Published online Nov 26, 2013. doi: 10.13105/wjma.v1.i3.121
Revised: October 16, 2013
Accepted: October 19, 2013
Published online: November 26, 2013
Processing time: 209 Days and 17.2 Hours
AIM: To perform a meta-analysis of the prevalence of anti-ribosomal P (aRP) antibodies in lupus psychosis, and the odds of psychosis in aRP-positive subjects.
METHODS: We identified articles by searching PubMed, PsychInfo, and ISI, and the reference lists of identified studies.
RESULTS: Twenty-four studies met the inclusion criteria. Positive aRP antibodies were found in 51% (91 of 179 total cases) of cases of lupus psychosis. There was an almost 3.5-fold increased odds of psychosis in aRP-positive patients (OR = 3.46, 95%CI: 1.97-6.09, P < 0.001). The population attributable risk percentage was 36% for aRP antibodies.
CONCLUSION: aRP antibodies are common in lupus psychosis, although the potential mechanism(s) underlying this association remain unclear. Given the overlap between the clinical presentation and risk factors for lupus psychosis and schizophrenia, further investigation of aRP antibodies in schizophrenia is warranted.
Core tip: In a meta-analysis of twenty-four studies, positive anti-ribosomal P (aRP) antibodies were found in 51% (91 of 179 total cases) of cases of lupus psychosis. There was an almost 3.5-fold increased odds of psychosis in aRP-positive patients (OR = 3.46, 95%CI: 1.97-6.09, P < 0.001). The population attributable risk percentage was 36% for aRP antibodies. aRP antibodies are common in lupus psychosis, although the potential mechanism(s) underlying this association remain unclear. Given the overlap between the clinical presentation and risk factors for lupus psychosis and schizophrenia, further investigation of aRP antibodies in schizophrenia is warranted.
- Citation: Linz K, Miller BJ. Meta-analysis of anti-ribosomal P antibodies in lupus psychosis. World J Meta-Anal 2013; 1(3): 121-129
- URL: https://www.wjgnet.com/2308-3840/full/v1/i3/121.htm
- DOI: https://dx.doi.org/10.13105/wjma.v1.i3.121
Neuropsychiatric manifestations occur in about half of patients with systemic lupus erythematosus (SLE)[1]. Psychosis is a rare, but well-documented neuropsychiatric sequelae of SLE. A systematic review of 9 studies, comprised of 1422 subjects with SLE, found a 5% point prevalence of lupus psychosis[2]. Lupus psychosis is defined as a severe disturbance in the perception of reality characterized by delusions and/or hallucinations[3]. The diagnostic criteria require that the disturbance (1) includes either delusions or hallucinations without insight; (2) causes clinical distress or impairment in social, occupational, or other relevant areas of functioning; (3) does not occur exclusively during the course of a delirium; and (4) is not better accounted for by another mental disorder. A primary psychotic disorder unrelated to SLE (e.g., schizophrenia), substance- or drug-induced psychosis, and a psychologically medicated reaction to SLE (e.g., brief reactive psychosis with a major stressor) are exclusionary to the diagnosis of lupus psychosis.
The clinical presentation of lupus psychosis may mimic that of schizophrenia. Schizophrenia is a heterogeneous psychotic disorder that requires the presence of two or more characteristic symptoms-delusions, hallucinations, disorganized speech, grossly abnormal psychomotor behavior (such as catatonia), or negative symptoms (i.e. restricted affect or avolition/asociality[4]. At least one of these two characteristic symptoms should include delusions, hallucinations, or disorganized speech. There is significant impairment in one or more major areas of functioning, including work, interpersonal relations, and self-care. It must also be established that the disturbance is not better accounted for by a primary mood disorder, schizoaffective disorder, substance intoxication or withdrawal, or another general medical condition.
A recent study found that 2 of 85 subjects hospitalized for a first-episode of schizophrenia had positive anti-nuclear (ANA) antibody titers and subsequently were found to have neuropsychiatric SLE[5]. Importantly, neither subject had signs or symptoms suggestive of rheumatologic disease, and presented only with psychiatric complaints.
A number of previous studies have found an association between anti-ribosomal P (aRP) antibodies and lupus psychosis[6-11]. aRP antibodies target P0, P1 and P2 proteins on the ribosomal sub-unit, and are capable of penetrating cells and inducing apoptotic changes. A previous meta-analysis investigated the accuracy of aRP antibody testing for the diagnosis of neuropsychiatric SLE[1]; however, this study did not consider psychosis separately from other neuropsychiatric manifestations of SLE, such as mood disorders and seizures. The purpose of the present study was to perform a meta-analysis of the prevalence of aRP antibodies in lupus psychosis, and the odds of psychosis in aRP-positive subjects.
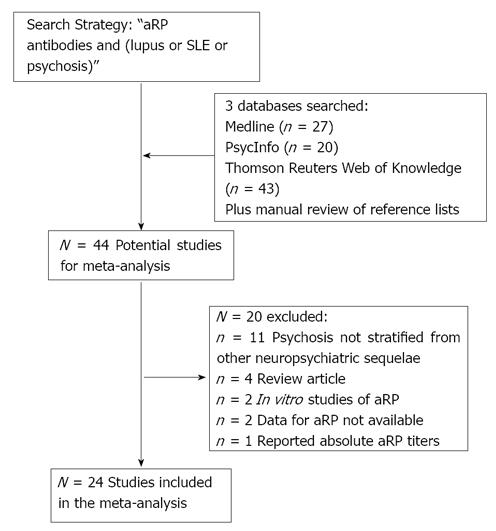
Studies of aRP antibodies in lupus psychosis were systematically searched using using Medline (PubMed, National Center for Biotechnology Information, United States National Library of Medicine, Bethesda, Maryland), PsycInfo (via Ovid, United States Psychological Association, Washington, DC), and Thomson Reuters (formerly ISI) Web of Knowledge (Science Citation Index and Social Sciences Citation Index, Thomson Reuters, Charlottesville, Virginia) in October 2011 and again in March 2013. The primary search strategy was “anti-ribosomal P antibodies and (lupus or SLE or psychosis).” Limiting results to studies in English, this search resulted in 27 citations from Medline, 20 from PsychInfo, and 43 from ISI. From these citations, as well as a manual review of their reference lists, we identified 44 potential studies, which are described in Table 1[1,6-48].
| Study | Assay method | Location | Included | Comment |
| Abdel-Nesser 2008 | ELISA | Egypt | Yes | Case-control study |
| Almeida 2002 | ELISA | Spain | Yes | |
| Arnett 1996 | ELISA | United States | Yes | Case-control study |
| Bonfa 1987 | Immunoblotting, RIA | United States | Yes | |
| Briani 2009 | Immunoblotting | Italy | Yes | Case-control study |
| Caponi 2002 | ELISA | Italy | Yes | Case-control study |
| Chan 1998 | ELISA, Western blot | China | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Conti 2004 | ELISA | Italy | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Derksen 1990 | ELISA | Netherlands | Yes | |
| Ebert 2005 | N/A | No | Review article | |
| Ghirardello 2001 | N/A | No | Review article | |
| Haddouk 2009 | Immunodot assay | Tunisia | Yes | Case-control study |
| Hanly 2008 | ELISA | Canada | Yes | Case-control study |
| Hanly 2011 | ELISA, Lupus anticoagulant | Canada | Yes | Case-control study |
| Hoffman 2004 | N/A | Europe | No | Data for aRP not available |
| Isshi 1996 | ELISA | Japan | Yes | Case-control study |
| Isshi 1998 | ELISA | Japan | No | Reported absolute titers |
| Jonsen 2003 | Immunoassays | Sweden | Yes | |
| Kao 1999 | N/A | China | Yes | |
| Karassa 2005 | N/A | Multicenter | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Magalhaes 2007 | ELISA | Brazil | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Mahler 2003 | Indirect immunofluorescence | No | Review article | |
| Massardo 2002 | Double immune diffusion, or Western blot and ELISA | Chile | Yes | Case-control study |
| Munoz 1999 | N/A | Spain | Yes | |
| Nagai 2005 | Flow cytometery | Japan | No | In vitro study |
| Nagai 2011 | ELISA | Japan | No | In vitro study |
| Nojima 1992 | western blot | Japan | Yes | Case-control study |
| Press 1996 | ELISA | Canada | Yes | Case-control study |
| Sanna 2000 | N/A | Italy | No | Data for aRP not available |
| Sato 1991 | FIEA | Japan | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Schneebaum 1991 | ELISA | United States | Yes | Case-control study |
| Shovman 2006 | ELISA | Israel | Yes | Case-control study |
| Teh 1992 | ELISA | United Kingdom | Yes | Case-control study |
| Teh 1993 | ELISA | United Kingdom | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Teh 1993b | ELISA | United Kingdom | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Toubi 2007 | N/A | No | Review article | |
| Tziousfas 2000 | Western blot | Isreal | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Van Dam 1991 | ELISA, Immunoblotting | Netherlands | Yes | Case-control study |
| Watanabe 1996 | ELISA | Japan | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Weiner 2000 | Not specified | Germany | No | Psychosis not stratified from other neuropsychiatric sequelae |
| West 1995 | ELISA | United States | Yes | |
| Williams 2004 | Western blot | United States | Yes | |
| Yalaoui 2002 | N/A | Tunisia | No | Psychosis not stratified from other neuropsychiatric sequelae |
| Yoshio 1995 | ELISA | Japan | Yes | Case-control study |
The inclusion criteria were (1) cross-sectional studies of the proportion of subjects with SLE positive for aRP antibodies, stratified by the presence or absence of psychosis; (2) cross-sectional studies of the proportion of subjects with lupus psychosis and positive aRP antibodies; or (3) longitudinal studies of aRP antibodies at multiple time points in subjects with lupus psychosis. The exclusion criteria were: (1) studies in which there were no cases of lupus psychosis; (2) studies which did not stratify psychosis from other neuropsychiatric sequelae of SLE, such as seizures and mood disorders; and (3) in vitro studies of aRP antibodies.
After independent searches, review of the study methods by two authors (BJM and KL) 24 studies met the inclusion criteria. There was universal agreement on the independent studies. 20 studies were excluded due: psychosis not stratified from other neuropsychiatric sequelae (n = 11), review articles (n = 4), in vitro studies of aRP (n = 2), data for aRP not available (n = 2), and reported absolute aRP titers (n = 1). A flow chart summarizing the study selection process is presented in Figure 1. For each of the 16 case-control studies identified, we also extracted descriptive data on subject age, gender, and illness duration.
Statistical analysis (prevalence of anti-ribosomal P antibodies)
For all 24 studies, we calculated the prevalence of positive aRP antibodies in lupus psychosis by dividing the number of subjects with psychosis and positive aRP antibodies by the total number of subjects with psychosis.
For each of the 16 case-control studies, we calculated odds ratios (OR) and 95% confidence intervals (95%CIs) for psychosis in aRP-positive patients, with odds set equal to 1.00 for psychosis in aRP-negative patients. We then performed a meta-analysis to estimate pooled OR (and 95%CI) for psychosis in aRP-positive patients, again with risk = 1.00 for psychosis in aRP-negative patients. Random effects pooled estimates and 95%CI were calculated using the method of DerSimion and Laird. Random effects models yield their actual first error rate while fixed effect models tend to inflate their first error rate. CIs obtained by fixed effect models are also biased and their actual coverage rate is smaller than their nominal coverage rate[49]. P-values were considered statistically significant at the χ2 = 0.05 level. A funnel plot and Egger’s test were generated to assess for publication bias. In case of significant heterogeneity in the overall result, we performed subgroup analysis and meta-regression, to explore possible reasons for the heterogeneity. The subgroup analysis included assay methodology (ELISA vs other). We conducted meta-regression analyses of four variables, year of publication, age, the proportion of female subjects, and illness duration. The statistical analyses were performed using Stata 10.0 (StataCorp LP, College Station, TX). The meta-analysis procedure also calculates a χ2 value for the heterogeneity in effect size (ES) estimates, which is based on Cochran’s Q-statistic[50]. Between-study heterogeneity χ2 was considered significant for P < 0.10[51].
Positive aRP antibodies were found in 51% (91 of 179 total cases) of cases of lupus psychosis.
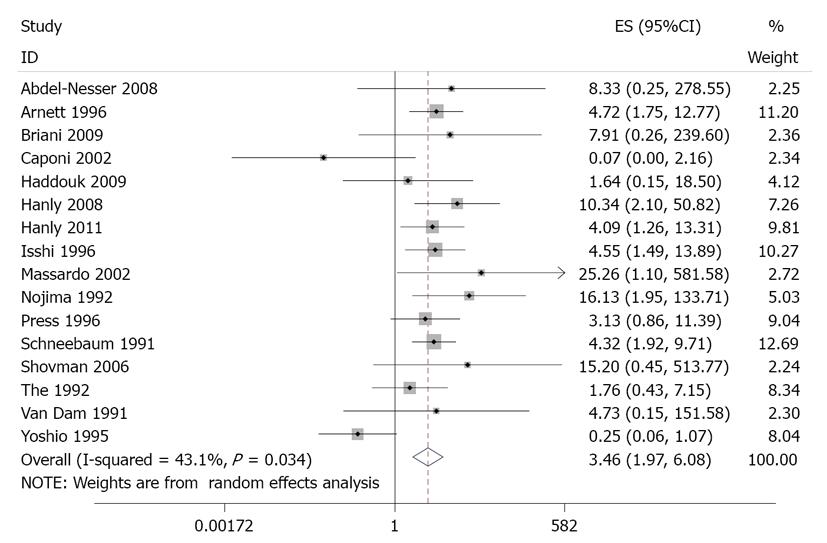
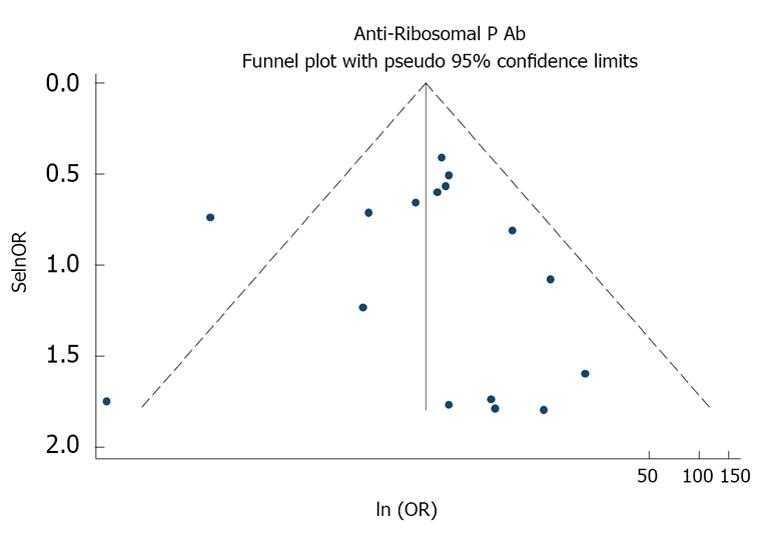
As described in Table 2, the case-control studies included a total of 3093 subjects. Table 2 and Figure 2 present the estimates of OR with 95%CIs from the meta-analysis. There was an almost 3.5-fold increased odds of psychosis in aRP-positive patients (OR = 3.46, 95%CI: 1.97-6.09, P < 0.001). There was significant heterogeneity in this effect size estimate, χ2 = 26.43, P = 0.03. In a post-hoc sensitivity analysis, the heterogeneity was no longer significant (χ2 = 12.63, P = 0.55) and the association was stronger (OR = 4.29, 95%CI: 2.90-6.36, P < 0.001) after excluding one study (Yoshio). A funnel plot showed no evidence of publication bias (Figure 3; Eggers test, P = 0.99).
| Study | Total (N) | Meanage (yr) | Female (%) | Mean illness duration (yr) | aRP(+) (n) | aRP(-) (n) | Psychosis (n) | Psychosis a-nd aRP(+) (n) | No psycho-sis and aRP(+) (n) | Psychosis and aRP(-) (n) | No psycho-sis and aRP(-) (n) | OR | 95%CI |
| Abdel-Nesser 2008 | 32 | 25.0 | 87.5 | 3.9 | 7 | 25 | 1 | 1 | 6 | 0 | 25 | 8.33 | 0.25-278.68 |
| Arnett 1996 | 364 | 63 | 301 | 17 | 8 | 55 | 9 | 292 | 4.72 | 1.74-12.76 | |||
| Briani 2009 | 219 | 28.0 | 84.5 | 45 | 174 | 1 | 1 | 44 | 0 | 174 | 7.91 | 0.26-239.58 | |
| Caponi 2002 | 149 | 37.1 | 93.3 | 9.3 | 18 | 131 | 1 | 0 | 18 | 1 | 130 | 0.07 | 0-2.6 × 107 |
| Haddouk 2009 | 200 | 30.5 | 86.5 | 47 | 153 | 3 | 1 | 46 | 2 | 151 | 1.64 | 0.15-18.51 | |
| Hanly 2008 | 214 | 34.9 | 87.4 | 0.4 | 17 | 197 | 7 | 3 | 14 | 4 | 193 | 10.34 | 2.10-50.82 |
| Hanly 2011 | 991 | 35.2 | 89.1 | 0.5 | 91 | 900 | 14 | 4 | 87 | 10 | 890 | 4.09 | 1.26-13.32 |
| Isshi 1996 | 75 | 21 | 54 | 19 | 10 | 11 | 9 | 45 | 4.55 | 1.49-13.88 | |||
| Massardo 2002 | 141 | 33.0 | 90.1 | 5.0 | 21 | 120 | 2 | 2 | 19 | 0 | 120 | 25.26 | 1.10-581.69 |
| Nojima 1992 | 91 | 80.0 | 38 | 53 | 10 | 9 | 29 | 1 | 53 | 16.45 | 1.98-136.36 | ||
| Press 1996 | 79 | 16 | 63 | 13 | 5 | 11 | 8 | 55 | 3.13 | 0.86-11.37 | |||
| Schneebaum 1991 | 269 | 51 | 218 | 29 | 13 | 38 | 16 | 202 | 4.32 | 1.92-9.71 | |||
| Shovman 2006 | 44 | 6 | 38 | 1 | 1 | 5 | 0 | 38 | 15.2 | 0.45-513.80 | |||
| The 1992 | 116 | 18 | 98 | 13 | 3 | 15 | 10 | 88 | 1.76 | 0.43-7.15 | |||
| Van Dam 1991 | 38 | 12 | 26 | 1 | 1 | 11 | 0 | 26 | 4.73 | 0.15-151.50 | |||
| Yoshio 1995 | 70 | 31.9 | 94.3 | 41 | 29 | 10 | 3 | 38 | 7 | 22 | 0.25 | 0.06-1.06 | |
| Total | 3093 | 512 | 2581 | 142 | 65 | 447 | 77 | 2504 |
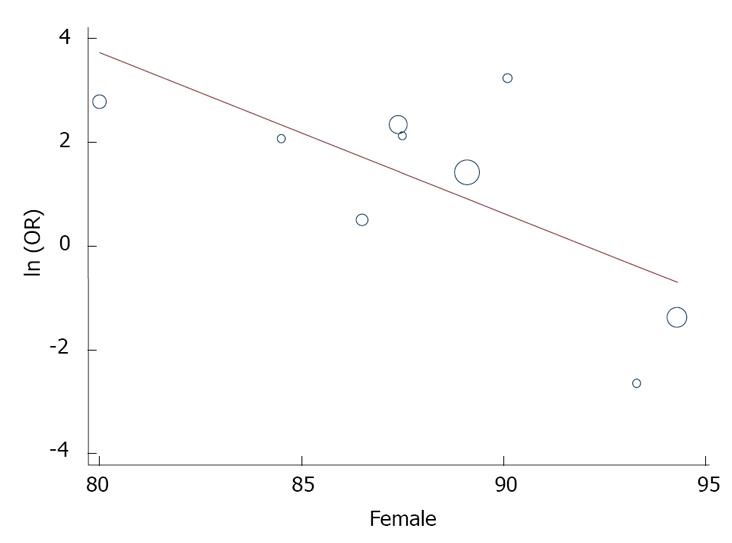
In the subgroup analysis, there was no change in the association when studies using ELISA to measure aRP antibodies were considered separately (OR = 3.00, 95%CI: 1.60-5.59, P < 0.001). In meta-regression analyses, year of publication (P = 0.55), age (0.55), and illness duration (P = 0.27) were unrelated to the association between aRP antibodies and lupus psychosis. However, there was a significant association with gender (slope = -0.31, 95%CI: -0.55 to -0.08, P = 0.02), with a stronger association in studies with a higher proportion of males (Figure 4).
We also estimated the population attributable risk percentage (PAR%) for aRP-positivity. The PAR% is the prevalence of the outcome (psychosis) in all subjects, minus the prevalence of the outcome among the unexposed (defined here as aRP negative patients), divided by the prevalence of outcome in the total population, and multiplied by 100%. The population PAR% was 36% for aRP antibodies.
Although psychosis is a rare neuropsychiatric manifestation of SLE, we found that more than half of subjects with lupus psychosis had positive aRP antibodies. Furthermore, there was an almost 3.5-fold increased odds of psychosis in aRP-positive patients. The association was not moderated by year of publication, age, or illness duration, but there was a significant association with gender. The PAR% was 36% for anti-ribosomal P antibodies.
An important strength of our study is that we included data from all case-control studies of this association. A previous meta-analysis aRP antibodies in SLE did not consider psychosis separately from other neuropsychiatric manifestations of SLE[1]. Our analysis differed from this study in several ways. First, we focused on psychosis as the outcome, rather than the broader category of neuropsychiatric SLE. Second, we were able to calculate the odds of psychosis in aRP-positive subjects, as well as the PAR% for aRP-positivity. Although we were able to perform subgroup and meta-regression analyses, an important limitation of the present study was that data on a number of potential confounding factors, including age, sex, and illness duration, were available for only a portion of studies. We were not able to control for other potential confounding factors including smoking status, rheumatologic symptoms, stage of illness (e.g., active vs inactive SLE), and medications.
We found a population attributable risk percentage (PAR%) of 36% for aRP-positivity. As the PAR% varies with both the risk (i.e. OR) associated with an exposure (i.e. aRP-positivity) and its prevalence, caution must be exercised in the interpretation of this result. The PAR% refers to a family of concepts. Greenland and Robins[52] distinguished between the etiologic and excess fraction. The etiologic fraction is the proportion of cases that the exposure had played a causal role in its development. The excess fraction is the proportion of cases among the exposed population that is in excess in comparison with the unexposed. Our results describe the excess fraction for aRP-positivity, as it is not possible to establish the causality of this association.
One longitudinal study found that IgA and IgM classes of aRP antibodies were elevated at the onset of psychosis, and titers decreased following a remission of psychosis[43]. Another longitudinal study of aRP activity in two patients with psychosis revealed that aRP levels increased before and during the active phases of psychosis[15]. This could possibly help predict efficacy of treatment and warrants further investigation into the possibility of monitoring disease activity by aRP titers.
The mechanism(s) underlying this association remain unclear and warrant further investigation. One possibility is that aRP antibodies may directly cross-react with central nervous system antigens, resulting in acute psychosis. Autoantibodies are also associated with increases in pro-inflammatory cytokines, such as interleukin-6 (IL-6) which can directly modulate dopaminergic neurotransmission[53], or indirectly modulate glutamatergic neurotransmission through tryptophan catabolism[54], which can also result in acute psychosis. Consistent with the latter, increased cerebrospinal fluid IL-6 is also associated with lupus psychosis, although the relationship with aRP antibodies is unknown[55].
A previous study found 2 of 85 subjects presenting with first-episode schizophrenia were subsequently diagnosed with neuropsychiatric SLE, and neither subject had other signs or symptoms of rheumatologic disease[5]. A systematic quantitative review also found an increased prevalence of autoantibodies associated with limbic encephalitis (NMDA receptor antibodies) in subjects with first-episode schizophrenia, in the absence of other neurologic signs or symptoms[56]. To our knowledge, only one previous study has measured aRP antibodies in subjects with schizophrenia[57]. Among 59 patients in this study, aRP antibody titers were below cutoff levels in 58 patients and borderline in 1 patient. One possibility for the negative finding is that the prevalence of potentially pathogenic central nervous system autoantibodies in schizophrenia is low, and this study was underpowered to detect an association. Another possibility is that serum autoantibodies are only present earlier in the course of the disorder.
In addition to overlapping clinical presentations, there are also shared risk factors for lupus psychosis and schizophrenia. There is bidirectional evidence for an association between schizophrenia and autoimmune disorders[58-60]. Single nucleotide polymorphisms in genes in the major histocompatibility complex on chromosome 6q, which are critical to immune system function and associated with autoimmune disorders, are also risk factors for schizophrenia[61-63]. Patients with schizophrenia may also have abnormal absolute levels of antibody-producing B-lymphocytes[64-66]. Thus, as identification of patients with autoantibody-mediated psychosis (vs schizophrenia) has important treatment-related implications, these findings suggest that future studies of aRP antibodies in patients with schizophrenia are warranted.
In conclusion, aRP antibodies are highly prevalent and significant predictors of lupus psychosis. Future studies of these antibodies will be important to an improved understanding of the pathophysiology of psychosis. Further investigation of these autoantibodies in patients with schizophrenia, which has largely been unexplored, are warranted.
The authors wish to thank Billy Houke for assistance with references.
Neuropsychiatric manifestations occur in about half of patients with systemic lupus erythematosus (SLE). Psychosis is a rare, but well-documented neuropsychiatric sequelae of SLE. A number of previous studies have reported an association between anti-ribosomal P (aRP) antibodies and lupus psychosis.
The purpose of the present study was to perform a meta-analysis of the prevalence of aRP antibodies in lupus psychosis, and the odds of psychosis in aRP-positive subjects.
A previous meta-analysis investigated the accuracy of aRP antibody testing for the diagnosis of neuropsychiatric SLE; however, this study did not consider psychosis separately from other neuropsychiatric manifestations of SLE, such as mood disorders and seizures. In a meta-analysis of 24 studies, we report that positive aRP antibodies were found in 51% (91 of 179 total cases) of cases of lupus psychosis. There was an almost 4-fold increased odds of psychosis in aRP-positive patients (OR = 3.75, 95%CI: 2.23-6.30, P < 0.001). The population attributable risk percentage was 36% for aRP antibodies.
aRP antibodies are common in lupus psychosis. Schizophrenia is associated with increased prevalence of autoantibodies and autoimmune disease. Given these associations, aRP warrants further investigation in schizophrenia.
aRP antibodies: Autoantibodies are immune molecules (proteins) that are directed against the body’s own tissues. aRP antibodies target proteins on the ribosome, and are capable of penetrating cells and inducing apoptosis, or programmed cell death.
Psychosis: Psychosis is a potential neuropsychiatric complication that occurs in some patients with systemic lupus erythematosus. Psychosis is a neuropsychiatric disorder that includes abnormalities in thinking, behavior, mood, and cognition. Common symptoms of psychosis included hallucinations, delusions, disorganized speech and behavior, and negative symptoms.
This is a very good study that supports a potential role for anti-ribosomal P antibodies in lupus psychosis. Findings strengthened previous reports in the literature about this association. The topic is up-to-date and the results are of high interest for other researchers.
P- Reviewers: Espinoza LR, Kambeitz JP S- Editor: Cui XM L- Editor: A E- Editor: Lu YJ
| 1. | Karassa FB, Afeltra A, Ambrozic A, Chang DM, De Keyser F, Doria A, Galeazzi M, Hirohata S, Hoffman IE, Inanc M. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum. 2006;54:312-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. J Clin Psychiatry. 2012;73:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599-608. [PubMed] |
| 4. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing 2013; . |
| 5. | Mantovani C, Louzada-Junior P, Nunes EA, de Figueiredo FP, Oliveira GR, Del-Ben CM. Antinuclear antibodies testing as a routine screening for systemic lupus erythematosus in patients presenting first-episode psychosis. Early Interv Psychiatry. 2012;6:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Arnett FC, Reveille JD, Moutsopoulos HM, Georgescu L, Elkon KB. Ribosomal P autoantibodies in systemic lupus erythematosus. Frequencies in different ethnic groups and clinical and immunogenetic associations. Arthritis Rheum. 1996;39:1833-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Hanly JG, Urowitz MB, Siannis F, Farewell V, Gordon C, Bae SC, Isenberg D, Dooley MA, Clarke A, Bernatsky S. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58:843-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Clarke A, Bernatsky S, Vasudevan A, Isenberg D, Rahman A. Autoantibodies as biomarkers for the prediction of neuropsychiatric events in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Isshi K, Hirohata S. Association of anti-ribosomal P protein antibodies with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 1996;39:1483-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Nojima Y, Minota S, Yamada A, Takaku F, Aotsuka S, Yokohari R. Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann Rheum Dis. 1992;51:1053-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Schneebaum AB, Singleton JD, West SG, Blodgett JK, Allen LG, Cheronis JC, Kotzin BL. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991;90:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 181] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Briani C, Lucchetta M, Ghirardello A, Toffanin E, Zampieri S, Ruggero S, Scarlato M, Quattrini A, Bassi N, Ermani M. Neurolupus is associated with anti-ribosomal P protein antibodies: an inception cohort study. J Autoimmun. 2009;32:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Abdel-Nasser AM, Ghaleb RM, Mahmoud JA, Khairy W, Mahmoud RM. Association of anti-ribosomal P protein antibodies with neuropsychiatric and other manifestations of systemic lupus erythematosus. Clin Rheumatol. 2008;27:1377-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Almeida D, Antolín J, Amérigo MJ, Cantabrana A, Roces A, Hayeck M. [Anti-ribosomal antibodies as activity markers in systemic lupus erythematosus]. An Med Interna. 2002;19:73-75. [PubMed] |
| 15. | Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 409] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Caponi L, Chimenti D, Pratesi F, Migliorini P. Anti-ribosomal antibodies from lupus patients bind DNA. Clin Exp Immunol. 2002;130:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Chan EY, Ko OK, Lawton JW, Lau CS. The use of anti-ribosomal P antibodies in the diagnosis of cerebral lupus---superiority of western blotting over enzyme-linked immunosorbent assay. Hong Kong Med J. 1998;4:145-150. [PubMed] |
| 18. | Conti F, Alessandri C, Bompane D, Bombardieri M, Spinelli FR, Rusconi AC, Valesini G. Autoantibody profile in systemic lupus erythematosus with psychiatric manifestations: a role for anti-endothelial-cell antibodies. Arthritis Res Ther. 2004;6:R366-R372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Derksen RH, van Dam AP, Gmelig Meyling FH, Bijlsma JW, Smeenk RJ. A prospective study on antiribosomal P proteins in two cases of familial lupus and recurrent psychosis. Ann Rheum Dis. 1990;49:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Eber T, Chapman J, Shoenfeld Y. Anti-ribosomal P-protein and its role in psychiatric manifestations of systemic lupus erythematosus: myth or reality? Lupus. 2005;14:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Ghirardello A, Doria A, Zampieri S, Gambari PF, Todesco S. Autoantibodies to ribosomal P proteins in systemic lupus erythematosus. Isr Med Assoc J. 2001;3:854-857. [PubMed] |
| 22. | Haddouk S, Marzouk S, Jallouli M, Fourati H, Frigui M, Hmida YB, Koubaa F, Sellami W, Baklouti S, Hachicha J. Clinical and diagnostic value of ribosomal P autoantibodies in systemic lupus erythematosus. Rheumatology (Oxford). 2009;48:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Hoffman IE, Peene I, Meheus L, Huizinga TW, Cebecauer L, Isenberg D, De Bosschere K, Hulstaert F, Veys EM, De Keyser F. Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann Rheum Dis. 2004;63:1155-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Isshi K, Hirohata S. Differential roles of the anti-ribosomal P antibody and antineuronal antibody in the pathogenesis of central nervous system involvement in systemic lupus erythematosus. Arthritis Rheum. 1998;41:1819-1827. [PubMed] |
| 25. | Jönsen A, Bengtsson AA, Nived O, Ryberg B, Truedsson L, Rönnblom L, Alm GV, Sturfelt G. The heterogeneity of neuropsychiatric systemic lupus erythematosus is reflected in lack of association with cerebrospinal fluid cytokine profiles. Lupus. 2003;12:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Kao CH, Ho YJ, Lan JL, Changlai SP, Liao KK, Chieng PU. Discrepancy between regional cerebral blood flow and glucose metabolism of the brain in systemic lupus erythematosus patients with normal brain magnetic resonance imaging findings. Arthritis Rheum. 1999;42:61-68. [PubMed] |
| 27. | Magalhães MB, da Silva LM, Voltarelli JC, Donadi EA, Louzada-Junior P. Lymphocytotoxic antibodies in systemic lupus erythematosus are associated with disease activity irrespective of the presence of neuropsychiatric manifestations. Scand J Rheumatol. 2007;36:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Mahler M, Ngo JT, Schulte-Pelkum J, Luettich T, Fritzler MJ. Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Res Ther. 2008;10:R131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Massardo L, Burgos P, Martínez ME, Pérez R, Calvo M, Barros J, González A, Jacobelli S. Antiribosomal P protein antibodies in Chilean SLE patients: no association with renal disease. Lupus. 2002;11:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Muñoz-Málaga A, Anglada JC, Páez M, Girón JM, Barrera A. [Psychosis as the initial manifestation of systemic lupus erythematosus: the role of lupus band test and anti-ribosomal antibodies]. Rev Neurol. 1999;28:779-781. [PubMed] |
| 31. | Nagai T, Arinuma Y, Yanagida T, Yamamoto K, Hirohata S. Anti-ribosomal P protein antibody in human systemic lupus erythematosus up-regulates the expression of proinflammatory cytokines by human peripheral blood monocytes. Arthritis Rheum. 2005;52:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Nagai T, Yanagida T, Hirohata S. Anti-ribosomal P protein antibody induces Th1 responses by enhancing the production of IL-12 in activated monocytes. Mod Rheumatol. 2011;21:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Press J, Palayew K, Laxer RM, Elkon K, Eddy A, Rakoff D, Silverman ED. Antiribosomal P antibodies in pediatric patients with systemic lupus erythematosus and psychosis. Arthritis Rheum. 1996;39:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Sanna G, Piga M, Terryberry JW, Peltz MT, Giagheddu S, Satta L, Ahmed A, Cauli A, Montaldo C, Passiu G. Central nervous system involvement in systemic lupus erythematosus: cerebral imaging and serological profile in patients with and without overt neuropsychiatric manifestations. Lupus. 2000;9:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Sato T, Uchiumi T, Ozawa T, Kikuchi M, Nakano M, Kominami R, Arakawa M. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J Rheumatol. 1991;18:1681-1684. [PubMed] |
| 36. | Shovman O, Zandman-Goddard G, Gilburd B, Blank M, Ehrenfeld M, Bardechevski S, Stojanovich L, Langevitz P, Shoenfeld Y. Restricted specificity of anti-ribosomal P antibodies to SLE patients in Israel. Clin Exp Rheumatol. 2006;24:694-697. [PubMed] |
| 37. | Teh LS. Antiribosomal P antibodies and lupus psychosis. Ann Rheum Dis. 1992;51:1104. [PubMed] |
| 38. | Teh LS, Hay EM, Amos N, Black D, Huddy A, Creed F, Bernstein RM, Holt PJ, Williams BD. Anti-P antibodies are associated with psychiatric and focal cerebral disorders in patients with systemic lupus erythematosus. Br J Rheumatol. 1993;32:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Teh LS, Lee MK, Wang F, Manivasagar M, Charles PJ, Nicholson GD, Hay EM, Isenberg DA, Amos N, Williams BD. Antiribosomal P protein antibodies in different populations of patients with systemic lupus erythematosus. Br J Rheumatol. 1993;32:663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Toubi E, Shoenfeld Y. Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmun Rev. 2007;6:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Tzioufas AG, Tzortzakis NG, Panou-Pomonis E, Boki KA, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM. The clinical relevance of antibodies to ribosomal-P common epitope in two targeted systemic lupus erythematosus populations: a large cohort of consecutive patients and patients with active central nervous system disease. Ann Rheum Dis. 2000;59:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | van Dam AP. Diagnosis and pathogenesis of CNS lupus. Rheumatol Int. 1991;11:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Watanabe T, Sato T, Uchiumi T, Arakawa M. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: diagnostic and predictive value of longitudinal examination of anti-ribosomal P antibody. Lupus. 1996;5:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Weiner SM, Otte A, Schumacher M, Klein R, Gutfleisch J, Brink I, Otto P, Nitzsche EU, Moser E, Peter HH. Diagnosis and monitoring of central nervous system involvement in systemic lupus erythematosus: value of F-18 fluorodeoxyglucose PET. Ann Rheum Dis. 2000;59:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | West SG, Emlen W, Wener MH, Kotzin BL. Neuropsychiatric lupus erythematosus: a 10-year prospective study on the value of diagnostic tests. Am J Med. 1995;99:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 163] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Williams RC, Sugiura K, Tan EM. Antibodies to microtubule-associated protein 2 in patients with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2004;50:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Yalaoui S, Gorgi Y, Hajri R, Goucha R, Chaabouni L, Kooli C, Ayed K. Autoantibodies to ribosomal P proteins in systemic lupus erythematosus. Joint Bone Spine. 2002;69:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Yoshio T, Masuyama J, Ikeda M, Tamai K, Hachiya T, Emori T, Mimori A, Takeda A, Minota S, Kano S. Quantification of antiribosomal P0 protein antibodies by ELISA with recombinant P0 fusion protein and their association with central nervous system disease in systemic lupus erythematosus. J Rheumatol. 1995;22:1681-1687. [PubMed] |
| 49. | Hunter J, Schmidt F. Fixed Effects vs. Random Effects Meta-Analysis Models: Implications for Cumulative Research Knowledge. Inter J Select Assess. 2000;8:275-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 50. | Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256-266. [PubMed] |
| 51. | Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126-151. [PubMed] |
| 52. | Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128:1185-1197. [PubMed] |
| 53. | Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, Greenberg AH. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res. 1994;643:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 309] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Hirohata S, Kanai Y, Mitsuo A, Tokano Y, Hashimoto H. Accuracy of cerebrospinal fluid IL-6 testing for diagnosis of lupus psychosis. A multicenter retrospective study. Clin Rheumatol. 2009;28:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Ezeoke A, Mellor A, Buckley P, Miller B. A systematic, quantitative review of blood autoantibodies in schizophrenia. Schizophr Res. 2013;150:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Gilat Y, Shoenfeld Y, Kotler M, Iancu I. Anti-ribosomal P antibody in schizophrenia. Isr J Psychiatry Relat Sci. 2011;48:275-279. [PubMed] |
| 58. | Chen SJ, Chao YL, Chen CY, Chang CM, Wu EC, Wu CS, Yeh HH, Chen CH, Tsai HJ. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry. 2012;200:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 59. | Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, Mortensen PB. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 378] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 60. | Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12:638-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 61. | Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3988] [Cited by in RCA: 3559] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 62. | Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 884] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 63. | Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen OP, Mors O, Mortensen PB. Common variants conferring risk of schizophrenia. Nature. 2009;460:744-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 1283] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 64. | McAllister CG, Rapaport MH, Pickar D, Podruchny TA, Christison G, Alphs LD, Paul SM. Increased numbers of CD5+ B lymphocytes in schizophrenic patients. Arch Gen Psychiatry. 1989;46:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Printz DJ, Strauss DH, Goetz R, Sadiq S, Malaspina D, Krolewski J, Gorman JM. Elevation of CD5+ B lymphocytes in schizophrenia. Biol Psychiatry. 1999;46:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Steiner J, Jacobs R, Panteli B, Brauner M, Schiltz K, Bahn S, Herberth M, Westphal S, Gos T, Walter M. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |