Published online Nov 26, 2013. doi: 10.13105/wjma.v1.i3.138
Revised: June 13, 2013
Accepted: August 4, 2013
Published online: November 26, 2013
Processing time: 248 Days and 23.4 Hours
AIM: To evaluate the association between apolipoprotein E (apoE) gene polymorphism and total cholesterol (TC) level in patients with kidney diseases.
METHODS: A predefined literature search was performed to collect data from the electronic databases of PubMed, Embase and the Cochrane Library and eligible relevant studies reporting the association of apoE gene polymorphism with TC level in patients with kidney diseases were recruited for meta-analysis.
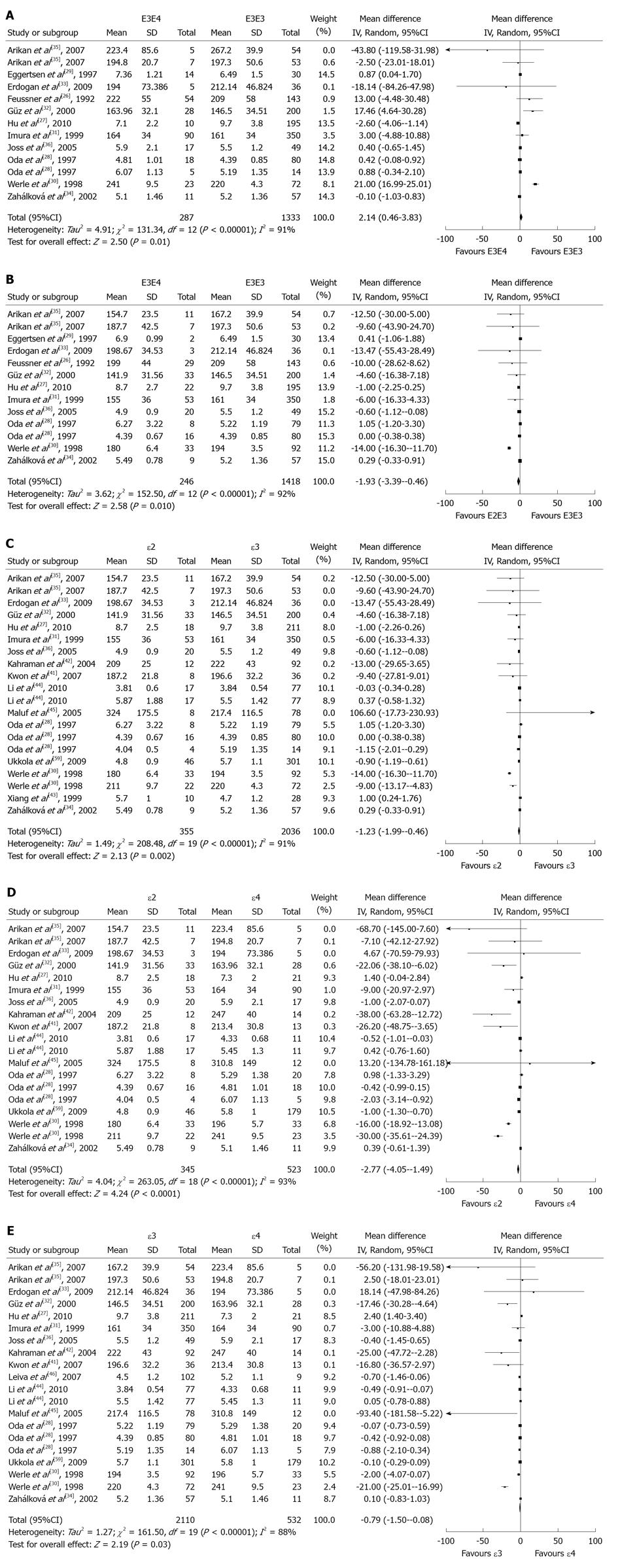
RESULTS: Twenty-one studies were identified for the analysis of association between apoE gene polymorphism and TC level in patients with kidney disease. Subjects with E3E4 had a higher TC than those with E3E3 [weighted mean differences (WMD) = 2.14, P = 0.01] and subjects with E2E3 had a lower TC than those with E3E3 (WMD = -1.93, P = 0.01). Subjects with ε2 had a lower TC than those with ε3 (ε2 vs ε3: WMD = -1.23, P = 0.002; ε2 vs ε4: WMD = -2.77, P ﹤0.0001) and subjects with 3 had a lower TC than those with 4 (WMD = -0.79, P = 0.03).
CONCLUSION: Subjects with apoE E3E4 and ε4 had a higher TC level and subjects with apoE E2E3 and ε2 had a higher TC level in patients with kidney disease. However, more well-designed studies should be performed in the future to confirm these findings.
Core tip: The available evidence for an association between apolipoprotein E (apoE) and total cholesterol (TC) in kidney disease is weak and has conflicting results. This meta-analysis based on 21 included studies indicated that subjects with apoE E3E4 and 4 had a higher TC level and subjects with apoE E2E3 and ε2 had a higher TC level in patients with kidney disease.
- Citation: Zhou TB, Jiang ZP, Yin SS, Qin YH. Relationship between apolipoprotein E gene polymorphism and total cholesterol level in patients with kidney diseases. World J Meta-Anal 2013; 1(3): 138-146
- URL: https://www.wjgnet.com/2308-3840/full/v1/i3/138.htm
- DOI: https://dx.doi.org/10.13105/wjma.v1.i3.138
Apolipoprotein E (apoE) is an important protein of the lipoprotein transport system and plays an important role in lipoprotein metabolism and lipid homeostasis[1-3]. It is associated with the metabolism of total cholesterol (TC) and triglyceride[4,5]. ApoE, a 229-amino-acid polypeptide, is classified into three major isoforms (ε2, ε3 and ε4) according to the differences in amino acids at positions 112 and 158, forming six genotypes: E2E2, E2E3, E2E4, E3E3, E3E3 and E4E4[6,7]. ε3 and E3E3 are the wild type of apoE and ε2, ε4, E2E2, E2E3, E2E4, E3E3 and E4E4 are the mutation type of apoE.
The represent one of the major systems that maintain the body homeostasis[8]. Abnormalities of lipid metabolism and homeostasis are commonly present in patients with kidney disease[9], such as nephrotic syndrome (NS). Most kidney diseases are associated with elevated serum/plasma concentration and the impaired clearance of very low density lipoprotein and their atherogenic remnants. Nephrotic dyslipidemia is a risk factor for the development of systemic atherosclerosis, it may aggravate the glomerulosclerosis lesion and enhance the progression of glomerular disease[10]. apoE lipoprotein plays an important role in lipid homeostasis and kidney diseases.
In renal tissue, apoE is mainly synthesized by mesangial cells under normal physiological conditions[11,12]. Investigators reported that apoE had a protective role for the kidney[13,14]. Some other investigations found that the apoE expression in renal glomerulus was elevated when compared with that of the normal group[15-19]. There were some investigations reporting that apoE polymorphism was associated with renal diseases. In our previous study[20], we reported that the apoE gene expression was associated with the NS susceptibility in experimental studies and the apoE ε3ε3, ε3ε4, ε3 and ε4 were associated with the onset of NS in human studies. Hayakawa et al[21] carried out an investigation on a patient with apoE ε3ε3 variant without lipoprotein glomerulopathy and illustrated that not all apoE variants resulted in lipoprotein glomerulopathy and that the location of mutations in the apoE protein was one of the important determinants for the development of lipoprotein glomerulopathy. Luo et al[22] reported that apoE mutation was associated with the lipoprotein glomerulopathy risk and found that the apoE mutation could cause a marked molecular conformational change of apoE and thus impaired its binding ability to lipids.
Some reports show that apoE gene polymorphism is associated with the expression of TC. However, some studies found that apoE gene polymorphism was not associated with the expression of TC. The available evidence for an association between apoE and TC in kidney disease is weak due to paucity of data or disagreements among the reported studies. Thus, evidence from a meta-analysis may be more powerful compared to single studies[23]. To date, no meta-analysis exists that determines the association between apoE gene polymorphism and TC level in patients with kidney diseases. This meta-analysis was performed to investigate the association of the apoE gene polymorphism with the TC level in patients with kidney diseases.
The relevant studies were screened from the search engines of PubMed, Embase and the Cochrane Library on March 1, 2012. “(apoE AND apolipoprotein E) AND (renal OR kidney)” was used in PubMed, Embase and the Cochrane Library. We also extended our search spectrum to the “related articles” and the bibliographies of all retrieved studies. If multiple publications from the same study group using the same data were reported, we only recruited the most complete study for our analysis.
Inclusion criteria: (1) the study had to be about kidney diseases; (2) provide detailed gene distribution of apoE; and (3) report on the level of TC.
Exclusion criteria: (1) Editorials, reviews, case reports; (2) data from multiple publications; (3) investigating the association of other genes in kidney diseases; and (4) investigating the role of apoE in diseases other than kidney diseases.
The following information was extracted from each study independently by the investigators: first author’s surname, year of publication, location of study, patient type, level of TC and the number of subjects. Study type, comparisons of apoE gene polymorphism and methodological quality assessment outcomes were also extracted. The results were compared and disagreements were resolved by discussion.
Available data was entered into Cochrane Review Manager (RevMan, Version 5) and analyzed. The pooled statistic was counted using the fixed effects model but a random effects model was conducted when the P value of heterogeneity test was less than 0.1. Results were expressed with weighted mean differences (WMD) for continuous data and 95%CI were also counted. P < 0.05 was required for the overall WMD to be deemed statistically significant. I2 was used to test the heterogeneity between the included studies. The Begg adjusted rank correlation test[24] and the Egger regression asymmetry test[25] were used for exploring publication bias (P < 0.1 was considered significant) when the number of the included studies was more than fifteen.
Twenty-one studies were included in the meta-analysis for the relationship between apoE gene polymorphism with TC expression (Figure 1 and Table 1). One study[26] was for the comparison of E2E2 vs E3E3. Eleven reports[26-36] were recruited into the study of E2E3 vs E3E3 (including 13 comparisons). Three reports[26,27,29] were recruited into the study of E2E4 vs E3E3 (including 3 comparisons). 11 reports[26-36] were recruited into the study of E3E4 vs E3E3 (including 13 comparisons). Three reports[26,27,29] were recruited into the study of E4E4 vs E3E3 (including 3 comparisons). Two reports[37] were included for the meta-analysis of ε2 vs non-ε2 (including 2 comparisons). Three studies[38-40] were recruited into our meta-analysis for the comparison of ε4 with non-ε4 (including 4 comparisons). 13 studies[27,28,30-36,41-45] were included into the study of ε2 vs ε3 (including 20 comparisons). Fourteen studies[27,28,30-36,41,42,44-46] were included into the study of ε4 vs ε4 (including 20 comparisons). Thirteen studies[27,28,30-36,41,42,44,45] were included into the study of ε2 vs ε4 (including 19 comparisons).
| Author | Study type | Location of study | Ethnicity | Patient type | Number of subjects | Comparisons of apoE gene polymorphism | Genotyping method reported | Blinding of genotyping |
| Feussner et al[26] | Prospective | Germany | Caucasian | ESRD | 141 males and 104 females | E2E2, E2E3, E2E4, E3E3, E3E4, E4E4 | No | Not mention |
| Eggertsen et al[29] | Prospective | Sweden | Caucasian | ESRD: 19 cases glomerulonephritis, 11 cases diabetic nephropathy, nine cases interstitial nephritis | 25 males and 26 females | E2E3, E2E4, E3E3, E3E4, E4E4 | Yes | Not mention |
| Oda et al[28] | Prospective | Japan | Asian | 107 with GN and 399 with ESRD | Patients with GN consisted of 42 men and 65 women | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Lim et al[38] | Prospective | China | Asian | ESRD: 85 patients with chronic GN, 30 patients with DN, 18 patients with chronic pyelonephritis, 3 patients with PKD | 96 males and 60 females | ε4, non-ε4 | Yes | Not mention |
| Kimura et al[39] | Prospective | Japan | Asian | DN | 88 men and 90 women | ε4, non-ε4 | Yes | Not mention |
| Werle et al[30] | Prospective | Germany | Caucasian | DN | 159 men and 129 women | E2E3, E3E3, ε2, ε3, ε4 | Yes | Not mention |
| Imura et al[31] | Prospective | Japan | Asian | ESRD | 287 men and 206 women | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Xiang et al[43] | Prospective | China | Asian | DN | 26 men and 20 women | ε2, ε3 | Yes | Not mention |
| Güz et al[32] | Prospective | Turkey | Caucasian | ESRD: GN (107 cases), hypertension nephropathy (37 cases), unknown (36 cases), pyelonephritis (29 cases), amyloidosis (20 cases), DN (15 cases), obstructive uropathy (9 cases), PKD (7 cases), toxic nephropathy (6 cases), and Alport’s syndrome (3 cases) | 149 men and 112 women | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Zahálková et al[34] | Prospective | Czech | Caucasian | ESRD | 53 males and 34 females | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Lehtinen et al[40] | Prospective | Finland | Caucasian | DN | Not mention | ε4, non-ε4 | Yes | Not mention |
| Kahraman et al[42] | Prospective | Turkey | Caucasian | Renal transplant recipients | 80 males and 38 females | ε2, ε3, ε4 | Yes | Not mention |
| Joss et al[36] | Prospective | United Kingdom | Caucasian | DN | Not mention | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Maluf et al[45] | Prospective | United States | Mix | Renal transplant recipients | 21 males and 18 females | ε2, ε3, ε4 | Yes | Not mention |
| Arikan et al[35] | Prospective | Turkey | Caucasian | ESRD | 84 males and 60 females | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Kwon et al[41] | Retrospective | Korea | Asian | DN | 32 males and 62 females | ε2, ε3, ε4 | No | Not mention |
| Leiva et al[46] | Retrospective | Chile | South America | DN | 53 males and 32 females | ε3, ε4 | Yes | Not mention |
| Ma et al[37] | Prospective | China | Asian | DN | 146 males and 259 females | ε2, non-ε2 | Yes | Not mention |
| Erdogan et al[33] | Prospective | Turkey | Caucasian | DN | 19 males and 27 females | E2E3, E3E3, E3E4, ε2, ε3, ε4 | Yes | Not mention |
| Hu et al[27] | Prospective | China | Asian | MCNS | 176 males and 74 females | E2E2, E2E3, E2E4, E3E3, E3E4, E4E4, ε2, ε3, ε4 | Yes | Not mention |
| Li et al[44] | Prospective | China | Asian | Renal transplant recipients | 59 males and 46 females | ε2, ε3, ε4 | Yes | Not mention |
In this meta-analysis, the number of included studies for some comparisons was more than ten (E2E3 vs E3E3, E3E4 vs E3E3, ε2 vs ε3, ε4 vs ε3, ε2 vs ε4) and the number of included studies for some comparisons was less ten (E2E2 vs E3E3, E2E4 vs E3E3, E4E4 vs E3E3, ε2 vs non-ε2, ε4 vs non-ε4). Those results from less than ten might be less robust. We presented those results independently.
Subjects with E3E4 had a higher TC than those with E3E3 (Figure 2A and Table 2) and subjects with E2E3 had a lower TC than those with E3E3 (Figure 2B and Table 2). Subjects with ε2 had a lower TC than those with ε3 or ε4 (Figure 2C for ε3 and Figure 2D for ε4; Table 2) and subjects with ε4 had a higher TC than those with ε3 (Figure 2E and Table 2). It seemed that E3E4 and ε4 were associated with higher level of TC and E2/E3 and ε2 were associated with lower level of TC. The number of included studies for some comparisons was more than ten and those results might be robust to some extent.
| Genetic comparisons | Q test | P contrasts | Model seclected | Weighted mean differences (95%CI) | P value |
| E2E2 vs E3E3 | 1 | - | Fixed | 109.00 (-32.07-250.07) | 0.13 |
| E2E3 vs E3E3 | 13 | < 0.00001 | Random | -1.93 (-3.39--0.46) | 0.01 |
| E2E4 vs E3E3 | 3 | 0.48 | Random | -2.48 (-4.23--0.72) | 0.006 |
| E3E4 vs E3E3 | 13 | < 0.00001 | Random | 2.14 (0.46-3.83) | 0.01 |
| E4E4 vs E3E3 | 3 | 0.02 | Random | 0.30 (-4.25-4.84) | 0.90 |
| ε2 vs non-ε2 | 2 | 0.57 | Fixed | -0.25 (-0.43--0.08) | 0.005 |
| ε4 vs non-ε4 | 4 | 0.0001 | Random | 0.20 (-1.42-1.83) | 0.81 |
| ε2 vs ε3 | 20 | < 0.00001 | Random | -1.23 (-1.99--0.46) | 0.002 |
| ε3 vs ε4 | 20 | < 0.00001 | Random | -0.79 (-1.50--0.08) | 0.03 |
| ε2 vs ε4 | 19 | < 0.00001 | Random | -2.77 (-4.05--1.49) | < 0.0001 |
Subjects with E2E4 had a much lower TC level when compared with those with E3E3. Subjects with ε2 had a lower level of TC than those with non-ε2. Subjects with E2E2 or E4E4 had a slightly higher TC than those with E3E3, although there was no statistical difference. Subjects with 4 had a similar level of TC than those with non-ε4 (Table 2). It seemed that E2E4 and ε2 was associated with lower level of TC. The number of included studies for some comparisons was less than ten and more studies should be performed in the future.
There was a publication bias test for the comparisons of E2E3 vs E3E3, E3E4 vs E3E3, ε2 vs ε3, ε4 vs ε3, ε2 vs ε4. There was no publication bias for the comparisons of E2E3 vs E3E3, E3E4 vs E3E3, ε2 vs ε3, ε4 vs ε3, ε2 vs ε4 (E2E3 vs E3E3: Begg P = 0.951, Egger P = 0.936; E3E4 vs E3E3: Begg P = 0.951, Egger P = 0.710; ε2 vs ε3: Begg P = 0.871, Egger P = 0.970; ε4 vs ε3: Begg P=0.315, Egger P = 0.251; ε2 vs ε4: Begg P=0.294, Egger P = 0.495).
High serum TC level is an important characteristic for many kidney diseases, such as NS[47], glomerulonephritis[48], end-stage kidney disease requiring dialysis[49], etc. Raised TC is a consequence of kidney disease but may accelerate progression to end-stage kidney disease. In our meta-analysis, we found that subjects with E3E4 had a higher TC than those with E3E3 and subjects with E2E3 had a lower TC than those with E3E3. Subjects with ε2 had a lower TC than those with ε3 or ε4, and subjects with ε4 had a higher TC than those with ε3. There was no publication bias test for the comparisons of E2E3 vs E3E3, E3E4 vs E3E3, ε2 vs ε3, ε4 vs ε3, ε2 vs ε4. E3E4 and ε4 were associated with higher level of TC and E2/E3 and ε2 were associated with lower level of TC. The conclusions for those comparisons were robust to some extent. Toms et al[50] reported that the ε2 allele was associated with the lowest and ε4 allele with the highest level of TC in rheumatoid arthritis patients. Alvim et al[51] reported that the ε4 allele was associated with higher TC value in healthy urban Brazilian individuals. Rahimi et al[52] found that ε4 allele resulted in a significant increase in the level of TC in sickle cell disease. Smart et al[53] reported that significantly higher TC was observed in apoE ε4 carriers compared to E3E3 homozygotes and ε2 carriers in 882 Greek children. The results from those studies mentioned above were similar to our results in this meta-analysis.
The roles of apoE in diseases were also complicated. Many studies reported that apoE could play a protective role against diseases. apoE can play an antioxidant role[54], has been demonstrated to play an important role in providing protection against mesangial cell injury[55] and also appears to be involved in the repair response to tissue injury; for example, markedly increased amounts of apoE are found at sites of peripheral nerve injury and regeneration[56]. apoE deficiency in mice leads to the development of atherosclerosis and re-expression of the protein reduces the extent of the disease[57]. It appears that the increased apoE is a protective factor against disease progression. However, more studies need to be performed to explore the role of apoE in diseases.
There were some meta-analyses to evaluate the relationship between apoE gene polymorphism and TC level. Anthopoulos et al[58] performed a meta-analysis to detect the association of apoE gene polymorphism with TC level in patients with type 2 diabetes and the meta-regression analysis provided some weak evidence that the risk conferred by ε2 allele was mediated through altering serum TC level. Our study reported the association of apoE gene polymorphism with increased TC levels in patients with kidney diseases and showed that apoE gene polymorphism was associated with the increased levels of TC in patients with kidney disease.
In this meta-analysis, it was difficult to conduct the methodological quality assessment of included studies for the reason that the information in the included studies was few. More well-designed studies should be performed in the future.
In conclusion, our meta-analysis shows that apoE gene polymorphism is associated with increased levels of TC. However, larger studies should be performed in the future to confirm this association.
Apolipoprotein E (apoE), one of the major plasma lipoproteins, plays a major role in the transport and metabolism of lipids by acting as a ligand. apoE gene contains three potential alleles: ε2, ε3 and ε4, forming six genotypes: E2E2, E2E3, E2E4, E3E3, E3E3 and E4E4. An association between apoE gene polymorphism and total cholesterol (TC) level is still controversial.
To date, no meta-analysis exists that determines the association between the apoE gene polymorphism and TC level in patients with kidney diseases. This meta-analysis was performed to investigate the association of apoE gene polymorphism with the TC level in patients with kidney diseases.
This paper performed a meta-analysis to investigate the relationship between apoE gene polymorphism and TC level in patients with renal diseases. The paper presents an interesting topic.
P- Reviewers: Bhimma R, Chen SJ S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Zheng XM
Table 2 Meta-analysis of the association of apoE gene polymorphism with total cholecterol level
| 1. | Annema W, Dikkers A, Freark de Boer J, Gautier T, Rensen PC, Rader DJ, Tietge UJ. ApoE promotes hepatic selective uptake but not RCT due to increased ABCA1-mediated cholesterol efflux to plasma. J Lipid Res. 2012;53:929-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Georgiadou D, Chroni A, Vezeridis A, Zannis VI, Stratikos E. Biophysical analysis of apolipoprotein E3 variants linked with development of type III hyperlipoproteinemia. PLoS One. 2011;6:e27037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. apoE expression in glomerulus and correlation with glomerulosclerosis induced by adriamycin in rats. Ren Fail. 2011;33:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Losonczi E, Bencsik K, Fricska Nagy Z, Honti V, Szalczer E, Rajda C, Illés Z, Mátyás K, Rózsa C, Csépány T. APOE epsilon status in Hungarian patients with primary progressive multiple sclerosis. Swiss Med Wkly. 2010;140:w13119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Brito DD, Fernandes AP, Gomes KB, Coelho FF, Cruz NG, Sabino AP, Cardoso JE, Figueiredo-Filho PP, Diamante R, Norton CR. Apolipoprotein A5-1131T& gt; C polymorphism, but not APOE genotypes, increases susceptibility for dyslipidemia in children and adolescents. Mol Biol Rep. 2011;38:4381-4388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | De Feo E, Cefalo C, Arzani D, Amore R, Landolfi R, Grieco A, Ricciardi W, Miele L, Boccia S. A case-control study on the effects of the apolipoprotein E genotypes in nonalcoholic fatty liver disease. Mol Biol Rep. 2012;39:7381-7388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Marrzoq LF, Sharif FA, Abed AA. Relationship between ApoE gene polymorphism and coronary heart disease in Gaza Strip. J Cardiovasc Dis Res. 2011;2:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Salem NA, Salem EA. Renoprotective effect of grape seed extract against oxidative stress induced by gentamicin and hypercholesterolemia in rats. Ren Fail. 2011;33:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Lee HS. Mechanisms and consequences of hypertriglyceridemia and cellular lipid accumulation in chronic kidney disease and metabolic syndrome. Histol Histopathol. 2011;26:1599-1610. [PubMed] |
| 10. | Gheith O, Sheashaa H, Abdelsalam M, Shoeir Z, Sobh M. Efficacy and safety of Monascus purpureus Went rice in children and young adults with secondary hyperlipidemia: a preliminary report. Eur J Intern Med. 2009;20:e57-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Lin CT, Xu YF, Wu JY, Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J Clin Invest. 1986;78:947-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Zhou TB. Signaling pathways of apoE and its role of gene expression in glomerulus diseases. J Recept Signal Transduct Res. 2013;33:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Chen G, Paka L, Kako Y, Singhal P, Duan W, Pillarisetti S. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem. 2001;276:49142-49147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Yasumoto Y, Ikeda T, Takenouchi S, Sogabe A, Nosaki T, Che X, Zheng C, Haraguchi M, Akiyama S. Apolipoprotein E regulates primary cultured human mesangial cell proliferation. Nephron Exp Nephrol. 2006;102:e62-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Calandra S, Gherardi E, Fainaru M, Guaitani A, Bartosek I. Secretion of lipoproteins, apolipoprotein A-I and apolipoprotein E by isolated and perfused liver of rat with experimental nephrotic syndrome. Biochim Biophys Acta. 1981;665:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. Patients with nephrotic-range proteinuria have apolipoprotein C and E deficient VLDL1. Kidney Int. 2000;58:1238-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Russi G, Furci L, Leonelli M, Magistroni R, Romano N, Rivasi P, Albertazzi A. Lipoprotein glomerulopathy treated with LDL-apheresis (Heparin-induced Extracorporeal Lipoprotein Precipitation system): a case report. J Med Case Rep. 2009;3:9311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. Less gelatinases is associated with apolipoprotein E accumulation in glomerulosclerosis rats. Histol Histopathol. 2012;27:249-256. [PubMed] |
| 19. | Zhou TB, Qin YH, Ou C, Lei FY, Su LN, Huang WF, Zhao YJ. All-trans retinoic acid can regulate the expressions of gelatinases and apolipoprotein E in glomerulosclerosis rats. Vascul Pharmacol. 2011;55:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Zhou TB, Qin YH, Xu HL. Association of apoE gene expression and its gene polymorphism with nephrotic syndrome susceptibility: a meta-analysis of experimental and human studies. Mol Biol Rep. 2012;39:9347-9354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Hayakawa M, Okubo M, Katori H, Nagahama K, Murase T, Kobayashi T, Tanaka S, Nakanishi K, Odawara M, Matsushita H. A patient with apolipoprotein E2 variant (Q187E) without lipoprotein glomerulopathy. Am J Kidney Dis. 2002;39:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Luo B, Huang F, Liu Q, Li X, Chen W, Zhou SF, Yu X. Identification of apolipoprotein E Guangzhou (arginine 150 proline), a new variant associated with lipoprotein glomerulopathy. Am J Nephrol. 2008;28:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Zhou TB, Liu YG, Lin N, Qin YH, Huang K, Shao MB, Peng DD. Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic lupus erythematosus/lupus nephritis: a systematic review and metaanalysis. J Rheumatol. 2012;39:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 25. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40550] [Article Influence: 1448.2] [Reference Citation Analysis (2)] |
| 26. | Feussner G, Wey S, Bommer J, Deppermann D, Grützmacher P, Ziegler R. Apolipoprotein E phenotypes and hyperlipidemia in patients under maintenance hemodialysis. Hum Genet. 1992;88:307-312. [PubMed] |
| 27. | Hu P, Qin YH, Lu L, Hu B, Jing CX, Lei FY, Li MF. Genetic variation of apolipoprotein E does not contribute to the lipid abnormalities secondary to childhood minimal change nephrotic syndrome. Int Urol Nephrol. 2010;42:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Oda H, Yorioka N, Ueda C, Nishida Y, Yamakido M. Apolipoprotein E phenotype and renal disease. Contrib Nephrol. 1997;120:22-29. [PubMed] |
| 29. | Eggertsen G, Heimbürger O, Stenvinkel P, Berglund L. Influence of variation at the apolipoprotein E locus on lipid and lipoprotein levels in CAPD patients. Nephrol Dial Transplant. 1997;12:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Werle E, Fiehn W, Hasslacher C. Apolipoprotein E polymorphism and renal function in German type 1 and type 2 diabetic patients. Diabetes Care. 1998;21:994-998. [PubMed] |
| 31. | Imura T, Kimura H, Gejyo F. Apolipoprotein E phenotypes in hemodialysis patients. Kidney Int Suppl. 1999;71:S245-S247. [PubMed] |
| 32. | Güz G, Nurhan Ozdemir F, Sezer S, Işiklar I, Arat Z, Turan M, Haberal M. Effect of apolipoprotein E polymorphism on serum lipid, lipoproteins, and atherosclerosis in hemodialysis patients. Am J Kidney Dis. 2000;36:826-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Erdogan M, Eroglu Z, Biray C, Karadeniz M, Cetinkalp S, Kosova B, Gunduz C, Topcuoglu N, Ozgen G, Yilmaz C. The relationship of the apolipoprotein E gene polymorphism Turkish Type 2 diabetic patients with and without nephropathy. J Endocrinol Invest. 2009;32:219-222. [PubMed] |
| 34. | Zahálková J, Vaverková H, Novotný D, Kosatíková Z. Impaired triglyceride tolerance in hemodialysis patients with different apolipoprotein E (apo E) isoforms. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2002;146:73-76. [PubMed] |
| 35. | Arikan H, Koc M, Sari H, Tuglular S, Ozener C, Akoglu E. Associations between apolipoprotein E gene polymorphism and plasminogen activator inhibitor-1 and atherogenic lipid profile in dialysis patients. Ren Fail. 2007;29:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Joss N, Jardine A, Gaffney D, Boulton-Jones JM. Influence of apolipoprotein E genotype on progression of diabetic nephropathy. Nephron Exp Nephrol. 2005;101:e127-e133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Ma SW, Benzie IF, Yeung VT. Type 2 diabetes mellitus and its renal complications in relation to apolipoprotein E gene polymorphism. Transl Res. 2008;152:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Lim PS, Liu CS, Hong CJ, Wei YH. Prevalence of apolipoprotein E genotypes in ischaemic cerebrovascular disease in end-stage renal disease patients. Nephrol Dial Transplant. 1997;12:1916-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Kimura H, Suzuki Y, Gejyo F, Karasawa R, Miyazaki R, Suzuki S, Arakawa M. Apolipoprotein E4 reduces risk of diabetic nephropathy in patients with NIDDM. Am J Kidney Dis. 1998;31:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Lehtinen S, Rantalaiho V, Wirta O, Pasternack A, Laippala P, Koivula T, Lehtimäki T. Apolipoprotein E gene polymorphism, hypercholesterolemia and glomerular filtration rate in type 2 diabetic subjects: a 9-year follow-up study. J Biomed Sci. 2003;10:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 41. | Kwon MK, Rhee SY, Chon S, Oh S, Woo JT, Kim SW, Kim JW, Kim YS, Jeong KH, Lee SH. Association between apolipoprotein E genetic polymorphism and the development of diabetic nephropathy in type 2 diabetic patients. Diabetes Res Clin Pract. 2007;77 Suppl 1:S228-S232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Kahraman S, Kiykim AA, Altun B, Gençtoy G, Arici M, Gulsun M, Erdem Y, Yasavul U, Turgan C, Cağlar S. Apolipoprotein E gene polymorphism in renal transplant recipients: effects on lipid metabolism, atherosclerosis and allograft function. Clin Transplant. 2004;18:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Xiang G, Xia B, He Y. [The relationship of Apo E2 and renal insufficiency lipid levels in NIDDM]. Zhonghua Yixue Zazhi. 1999;79:339-341. [PubMed] |
| 44. | Li HF, Han CF, Wang YX, Lu YS, Zou HQ, Xu QQ. Effect of apolipoprotein E gene polymorphism on serum lipid level before and after renal transplantation. Transplant Proc. 2010;42:2513-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Maluf DG, Mas VR, Archer KJ, Yanek K, King A, Ferreira-Gonzalez A, Fisher RA, Posner M. Apolipoprotein E genotypes as predictors of high-risk groups for developing hyperlipidemia in kidney transplant recipients undergoing sirolimus treatment. Transplantation. 2005;80:1705-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Leiva E, Mujica V, Elematore I, Orrego R, Díaz G, Prieto M, Arredondo M. Relationship between Apolipoprotein E polymorphism and nephropathy in type-2 diabetic patients. Diabetes Res Clin Pract. 2007;78:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Stoycheff N, Stevens LA, Schmid CH, Tighiouart H, Lewis J, Atkins RC, Levey AS. Nephrotic syndrome in diabetic kidney disease: an evaluation and update of the definition. Am J Kidney Dis. 2009;54:840-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. All-trans retinoic acid regulates the expression of apolipoprotein E in rats with glomerulosclerosis induced by Adriamycin. Exp Mol Pathol. 2011;90:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Makówka A, Dryja P, Chwatko G, Bald E, Nowicki M. Treatment of chronic hemodialysis patients with low-dose fenofibrate effectively reduces plasma lipids and affects plasma redox status. Lipids Health Dis. 2012;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Toms TE, Smith JP, Panoulas VF, Blackmore H, Douglas KM, Kitas GD. Apolipoprotein E gene polymorphisms are strong predictors of inflammation and dyslipidemia in rheumatoid arthritis. J Rheumatol. 2012;39:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Alvim RO, Freitas SR, Ferreira NE, Santos PC, Cunha RS, Mill JG, Krieger JE, Pereira AC. APOE polymorphism is associated with lipid profile, but not with arterial stiffness in the general population. Lipids Health Dis. 2010;9:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Rahimi Z, Vaisi-Raygani A, Pourmotabbed T. Association between apolipoprotein ε4 allele, factor V Leiden, and plasma lipid and lipoprotein levels with sickle cell disease in Southern Iran. Mol Biol Rep. 2011;38:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Smart MC, Dedoussis G, Louizou E, Yannakoulia M, Drenos F, Papoutsakis C, Maniatis N, Humphries SE, Talmud PJ. APOE, CETP and LPL genes show strong association with lipid levels in Greek children. Nutr Metab Cardiovasc Dis. 2010;20:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Arora S, Husain M, Kumar D, Patni H, Pathak S, Mehrotra D, Reddy VK, Reddy LR, Salhan D, Yadav A. Human immunodeficiency virus downregulates podocyte apoE expression. Am J Physiol Renal Physiol. 2009;297:F653-F661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 2919] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 57. | Greenow K, Pearce NJ, Ramji DP. The key role of apolipoprotein E in atherosclerosis. J Mol Med (Berl). 2005;83:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 58. | Anthopoulos PG, Hamodrakas SJ, Bagos PG. Apolipoprotein E polymorphisms and type 2 diabetes: a meta-analysis of 30 studies including 5423 cases and 8197 controls. Mol Genet Metab. 2010;100:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | Ukkola O, Kunnari A, Jokela M, Päivänsalo M, Kesäniemi YA. ApoE phenotype is associated with inflammatory markers in middle-aged subjects. Inflamm Res. 2009;58:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |