Published online Nov 29, 2018. doi: 10.5662/wjm.v8.i3.40
Peer-review started: August 5, 2018
First decision: August 24, 2018
Revised: September 30, 2018
Accepted: October 17, 2018
Article in press: October 18, 2018
Published online: November 29, 2018
Processing time: 117 Days and 17.4 Hours
Transcranial Doppler (TCD) is useful for investigation of intracranial arterial blood flow and can be used to detect a real-time embolic signal. Unfortunately, artefacts can mimic the embolic signal, complicating interpretation and necessitating expert-level opinion to distinguish the two. Resolving this situation is critical to achieve improved accuracy and utility of TCD for patients with disrupted intracranial arterial blood flow, such as stroke victims. A common type of stroke encountered in the clinic is cryptogenic stroke (or stroke with undetermined etiology), and patent foramen ovale (PFO) has been associated with the condition. An early clinical trial of PFO closure effect on secondary stroke prevention failed to demonstrate any benefit for the therapy, and research into the PFO therapy generally diminished. However, the recent publication of large randomized control trials with demonstrated benefit of PFO closure for recurrent stroke prevention has rekindled the interest in PFO in patients with cryptogenic stroke. To confirm that emboli across the PFO can reach the brain, TCD should be applied to detect the air embolic signal after injection of agitated saline bubbles at the antecubital vein. In addition, the automated embolic signal detection method should further facilitate use of TCD for air embolic signal detection after the agitated saline bubbles injection in patients with cryptogenic stroke and PFO.
Core tip: Patent foramen ovale (PFO) is an emerging etiology of cryptogenic stroke, and PFO closure therapy has been shown to reduce the rate of recurrent stroke. Detection of the air embolic signal by transcranial Doppler (TCD) after injection of agitated saline bubbles at the antecubital vein will help to confirm the importance of PFO as the cause of a concurrent stroke. In addition, the automated embolic signal detection method should further facilitate use of TCD for air embolic signal detection after the agitated saline bubbles injection in patients with cryptogenic stroke and PFO.
- Citation: Muengtaweepongsa S, Tantibundhit C. Microembolic signal detection by transcranial Doppler: Old method with a new indication. World J Methodol 2018; 8(3): 40-43
- URL: https://www.wjgnet.com/2222-0682/full/v8/i3/40.htm
- DOI: https://dx.doi.org/10.5662/wjm.v8.i3.40
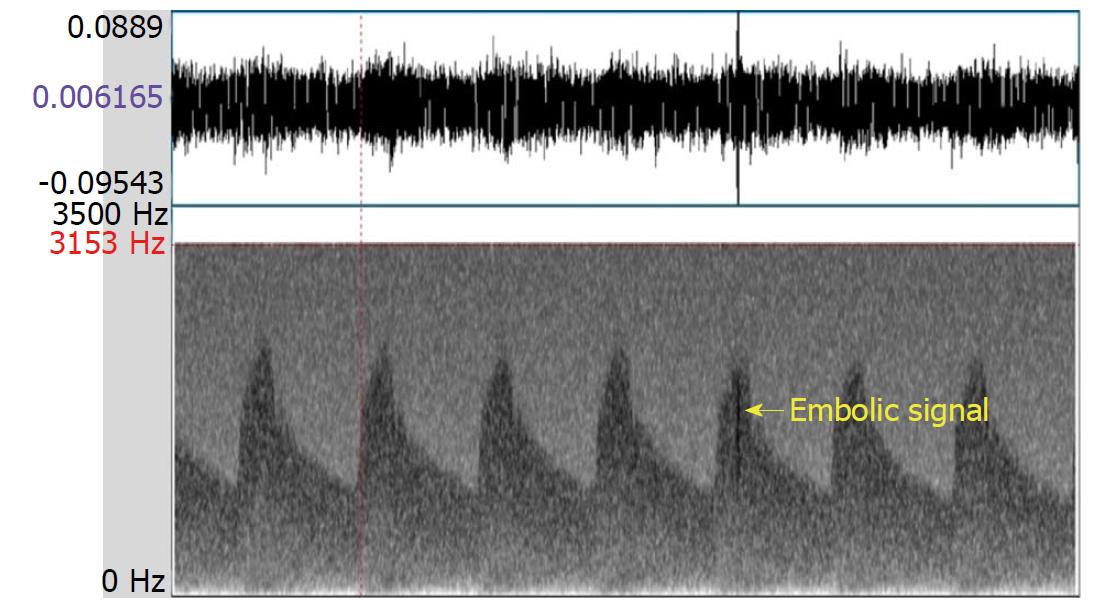
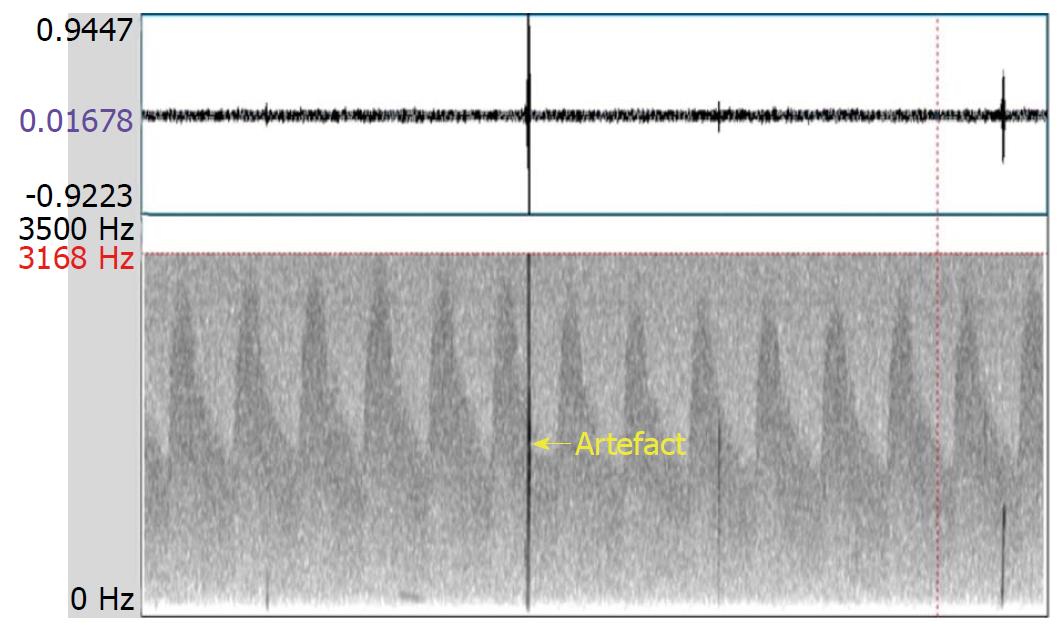
Transcranial Doppler (TCD) is a noninvasive method for evaluating blood flow velocities in intracranial arteries. The TCD instrument is also used to detect emboli in real time, as they emerge in the main intracranial circulation. In general, an embolic signal representing an embolus has some characteristics that are distinctive from the signal that represents normal blood flow. Specifically, the embolic signal is classified as high intensity transient signals (commonly referred to as “HITS”) lying on top of the Doppler signal, deflected through an angle of 180 degrees by red blood cells. However, artefacts, caused by a variety of situations (e.g., probe motion, patient movement, and sound waves from the patient speaking) are sometimes detected as HITS. Distinguishing embolic signals from artefacts requires an expert-level evaluation of the morphology of the signals, resulting in a subjective finding[1,2]. The multigated method was developed to improve the objective differentiation between embolic signals and artefacts. This technique samples signals from different depths of a similar vessel to demonstrate the motion of the following embolus from proximal to distal (Figure 1); in contrast, an artefact shows no movement property but appears in all depths simultaneously (Figure 2)[3].
Cryptogenic stroke is described as a cerebral infarct of unclear or undetermined etiology, according to the Trial of Org 10172 in Acute Stroke Treatment stroke-subtype categorization system (TOAST). The source of cryptogenic stroke remains unclear mainly because the episode itself is temporary or reversible, and the available forms of clinical investigation do not address all possible etiologies. It is also important to consider that there may be additional etiologies that have yet to be recognized[4]. The finding that more than one-third of reported cerebral infarcts are categorized as cryptogenic etiology highlights the need for technologies and procedures to better investigate them[5].
Patent foramen ovale (PFO) is a possible etiology of cryptogenic stroke, especially in young adults. Prevalence of PFO is considerable, with estimates as high as 25% for the overall population. Moreover, it has been reported that around 50% of cryptogenic stroke patients of age less than 55 have a PFO[6]. The consideration of PFO as an etiology of consecutive cryptogenic stroke has been controversial, however, since early clinical trials of PFO closure therapy did not show any benefit for prevention of recurrent stroke[7-9]. Moreover, due to this reported lack of benefit, a routine practice of investigating cryptogenic stroke patients for PFO was not included among the recommendations in standard guidelines[10].
Three recent large randomized control trials (i.e., RESPECT[11], REDUCE[12] and CLOSURE[13]) demonstrated benefit of PFO closure for secondary stroke prevention in selected cases of patients with cryptogenic stroke[11-13]. Of the three, the RESPECT study had the longest follow-up time, at 6 years[11]. The REDUCE study showed the benefit of PFO closure therapy over antiplatelet therapy, at 3 years after treatment[12]. The CLOSURE study included high-risk PFO cases with an atrial septal aneurysm or large interatrial shunt[13]. Data on device-related atrial fibrillation was reported in the REDUCE[12,13] and CLOSURE[12,13] studies. Furthermore, other recent studies carried out as meta-analyses also confirmed the benefit of PFO closure for secondary stroke prevention[14,15]. Considering these studies and their findings collectively, the next step would be carrying out systematic investigation of the potential for routinely seeking PFO in patients with cryptogenic stroke, particularly since the PFO itself holds promise as a target of therapy.
Echocardiography plays a major role in diagnosis of PFO. In some cases, the PFO is detectable with color flow Doppler imaging in the echocardiogram. In most cases, the agitated saline bubbles test is mandatory for the diagnosis of PFO. In this procedure, a bolus of agitated saline is injected into an antecubital vein, after which air bubbles appear in the right atrium. A positive PFO finding is indicated when the air bubbles appear in the left atrium within three cardiac cycles of their initial appearance in the right atrium. The mechanism underlying this finding is the Valsalva maneuver, which increases right atrial pressure and facilitates right-to-left shunting[16].
Use of TCD for detection of the air embolic signal after injection of agitated saline bubbles into an antecubital vein is an alternative procedure to confirm right-to-left shunting. Detection of an air embolic signal in intracranial arteries should affirm that an embolus from the heart is able to reach the brain and cause the ischemic lesions. With the new indication for PFO closure, the use of TCD for air embolic signal detection with agitated saline bubbles test will be increased. The number of air embolic signals may be related to the size of the PFO, and such would help to strengthen the interpretation of clinical significance for the shunting.
Furthermore, the automated embolic signal detection method should improve the differentiation between artefacts and real emboli, and allow for counting the number of emboli[17,18]. The sensitivity and specificity of the automated system for differentiation between real emboli and artefacts were demonstrated to be as high as those of experts’ opinions[17,18]. With this automated method, TCD for air embolic signal detection with agitated saline bubbles test should be more useful in patients with cryptogenic stroke with PFO. Moreover, the automated method may extend use of TCD for embolic signal detection in other indications, such as emboli detection during invasive cardiac or great vessels procedure and microembolic monitoring during the first 48 h after onset of stroke[19,20].
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Sharma P, Vieyra JP, Weng CF S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Semin Neurol. 2012;32:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | D’Andrea A, Conte M, Cavallaro M, Scarafile R, Riegler L, Cocchia R, Pezzullo E, Carbone A, Natale F, Santoro G. Transcranial Doppler ultrasonography: From methodology to major clinical applications. World J Cardiol. 2016;8:383-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 3. | Ringelstein EB, Droste DW, Babikian VL, Evans DH, Grosset DG, Kaps M, Markus HS, Russell D, Siebler M. Consensus on microembolus detection by TCD. International Consensus Group on Microembolus Detection. Stroke. 1998;29:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 367] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7474] [Cited by in RCA: 8854] [Article Influence: 276.7] [Reference Citation Analysis (0)] |
| 5. | Weimar C. Stroke of undetermined cause: workup and secondary prevention. Curr Opin Neurol. 2016;29:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, Coste J, Mas JL. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002;33:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 667] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 8. | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 710] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 9. | Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 631] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 10. | O’Gara PT, Messe SR, Tuzcu EM, Catha G, Ring JC; American Heart Association; American Stroke Association; American College of Cardiology Foundation. Percutaneous device closure of patent foramen ovale for secondary stroke prevention: a call for completion of randomized clinical trials. A science advisory from the American Heart Association/American Stroke Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2009;53:2014-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 711] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 12. | Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2017;377:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 760] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 13. | Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med. 2017;377:1011-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 757] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 14. | Tsivgoulis G, Katsanos AH, Mavridis D, Frogoudaki A, Vrettou AR, Ikonomidis I, Parissis J, Deftereos S, Karapanayiotides T, Palaiodimou L. Percutaneous patent foramen ovale closure for secondary stroke prevention: Network meta-analysis. Neurology. 2018;91:e8-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Vaduganathan M, Qamar A, Gupta A, Bajaj N, Golwala HB, Pandey A, Bhatt DL. Patent Foramen Ovale Closure for Secondary Prevention of Cryptogenic Stroke: Updated Meta-Analysis of Randomized Clinical Trials. Am J Med. 2018;131:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Schuchlenz HW, Weihs W, Beitzke A, Stein JI, Gamillscheg A, Rehak P. Transesophageal echocardiography for quantifying size of patent foramen ovale in patients with cryptogenic cerebrovascular events. Stroke. 2002;33:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Sombune P, Phienphanich P, Muengtaweepongsa S, Ruamthanthong A, Tantibundhit C. Automated embolic signal detection using adaptive gain control and classification using ANFIS. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:3825-3828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Sombune P, Phienphanich P, Phuechpanpaisal S, Muengtaweepongsa S, Ruamthanthong A, Tantibundhit C. Automated embolic signal detection using Deep Convolutional Neural Network. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:3365-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | von Bary C, Deneke T, Arentz T, Schade A, Lehrmann H, Schwab-Malek S, Fredersdorf S, Baldaranov D, Maier L, Schlachetzki F. Clinical Impact of the Microembolic Signal Burden During Catheter Ablation for Atrial Fibrillation: Just a Lot of Noise? J Ultrasound Med. 2018;37:1091-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Iguchi Y, Kimura K, Kobayashi K, Ueno Y, Shibazaki K, Inoue T. Microembolic signals at 48 hours after stroke onset contribute to new ischaemia within a week. J Neurol Neurosurg Psychiatry. 2008;79:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |