Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12617
Peer-review started: May 22, 2022
First decision: August 21, 2022
Revised: October 1, 2022
Accepted: November 8, 2022
Article in press: November 8, 2022
Published online: December 6, 2022
Processing time: 186 Days and 1 Hours
Although coronavirus disease 2019 (COVID-19) vaccines have been effective in controlling the COVID-19 pandemic, a variety of post-vaccination neurological complications have been reported worldwide. Amyloid β-related angiitis (ABRA) is a rare neurological disease. The underlying cause of ABRA is unknown, but several studies suggest that it is caused by an excessive immune response to amyloid-β deposited in blood vessels. In addition, limited attention has been paid to potential triggers of ABRA, such as infection or vaccination.
We report a case of ABRA that developed 2 wk after COVID-19 vaccination. A 75-year-old woman developed a frontal headache after receiving a second dose of COVID-19 BNT162b2 vaccine (Pfizer-BioNTech). Diffusion-weighted magnetic resonance imaging (DW-MRI) of the head showed abnormal hyperintensity, suggesting cerebral infarctions in the left parietal and occipital lobes. We diagnosed her condition as ABRA based on a brain biopsy. We administered steroid pulse therapy and the patient’s symptoms and DW-MRI abnormalities improved. This case had a good outcome due to prompt diagnosis and treatment.
We report a case of ABRA that may have been triggered by COVID-19 vaccina
Core Tip: Amyloid β-related angiitis (ABRA) is a rare neurological disease with overlapping features of cerebral amyloid angiopathy and primary angiitis of the central nervous system. We present a case of ABRA that appeared 2 wk after coronavirus disease 2019 (COVID-19) vaccination. The patient was diagnosed with ABRA based on a brain biopsy. Steroid pulse therapy was administered, and, the patient’s symptoms and diffusion-weighted magnetic resonance imaging abnormalities improved. This case had a good outcome due to prompt diagnosis and treatment. Although the relationship between ABRA and COVID-19 vaccination is unclear, this case contributes to the literature on adverse neurological events following COVID-19 vaccination.
- Citation: Kizawa M, Iwasaki Y. Amyloid β-related angiitis of the central nervous system occurring after COVID-19 vaccination: A case report. World J Clin Cases 2022; 10(34): 12617-12622
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12617.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12617
Severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that gave rise to the coronavirus disease 2019 (COVID-19) pandemic[1,2]. COVID-19 vaccines have been effective in controlling the pandemic; however, a wide variety of neurological complications have been reported worldwide following COVID-19 vaccination[1,2].
Several types of vaccines are associated with a risk of a variety of serious neurological complications[1-3]. Neurological complications after vaccination can be explained by several pathogenic mechanisms, including molecular mimicry, direct neurotoxicity, and an abnormal immune response[1].
Amyloid β-related angiitis (ABRA), also known as amyloid β-related vasculitis, is a rare neurological disorder. Although the causes of ABRA have not been clearly elucidated[4-7], several studies suggest that ABRA is caused by an excessive immune response to amyloid-β deposited in the blood vessels[4,8,9].
However, limited attention had been paid to the triggers of ABRA, such as infection or vaccination. Currently, there is no evidence showing that COVID-19 vaccine triggers ABRA. Herein, we report a case of ABRA that developed 2 wk after COVID-19 vaccination. Although the causal relationship between COVID-19 vaccines and ABRA is unclear, we report this case to add to the current literature in order to enable a better understanding of the etiology and triggers of ABRA.
A 75-year-old Japanese woman weighing 47 kg received the BNT162b2 COVID-19 vaccine (Pfizer-BioNTech) in late June 2021, and did not experience any adverse effects. Three weeks later, in early July 2021, she received a second dose of the vaccine. She developed a frontal headache after receiving the second vaccination. This post-vaccination headache improved within a few days.
Two weeks after the second vaccination, the patient’s headache worsened, and she subsequently developed progressive depression, aphasia, apraxia, and a gait disturbance. She was admitted to the hospital for further investigation and treatment.
The patient’s medical history included hypertension, hyperlipidemia, and osteoarthritis. Her drug history included chronic use of amlodipine (5 mg) per day, for the treatment of hypertension. Additionally, the patient took one tablet of paracetamol (200 mg) as needed, for back pain.
The patient’s brother had a history of cerebral infarction. There was no known family history of vasculitis or autoimmune disease.
On admission, the patient had a temperature of 36.7 °C, a heart rate of 67 bpm, a blood pressure of 167/91 mmHg, and an oxygen saturation of 98% at room air. A neurological examination showed that she had weakness in her right arm, and hyperreflexia in both arms, both of which are pyramidal signs, and confirmed that she had aphasia and apraxia.
No abnormalities were found in the complete blood count, blood biochemistry, or coagulation tests. The complete blood count results were: White blood cell count, 5.15 × 103/μL (reference: 3.3–8.6 × 103/μL); red blood cell count, 4.52 × 106/μL (reference: 3.8–5.0 × 106/μL); and platelet count of 150 × 103/μL (reference: 150–350 × 103/μL). The differential leukocyte count results were: Neutrophils, 3.31 × 103/μL (reference: 1.20-6.60 × 103/μL); lymphocytes, 1.09 × 103/μL (reference: 0.50–4.30 × 103/μL); eosinophils 0.34 × 103/μL (reference: ≤ 0.80 × 103/μL), and basophils 0.03 × 103/μL (reference: ≤ 0.03 × 103/μL). The leukocyte percentages were 64.2% neutrophils (reference: 38.5%–76.5%), 21.1% lymphocytes (reference: 16.5%–49.5%), 5.7% monocytes (reference: 2.0%–10.0%), 6.6% eosinophils (reference: ≤ 8.5%), 0.6% basophils (reference: ≤ 2.5%) and 1.7% large non-pigmented cells (reference: ≤ 4.0%).
Blood biochemistry showed a C-reactive protein level of 0.04 mg/dL (reference: ≤ 0.14 mg/dL). Autoantibody test results for antinuclear antibody, antiribonucleoprotein antibody, anti-Smith antibody, anti-Ro (anti-SSA antibodies) and anti-La (Anti-SSB antibody) antibodies, cytoplasmic-antineutrophil cytoplasmic antibody (C-ANCA), and perinuclear-antineutrophil cytoplasmic antibody (P-ANCA) were negative. The reference values for the autoantibody tests were as follows: Anti-Smith antibody, ≤ 10.0 U/mL; anti-SSA antibody, ≤ 10.0 U/mL; anti-SSB antibody, ≤ 10.0 U/mL; C-ANCA, ≤ 3.5 IU/mL; P-ANCA, ≤ 3.5 IU/mL). Cerebrospinal fluid tests were not performed because the patient’s neurological symptoms and imaging findings suggested increased intracranial pressure.
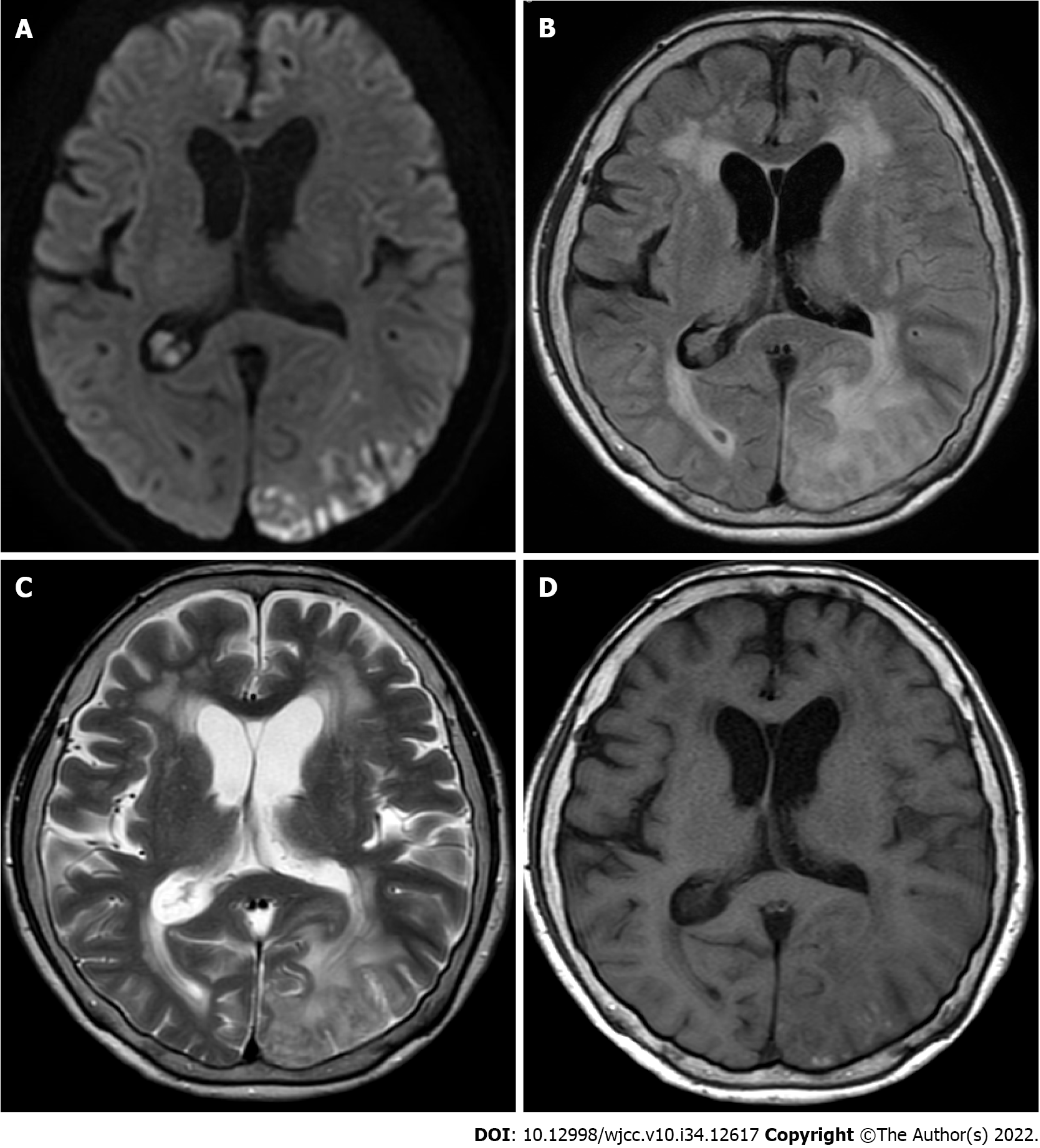
Magnetic resonance imaging (MRI) showed abnormal hyperintensity, suggesting cerebral infarctions in the left parietal and occipital lobes. These lesions were not consistent with the vascular territory (Figure 1).
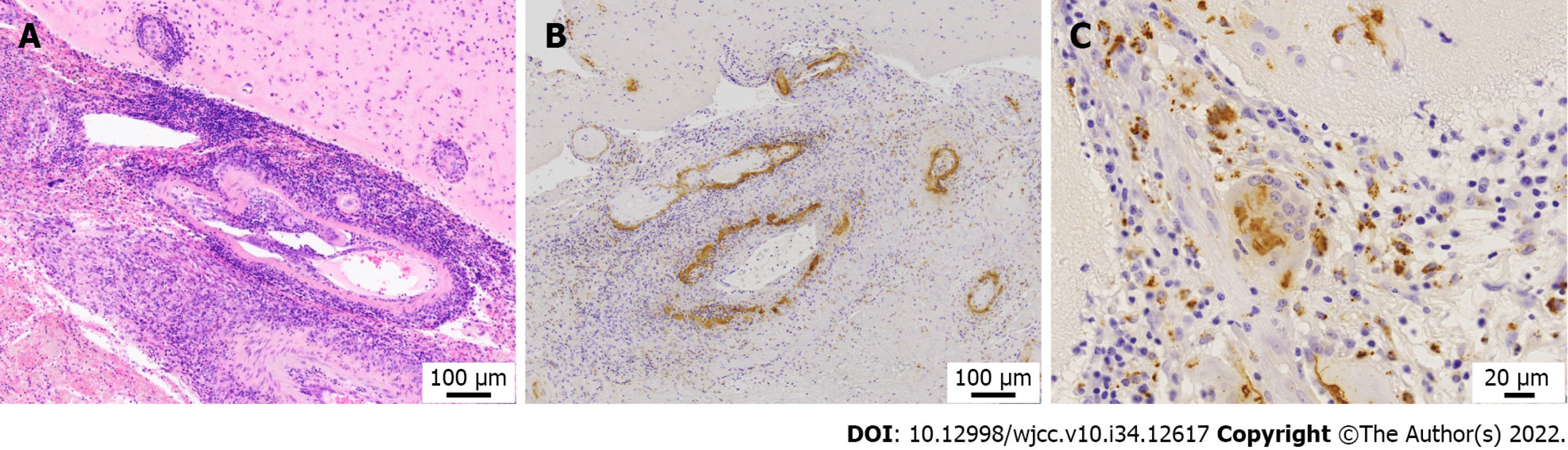
Brain biopsy of the left occipital lobe revealed granulomatosis vasculitis with multinucleated giant cells in the leptomeningeal small vessels, fibrinoid necrosis of the vessel wall, and microhemorrhages in the subarachnoid space (Figure 2A). Immunohistochemical staining for amyloid-β revealed amyloid-β deposits in the blood vessel wall (Figure 2B), and multinucleated macrophages phagocytosing amyloid-β (Figure 2C), consistent with a diagnosis of amyloid β-related vasculitis.
According to the modified Boston criteria for cerebral amyloid angiopathy (CAA)[10], these findings correspond to probable CAA with supporting pathology. However, the strong reaction of lymphocytes and histiocytes to amyloid-β in the vascular wall led to the diagnosis of ABRA.
The diagnosis of ABRA was made immediately after the brain biopsy, and steroid pulse therapy was initiated on the same day. Prednisolone (1000 mg/day) was administered intravenously for 3 d followed by prednisolone (80 mg/day) intravenously on the 4th day. On day 5, the steroid was switched to oral prednisone (45 mg/day) for 2 wk, and then tapered by 5 mg each wk until the dose was 20 mg/day. The patient was maintained on a prednisolone dose of 20 mg/day. The patient’s headache, gait disturbance, weakness in the arms, and the MRI abnormalities improved, but her aphasia and apraxia persisted.
The patient was discharged 90 d after the onset of her headache. Since her discharge 3 mo ago, she has had monthly follow-up visits to our hospital, and her condition has remained stable.
Several types of vaccines are associated with a risk of a variety of serious neurological complications[1-3]. Neurological complications after vaccination can be explained by several pathogenic mechanisms, including molecular mimicry, direct neurotoxicity, and an abnormal immune response[1,2].
ABRA is a rare neurological disease that is classified as a primary angiitis of the central nervous system (PACNS). It shares characteristics of both PACNS and CAA[4-7,11]. CAA is characterized by deposition of amyloid-β in the cortical and leptomeningeal vessels[4-7,11]. Vascular inflammation can also be present in the affected vessels. Two types of inflammatory responses have been reported: ABRA and CAA-associated inflammation (CAA-RI). CAA-RI is characterized by an inflammatory response surrounding amyloid-laden vessels, without vasodestructive features[4-7,11]. ABRA is a granulomatous, vasodestructive vasculitis that affects the subarachnoid and cortical blood vessels, and is characterized by abundant amyloid-β deposition in the vessel wall[4-7,11]. Although ABRA has features similar to CAA with age-related changes, the excessive immune response to amyloid-β cannot be attributed to aging.
The causes of ABRA are currently unknown; however, several studies suggest an abnormal immune response to amyloid-β as the primary cause[8,9,11,12]. It has been hypothesized that ABRA, which has been implicated in subarachnoid and cortical vasculitis with amyloid-β deposition and increased clearance[4,12], is caused by inflammation that occurs as a result of an excessive immune response to amyloid-β deposition in the blood vessels[8,9,12]. The pathology of this case showed that the angiopathy met the Boston criteria for CAA with age-related changes, but the destructive vasculitis and phagocytosis of amyloid-β by macrophages could not be attributed solely to age[10].
Currently, limited research has been conducted on triggers for ABRA, such as infection or vaccination. The lack of knowledge about factors that may trigger ABRA makes this case difficult to explain.
Furthermore, to the best of our knowledge, there have been no previous reports of ABRA following vaccination, making it difficult to infer a causal relationship between the vaccination and ABRA in the present case. Moreover, no similar closely related diseases, such as CAA and CAA-RI, have been reported following vaccination. Additionally, ABRA has not been reported in association with SARS-CoV-2 infection. Nevertheless, an excessive immune response may occur following vaccination, which could explain the temporal association between ABRA and COVID-19 vaccination in this case.
In this case, there were no factors identified other than the vaccination, that could have triggered an immune disorder; therefore, the diagnosis of ABRA is consistent with an abnormal response to a COVID-19 vaccine. However, identifying the cause of ABRA requires a detailed analysis of the immune mechanism at a molecular level, which was beyond the scope of investigations available in the community hospital. Therefore, we were unable to determine the exact cause in this case. This patient was diagnosed and treated promptly resulting in a relatively good outcome. We are reporting this case of a suspected adverse reaction to a COVID-19 vaccine as this could aid in timely diagnosis and treatment of patients with similar reactions following COVID-19 vaccination in the future.
In this case of ABRA, there were no factors other than the COVID-19 vaccine that may have led to an abnormal immune response; therefore, we suspect that ABRA was triggered by the second COVID-19 vaccination.
Ongoing surveillance of adverse reactions is warranted to confirm a causal relationship between adverse neurological events and COVID-19 vaccination, which could facilitate timely diagnosis and treatment of individuals with similar events in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pitton Rissardo J, Brazil; Zhang JX, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43:3-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 2. | Finsterer J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol Scand. 2022;145:5-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 3. | Toussirot É, Bereau M. Vaccination and induction of autoimmune diseases. Inflamm Allergy Drug Targets. 2015;14:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Salvarani C, Hunder GG, Morris JM, Brown RD Jr, Christianson T, Giannini C. Aβ-related angiitis: comparison with CAA without inflammation and primary CNS vasculitis. Neurology. 2013;81:1596-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Scolding NJ, Joseph F, Kirby PA, Mazanti I, Gray F, Mikol J, Ellison D, Hilton DA, Williams TL, MacKenzie JM, Xuereb JH, Love S. Abeta-related angiitis: primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain. 2005;128:500-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Giannini C, Salvarani C, Hunder G, Brown RD. Primary central nervous system vasculitis: pathology and mechanisms. Acta Neuropathol. 2012;123:759-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140:1829-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 8. | Yamada M, Itoh Y, Shintaku M, Kawamura J, Jensson O, Thorsteinsson L, Suematsu N, Matsushita M, Otomo E. Immune reactions associated with cerebral amyloid angiopathy. Stroke. 1996;27:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Melzer N, Harder A, Gross CC, Wölfer J, Stummer W, Niederstadt T, Meuth SG, Marziniak M, Grauer OM, Wiendl H. CD4(+) T cells predominate in cerebrospinal fluid and leptomeningeal and parenchymal infiltrates in cerebral amyloid β-related angiitis. Arch Neurol. 2012;69:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Greenberg SM, Charidimou A. Diagnosis of Cerebral Amyloid Angiopathy: Evolution of the Boston Criteria. Stroke. 2018;49:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 309] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 11. | Nouh A, Borys E, Gierut AK, Biller J. Amyloid-Beta related angiitis of the central nervous system: case report and topic review. Front Neurol. 2014;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bogner S, Bernreuther C, Matschke J, Barrera-Ocampo A, Sepulveda-Falla D, Leypoldt F, Magnus T, Haag F, Bergmann M, Brück W, Vogelgesang S, Glatzel M. Immune activation in amyloid-β-related angiitis correlates with decreased parenchymal amyloid-β plaque load. Neurodegener Dis. 2014;13:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |