Published online Jun 26, 2016. doi: 10.5662/wjm.v6.i2.163
Peer-review started: June 18, 2015
First decision: September 23, 2015
Revised: March 16, 2016
Accepted: March 22, 2016
Article in press: March 23, 2016
Published online: June 26, 2016
Processing time: 372 Days and 17.4 Hours
AIM: To evaluate telomere length in sperm DNA and its correlation with oxidative stress (normal, mild, severe).
METHODS: The study included infertile men (n = 112) and age matched fertile controls (n = 102). The average telomere length from the sperm DNA was measured using a quantitative real time PCR based assay. Seminal reactive oxygen species (ROS) and 8-Isoprostane (8-IP) levels were measured by chemiluminescence assay and ELISA respectively.
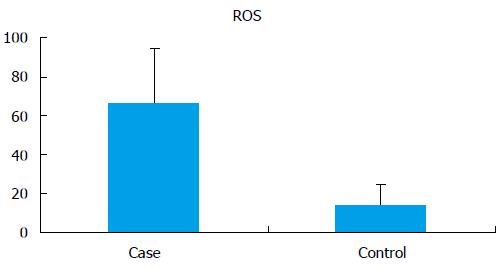
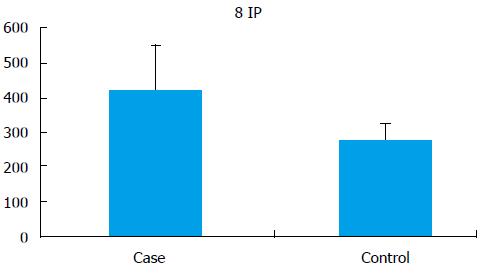
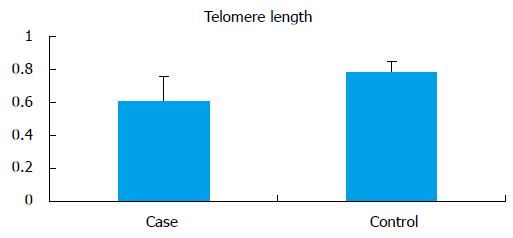
RESULTS: Average sperm telomere length in infertile men and controls was 0.609 ± 0.15 and 0.789 ± 0.060, respectively (P < 0.0001). Seminal ROS levels in infertile was higher [66.61 ± 28.32 relative light units (RLU)/s/million sperm] than in controls (14.04 ± 10.67 RLU/s/million sperm) (P < 0.0001). The 8-IP level in infertile men was significantly higher (421.55 ± 131.29 pg/mL) than in controls (275.94 ± 48.13 pg/mL) (P < 0.001). When correlated to oxidative stress, in normal range of oxidative stress (ROS, 0-21.3 RLU/s/million sperm) the average telomere length in cases was 0.663 ± 0.14, in mild oxidative stress (ROS, 21.3-35 RLU/s/million sperm) it was elevated (0.684 ± 0.12) and in severe oxidative stress (ROS > 35 RLU/s/million sperm) average telomere length was decreased to 0.595 ± 0.15.
CONCLUSION: Mild oxidative stress results in lengthening of telomere length, but severe oxidative stress results in shorter telomeres. Although telomere maintenance is a complex trait, the study shows that mild oxidative stress is beneficial in telomere length maintenance and thus a delicate balance needs to be established to maximize the beneficial effects of free radicals and prevent harmful effects of supra physiological levels. Detailed molecular evaluation of telomere structure, its correlation with oxidative stress would aid in elucidating the cause of accelerated telomere length attrition.
Core tip: In the present study we found that infertile men experienced oxidative stress evident from increased seminal reactive oxygen species and 8-Isoprostane levels. Infertile men also had shorter telomeres as compared to the controls. Severe oxidative stress negatively affected sperm telomere length but surprisingly mild oxidative stress resulted in lengthening of telomere and thus may aid in maintaining genomic integrity. To the best of our knowledge we are the first to report positive effect of mild oxidative stress on sperm telomere length.
- Citation: Mishra S, Kumar R, Malhotra N, Singh N, Dada R. Mild oxidative stress is beneficial for sperm telomere length maintenance. World J Methodol 2016; 6(2): 163-170
- URL: https://www.wjgnet.com/2222-0682/full/v6/i2/163.htm
- DOI: https://dx.doi.org/10.5662/wjm.v6.i2.163
Infertility is a complex life style disorder and affects about 1 in 5 couples. Oxidative damage to sperm DNA is one of the major causes of infertility especially in men with normal sperm parameters. Recent studies have shown that sperm DNA integrity, mitochondrial genome, gene expression, and sperm telomere length along with the interplay of more than 2000 genes and gene environment interaction are the key players in male infertility. Studies have shown that oxidative stress is a major player in male infertility. Oxidative stress affects all the bio molecules, membrane lipids, nuclear and mitochondrial genome. Various studies have explored the role of oxidative stress and DNA damage in male infertility but there are few studies to understand the role of sperm telomeres and its length in the aetiology of male infertility. Shorter telomeres may result in impaired spermatogenesis and fewer cell divisions which may ultimately manifest as oligozoospermia or azoospermia.
Telomeres are hexameric guanine rich repeats present at the ends of chromosomes in all eukaryotic cells[1]. Human telomeres are composed of long stretches of the repetitive sequence TTAGGG and a telomere-specific protein complex, Shelterin. TRF1, TRF2 and POT1 are the three proteins that impart Shelterin the specificity for telomeres[2]. TIN2 and TPP1 link POT1 to TRF1 and TRF2. The t-loop of the telomeric lariats are formed due to the invasion of the 3′ single-stranded overhang into the double-stranded telomeric DNA. Shelterin caps most of the double stranded telomeric region and POT1 covers the single-stranded telomeric DNA in the 3′ overhang or in the D loop. Various shelterin associated proteins and nucleosomes are also found associated to telomeric DNA[3].
In mammalian sperm nuclei, all telomeres exist in the form of dimers. In humans, these dimers are localized at the nuclear periphery and, most probably, interact with the nuclear membrane. The human telomere sequence varies in length from 5 to 10 kb in somatic cells and 10 to 20 kb in germ cells. Telomere length is critical for chromosome stability, cell proliferation and survival. Telomere length in spermatozoa is substantially longer compared to normal somatic cells[4]. Initial telomere length setting happens in the sperm cells before fertilization. Sperm cells are the carriers of intact chromosomes to the progeny. Yet only limited reports are available regarding the role of telomere dynamics in germ cells.
During cell division telomeres are not fully replicated because of end replication problem, an inability of the DNA polymerase to completely replicate the DNA ends resulting in telomere shortening with every cell division. Telomerase, an enzyme consisting of the telomerase RNA template and telomerase reverse transcriptase maintains telomere length by adding nucleotide repeats to the chromosome ends.
Other causes of telomere shortening apart from normal cell division include oxidative stress, genotoxic insults, and genetic predisposition. Telomere shortening has been associated with oxidative stress[5]. Reactive oxygen species (ROS) resulting endogenously from normal cellular metabolism and due to exogenous genotoxic insults like environmental exposure, exposure to electromagnetic radiations, exhaustive exercise, poor life style habits also shorten telomeres by oxidizing guanine residues in the telomeric DNA and thereby initiating a DNA damage response. DNA damage response results in excision of telomere repeats[6]. The high content of guanine nucleotides - in telomeric DNA makes telomere a preferred target for oxidative damage[7]. Thus this study was planned with an aim to study impact of oxidative stress on telomere length. Though sperm maintain longer average telomere length, sperm telomere length does vary among individual men and individual spermatozoa. Variability in sperm telomere length is due to telomere shortening as a result of differential telomerase activity and oxidative stress[8]. Cellular environment also plays an important role in regulating telomere length and telomerase activity. Most notably, oxidative stress can shorten telomeres and antioxidants can decelerate shortening[9]. Studies by Kumar et al[10,11] have shown upregulation in telomerase activity and decline in free radical levels, oxidized mutagenic bases following practice of yoga and meditation. Telomere shortening in sperm cells results in segregation errors, generation of unbalanced gametes, reduced sperm count due to apoptosis which ultimately leads to loss of fertility potential and abortions. There are few studies on the role of telomere in reproduction and since oxidative stress has such a marked effect on telomere phenotype, it is important to evaluate the effect of oxidative stress (normal, mild, severe) on telomere length. It is important to establish cut off levels of free radicals which are beneficial to telomeres and cause telomere lengthening as compared to very high ROS levels which induce DNA damage response in telomeres and cause telomere shortening. In this study we found longer telomere length in cases with mild oxidative stress levels [21.3-35 relative light units (RLU)/s/million sperm] as compared to cases with normal ROS levels (0-21.3 RLU/s/million sperm). Thus it would be more valuable to establish cut off free radical levels which are beneficial/detrimental to telomere length maintenance. In this study we have investigated the role of oxidative stress in sperm telomere length maintenance in idiopathic male infertility.
The study was initiated after institutional ethical clearance and written informed consent from patients and controls. The female partners of all the cases were normal after complete clinical, gynaecological, hormonal and radiological examination. Human ejaculates were obtained from 102 healthy volunteers of proven fertility from family planning OPD of Obstetrics and Gynaecology Department, and 112 male partners of couples experiencing primary infertility within age group of 18-45 years. A detailed family history was recorded in a pre-designed proforma. Cytogenetic analysis was done for all cases to exclude cases with abnormal chromosome complement. All cases with recent history of fever, drug intake, any inflammatory disorders, or infections were excluded. Semen analysis was assessed by World Health Organization (WHO) (1999) criteria. These patients after thorough clinical examination were referred from the Department of Gynaecology and Obstetrics and Department of Urology, AIIMS, New Delhi.
Semen analysis was done twice at 2 wk interval. Samples were collected after minimum of 48 h and not longer than 7 d of sexual abstinence. The name of the patient, period of abstinence and time of collection were recorded on the form accompanying each semen analysis. Samples were collected in a private room near the laboratory and were delivered to the laboratory within 1 h after collection. The samples were obtained by masturbation and ejaculated into a clean, wide-mouthed glass or plastic container. The procedure for sample collection was explained to the patients and controls. Semen analysis was done as per WHO guidelines (1999).
The ROS production in 400 μL of liquefied neat semen was measured after addition of 10 μL of 5 mmol solution of luminol in DMSO (dimethylsulphoxide, Sigma Chemical Co.). A tube containing 10 μL of 5 mmol luminol (5-amino-2,3-dihydro-1,4-phthalazinedione, Sigma Chemical Co., St. Louis, MO, United States) solution in DMSO was used as a blank. Chemiluminescence was measured in duplicate for 10 min using the Berthold detection luminometer (United States). Sample analysis was done along with blank, positive control (H2O2 + PBS + Luminol) and negative control (PBS + luminol). Results were expressed in RLU per second and per 1 × 106 spermatozoa.
8-Isoprostane (8-IP) is a stable marker of oxidative stress which is indicative of lipid peroxidation. 8-IP levels are independent of dietary intake of lipids hence its level gives the absolute measurement of lipid peroxidation due to oxidative stress. 8-IP levels were estimated by ELISA. The quantification was done by Cayman’s 8-IP EIA Kit. Protocol was followed as described by the manufacturer for the quantification.
Real time PCR: Sperm telomere length was determined from the sperm DNA by a quantitative real-time PCR-based method. Briefly, the relative mean telomere length was determined by comparing the value from absolute quantification of telomere DNA with a single copy reference gene, 36B4 (T/S ratio). These two assays were carried out as separate reactions on separate plates maintaining the sample positions between the two plates. Amplification signals were quantified by the standard curve method using a DNA template series (100 ng, 10 ng, 1 ng, 0.1 ng, 0.01 ng/μL) on every plate. All randomized DNA samples (20 ng) and standard dilution was processed as triplicates on 96-well plates using Bio-Rad CFX 96 (Hercules, CA, United States). The purpose of the standard curve was to assess and compensate for inter-plate variations in PCR efficiency. Amplification of the telomeric repeat region was expressed relative to amplification of 36B4, a single copy gene (SCG) encoding acidic ribosomal phosphoprotein located on chromosome 12. Real time kinetic quantitative PCR determines, for each sample well, the Ct, i.e., the fractional cycle number at which the well’s accumulating fluorescence crosses a set threshold that is several standard deviations above baseline fluorescence. A plot of Ct vs log (amount of input target DNA) is linear, allowing simple relative quantitation of unknowns in comparison to a standard curve derived from amplification, in the same plate, of serial dilutions of a known reference DNA sample. For this study, telomere (T) PCRs and SCG PCRs were always performed in separate 96-well plates.
The sperm parameters, sperm count (P = 0.0007) and forward motility (P < 0.0001) were significantly lower in infertile men compared to controls and no significant difference in the seminal volume and pH was observed between infertile men and controls. Out of 112 cases, 70 men had normal semen parameters as per WHO 1999 guidelines (Table 1).
| Category | Age (mean ± SD, yr) | SC (mean ± SD) | FM (mean ± SD) | pH (mean ± SD) | Volume (mean ± SD, mL) |
| Infertile | 31.71 ± 4.45 | 41.5 ± 36.6 | 44.25 ± 19.94 | 7.5 ± 0.55 | 3.04 ± 1.23 |
| n = 112 | |||||
| Controls | 32.22 ± 4.0 | 70.66 ± 57.9 | 60.14 ± 18.33 | 7.61 ± 0.38 | 2.96 ± 1.07 |
| n = 102 |
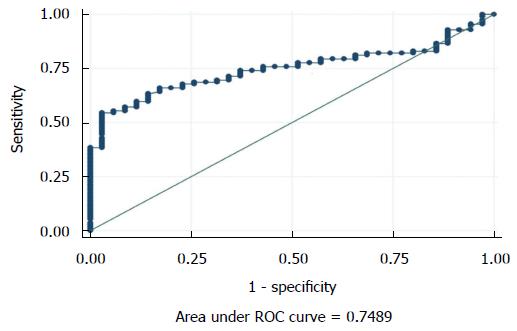
The seminal ROS levels in cases were significantly higher (66.61 ± 28.32 RLU/s/million sperm) than controls (14.04 ± 10.67 RLU/s/million sperm) (P < 0.0001) (Figure 1). Receiver operating curve analysis was done to establish a cut off value for seminal ROS levels. The cut off obtained was 21.3 RLU/s/million sperm (Figure 2).
Seminal 8-IP levels were significantly (P < 0.001) elevated in the cases (421.55 ± 131.29 pg/mL) as compared to controls (275.94 ± 48.13 pg/mL) (Figure 3).
The mean telomere length (T/S) in the infertile men (0.609 ± 0.15) was significantly lower (P < 0.0001) when compared to controls (0.789 ± 0.060) (Figure 4). No significant difference in the average mean age of infertile men and control was observed. All analysis were adjusted for age. For the normalization of telomere length with age, cases and controls were divided into 2 groups according to age (group A: 18-30 years; group B: 31-45 years). There was no significant difference between the mean age of the cases compared to controls in the respective groups, but there was significant difference between telomere length in cases as compared to controls in both of these groups (group A cases: 0.51 ± 0.15 vs controls: 0.79 ± 0.064, P < 0.0001; group B cases: 0.50 ± 0.11 vs controls: 0.77 ± 0.05, P < 0.0001).
All the cases (n = 112) were classified into three groups on the basis of seminal ROS levels. group I (n = 17) had normal ROS levels (0-21.3 RLU/s/million sperm), group II (n = 40) had mild ROS levels (21.3-35 RLU/s/million sperm) and group III (n = 55) had severe ROS levels (> 35 RLU/s/million sperm). The mean telomere length was analysed in these three groups and we observed a significant (P < 0.001) shortening of telomere length in the group (0.595 ± 0.15) with ROS levels more than 35 RLU/s/million sperm as compared to other two groups (group I: 0.663 ± 0.14 and group II: 0.684 ± 0.12). Interestingly, it was observed that the mean telomere length was elevated in the patient group with mild oxidative stress (0.684 ± 0.12) as compared to the patient group with normal oxidative stress (0.663 ± 0.14) (P < 0.001) (Table 2).
| ROS (RLU/s/million sperm), (mean ± SD) | 0-21.3 (normal) | 21.3-35 (mild) | > 35 (severe) |
| RLU/s/million sperm | RLU/s/million sperm | RLU/s/million sperm | |
| Mean telomere length (mean ± SD) | 0.663 ± 0.14 | 0.684 ± 0.12 | 0.595 ± 0.15 |
| 8-IP (pg/mL) (mean ± SD) | 282.41 ± 61.97 | 440.54 ± 23.78 | 560.66 ± 43.82 |
There were 23 (22.6%) controls that had ROS levels in the mild oxidative stress level range. When the mean telomere length in this group was compared with rest of the controls a similar pattern of increased telomere length was observed in the group of controls with mild oxidative stress (0.79 ± 0.06) as compared to the rest of the controls with normal oxidative stress levels (0.73 ± 0.04) (P = 0.01).
8-IP levels also showed an increasing trend towards increasing ROS levels (Table 2). There was a negative correlation of sperm telomere length with ROS (r = -0.63, P = 0.02) and 8-IP (r = -0.33, P = 0.03) (Spearman’s correlation). Also, telomere length was found to be negatively correlated with age (r = -0.4, P = 0.035).
Telomeres and telomere associated proteins play an important role in the maintenance of genomic integrity in the eukaryotic cells. They also participate in the cellular DNA damage response pathways. Additionally, telomeres aid in the movement, localization, and anchoring of the chromosomes to the nuclear membrane. During cell division, telomeres mediate the pairing of homologous chromosomes, synapsis formation, and homologous recombination[12]. Telomere length shortens with every cell division due to the inefficiency of the DNA polymerases to replicate the chromosome ends. Besides this mechanism of telomere shortening, oxidative stress is another important factor affecting the telomere dynamics in the nucleus. ROS induced telomere shortening may be due to direct oxidative injury to guanine bases in telomeric DNA.
Oxidative stress induces accelerated telomere shortening by the accumulation of oxidized DNA base products (8-OHdG) in the guanine rich telomeres which further recruit DNA damage response machinery that ultimately cause telomere attrition during the course of DNA damage repair. Accumulation of these base adducts cause single-strand breaks in the telomeric regions and these breaks are less efficiently repaired at telomeres than in the rest of the genome[13]. In this study we have observed elevated ROS levels in the sperm of infertile men as compared to the fertile controls. Limited studies exist exploring the effect of oxidative stress on the sperm telomere and all the studies so far direct towards a negative correlation between oxidative stress and telomere length.
It is now well documented that basal levels of ROS are essential for cell survival and subsevere several physiological functions. It is evident from previous studies that severe oxidative stress leads to extensive damage to biomolecules and causes cell death[14] but moderate levels of ROS are beneficial to cellular homeostasis especially in cellular responses during pathological challenges in aging and aging-associated diseases[15]. ROS is generated as a part of normal aerobic cellular metabolism[16] and can be generated from various metabolic pathways[17], including electron transport during oxidative phosphorylation in the mitochondria[18], dihydrolipoamide dehydrogenase in the α-keto acid dehydrogenase complexes[19], NADPH oxidase and, xanthine oxidase and, monoamine oxidase, and cytochrome P450 proteins[20].
Although basal levels of ROS are pivotal for redox signalling[21] and cell survival, high levels of ROS would be lethal to normal cellular processes. High levels of oxidative stress are believed to contribute a major part in cellular aging and several aging-related diseases. A moderate level of oxidative stress is needed for various cellular responses beneficial for cell survival[22].
As ROS are transient molecules with very short half lives they execute beneficial effects by activating downstream molecules. These downstream molecules include oxidative products such as lipid peroxidation by-products and protein oxidation adducts. These modifications include disulfide formation, S-glutathionylation, S-sulfenation, and S-nitrosylation[23]. These modifications induced by ROS regulate protein function and thus protect them from severe stress conditions. These effectors of moderate oxidative stress can actually execute the ultimate function of positive oxidative stress by redox signaling and activation of transcription factors[24]. Since oxidative stress plays a major role in chronic inflammatory diseases, telomere attrition may be involved in the pathophysiology of these diseases. Several studies have linked telomere shortening to various chronic metabolic and inflammatory diseases such as atherosclerosis, diabetes, inflammatory bowel disease, and chronic obstructive pulmonary disease conditions that are all characterized by the presence of systemic oxidative stress[13]. However, the exact underlying mechanisms of telomere shortening under conditions of chronic oxidative stress have not been elucidated. In the present study we found that the infertile group with mild oxidative stress (21.3-35) had longer telomeres as compared to the group with normal free radical levels and group with severe oxidative stress emphasizing that the slightly elevated free radical levels are beneficial for telomere length maintenance[25]. The mean telomere length decreased in the patients as compared to the controls but it was increased in the patient group that had mild oxidative stress. The result of our study can be explained by findings of Wang et al[26]. It was documented that, when few base lesions affect telomeric DNA repeats, they reduce binding of proteins in telomeres which liberate the negative regulation of telomere binding proteins on telomerase and consequently increase telomerase-dependent telomere repeat additions such that telomere lengthening occurs. However severe oxidative stress causes complete uncapping of telomeric DNA with oxidized bases and consequently activate DNA damage response and results in telomere shortening. As already documented, physiological levels of free radical maintain homeostasis and positive oxidative stress can be induced by non lethal free radicals[27]. There are also positive effects of physiological level of ROS in in vivo conditions and cellular environment also plays an important role in regulating telomere length and telomerase activity[28].
Recently, it has been shown that telomeric regions are favoured targets of a persistent DNA damage response induced by genotoxic and oxidative stress, both in vitro and in vivo[29]. Oxidative stress induces single-strand breaks both directly and indirectly. These are less efficiently repaired in telomeric DNA as compared to genomic DNA[30]. Kawanishi et al[31] also stated that, formation of 8-OHdG at the GGG triplet in telomere sequence induced by oxidative stress could accelerate telomere shortening. Accumulation of oxidized bases beyond a certain level in telomeres may severely deplete telomere binding proteins in telomeres and result in telomere uncapping. Uncapped telomeres can become targets for ATM or ATR kinases and nucleolytic degradation that eventually cause telomere shortening and cell cycle arrest[26]. Therefore, very high oxidative stress levels are detrimental to telomere length maintenance as is evident from the study. Infertility may thus be accelerated with oxidative stress, DNA damage and shorter telomeres. However mild oxidative stress is beneficial for telomere length and results in longer telomeres and thus may aid in maintainenece of genomic integrity. Thus severe oxidative stress and resultant shorter telomeres may impair spermatogenesis and result in hypospermatogenesis and may manifest as oligozoospermia and later as azoospermia however mild oxidative stress aids in maintainece of telomeres and thus aid in mitotic division of germ cells. Thus one should not indiscriminately take antioxidants and levels of seminal free radical levels should be monitored regularly when one is on antioxidants. Recent studies from our laboratory have documented the beneficial effect of yoga and meditation in reducing oxidative stress and upregulating activity of telomerase enzyme. Thus we believe that infertility is actually accelerated testicular aging characterised by oxidative stress, DNA damage and shorter telomeres and it could be reversed by adopting yoga and meditation into daily lifestyle.
With available evidence that physiological levels of free radical is important for various cellular, physiological and biochemical mechanisms including regulation of gene expression and cell signalling a delicate balance for free radical levels needs to be established to maximize the beneficial physiological effects of free radicals and minimize the detrimental effects of supra physiological levels.
Infertility is a complex life style disorder. Oxidative damage to sperm DNA is one of the major causes of infertility especially in men with normal sperm parameters. Recent studies have shown that loss of sperm DNA integrity, hypermutability of mitochondrial genome, altered gene expression, short telomere length and environmental/lifestyle factors like smoking, psychological stress are the key players in male infertility. DNA damage is chiefly oxidative and one of the leading causes of defective sperm function. Simple lifestyle modifications can significantly improve DNA integrity.
Various studies have explored the role of oxidative stress and DNA damage in male infertility but there are few studies to understand the role of sperm telomeres and its length in the aetiology of male infertility. Numerous studies have addressed the detailed description of telomere in various diseases but the telomere length in the sperm and their correlation with seminal oxidative stress has not been intensely investigated. There are few studies on the role of telomere in reproduction and since oxidative stress has such a marked effect on telomere phenotype, it is important to evaluate the effect of oxidative stress (normal, mild, severe) on telomere length. It is important to establish cut off levels of free radicals which are beneficial to telomeres and cause telomere lengthening as compared to very high reactive oxygen species (ROS) levels which induce DNA damage response in telomeres and cause telomere shortening.
In this study the authors found longer telomere length in cases with mild oxidative stress levels [21.3-35 relative light units (RLU)/s/million sperm] as compared to cases with normal ROS levels (0-21.3 RLU/s/million sperm). Thus it would be more valuable to establish cut off free radical levels which are beneficial/detrimental to telomere length maintenance. The authors have investigated the role of oxidative stress in sperm telomere length maintenance in idiopathic male infertility.
Thus severe oxidative stress and resultant shorter telomeres may impair spermatogenesis and result in hypospermatogenesis and may manifest as oligozoospermia and later as azoospermia however mild oxidative stress aids in maintainece of telomeres and thus aid in mitotic division of germ cells. One should not indiscriminately take antioxidants and levels of seminal free radical levels should be monitored regularly when one is on antioxidants.
This is a quite well designed study which is worth to be published in scientific journal. Language is communicative, manuscript is concise, methods are well chosen, results transparent and discussion sufficient.
P- Reviewer: Dziegiel P, El Sherbini MAH S- Editor: Kong JX L- Editor: Cant MR E- Editor: Jiao XK
| 1. | Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114-10118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1536] [Cited by in RCA: 1537] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 2. | Cooke HJ, Smith BA. Variability at the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harb Symp Quant Biol. 1986;51 Pt 1:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 490] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Kozik A, Bradbury EM, Zalensky A. Increased telomere size in sperm cells of mammals with long terminal (TTAGGG)n arrays. Mol Reprod Dev. 1998;51:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Krishna BH, Keerthi GS, Kumar CK, Reddy NM. Association of leukocyte telomere length with oxidative stress in yoga practitioners. J Clin Diagn Res. 2015;9:CC01-CC03. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Kim SY, Velando A. Antioxidants safeguard telomeres in bold chicks. Biol Lett. 2015;11:20150211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Coluzzi E, Colamartino M, Cozzi R, Leone S, Meneghini C, O’Callaghan N, Sgura A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS One. 2014;9:e110963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Li H, Hedmer M, Wojdacz T, Hossain MB, Lindh CH, Tinnerberg H, Albin M, Broberg K. Oxidative stress, telomere shortening, and DNA methylation in relation to low-to-moderate occupational exposure to welding fumes. Environ Mol Mutagen. 2015;56:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312-17315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 1977] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 10. | Kumar SB, Yadav R, Yadav RK, Tolahunase M, Dada R. Telomerase activity and cellular aging might be positively modified by a yoga-based lifestyle intervention. J Altern Complement Med. 2015;21:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kumar SB, Gautam S, Tolahunase M, Chawla B, Yadav RK, Kumar P, Mitra DK, Dada R. Improvement in sperm DNA quality following simple life style intervention: a study in fathers of children with non familial sporadic heritable retinoblastoma. J Clin Case Rep. 2015;5:509. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Scherthan H. Telomere attachment and clustering during meiosis. Cell Mol Life Sci. 2007;64:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Boesten DM, de Vos-Houben JM, Timmermans L, den Hartog GJ, Bast A, Hageman GJ. Accelerated aging during chronic oxidative stress: a role for PARP-1. Oxid Med Cell Longev. 2013;2013:680414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 742] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 15. | Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2009;11:2655-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Knoefler D, Thamsen M, Koniczek M, Niemuth NJ, Diederich AK, Jakob U. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol Cell. 2012;47:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Dröse S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol. 2012;748:145-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 376] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 18. | Yan LJ, Sumien N, Thangthaeng N, Forster MJ. Reversible inactivation of dihydrolipoamide dehydrogenase by mitochondrial hydrogen peroxide. Free Radic Res. 2013;47:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Ambrus A, Adam-Vizi V. Molecular dynamics study of the structural basis of dysfunction and the modulation of reactive oxygen species generation by pathogenic mutants of human dihydrolipoamide dehydrogenase. Arch Biochem Biophys. 2013;538:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Agarwal A, Allamaneni SS. Free radicals and male reproduction. J Indian Med Assoc. 2011;109:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1944] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 22. | Pickering AM, Vojtovich L, Tower J, A Davies KJ. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic Biol Med. 2013;55:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Pimentel D, Haeussler DJ, Matsui R, Burgoyne JR, Cohen RA, Bachschmid MM. Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system. Antioxid Redox Signal. 2012;16:524-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Milisav I, Poljsak B, Suput D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci. 2012;13:10771-10806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 657] [Cited by in RCA: 598] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Rhee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6:e1000951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Yan LJ. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014;2C:165-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Espejel S, Martín M, Klatt P, Martín-Caballero J, Flores JM, Blasco MA. Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep. 2004;5:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Rhee DB, Ghosh A, Lu J, Bohr VA, Liu Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair (Amst). 2011;10:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 537] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 31. | Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 377] [Article Influence: 18.0] [Reference Citation Analysis (0)] |