Copyright
©The Author(s) 2016.
World J Meta-Anal. Jun 26, 2016; 4(3): 69-76
Published online Jun 26, 2016. doi: 10.13105/wjma.v4.i3.69
Published online Jun 26, 2016. doi: 10.13105/wjma.v4.i3.69
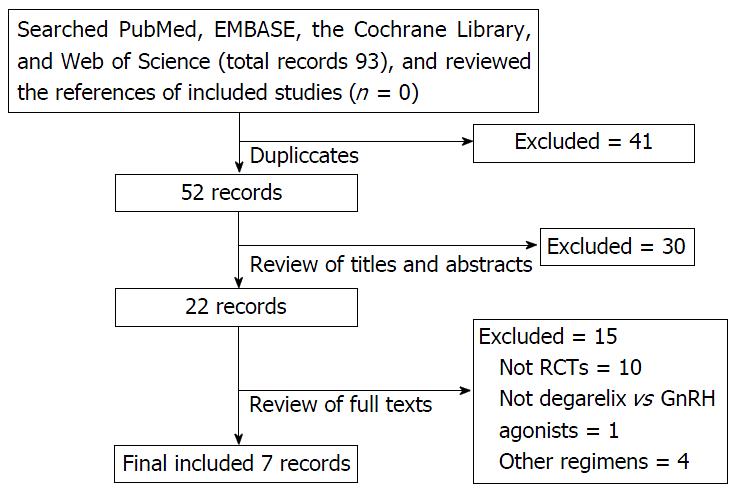
Figure 1 The details of identifying studies.
RCT: Randomized controlled trials; GnRH: Gonadotropin-releasing hormone.
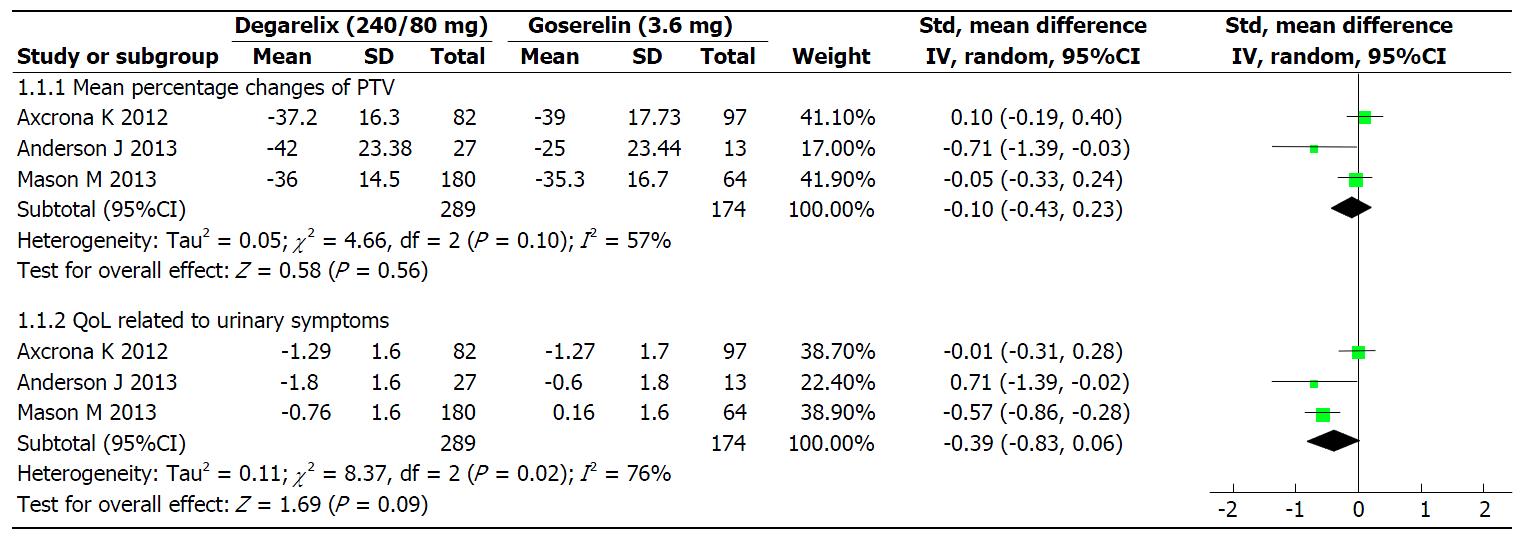
Figure 2 The effects of degarelix (240/80 mg) and goserelin (3.
6 mg) on mean percentage changes of total prostate volume and quality of life related to urinary symptoms within included studies. QoL: Quality of life.
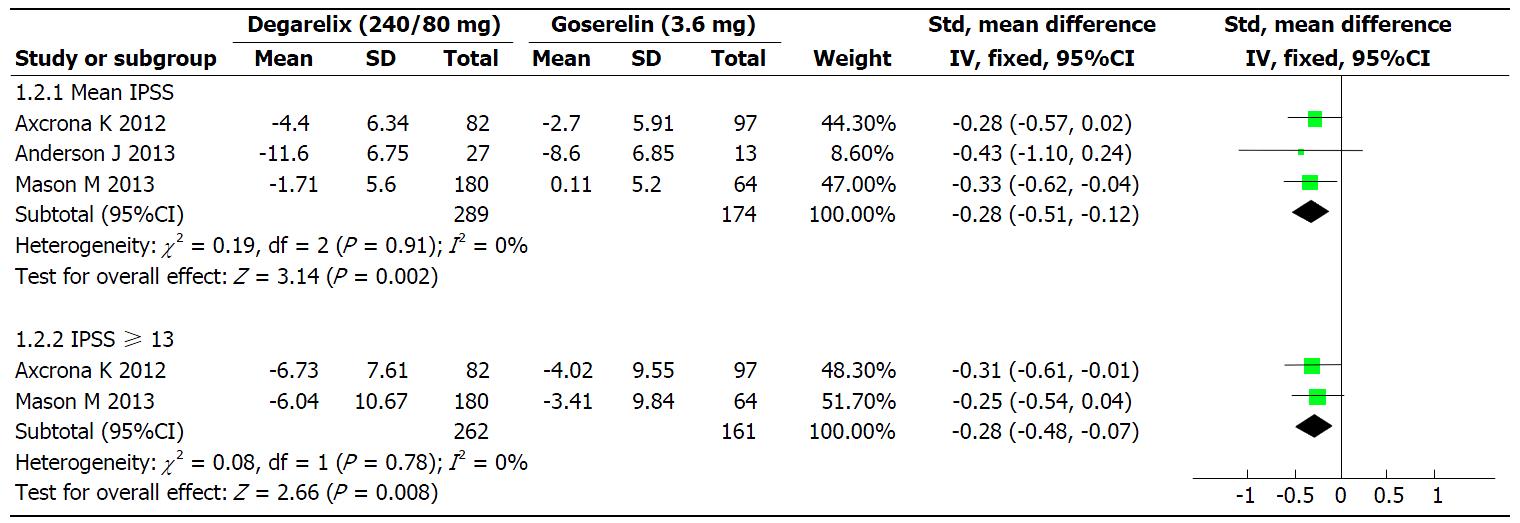
Figure 3 The effects of degarelix (240/80 mg) and goserelin (3.
6 mg) on mean International Prostate Symptom Score and International Prostate Symptom Score ≥ 13 within included studies. IPSS: International Prostate Symptom Score.
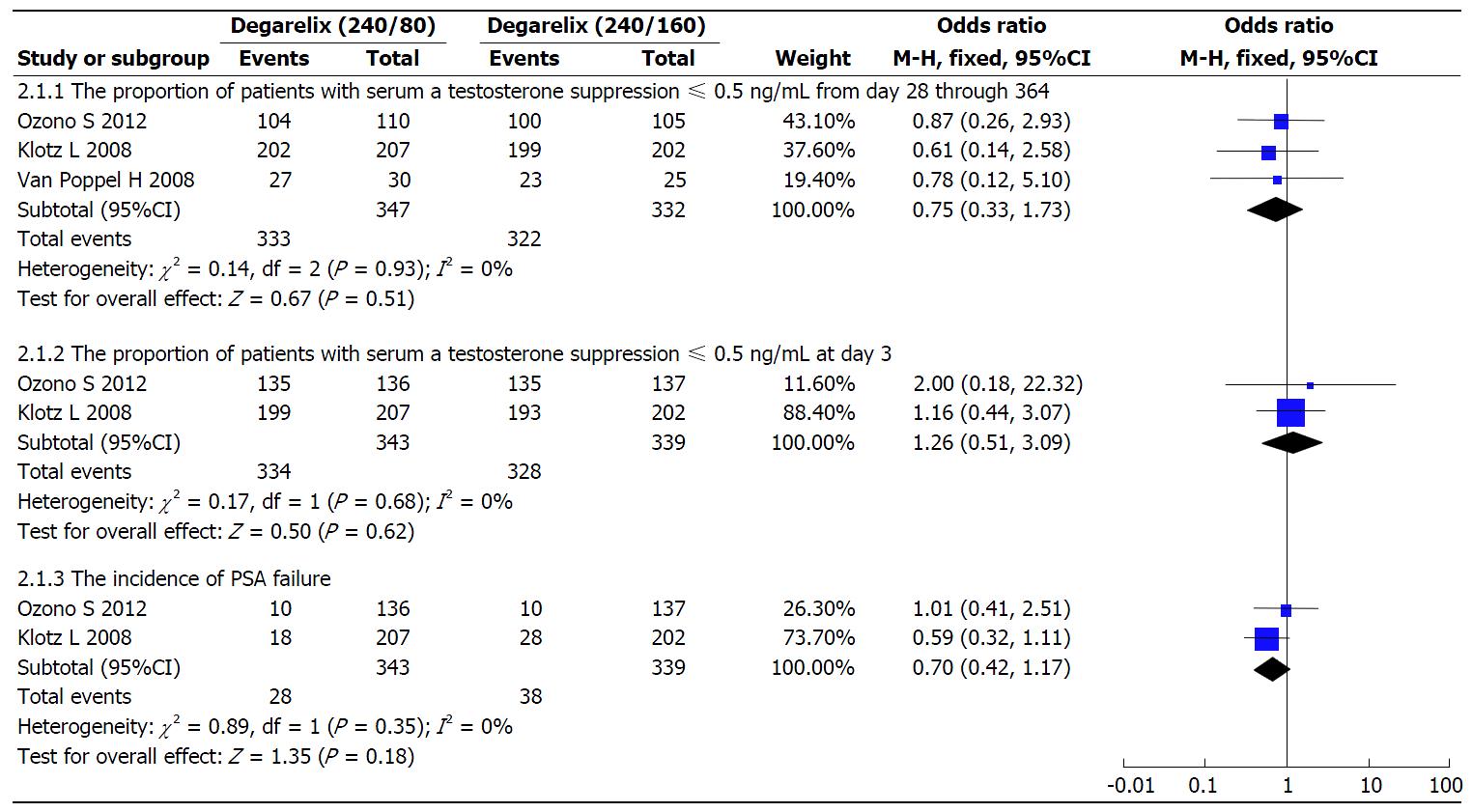
Figure 4 The effect of degarelix (240/80 mg vs 240/160 mg) on serum testosterone and prostate-specific antigen within included studies.
PSA: Prostate-specific antigen.
- Citation: Fang C, Wu CL, Liu SS, Ge L, Bai JL. Efficacy, safety, and dose comparison of degarelix for the treatment of prostate cancer: A systematic review and meta-analysis. World J Meta-Anal 2016; 4(3): 69-76
- URL: https://www.wjgnet.com/2308-3840/full/v4/i3/69.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i3.69