Copyright
©The Author(s) 2024.
World J Meta-Anal. Dec 18, 2024; 12(4): 98508
Published online Dec 18, 2024. doi: 10.13105/wjma.v12.i4.98508
Published online Dec 18, 2024. doi: 10.13105/wjma.v12.i4.98508
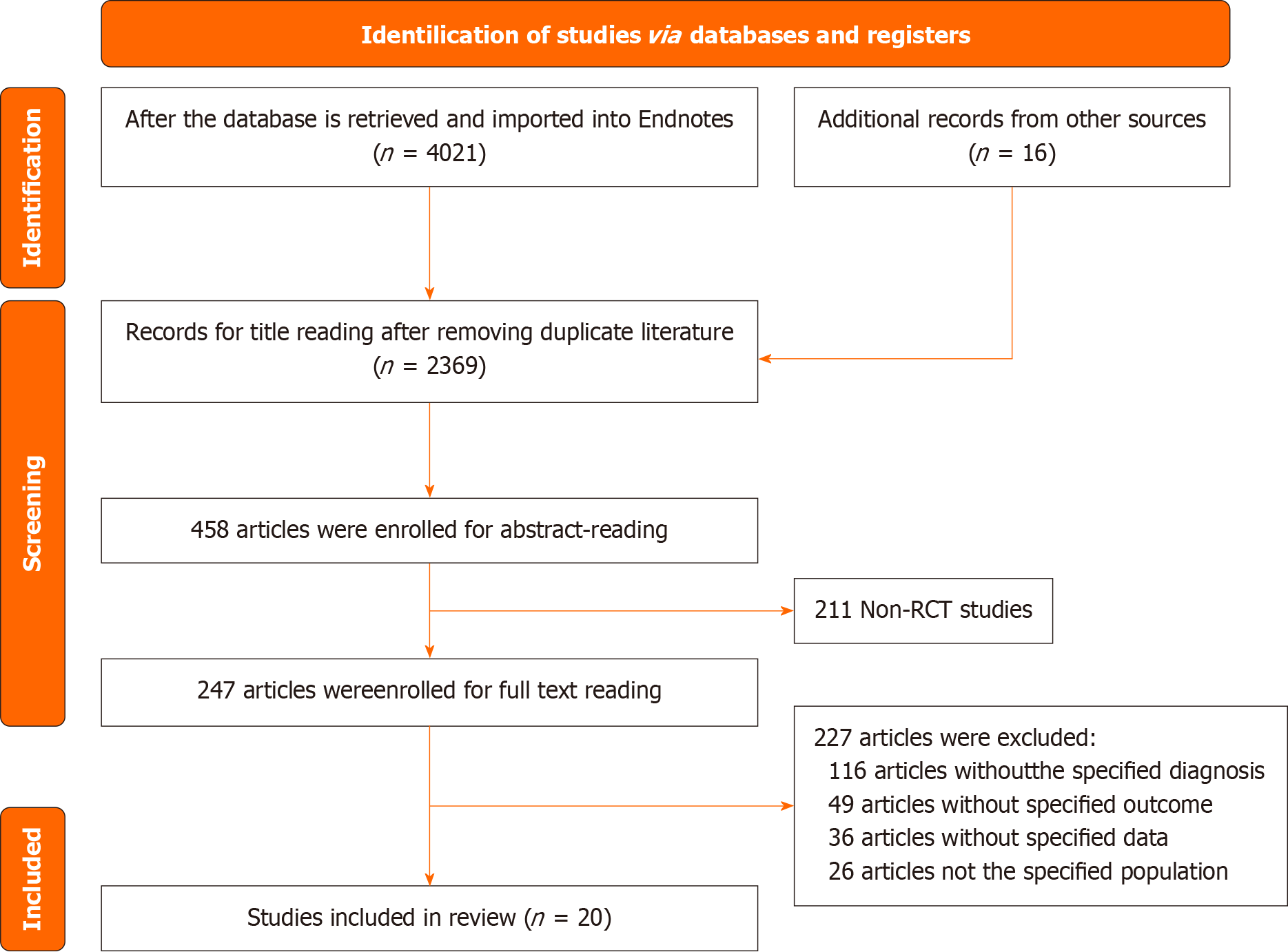
Figure 1 Literature screening flowchart.
RCT: Randomized controlled trial.
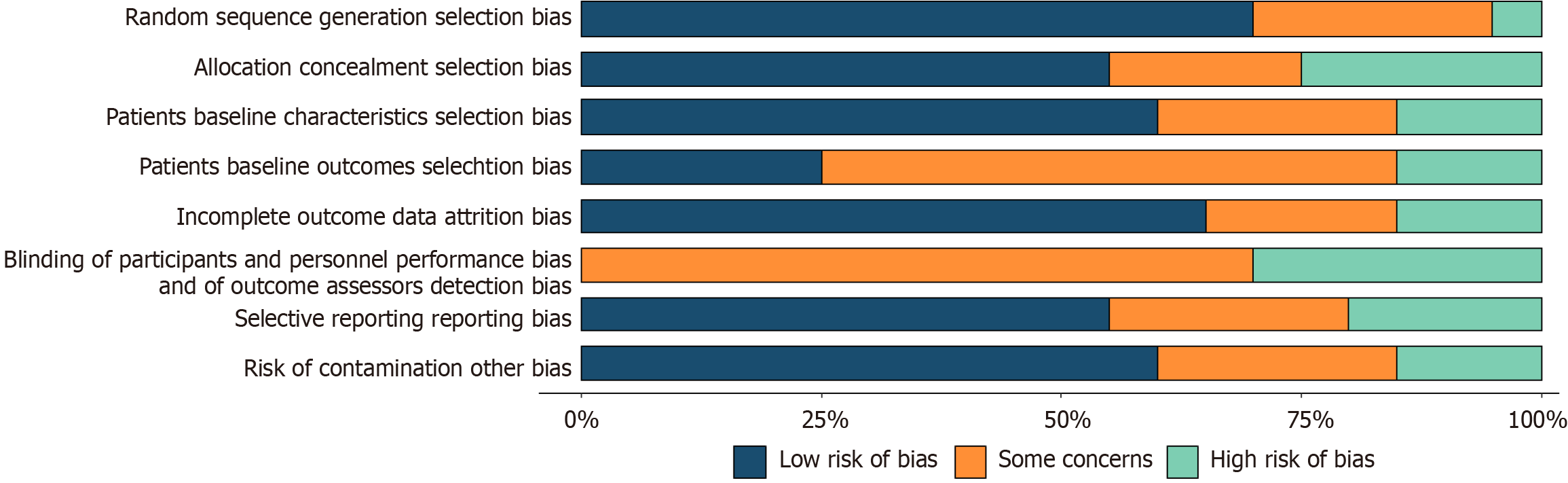
Figure 2
Risk of bias chart.
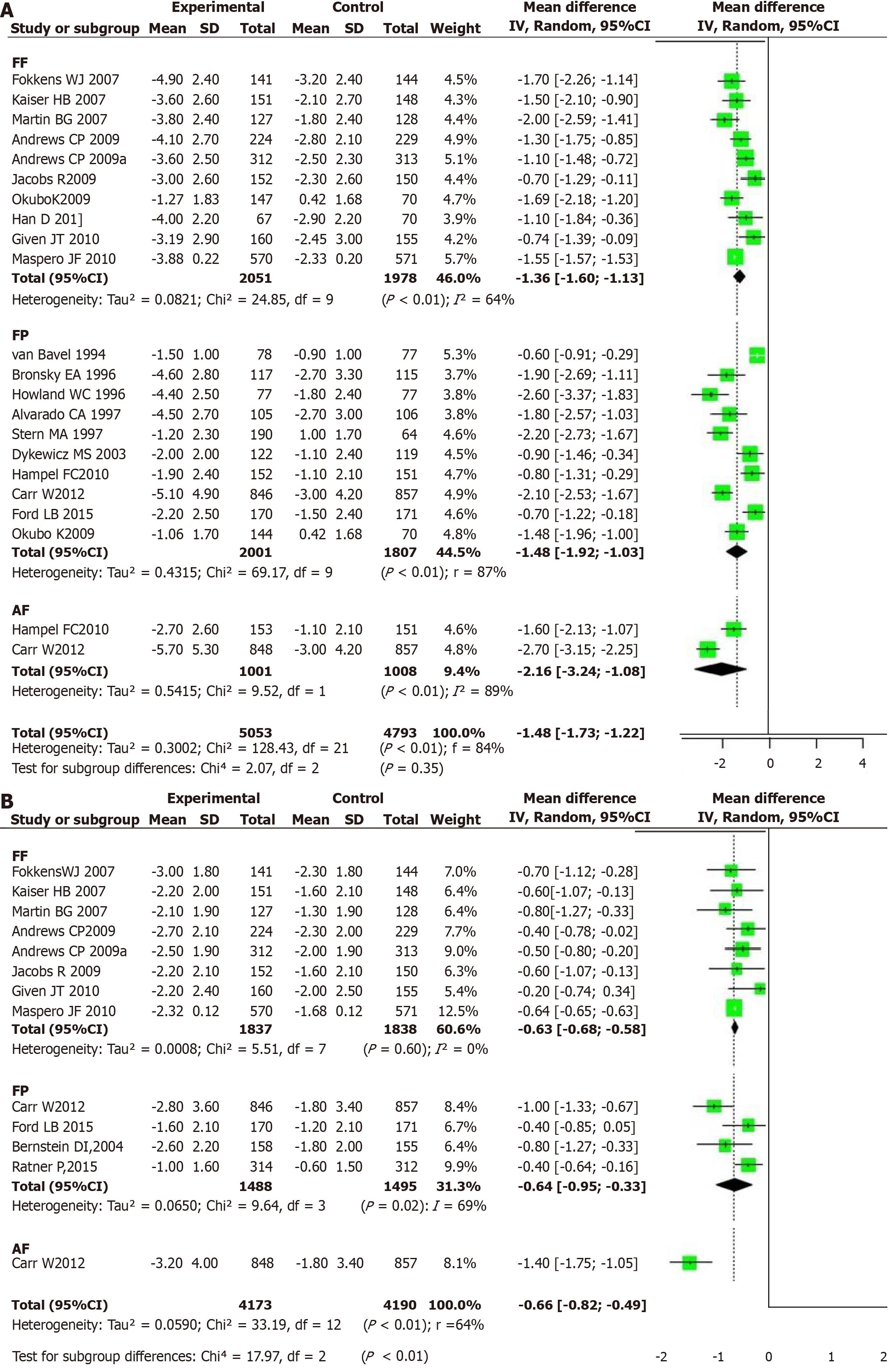
Figure 3 Forest plot.
A: Forest plot of relative Total Nasal Symptom Score for different intervention methods; B: Forest plot of relative Total Ocular Symptom Score for different intervention methods. AF: Azelastine-fluticasone; FP: Fluticasone propionate; FF: Fluticasone furoate.
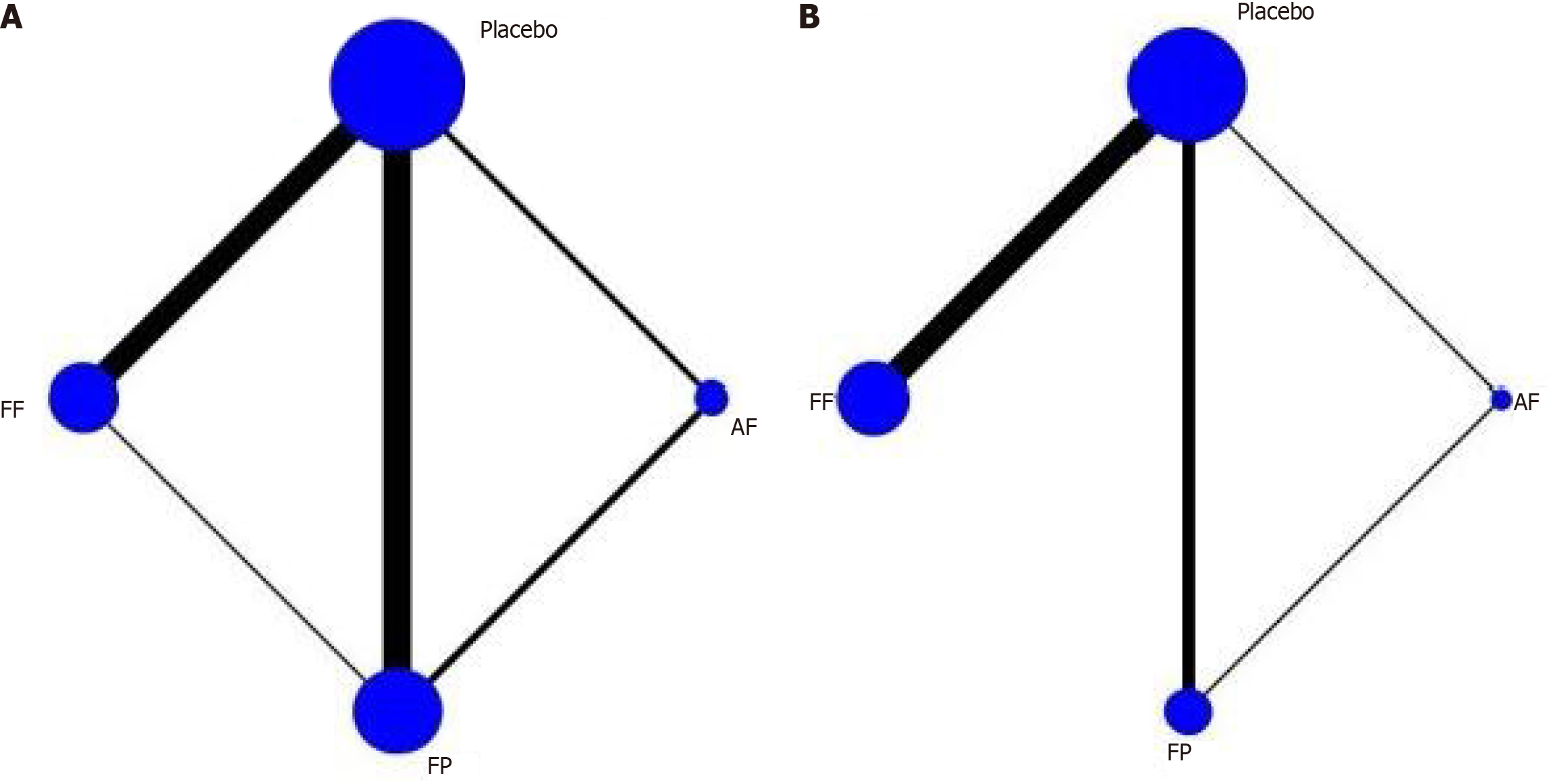
Figure 4 Network relationship diagram.
A: Network diagram illustrating the relationships among different treatment modalities for relative Total Nasal Symptom Score (rTNSS). For the rTNSS measure, direct comparisons were available between FF and fluticasone propionate (FP), as well as between FP and azelastine-fluticasone (AF); however, no direct comparison existed between FF and AF; B: Network diagram for rTOSS. A direct comparison was available between FP and AF, but no direct comparisons were found between FF and FP or between FF and AF. AF: Azelastine-fluticasone; FP: Fluticasone propionate; FF: Flutica
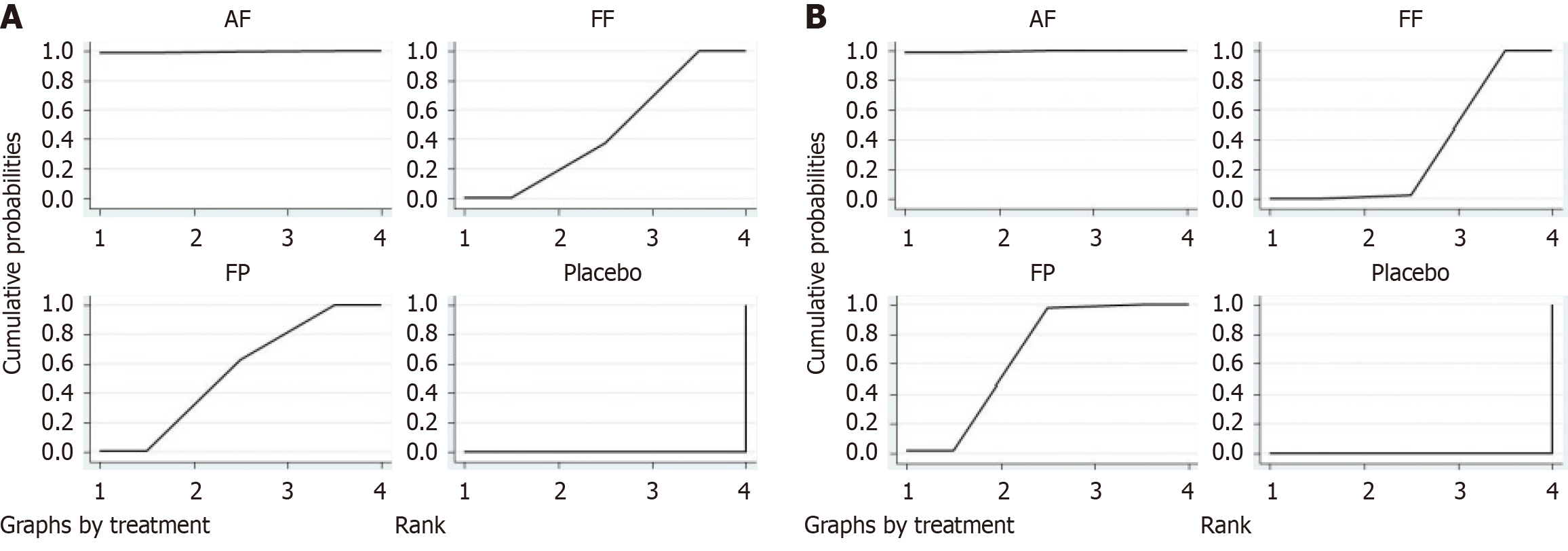
Figure 5 SUCRA comparison of different treatment methods.
A: The results of the network meta-analysis indicated that the SUCRA values ranked from high to low as follows: Azelastine-fluticasone (AF) (99.6%), fluticasone propionate (FP) (54.4%), fluticasone furoate (FF) (46.0%), and placebo (0.0%); B: The network meta-analysis results indicated SUCRA values ranked from high to low as follows: AF (99.5%), FP (66.4%), FF (34.1%), and placebo (0.0%). AF: Azelastine-fluticasone; FP: Fluticasone propionate; FF: Fluticasone furoate.
- Citation: Qin JY, Huang G, Pan ZH, Liao LF, Hu HF. Different medications for seasonal allergic rhinitis in adults: A systematic review and meta-analysis. World J Meta-Anal 2024; 12(4): 98508
- URL: https://www.wjgnet.com/2308-3840/full/v12/i4/98508.htm
- DOI: https://dx.doi.org/10.13105/wjma.v12.i4.98508