Published online Dec 26, 2017. doi: 10.5662/wjm.v7.i4.139
Peer-review started: May 17, 2017
First decision: June 16, 2017
Revised: July 2, 2017
Accepted: September 12, 2017
Article in press: September 12, 2017
Published online: December 26, 2017
Processing time: 226 Days and 23.8 Hours
To describe the development and validation of a novel neuronavigation-based method, which allows the quantification of the anatomical features that define an approach, as well as real-time visualization of the surgical pyramid.
The method was initially developed with commercially-available hardware for coordinate collection (a digitizer and a frameless navigation system) and software for volume rendering; dedicated neuronavigation software (ApproachViewer, part of GTx-UHN) was then developed. The accuracy of measurements and the possibility of volumetric rendering of surgical approaches simulated in a phantom were compared among three different methods and commercially-available radiological software. In the anatomy laboratory, ApproachViewer was applied to the comparative quantitative analysis of multiple neurosurgical approaches and was used by many surgeons who were untrained for the research method.
The accuracy of ApproachViewer is comparable to commercially-available radiological software. In the anatomy laboratory, the method appears versatile. The system can be easily used after brief training. ApproachViewer allows for real-time evaluation and comparison of surgical approaches, as well as post-dissection analyses of collected data. The accuracy of the method depends on the navigation registration: with a 1-2 mm registration error, it is adequate for evaluation and comparison of most neurosurgical approaches.
This new research method and software allows semi-automated visualization, quantification, and comparison of neurosurgical approaches in the anatomy laboratory.
Core tip: Herein, the authors describe the development and validation of a novel research method that allows quantification of the essential anatomical features of a neurosurgical approach and real-time rendering of its surgical volume. The measurements of ApproachViewer in a phantom with simulated approaches were noted to be largely homogeneous and comparable with those of other research methods. The authors further demonstrated the actual application of ApproachViewer in anatomical dissections to elaborate the advantages of real time 3D rendering and quantification. ApproachViewer provides a good alternative solution for fast 3D rendering and post-dissection analyses in a preclinical setting.
- Citation: Doglietto F, Qiu J, Ravichandiran M, Radovanovic I, Belotti F, Agur A, Zadeh G, Fontanella MM, Kucharczyk W, Gentili F. Quantitative comparison of cranial approaches in the anatomy laboratory: A neuronavigation based research method. World J Methodol 2017; 7(4): 139-147
- URL: https://www.wjgnet.com/2222-0682/full/v7/i4/139.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i4.139
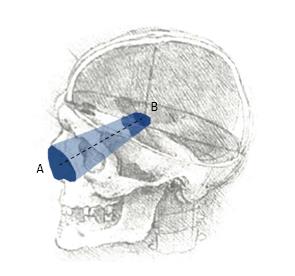
Anatomical research is an essential phase of modern surgical innovation and evaluation[1], especially in an era of technological advancements, leading to the continuous improvement of different surgical approaches and techniques[2,3]. In the last two decades, different methods have been described for the quantification and comparison of neurosurgical approaches in the anatomy laboratory[4]. A common limitation of currently available research methods is the lack of visualization of the surgical pyramid that anatomically defines a neurosurgical approach (Figure 1)[4,5].
Herein, we present a novel neuronavigation based method that allows real-time visualization of the surgical pyramid, as well as quantification of its features and various post-dissection analyses. The development and validation process is described, together with some examples of the application of the method to evaluation and comparison of neurosurgical approaches in the anatomy laboratory.
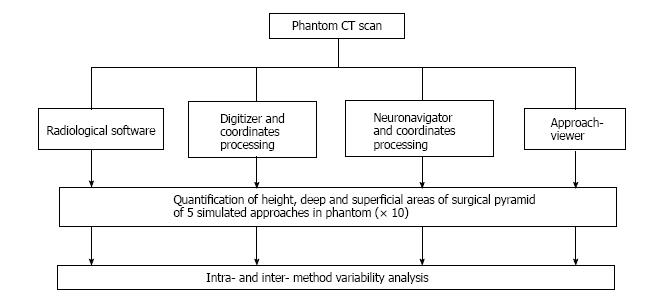
Four image-based systems were used to quantify and visualize the surgical pyramid[4,5] (Figure 1) in five simulated approaches (Figure 2). Every simulated approach was quantified 10 times with each research method. Data were then analyzed for intra- and inter-method variability (Figure 3). Two of the methods, based on neuronavigation systems, were also used to quantify surgical approaches in the anatomy laboratory.
Computed tomography (CT) scans of phantom and anatomical specimens were performed using a 1 × 1 frame with contiguous slices, both at 1 and 3 mm (Somatom Definition Flash®, Siemens, Forcheim, Germany). CT was performed at a gantry of 0°, with a scan window diameter of 225 mm and a pixel size of more than 0.44 × 0.44. Images were recorded on a CD in DICOM format.
The four research methods that were used to quantify and visualize the simulated surgical approaches are: (1) Radiological software (eFilm™); (2) digitizer and 3D rendering software (Autodesk Maya®); (3) navigation system and Autodesk Maya®; and (4) navigation hardware and a dedicated, new navigation software (ApproachViewer, part of GTx-UHN - Guided-Therapeutics software developed at University Health Network - Toronto, Canada).
Radiological quantification: Commercially available radiological software that allows visualization in 2D and 3D of DICOM images was used (eFilm™, Merge Healthcare Incorporated)[6] to visualize and quantify the simulated approaches. The superficial and deep surfaces were calculated by dividing them in eight triangles; each triangular area was measured using Heron’s formula (Figure 1 of online additional material). The height of the surgical pyramid was measured with the tool for linear distances (Figure 2 of online additional material)[6]. Three-D rendering of the approaches was performed, when feasible, using the volume rendering tools[6] (Figure 3A of online additional material).
Digitizer and Autodesk Maya®: MicroscribeTM 3DX Digitizer (Immersion Corporation, San Jose, CA, United States) consists of a base and digitizing arm with five degrees of freedom that terminates in a fine tip stylus (Figure 4 of online additional material). When the stylus tip is placed on a surface and a button is pressed, the Cartesian coordinates (x, y, z) of the point are recorded and stored in a computer as a text file. The digitizer mean accuracy is 0.23 mm[7]-0.5 mm[8].
Commercially-available software for image-based modelling and rendering (Autodesk Maya®) was used to build a 3D model of surgical approaches using collected coordinates (see Figure 3B of online additional material). MEL Script and Python programming languages were used to create a plug-in to import the coordinates into Autodesk Maya®. The resulting model could be manipulated in 3D and allowed visualization from any view. Using MEL, a script was created to measure: (1) Distance: The height of the truncated pyramid was calculated as the distance between the center points of the superficial and deep areas. The center points were determined by the centre of mass equation, using the 8 coordinates collected along the border of both superficial and deep surfaces; and (2) Area: The area was calculated using the triangulation method. A vector was drawn to each of the 8 registered points from the center of mass equation. Two adjacent points along with the center formed a triangle, for a total of 8 triangles. Each triangular area was measured using Heron’s formula.
Neuronavigation system and Autodesk Maya®: In the first phase of this study, we used a prototype created by Storz Company for ENT surgery (Karl Storz® ENT navigation system - Tuttlingen, Germany). The registration was based on four-point collection and neuronavigation was allowed when the mean registration error was less than 2 mm. This neuronavigation system provided the on-screen coordinates of a given point in millimeter values, including decimals (Figures 5 and 6 of online additional material). A screenshot of each point of interest was recorded using a commercially-available frame grabber (VGA2USB LR frame grabber, Epiphan, Ottawa, Canada) (see Figure 6 of online additional material). The coordinates were recorded on an Excel sheet, and then imported and elaborated in Autodesk Maya®, as described above.
ApproachViewer: Dedicated software, called ApproachViewer, part of GTx-UHN (Guided-Therapeutics software developed at University Health Network - Toronto, Canada), was developed to visualize the surgical pyramid inside the head in which the approach was being performed.
The used navigation hardware by NDI (Northern Digital Imaging®, Waterloo, Ontario, Canada) included: (1) A passive rigid body (Rigid body 2®); (2) a passive probe with 4 markers; and (3) Polaris Vicra Optical Tracking System.
Circumferential points of the superficial and deep surfaces of each funnel were collected using the pointer tool. The surface points were then projected to the best plane fit using linear least squares. Delaunay triangulation was used to generate triangles on the plane and Heron’s formula applied. The height of each funnel was measured as the distance between the center of the superficial and deep surfaces.
Each simulated approach was quantified 10 times with each research method. Mean values and standard deviation of each value were calculated. Data were collected in Excel (Microsoft®, United States) and analyzed with OriginPro® (OriginLab®, Northampton, MA, United States). Standard deviations of the different methods were compared calculating the coefficients of variation (CVs) for each, in order to test their precision[9]. In addition, Brown-Forsythe tests for homogeneity of variances (HOV) were performed by comparing ApproachViewer to the other quantification methods. The level of significance for Brown-Forsythe test was set at 0.05.
The statistical methods of this study were reviewed by Elena Raffetti from Epidemiology and Public Health Intervention Research group (EPHIR), Department of Public Health Sciences, Karolinska Institutet.
Anatomical dissections and data collection were performed at the University of Toronto Surgical Skills Centre at Mount Sinai Hospital. REB approval was obtained (UHN REB number: 09-0130-T) (Figure 6 of online additional material).
Dissections were performed under 3 to 40 microscopic magnifications (Carl Zeiss® Surgical Microscope) and with 0º and 30º, 4 mm endoscopes (Karl Storz® - Tuttlingen, Germany). Various microsurgical and endoscopic approaches to the skull base and to certain intracranial areas were performed. In the first phase of this study, they were quantified with a commercially-available neuronavigation system connected to a frame grabber and coordinate elaboration with Autodesk Maya®, as described above. In the second phase of the study, ApproachViewer was always used.
Table 1 summarizes the results of the quantification of height, deep, and superficial areas of the phantom funnels with all 4 methods. CVs were less than one in all cases, indicating that all methods have low variance distributions.
| e-FilmTM | Microscribe 3DX Digitizer® | Storz® navigator + Maya® reconstructions | ApproachViewer - GTx-UHN | ||||||
| mean | SD | mean | SD | mean | SD | Mean | SD | ||
| Height (mm) | Large MS | 67 | 0.67 | 67.8 | 0.79 | 66.36 | 0.29 | 69.19 | 0.26 |
| Medium | 48.3 | 0.48 | 52.5 | 0.23 | 47.51 | 0.31 | 48.7 | 0.55 | |
| Small | 38.3 | 0.67 | 40.46 | 1.11 | 36.98 | 0.56 | 38.9 | 1.03 | |
| Area (mm2) | Large - deep | 5310.19 | 43.50 | 4877.78 | 52.82 | 5461.03 | 35.56 | 6003.2 | 49.42 |
| Large - deep mid | 1805.37 | 19.64 | 1548.06 | 15.45 | 1836.83 | 32.66 | 2073.4 | 40.85 | |
| Large - superficial | 441.87 | 21.22 | 416.81 | 12.11 | 409.05 | 12.62 | 533.3 | 16.44 | |
| Medium - deep | NA | NA | 128.24 | 5.45 | 136.89 | 4.73 | 201.4 | 7.41 | |
| Medium - middle | NA | NA | 498.69 | 14.01 | NA | NA | 470.4 | 21.80 | |
| Medium - superficial | 2425.57 | 46.34 | 2375.56 | 35.35 | 2555 | 20.86 | 2955.7 | 58.89 | |
| Small - deep | 82.49 | 6.37 | 108.34 | 4.92 | 90 | 4.24 | 125.4 | 6.88 | |
| Small - superficial | 150.84 | 12.70 | 161.43 | 5.54 | 168.3 | 11.36 | 256.1 | 11.40 | |
The Brown-Forsythe tests showed that variances of ApproachViewer quantifications were homogeneous with those of the other methods, with two exceptions. Homogeneity of variances (HOV) was not verified comparing ApproachViewer to the digitizer for either medium or large funnels heights. On the contrary, HOV was statistically valid comparing ApproachViewer to e-Film and Storz navigation quantifications (P < 0.05).
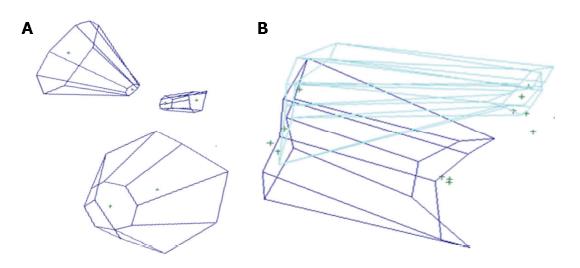
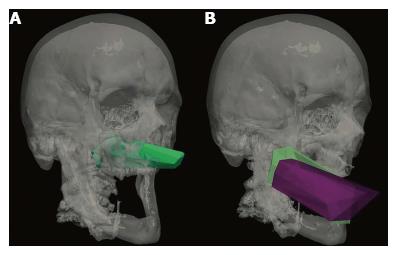
Figures 4A and 5 show volume rendering of the simulated approaches, obtained with newly developed system (i.e., navigation system and Maya® software; ApproachViewer - GTx-UHN). The results are visually comparable to the those obtained with commercially-available radiological software (eFilmTM) (Figure 3 of online additional material).
Figures 4B and 6 show 3D rendering of surgical approaches that were quantified with both navigation-based research methods (i.e., navigation system and Maya® software; ApproachViewer - GTx-UHN) in the anatomy laboratory (see also Figures 7 and 8 of online additional material for examples of rendering with the navigation system and Maya® software).
The mean time to obtain the 3D rendering of an approach with the navigation system and Maya® software was approximately 2-4 min for coordinate collection (8 points for each of the surfaces were collected; 2 people were needed), 3-8 min for coordinates registration in an Excel file (whether performed by one or two people), and 5 min for rendering with Maya®. The mean time to obtain the 3D rendering of an approach with ApproachViewer was 2 min, i.e., the time needed to collect multiple points both at the deep and superficial surface. Rendering was therefore immediate with ApproachViewer, while it was possible only after the laboratory session with the previously developed system.
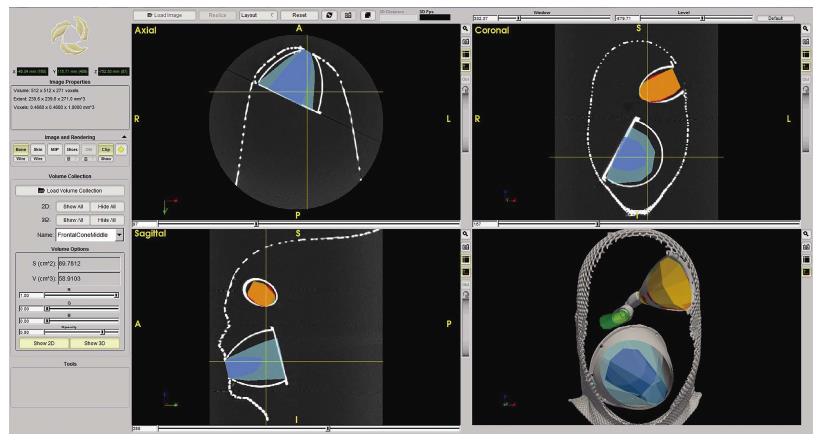
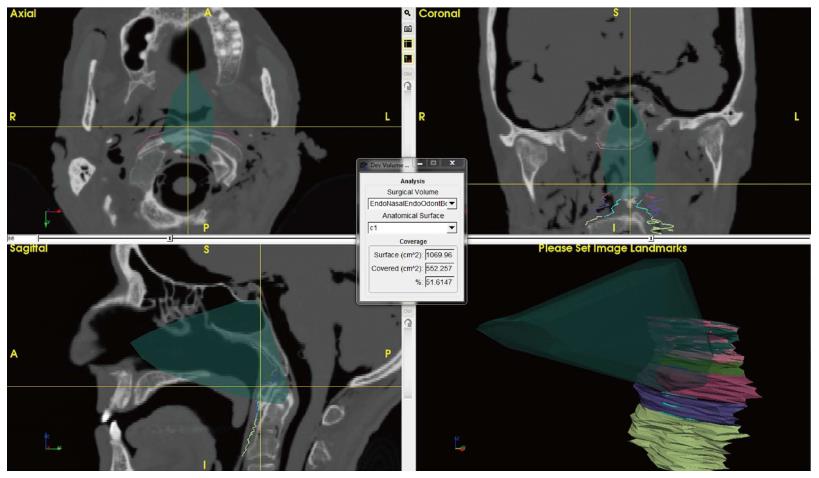
Additional features were developed with ApproachViewer, which was the only system that allowed visualization of approaches taking place inside the head in which they were performed. Areas of interest were contoured: ApproachViewer automatically calculated the absolute and percentage value of the area included in a specific approach (Figure 7). This feature was used in the post-dissection phase of anatomical studies (ApproachViewer guide 1.0 for further details, available from the corresponding author).
The comparative analyses documented a satisfactory, limited variation in the calculated data for each of the research methods, according to their CVs. Variation was more significant when different research methods were compared. We believe that this variability is due to different reasons, as recently reported by Ammirati’s group[9], possibly including different registration errors, varying methods to calculate the areas, and differing methods to collect the coordinates.
A digitizer is possibly the instrument for coordinate collection with the lowest error (0.23 mm[7] - 0.5 mm[8]). Its role in the analysis of surgical approaches is, however, limited by the physical constraints caused by its dimensions and desktop-based nature. The only statistically significant differences between the different methods were most probably due to the difference in coordinate collection, due to the physical constraints caused by the digitizer arm. Frameless navigation systems might have relatively higher errors, but have the advantage of high flexibility for coordinate collection, and have therefore been used extensively in recent anatomical studies[4]. The registration error of neuronavigation can be decreased using well recognizable registration points, such as screws that can be positioned around the area of interest[9].
The research method based on the use of a commercially-available navigation system and Maya® 3D rendering software provided 3D visualization and quantification of the truncated surgical pyramid that defines a surgical approach. A visual comparison of different approaches was therefore possible (Figure 4B), but to provide the correct orientation in space of each surgical pyramid a common registration had to be provided (i.e., three fixed points were registered during each session). This system, which was developed in 2009, provided for the first time the visualization in 3D of the surgical approach. The method is similar to others that have been recently published[4]. An evident practical limit of the method was the lengthy data elaboration process, which included manually copying the coordinates from each screenshot in an Excel sheet and manually superimposing the registration points in the 3D rendering of the pyramid. These issues may have been partially resolved, but the main limit that stopped us from further developing this research method was the impossibility of automatically visualizing the head in which the coordinates had been collected. It would have been possible to manually superimpose surgical volumes and CT images, but this would have introduced another possible cause of error and bias.
ApproachViewer was therefore developed as it allows visualization of the surgical volume inside the specimen in which the approach has been performed.
A new feature was then developed to further advance the objective evaluation of neurosurgical approaches. Once dissection sessions are over, areas of interest can be contoured. The software then allows the automatic quantification of absolute and percentage values of the area of interest that is included in each surgical approach (Figure 7 and ApproachViewer guide 1.0). We believe that this brings to reality a research method that was foreseen by Andaluz et al[5] more than 10 years ago: “We envision the ideal comparison between approaches as a volumetric analysis of truncated pyramids with polyhedral bases, with a special interest in the top of the pyramid. The incremental presence of computer-assisted devices in cranial base laboratories may some day bring this near-science-fiction concept into real numbers”.
When compared to other research methods based on navigation systems, ApproachViewer provides quantifications with the same precision, direct visualization of the surgical pyramids, and the possibility of using tools to measure areas of sections of the pyramids and to measure the distances between them to obtain heights (such as the middle surface of the medium funnel in the present study, which could not always be obtained with other methods). Compared to how we started, when we had to manually transcribe each coordinate into a separate database, which would then be elaborated, ApproachViewer is incredibly more user-friendly and faster, with automatic recording and calculations of pointer movements and positions, and thus automatic determination of pyramid dimensions and volumes. Use of the system should be intuitive to neurosurgeons who use any neuronavigation system in their surgical practice, as the same instruments, reflective spheres, and stereoscopic camera are used. Moreover, the system was used extensively by surgeons and medical students at the University of Brescia: Its use appears to be intuitive and easy to use (see ApproachViewer guide 1.0), although further studies are necessary to confirm these preliminary data.
The group led by Ammirati has recently described a method that allows visualization and quantification of the volume of a surgical approach[4,10]. The method is based on filling the surgical cavity with fat and CT scanning after dissection; the volume is then quantified with neuronavigation software. The main limit of the method is the need for post-dissection CT after each approach is performed[4], while ApproachViewer allows the collection of multiple data in a dissecting session without the need for multiple CT scans. On the other hand, it quantifies the surgical pyramid defined by a deep area and superficial one, which need to be defined before the dissections are performed.
The potential applications of ApproachViewer are multiple: (1) It provides a user-friendly method to quantify and therefore compare different surgical approaches; (2) as the working volume of the approach is immediately visualized on a screen, it can provide immediate feedback to a surgeon in training; and (3) the quantification of the working space has important implications for the development of angled instruments, especially for endoscopic skull base surgery.
In the past few years ApproachViewer has been systematically applied to the analysis of multiple skull base approaches: Some results have already been published[11-14]. All these potential applications require specific studies, possibly by multiple centers.
We have developed and validated a new, navigation-based tool for anatomical research in neurosurgery. ApproachViewer (part of part of GTx-UHN - Guided-Therapeutics software developed at University Health Network - Toronto, Canada) provides the possibility of multiple data collection during dissection sessions, as well as various post-dissections analyses, including 3D rendering of the surgical volume, quantification of the anatomical features that define a surgical approach, and of the area of interest exposed.
Anatomical studies are an essential part of the preclinical phase of surgical research. They can provide objective, quantitative, comparative data that can be used to evaluate novel surgical approaches and techniques.
Different research methods have been described to quantify and compare different surgical approaches and most of them do not provide visual rendering and quantification of the surgical pyramid, which is the working volume that defines a neurosurgical approach.
This study describes the development and validation of a novel neuronavigation-based method, which allows the quantification of the anatomical features that define an approach, as well as real-time visualization of its surgical pyramid.
ApproachViewer has already been extensively used in the anatomy laboratory to quantify and compare different neurosurgical approaches. It provides a real-time visualization and quantification of the surgical pyramid. Possible applications include not only the preclinical quantification of surgical approaches, but also: (1) Immediate feedback to surgeons in training in the anatomy laboratory during dissections; (2) Detailed analysis of the surgical volume for the development of new surgical instruments; and (3) Quantification of the working volume in clinical scenarios.
Neuronavigation: Computer-assisted technologies used to guide or “navigate” within the intracranial space.
The exposition is well conducted and the topic is very interesting.
The authors would like to thank Lisa Satterthwaite and her team at the University of Toronto Surgical Skills Centre at Mount Sinai Hospital for the invaluable support during the anatomical studies.
Manuscript source: Unsolicited manuscript
Specialty type: Medical laboratory technology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Neri V, Ni Y S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J; Balliol Collaboration, Aronson JK, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Feldman LS, Flum DR, Maddern GJ, Nicholl J, Reeves BC, Seiler CM, Strasberg SM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Ergina PL, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Meakins J, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1317] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 2. | Bernstein M, Khu KJ. Is there too much variability in technical neurosurgery decision-making? Virtual Tumour Board of a challenging case. Acta Neurochir (Wien). 2009;151:411-412; discussion 412-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Couldwell W. Comment to: Bernstein M, Khu KJ: Is there too much variability in technical neurosurgery decision-making? Virtual Tumour Board of a challenging case. Acta Neurochirurgica (Wien). 2009;151:412-413. |
| 4. | Doglietto F, Radovanovic I, Ravichandiran M, Agur A, Zadeh G, Qiu J, Kucharczyk W, Fernandez E, Fontanella MM, Gentili F. Quantification and comparison of neurosurgical approaches in the preclinical setting: literature review. Neurosurg Rev. 2016;39:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Andaluz N, Van Loveren HR, Keller JT, Zuccarello M. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery. 2003;52:1140-1148; discussion 1148-1149. [PubMed] |
| 6. | eFilmTM 3. 0 User’s Guide. Mississauga, ON, Canada: Merge Heathcare 2007; Available from: https://estore.merge.com/na/resources/efilm30userguide.pdf. |
| 7. | MicroscribeTM 3D user manual. San Jose, CA, USA: Immersion Corporation, 2000. Available from: http://www.3d-microscribe.com/msg2_0704_v1.pdf. |
| 8. | Ravichandiran K, Ravichandiran M, Oliver ML, Singh KS, McKee NH, Agur AM. Determining physiological cross-sectional area of extensor carpi radialis longus and brevis as a whole and by regions using 3D computer muscle models created from digitized fiber bundle data. Comput Methods Programs Biomed. 2009;95:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Salma A, Makiese O, Sammet S, Ammirati M. Effect of registration mode on neuronavigation precision: an exploration of the role of random error. Comput Aided Surg. 2012;17:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Salma A, Alkandari A, Sammet S, Ammirati M. Lateral supraorbital approach vs pterional approach: an anatomic qualitative and quantitative evaluation. Neurosurgery. 2011;68:364-372; discussion 371-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Ferrari M, Schreiber A, Mattavelli D, Belotti F, Rampinelli V, Lancini D, Doglietto F, Fontanella MM, Tschabitscher M, Rodella LF. The Inferolateral Transorbital Endoscopic Approach: A Preclinical Anatomic Study. World Neurosurg. 2016;90:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Schreiber A, Ferrari M, Rampinelli V, Doglietto F, Belotti F, Lancini D, Ravanelli M, Rodella LF, Fontanella MM, Nicolai P. Modular Endoscopic Medial Maxillectomies: Quantitative Analysis of Surgical Exposure in a Preclinical Setting. World Neurosurg. 2017;100:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Mattavelli D, Bolzoni Villaret A, Ferrari M, Ravanelli M, Rampinelli V, Lancini D, Rodella LF, Fontanella M, Maroldi R, Nicolai P. Different Perspectives of Internal Carotid Artery in Transnasal Endoscopic Surgery. World Neurosurg. 2016;95:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Jägersberg M, Brodard J, Qiu J, Mansouri A, Doglietto F, Gentili F, Kucharczyk W, Fasel J, Schaller K, Radovanovic I. Quantification of Working Volumes, Exposure, and Target-Specific Maneuverability of the Pterional Craniotomy and Its Minimally Invasive Variants. World Neurosurg. 2017;101:710-717.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |