Copyright
©The Author(s) 2015.
World J Meta-Anal. Dec 26, 2015; 3(6): 254-283
Published online Dec 26, 2015. doi: 10.13105/wjma.v3.i6.254
Published online Dec 26, 2015. doi: 10.13105/wjma.v3.i6.254
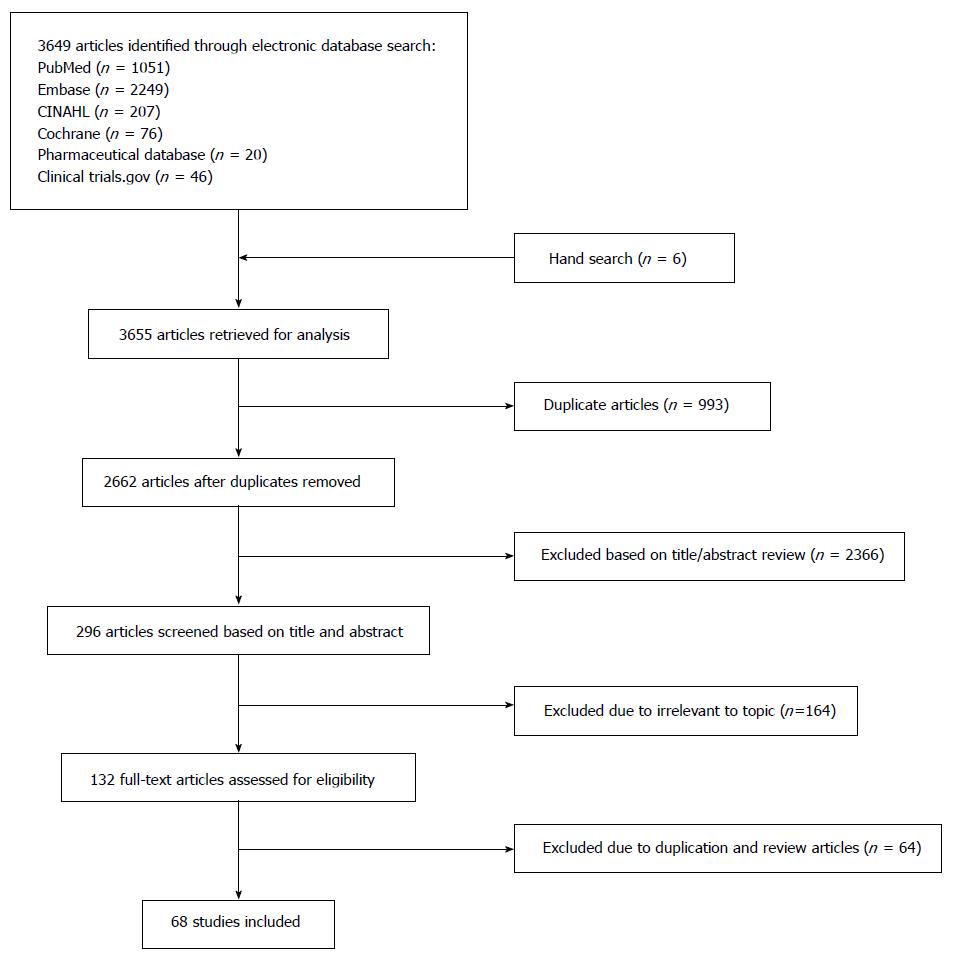
Figure 1 Flow diagram of studies identified and selected.
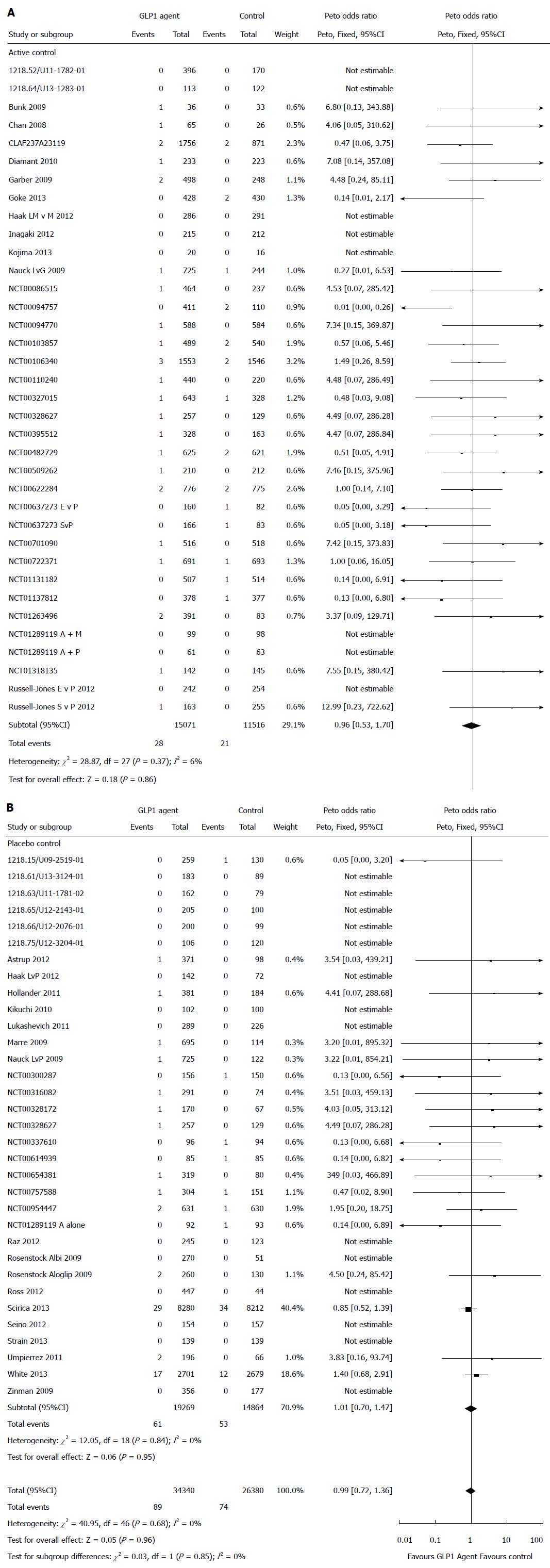
Figure 2 Risk of pancreatic adverse events in patients treated with glucagon-like peptide-1 based therapies (A and B).
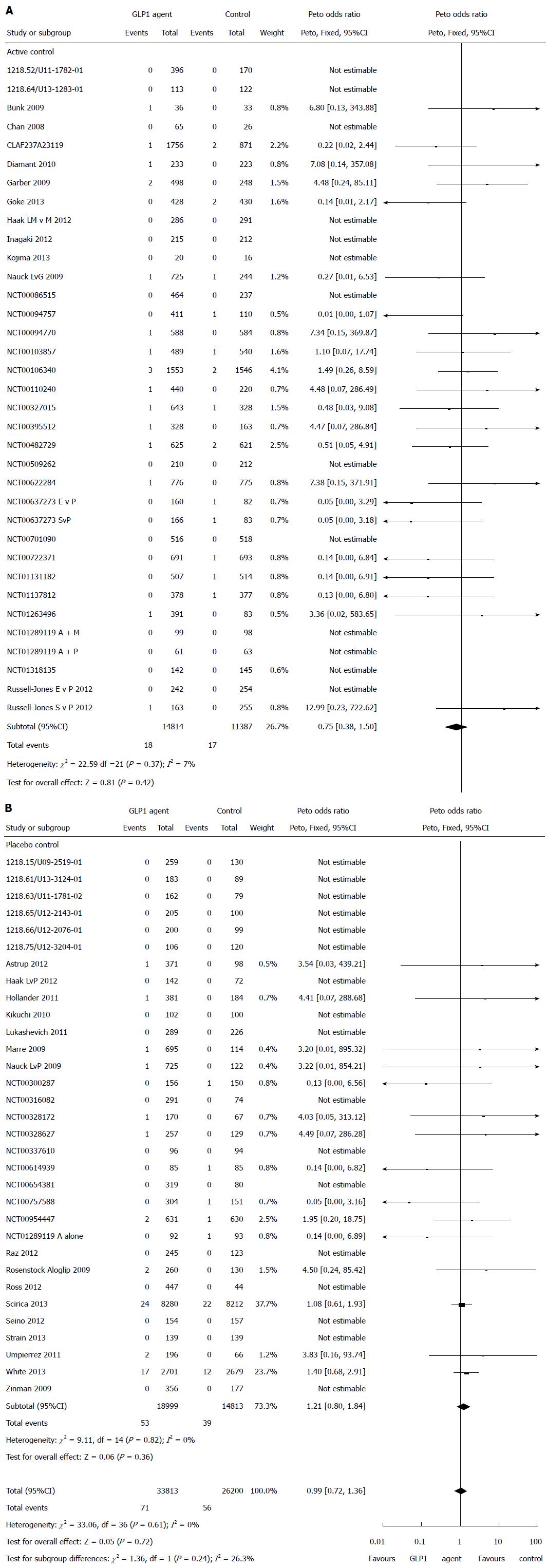
Figure 3 Risk of pancreatitis in patients treated with glucagon-like peptide-1 based therapies (A and B).
Figure 4 Risk of elevated pancreatic enzymes for glucagon-like peptide-1 based agents.
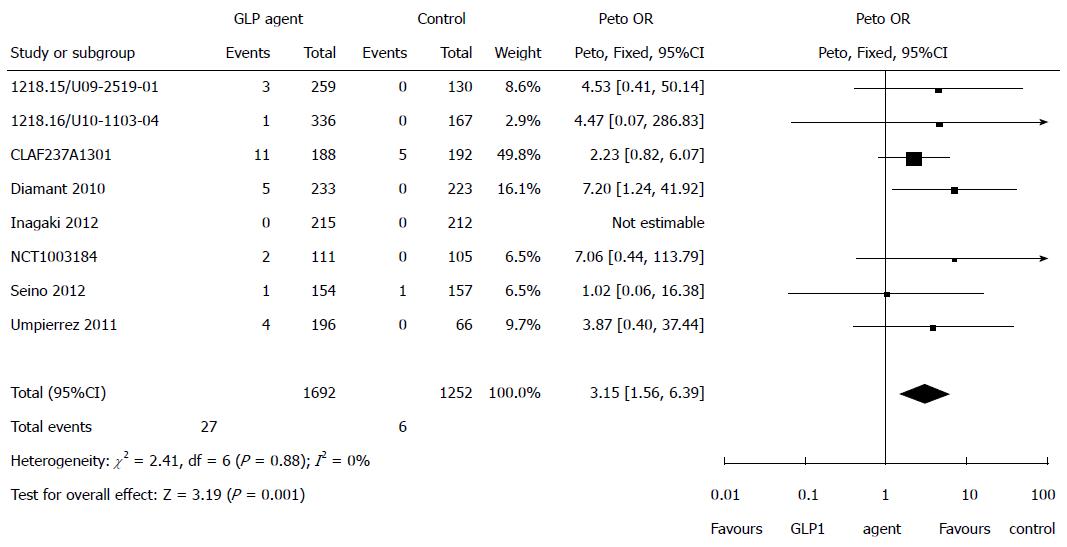
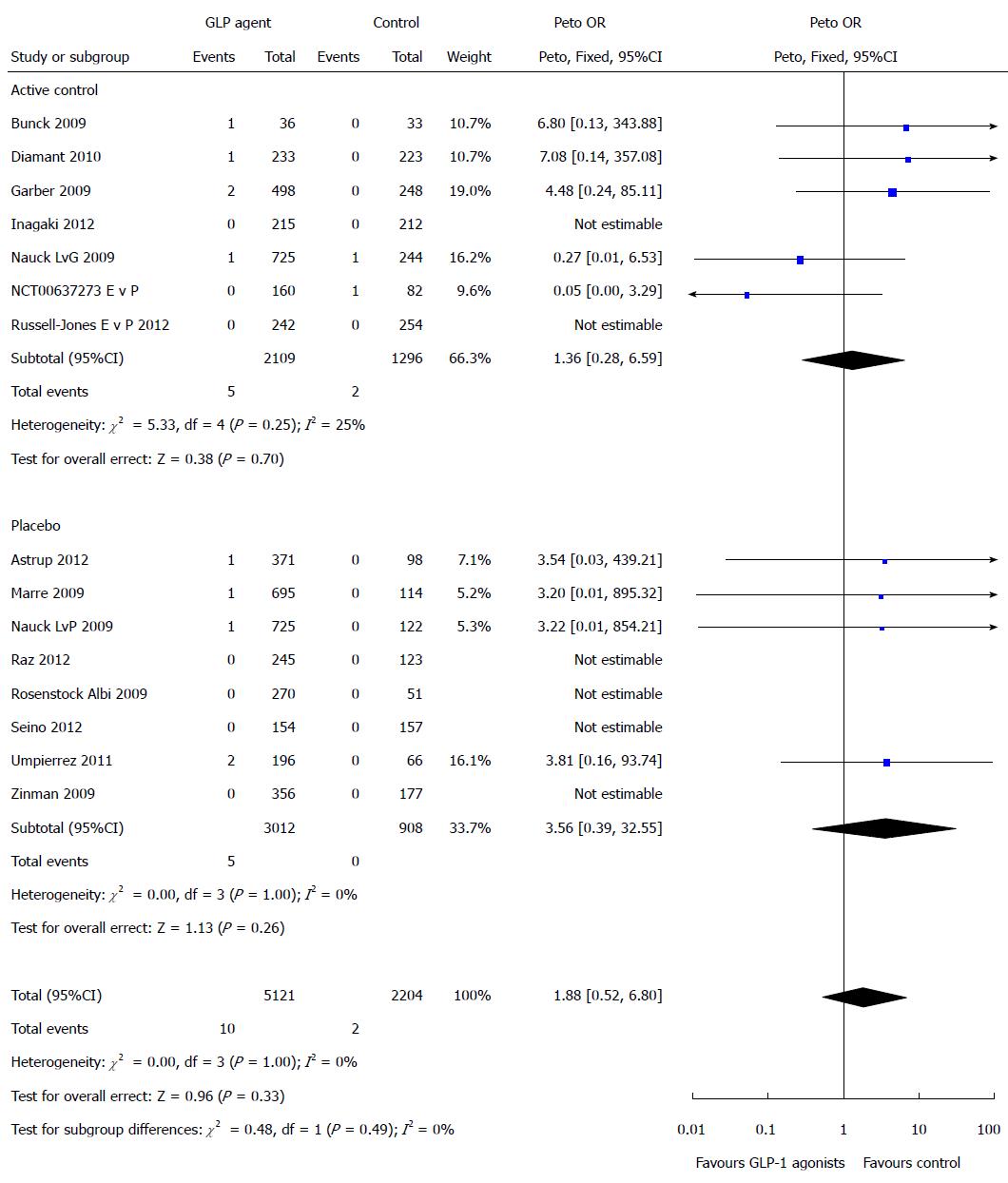
Figure 5 Risk of pancreatic events for glucagon-like peptide-1 receptor agonist drugs only.
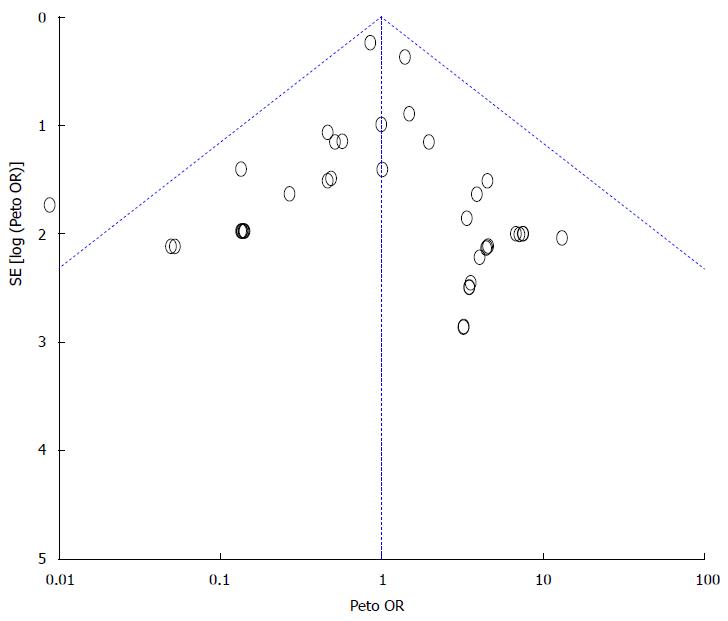
Figure 6 Funnel plot for risk of pancreatic adverse events.
- Citation: Shihab HM, Akande T, Armstrong K, Singh S, Loke YK. Risk of pancreatic adverse events associated with the use of glucagon-like peptide-1 receptor agonist and dipeptidyl peptidase-4 inhibitor drugs: A systematic review and meta-analysis of randomized trials. World J Meta-Anal 2015; 3(6): 254-283
- URL: https://www.wjgnet.com/2308-3840/full/v3/i6/254.htm
- DOI: https://dx.doi.org/10.13105/wjma.v3.i6.254