [Article Influence: 2115.3] [Reference Citation Analysis (3)] |
| 20. | Montgomery SR, Petrigliano FA, Gamradt SC. Biologic augmentation of rotator cuff repair. Curr Rev Musculoskelet Med. 2011;4:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear Rates After Arthroscopic Single-Row, Double-Row, and Suture Bridge Rotator Cuff Repair at a Minimum of 1 Year of Imaging Follow-up: A Systematic Review. Arthroscopy. 2015;31:2274-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181-190. [PubMed] |
| 24. | James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 25. | Betsch M, Schneppendahl J, Thuns S, Herten M, Sager M, Jungbluth P, Hakimi M, Wild M. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One. 2013;8:e71602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |