Published online Mar 26, 2016. doi: 10.5662/wjm.v6.i1.126
Peer-review started: August 6, 2015
First decision: September 28, 2015
Revised: December 12, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: March 26, 2016
Processing time: 233 Days and 21.2 Hours
AIM: To seek the cause of Burkholderia cepacia complex (Bcc) infection outbreak and evaluate the efficacy of new methods for nebulizer maintenance.
METHODS: We investigated the annual number of Bcc isolates recovered from clinical samples in our hospital between 1999 and 2013. Swab samples were randomly collected for bacterial culture before patient use from 10 each of the two machine types in August 2001; these included 20 samples from each of the following: Drain tubes, operating water chambers, oscillators, and nebulizing chambers. In addition, 10 samples each of nebulizer solutions before and after use were cultured. For environmental investigation, 10 samples were collected from sinks in the nurse stations of the wards where patients positive for Bcc were hospitalized. Numbers of Bcc isolates were compared before and after introduction of new methods for nebulizer maintenance in October 2001. In addition, randomly amplified polymorphic DNA (RAPD) assay was applied to find the genetic divergence of the Bcc isolates obtained from clinical samples and nebulizers.
RESULTS: From January 1999 to December 2013, a total of 487 Bcc isolates were obtained from clinical specimens from 181 patients. Notably, 322 (66.1%) Bcc isolates were obtained from clinical specimens from 1999 to 2001, including 244 (115 patients) from sputum and 34 (11 patients) from blood. During this period, 14 isolates were obtained from nebulizer components. Among these, six were derived from nebulizer drain tubes, five from operating water chambers, and one from the oscillator before patient use, and two from nebulizer solutions after patient use. When Bcc was isolated from the nebulizer solution after patient use, Bcc was simultaneously detected in other parts of the nebulizer. Bcc was not isolated from any nebulizer solution before use. RAPD assays revealed similar DNA profiles in isolates obtained from patients and nebulizers. Investigation revealed damaged diaphragms in many nebulizers. The new maintenance methods for nebulizers, including restriction of the usage period, thorough disinfection, and routine check for diaphragm breakage, remarkably reduced Bcc isolation (165 isolates from patients in 12 years and 0 isolate from nebulizers in periodical sampling). In particular, Bcc has been isolated from blood from only one patient since the new methods were introduced.
CONCLUSION: Appropriate maintenance of ultrasonic nebulizers is crucial for preventing Bcc contamination of nebulizers and subsequent respiratory tract and blood infections.
Core tip: In this study, we sought the cause of an outbreak of Burkholderia cepacia complex (Bcc) infection among inpatients using ultrasonic nebulizers and evaluated the efficacy of new methods for nebulizer maintenance introduced following the outbreak. Precise investigation revealed damaged diaphragms in many nebulizers, which we speculated would be the major cause of Bcc contamination of nebulizers and subsequent Bcc infection. The new maintenance methods for nebulizers, including restriction of the usage period, thorough disinfection, and routine check for diaphragm breakage, remarkably reduced Bcc isolation from nebulizers and patients’ samples.
- Citation: Ida Y, Ohnishi H, Araki K, Saito R, Kawai S, Watanabe T. Efficient management and maintenance of ultrasonic nebulizers to prevent microbial contamination. World J Methodol 2016; 6(1): 126-132
- URL: https://www.wjgnet.com/2222-0682/full/v6/i1/126.htm
- DOI: https://dx.doi.org/10.5662/wjm.v6.i1.126
Nebulizer devices are widely used to deliver aerosol therapy, especially in patients with respiratory disease[1,2]. However, nebulizers are potential sources of microbial contamination of the respiratory tract[3,4]. Small-volume medication nebulizers for administering bronchodilators, including hand-held nebulizers, can produce bacterial aerosols[5]. Both jet and ultrasonic hand-held nebulizers have been associated with nosocomial pneumonia[6-9].
The Centers for Disease Control and Prevention (CDC) established guidelines for preventing nosocomial pneumonia in 1997[10]. The guidelines specified that small-volume medication nebulizers should be disinfected, rinsed with sterile water, or air-dried between treatments on the same patient. These guidelines were substantially revised in 2003 to make these procedures (cleaning, disinfecting, rinsing, and air-drying) mandatory for maintaining medication nebulizers between treatments on the same patient[11]. However, there have been no subsequent guidelines offering more detail regarding cleaning and disinfection of nebulizers.
From 1995 to 1996, our tertiary care university hospital experienced two Burkholderia cepacia complex (Bcc) outbreaks associated with microbial contamination of ultrasonic nebulizer solutions[12]. Because the nebulizer solution was identified as the source of contamination, we controlled the outbreaks by replacing the nebulizer solution after each use. However, we again experienced increased numbers of Bcc isolates from sputum and blood culture from 1999 to 2001. This Bcc re-emergence forced us to re-examine the source of microbial contamination in the infected patients and to develop new methods to control the infection.
For this purpose, we compared the number of Bcc isolates from patients and nebulizers before and after introduction of a new disinfection method and analysed the genetic association between these isolates. In addition, we evaluated the efficacy of these new methods to prevent microbial contamination of ultrasonic nebulizers.
Kyorin University Hospital is a tertiary care hospital in Tokyo, Japan, with 1153 beds. From January 1999 to December 2013, we investigated the annual number of Bcc isolates recovered from clinical samples (sputum, blood, catheter, pus, and urine) sent to our clinical laboratory in routine clinical practice. After detecting a yearly increase in Bcc isolates from 1999 to 2001, we used microbial and genetic analyses to examine routes of contamination. In many cases, nebulizers were suspected as probable sources of contamination; we therefore thoroughly investigated nebulizers to discover the main cause of microbial contamination. Furthermore, we developed new methods for maintaining nebulizers and compared the number of Bcc isolates detected before and after introducing the new methods.
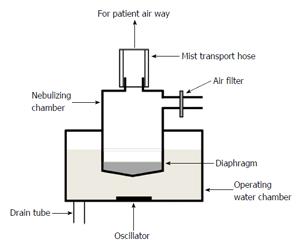
In our hospital, two types of ultrasonic nebulizers, the SONICLIZER 305 (ATOM Co., Tokyo, Japan) and ULTRASONIC NEBULIZER UN-701 (Alfresa Co., Tokyo, Japan), are used for respiratory care. Both nebulizers consist of a mouthpiece, mist transport hoses, nebulizing chamber, diaphragm, operating water chamber, oscillators, drain tubes, and air filter (Figure 1). The nebulizer solution and mouthpiece are single-use, but the other parts are reused. We checked patient medical records to determine whether an ultrasonic nebulizer was used for patients from whom Bcc was isolated. We also investigated the frequency of ultrasonic nebulizer usage and maintenance from 1999 to 2005.
Before August 2001, nurses or helper staff disinfected ultrasonic nebulizer components, including the diaphragm, mist transport hoses, and mouthpiece, according to the manufacturer’s operation instructions. The nebulizer solution was replaced with each use. All ultrasonic nebulizers were maintained in each ward and returned to the medical engineering section only when damaged.
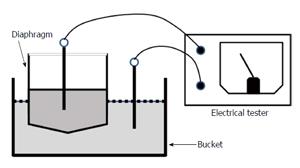
With some modification, the CDC guidelines for preventing nosocomial pneumonia were applied from September 2001[11]. In addition to nurses or helper staff cleaning, disinfecting, rinsing with sterile water, and air-drying nebulizer components, new contamination control measures were implemented as follows: (1) Availability of ultrasonic nebulizers was tightly limited to < 5 d. At that time, the devices were returned to the medical engineering section for maintenance; (2) Nebulizer drain tubes and oscillators were completely disinfected once every 24 h using 85% ethanol; and (3) After each use, nebulizers were surveyed for diaphragm breakage or pinholes using a device that measured electrical resistance (Figure 2).
Swab samples were randomly collected for bacterial culture before patient use from 10 each of the 2 machine types in August 2001; these included 20 samples from each of the following: Drain tubes, operating water chambers, oscillators, and nebulizing chambers. In addition, 10 samples each of nebulizer solutions before and after use were cultured. After the new ultrasonic nebulizer disinfection measures were implemented in September 2001, we performed a bacterial culture survey of drain tubes and oscillators of 10 nebulizer machines before patient use three times between January 2002 and December 2004. For environmental investigation, 10 samples were collected from sinks in the nurse stations of the wards where patients positive for Bcc were hospitalized. Sample solutions were centrifuged for 10 min at 3000 rpm and the resultant pellets processed for culture.
All clinical samples and pellets were inoculated onto 5% sheep blood agar (Oriental Yeast Co., Tokyo Japan) and incubated for 48 h at 35 °C in a humidified atmosphere. Bcc isolates were identified by an analytical profile index procedure using the API 20NE system (API-BioMerieux, La Balme les Grottes, France).
The genetic profiles of Bcc isolates obtained from clinical samples and nebulizers were compared using the random amplified polymorphic DNA (RAPD) assay as described previously[12]. Briefly, total DNA was prepared by boiling, and 50 ng of DNA was subjected to random polymerase chain reaction (PCR) using two PCR primers, RPKHM1 and RPKHM2, synthesized in-house. PCR products were electrophoresed in a 3% agarose gel, and the bands visualized by ultraviolet light.
From January 1999 to December 2013, a total of 487 Bcc isolates were obtained from clinical specimens from 181 patients (Table 1). Retrospective review of medical records revealed that > 90% had used a nebulizer. Notably, 322 (66.1%) Bcc isolates were obtained between January 1999 and December 2001. These included 244 isolates from sputum specimens (115 patients) and 34 from blood specimens (11 patients). After introduction of the new methods of operational management and ultrasonic nebulizer maintenance in 2001, the number of Bcc isolates from clinical specimens decreased dramatically (165 isolates in 12 years). In particular, Bcc has been isolated from blood from only one patient since the new methods were introduced. During the entire study period, the annual number of inpatients did not change remarkably, with a minimum of 291551 and a maximum of 309127 patients.
| No. of isolates in each year | ||||||||||||||||
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total | |
| Sputum | 55 | 66 | 123 | 80 | 18 | 12 | 6 | 10 | 0 | 4 | 5 | 1 | 6 | 3 | 3 | 392 |
| Blood | 7 | 27 | 3 | 37 | ||||||||||||
| Catheter | 3 | 23 | 2 | 1 | 29 | |||||||||||
| Pus | 3 | 1 | 10 | 4 | 2 | 2 | 2 | 1 | 25 | |||||||
| Urine | 4 | 4 | ||||||||||||||
| Total | 65 | 74 | 183 | 82 | 26 | 12 | 8 | 10 | 0 | 4 | 7 | 3 | 6 | 3 | 4 | 487 |
In the August 2001 investigation, 14 Bcc isolates were obtained from 5 nebulizers. Among these, 6 were derived from nebulizer drain tubes, 5 from operating water chambers, 1 from the oscillator before patient use, and 2 from nebulizer solutions after patient use. When Bcc was isolated from the nebulizer solution after patient use, Bcc was simultaneously detected in other parts of the nebulizer. Bcc was not isolated from any nebulizer solution before use. In contrast, after introducing the new ultrasonic nebulizer maintenance methods, Bcc was not isolated from any ultrasonic nebulizer components during three separate time points between January 2002 and December 2004. Bcc was also not isolated from environmental samples from the wards.
Before introducing the new rules in September 2001, the frequency of ultrasonic nebulizer maintenance by medical engineers was < 900 times per year. The average duration of ultrasonic nebulizer usage in each ward was 34.9 d. After introducing the new rules in September 2001, the maintenance frequency increased to > 3500 times annually, and the average duration of usage in wards decreased to 5.1 d.
After September 2001, visual examination of individual nebulizer components during routine maintenance showed obvious breakage in 2 of 20 diaphragms. No remarkable defects were found in other nebulizer components. In addition, routine testing of diaphragms by electrical resistance revealed damage in 34 of 140 nebulizers. After introduction of the new rules, these damaged diaphragms were not used on patients.
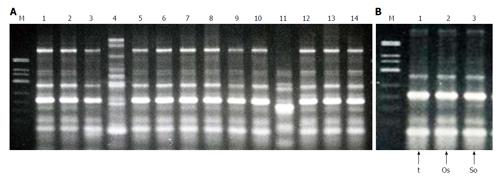
RAPD analysis revealed 15 fingerprint patterns, designated R6 to R20, among 140 isolates randomly chosen from patient specimens collected between January 2000 and July 2003. The genetic profiles of endemic strains from the most recent outbreak differed from the previous outbreak (RAPD profiles R1 to R5) from November 1995 to September 1996 (data not shown)[12]. Among DNA profiles R6 to R20, R6 was most prevalent, detected in 102 isolates (71.4%). The next most prevalent pattern, R7, was detected in 15 isolates (10.7%). Patterns R8 to R10 were detected in 3 isolates each, R11 to R14 in 2 each, and R15 to R20 in 1 each.
Among 14 isolates from nebulizers, 6 fingerprint patterns, R6, R7, R8, R21, R22 and R23, were observed. Four isolates had an R6 pattern; 1 was R7; 6 were R8; and 1 each were R21, R22, and R23 (Figure 3). DNA fingerprint patterns of each component matched those from nebulizer solutions when Bcc was isolated from both samples. Since 2003, we have not performed genotypic analyses of Bcc isolates because isolates were only sporadically recovered from patients in different wards.
This investigation, together with our previous reports regarding a Bcc outbreak, clearly showed that Bcc was harboured in ultrasonic nebulizers and caused respiratory tract infections in patients using them. Bcc, a ubiquitous bacterial species in the natural environment, is capable of surviving and growing in nutrient-poor water[13]. Bcc has been recovered from hospital environments, including sink drains and hospital tap water, medical devices including nebulizers, and a variety of solutions used in clinical practice[14-17]. Therefore, disinfection of ultrasonic nebulizer components after use is important for preventing Bcc contamination. To date, public guidelines contain no precise details regarding methods for disinfecting nebulizers. According to the manufacturers’ instructions, the components, including oscillators, should be wiped and disinfected with a 0.1%-0.5% benzalkonium chloride aqueous solution. A previous study demonstrated that disinfection at 24-h interval is indispensable when nebulization solutions not containing preservatives are used[4]. However, because of their complicated structures, nebulizer components are difficult to keep dry, and wiping with disinfectant detergent might lead to incomplete disinfection. In addition, nebulizers may be used on additional patients before the oscillator is completely dried and disinfected, as is the situation in our tertiary care hospital where frequent nebulizer use is required. Furthermore, Bcc can form biofilms[18]. Biofilms on nebulizer components may interfere with effective disinfection of Bcc.
In the present study, prior to introducing new measures for operational management and maintenance of nebulizers, Bcc was isolated from drain tubes and oscillators before use and from the nebulizer solution after use, but not from the nebulizer solution before use. These data suggest that Bcc initially contaminated nebulizer components, with secondary contamination of the nebulizer solution. Notably, diaphragm breakage or pinholes were found in multiple nebulizers using a precise investigation. Diaphragm breakage can allow microorganisms to invade the nebulizing chamber and contaminate the nebulizer solution. Thus, we speculated that small diaphragm breakage or pinholes led to Bcc contamination of the nebulizer solution, causing respiratory tract infection in patients using the nebulizers. The diaphragm in an ultrasonic nebulizer is typically reused, and the thickness of its plastic bottom decreases with continuous ultrasonic wave pressure. Small diaphragm breakage and pinholes may be overlooked by visual inspection. Therefore, we introduced new methods using electrical devices to check for diaphragm breakage. In addition, the average routine medical engineer maintenance interval was shortened from 34.9 to 5.1 d. Since introducing these new methods, the number of Bcc isolates from clinical specimens has decreased dramatically. These findings suggest that our new rules for maintaining nebulizers are effective in preventing nosocomial respiratory infection by Bcc.
While Bcc is considered of relatively low virulence and believed to rarely cause invasive disease, several studies have reported this microorganism to be an important infectious agent, causing bacteraemia with substantial clinical impact[19]. Jang et al[20] performed a prospective epidemiologic analysis of 147 nosocomial gram-negative bacteraemia episodes among intensive care units patients. The Bcc isolation rate was second only to Acinetobacter baumannii, and the most frequent primary infection site was the lower respiratory tract in the patients with bacteraemia. Although we could not clarify the source of Bcc bacteraemia in the present study, a substantial number of isolates were recovered from blood culture along with increased sputum isolates and Bcc isolation from multiple nebulizer components between 1999 and 2001. After introducing new methods for maintaining nebulizers, no Bcc bacteraemia was detected, in concordance with the absence of Bcc in nebulizer samples. These results suggest that disinfecting nebulizers is crucial for preventing Bcc bacteraemia and subsequent respiratory tract infections in patients using nebulizers.
Previous studies have verified that RAPD is a powerful tool for identifying routes of microbial infection, including Bcc, in nosocomial infections[21,22]. In the present study, genotypes of Bcc isolated from nebulizer components were similar to isolates from patients using nebulizers, but distinct from those from the previously recognized Bcc outbreak. These data suggest that contamination of nebulizer components is responsible for respiratory and bloodstream infections by Bcc in these patients. This observation confirms our previous report that the RAPD assay is useful for identifying the source of nosocomial Bcc infection.
Our investigation confirmed that nebulizers are important sources of Bcc contamination, which causes respiratory tract infection and subsequent bacteraemia. Our findings suggest that appropriate operational management and ultrasonic nebulizer maintenance are crucial for preventing microbial contamination of nebulizers and subsequent respiratory tract and bloodstream infections. Furthermore, RAPD is a powerful tool for identifying routes of nosocomial Bcc infection.
We are grateful to Dr. Mitsuhiro Okazaki for his excellent technical assistance.
Nosocomial infection is a ubiquitous problem in healthcare facilities. An ultrasonic nebulizer is one of the potential sources of microbial contamination of the respiratory tract and subsequent infection of lung and blood stream among the patients using this equipment. However, public guidelines containing precise details regarding methods for disinfecting nebulizers have yet been established to date. Therefore, proper methods for management of usage and disinfection of nebulizers need to be developed.
The Centers for Diseases Control and Prevention established guidelines for preventing nosocomial pneumonia in 1997 and revised them in 2003. In the guidelines, cleaning, disinfecting, rinsing, and air-drying of the nebulizers are prescribed to be mandatory between treatments on the same patient. However, the clinical relevance of these procedures has yet been tested in clinical settings.
A few studies have addressed the issues regarding bacterial contamination of nebulizers, and no detailed procedure except for the frequent disinfection has been emphasized in the previous studies. The present study specified breakage of diaphragm as an important cause for bacterial contamination of nebulizers. Furthermore, the efficient method for detecting breakage of diaphragm using an electrical device has been developed in this study.
This study allows readers to perform appropriate maintenance and disinfection of ultrasonic nebulizers, and will contribute to the decrease of nosocomial infection of respiratory tract and blood stream, at least that by Burkholderia cepacia complex (Bcc) which is described as a main pathogen transmitted by nebulizers in this study.
Burkholderia cepacia is a gram-negative rod previously known as Pseudomonas cepacia. While Bcc is considered of relatively low virulence and believed to rarely cause invasive disease, several studies have reported this microorganism to be an important infectious agent, causing bacteraemia with substantial clinical impact.
This is interesting and well written article, which may be a useful source of knowledge for all clinicians, because nosocomial infections are an important problem of contemporary clinical practice. The research is well designed and experimental part is described in detail.
P- Reviewer: Bugaj AM, Perrault LP S- Editor: Kong JX L- Editor: A E- Editor: Liu SQ
| 1. | Hess D, Fisher D, Williams P, Pooler S, Kacmarek RM. Medication nebulizer performance. Effects of diluent volume, nebulizer flow, and nebulizer brand. Chest. 1996;110:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Michalopoulos A, Metaxas EI, Falagas ME. Aerosol delivery of antimicrobial agents during mechanical ventilation: current practice and perspectives. Curr Drug Deliv. 2011;8:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Mastro TD, Fields BS, Breiman RF, Campbell J, Plikaytis BD, Spika JS. Nosocomial Legionnaires’ disease and use of medication nebulizers. J Infect Dis. 1991;163:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Oie S, Makieda D, Ishida S, Okano Y, Kamiya A. Microbial contamination of nebulization solution and its measures. Biol Pharm Bull. 2006;29:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Guideline for prevention of nosocomial pneumonia. Centers for Disease Control and Prevention. Respir Care. 1994;39:1191-1236. [PubMed] |
| 6. | Ringrose RE, McKown B, Felton FG, Barclay BO, Muchmore HG, Rhoades ER. A hospital outbreak of Serratia marcescens associated with ultrasonic nebulizers. Ann Intern Med. 1968;69:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Cobben NA, Drent M, Jonkers M, Wouters EF, Vaneechoutte M, Stobberingh EE. Outbreak of severe Pseudomonas aeruginosa respiratory infections due to contaminated nebulizers. J Hosp Infect. 1996;33:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Monforte V, Román A, Gavaldà J, Bravo C, Rodriguez V, Ferrer A, Pahissa A, Morell F. Contamination of the nebulization systems used in the prophylaxis with amphotericin B nebulized in lung transplantation. Transplant Proc. 2005;37:4056-4058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Schultsz C, Meester HH, Kranenburg AM, Savelkoul PH, Boeijen-Donkers LE, Kaiser AM, de Bree R, Snow GB, Vandenbroucke-Grauls CJ. Ultra-sonic nebulizers as a potential source of methicillin-resistant Staphylococcus aureus causing an outbreak in a university tertiary care hospital. J Hosp Infect. 2003;55:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Guidelines for prevention of nosocomial pneumonia. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997;46:1-79. [PubMed] |
| 11. | Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1-36. [PubMed] |
| 12. | Okazaki M, Watanabe T, Morita K, Higurashi Y, Araki K, Shukuya N, Baba S, Watanabe N, Egami T, Furuya N. Molecular epidemiological investigation using a randomly amplified polymorphic DNA assay of Burkholderia cepacia isolates from nosocomial outbreaks. J Clin Microbiol. 1999;37:3809-3814. [PubMed] |
| 13. | Goldmann DA, Klinger JD. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986;108:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Martone WJ, Tablan OC, Jarvis WR. The epidemiology of nosocomial epidemic Pseudomonas cepacia infections. Eur J Epidemiol. 1987;3:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Döring G, Jansen S, Noll H, Grupp H, Frank F, Botzenhart K, Magdorf K, Wahn U. Distribution and transmission of Pseudomonas aeruginosa and Burkholderia cepacia in a hospital ward. Pediatr Pulmonol. 1996;21:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Nasser RM, Rahi AC, Haddad MF, Daoud Z, Irani-Hakime N, Almawi WY. Outbreak of Burkholderia cepacia bacteremia traced to contaminated hospital water used for dilution of an alcohol skin antiseptic. Infect Control Hosp Epidemiol. 2004;25:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Lucero CA, Cohen AL, Trevino I, Rupp AH, Harris M, Forkan-Kelly S, Noble-Wang J, Jensen B, Shams A, Arduino MJ. Outbreak of Burkholderia cepacia complex among ventilated pediatric patients linked to hospital sinks. Am J Infect Control. 2011;39:775-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Huber B, Riedel K, Köthe M, Givskov M, Molin S, Eberl L. Genetic analysis of functions involved in the late stages of biofilm development in Burkholderia cepacia H111. Mol Microbiol. 2002;46:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Lu DC, Chang SC, Chen YC, Luh KT, Lee CY, Hsieh WC. Burkholderia cepacia bacteremia: a retrospective analysis of 70 episodes. J Formos Med Assoc. 1997;96:972-978. [PubMed] |
| 20. | Jang TN, Kuo BI, Shen SH, Fung CP, Lee SH, Yang TL, Huang CS. Nosocomial gram-negative bacteremia in critically ill patients: epidemiologic characteristics and prognostic factors in 147 episodes. J Formos Med Assoc. 1999;98:465-473. [PubMed] |
| 21. | Cartelle M, del Mar Tomas M, Pertega S, Beceiro A, Dominguez MA, Velasco D, Molina F, Villanueva R, Bou G. Risk factors for colonization and infection in a hospital outbreak caused by a strain of Klebsiella pneumoniae with reduced susceptibility to expanded-spectrum cephalosporins. J Clin Microbiol. 2004;42:4242-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Bonfim-Mendonça Pde S, Fiorini A, Shinobu-Mesquita CS, Baeza LC, Fernandez MA, Svidzinski TI. Molecular typing of Candida albicans isolates from hospitalized patients. Rev Inst Med Trop Sao Paulo. 2013;55:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |