Published online Dec 26, 2015. doi: 10.5662/wjm.v5.i4.223
Peer-review started: January 30, 2015
First decision: March 6, 2015
Revised: March 24, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: December 26, 2015
Processing time: 319 Days and 17.6 Hours
The transcription factor forkhead box protein A2 (FOXA2, also known as hepatocyte nuclear factor 3β or transcription factor 3β), has been found to play pivotal roles in multiple phases of mammalian life, from the early development to the organofaction, and subsequently in homeostasis and metabolism in the adult. In the embryonic development period, FOXA2 is require d for the formation of the primitive node and notochord, and its absence results in embryonic lethality. Moreover, FOXA2 plays an important role not only in lung development, but also in T helper type 2 (Th2)-mediated pulmonary inflammation and goblet cell hyperplasia. In this article, the role of FOXA2 in lung development and Th2-mediated pulmonary inflammation, as well as in goblet cell hyperplasia, is reviewed. FOXA2 deletion in airway epithelium results into Th2-mediated pulmonary inflammation and goblet cell hyperplasia in developing lung. Leukotriene pathway and signal transducers and activators of transcription 6 pathway may mediate this inflammation through recruitment and activation of denditric cell during lung developments. FOXA2 is a potential treatment target for lung diseases with Th2 inflammation and goblet cell hyperplasia, such as asthma and chronic obstructive pulmonary disease.
Core tip: The transcription factor forkhead box protein A2 (FOXA2) plays pivotal roles in embryonic development and organogenesis. Conditional deletion of FOXA2 in airway epithelial cells during the early stage of lung development will result in abnormal morphology of the lung and T helper type 2-mediated pulmonary inflammation. In addition, FOXA2 regulates the goblet cell differentiation during lung development and in pulmonary diseases such as asthma and chronic obstructive pulmonary disease. FOXA2 may be a new target for the treatment of lung disease.
- Citation: Sun L, Tang XJ, Luo FM. Forkhead box protein A2 and T helper type 2-mediated pulmonary inflammation. World J Methodol 2015; 5(4): 223-229
- URL: https://www.wjgnet.com/2222-0682/full/v5/i4/223.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i4.223
The transcription factor forkhead box protein A2 (FOXA2), also known as hepatocyte nuclear factor 3β (HNF3β), is first identified by its ability to regulate liver-specific gene expression[1]. FOXA2 is a part of the large Forkhead box (FOX) gene family that all members have the DNA binding “winged helix domain”[2]. The gene of FOXA2 is located in chromosome 20p11.21. FOXA2 is able to bind to specific DNA sequence[3], activate or inhibit the transcriptional activity of target genes, and also participate in cellular signal transduction[4] and metabolism regulation[5]. Meanwhile, it plays a key role in the development[6] and mature of tissues and organs[7].
With the development of mouse embryos, the first active FOXA gene is FOXA2 whose RNA and protein are detected on day 6.5 of gestation in the primitive streak and node[8], suggesting that FOXA2 plays an essential role in the formation of the primitive streak and endoderm[9]. The research has indicated that FOXA2 is required for the maintenance of dopaminergic properties in ventral midbrain neurons at late embryonic stage[10]. The expression of FOXA2 is also found in the liver, pancreas, lung, intestine, thyroid gland and prostate[11], implying that FOXA2 not only regulates the organogenesis and development of liver[7,12,13] and lung[14,15], but also participates in the process of glucose[16] and lipid metabolism[17]. Many studies have also shown that FOXA2 has a close relationship with the occurrence and metastasis of tumor[18-20]. For the past few years, FOXA2 is found to participate in regulating the lung development, Th2-mediated pulmonary inflammation and goblet cell hyperplasia[21,22].
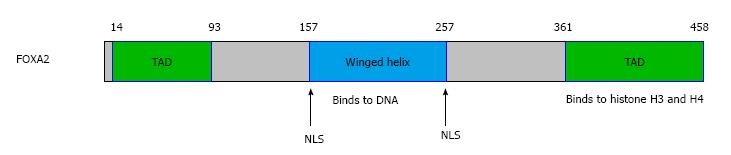
FOXA2 gene is located in chromosome 20p11.21 and its length is 2242 bp. As a member of the FOXA family, FOXA2 has a forkhead domain (FHD) complexed to a target DNA. The first 3D structure of FHD resolved by X-ray crystallography was that of FOXA3/HNF3γ in 1993[3]. Subsequently, the FHD structures of other members were resolved, which are similar to that of FOXA3[23], so is FOXA2. The FHD contains three N-terminal α-helices (H1-3), three β-strands and two loops (W1-2) towards its C-terminal region[24]. The recent data about the FOXA function have identified the FOXA proteins as “pioneer factors” whose binding to promoters and enhancers enable chromatin access for other factors[25-27]. It is unique in that FOXA2 is the only one in the family which contains an AKT2/PKB phosphorylation site at the N terminus of the FHD[27]. FOXA2 has two nuclear localization sequences (NLS) which are located at both ends of the FHD[28], one of the two NLS in H1 while the other in W2[24] (Figure 1).
Research has shown that the FOX superfamily express in many kinds of organism from invertebrate to vertebrate and its subfamily FOXA participants in the whole process of the embryonic development[29]. FOXA2, a member of FOXA family, is the first gene of this family to be expressed in the progress of embryogenesis[30]. In the study of mouse embryogenesis, the expression of the FOXA2 gene appears first at the anterior of the primitive streak. After the primitive node has formed, FOXA2 expression is localized in the primitive node, notochord and neural plate[31]. Mice lacking FOXA2 die by E10 to E11 and show marked defects in structures related to embryogenesis, without forming a distinct primitive node, aberrant somites and neural tube resulting from the absence of the notochord, and failure to form the gut tube, although endoderm cells are present[6]. The defects of the notochord and neural tube, can be ascribed to a deficiency of Sonic hedgehog, as FOXA2 cooperates with the homeobox gene Goosecoid in the activation of this gene[32]. Furthermore, FOXA2 can activate the canonical WNT-β-catenin pathway and subsequently induce the primitive extraembryonic endoderm by directly upregulating the Wnt6[33].
With the embryonic development, the expression of FOXA2 is also detected in definitive endoderm and endoderm-derived apparatus such as liver, pancreas, and prostatic gland, where it persists through development to adulthood[30,31,33-36]. As to the lung, FOXA2 expresses in the endoderm which later differentiates into the lung buds, where it expresses continuously in the pulmonary epithelium to adulthood[37]. FOXA2 is found in specific subsets of respiratory epithelial cells. In the respiratory epithelium, FOXA2 can activate the transcription of thyroid transcription factor-1 (TTF-1), clara cell secretary protein and surfactant proteins (A-D), which mark the differentiation of epithelial cells[38,39]. Moreover, the surfactant proteins A-D play critical roles in surfactant function and homeostasis[40]. In the mice lacking FOXA2 in conducting airways, pulmonary abnormalities are not observed by light microscopy at E18.5. However, the decreased alveolar septation and peripheral saccules appear at PN3. At PN15 and later, pulmonary abnormalities including emphysema in distal airways and goblet cell hyperplasia in bronchi and bronchioles are observed in the FOXA2∆/∆ mice[15,41]. FOXA2 is indeed a positive regulator for E-cadherin gene[42], a cell adhesion molecule required for normal lung branching morphogenesis and cell differentiation[43,44]. Mildred et al[45] verified that the temporal-spatial expression patterns of FOXA2 in the developing and regenerating of lung fit in with their proposed function in epithelial cell differentiation and regeneration, and surfactant protein gene expression. In summary, FOXA2 plays a critical role in the development of lung.
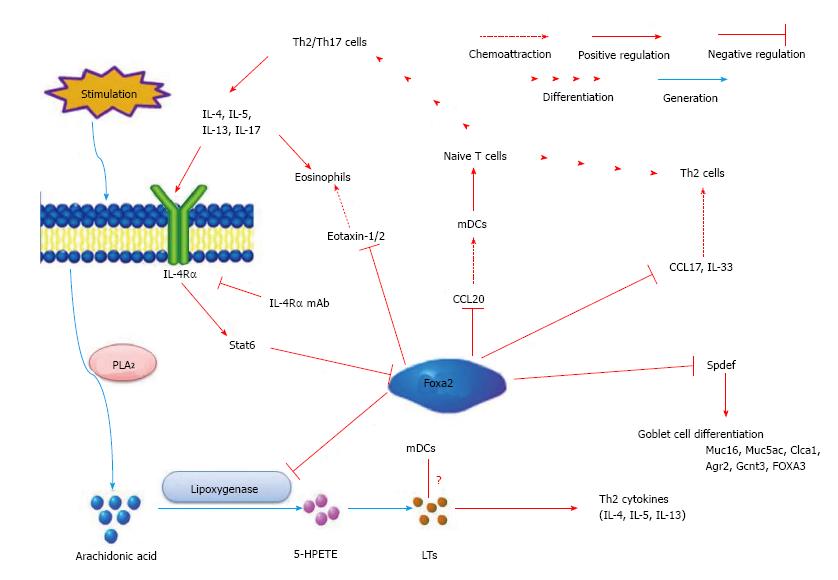
Evidences showed that respiratory epithelial cells lining conducting airways regulate the inflammatory responses caused by allergens, pathogens and injurious agents[46,47]. Disruption of FOXA2 in respiratory epithelial cells results in airspace enlargement, neutrophilic pulmonary infiltrates, mucus hypersecretion, goblet cell hyperplasia and metaplasia (GCHM)[15], associated with the activation of pro-GCHM Stat6 and epidermal growth factor receptor signaling pathways[15,21,48]. Further study demonstrates that lack of FOXA2 in airway epithelial cells results in Th2-mediated pulmonary inflammation, including infiltration of eosinophiles, up-regulation of Th2 cytokines and chemokines, goblet cell hyperplasia and mucus hypersecretion, accompanied by the activation of leukotriene pathway at PN15[21]. All these findings are common in the lung of asthma patients. Therefore, FOXA2 expression in the lung may be disturbed in asthma. In fact, decreased expression of FOXA2 in the lung is found in asthma patients compared with control subjects[49]. The decreased expression of FOXA2 was negatively correlated with increased expression of mucin-5ac (MUC5ac) and chloride channel accessory 1[49]. Furthermore, the expression of FOXA2 in airway epithelial cell is inhibited by allergen challenge, and by over-expression of Th2 cytokines such as interleukin (IL)-4 and IL-13 in mouse airway epithelium[15], and also by IL-13 stimulation in human bronchial epithelial cells[48]. All these results indicate that FOXA2 plays a critical role in Th2-mediated pulmonary inflammation in developing lung. Although it inhibits goblet cell hyperplasia and metaplasia, conditional over-expression of FOXA2 in the respiratory epithelium in adult mice prior to ovalbumin (OVA) sensitization cannot alter Th2 cytokine production or inflammation in the lung[21]. Inflammatory cell counts, as well as IL-4, IL-5, IL-13, IL-10, and interferon-γ concentrations, are similar in bronchoalveolar lavage fluid (BALF) from FOXA2 over-expressing and control mice after OVA exposure[21]. Tang et al[22] also demonstrate that in the very early stage (from PN0 to PN10) of lung development after birth, Th2-mediated inflammation is missing in the lung of mice with FOXA2 deletion in airway epithelium. The Th2 related cytokines and chemokines are up-regulated from PN7 and Th2 inflammation in the lung is obvious on PN15. All the results indicate that Th2-mediated pulmonary inflammation induced by deletion of FOXA2 in airway epithelial cells is development-depended.
The mechanism of Th2-mediated pulmonary inflammation induced by deletion of FOXA2 in airway epithelial cells remains unknown at present. Dendritic cell (DC) plays a very important role in Th2-mediated pulmonary inflammation. Chen et al[21] investigates the role of myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) in this inflammation. They found that the frequency of both DCs and mDC/pDC ratio are significant increased in the lung of FOXA2∆/∆ mice[21]. They also found that frequencies of mDCs expressing B7-DC, B7-H1, and CD86 are significantly elevated[21]. The results indicate that increased recruitment and activation of pulmonary mDCs may mediate the Th2 inflammation in the lung in FOXA2∆/∆ mice during development. However, the mechanism of the recruitment and activation of pulmonary mDCs after FOXA2 deletion in airway epithelial cells is not clear. Previous studies have provided direct evidence that cysteinyl leukotrienes (cys-LTs) plays an important role in regulating Th2 cell-dependent pulmonary inflammation[50,51]. Further study discloses that FOXA2 regulates 15-lipoxygenase (Alox15) and Alox5 gene transcription associating with leukotrienes (LTs) biosynthesis and lung inflammation[52,53]. Montelukast, a selective inhibitor of the CysLT1 receptor[54,55], suppresses the Th2-mediated inflammation arising from the ablation of FOXA2 in the developing mice lung. In developing FOXA2∆/∆ mice, the increased expression of Th2 cytokines followed the activation of LT pathway. In brief, these findings uncover that FOXA2 is required for the repression of Th2-mediated pulmonary inflammation during lung development via its regulation to CysLT pathway[22]. Therefore, deletion of FOXA2 in the early stage of lung development leads to the spontaneous activation of LTs pathway. The activated LTs pathway may increase the recruitment and activation of pulmonary mDCs and then mediate the Th2 inflammation in the lung of FOXA2∆/∆ mice during development. However, this hypothesis needs more direct evidences (Figure 2).
IL-13/IL-4-STAT6 pathway plays a critical role in Th2-mediated pulmonary inflammation[56,57]. Chen et al[21] tests whether Th2-mediated pulmonary inflammation and goblet cell differentiation caused by conditional deletion of FOXA2 in the airway epithelium is depend upon IL-4R–mediated signaling. The results indicate that administration of IL-4Rα mAb, an antibody which blocks IL-4Rα (a key molecular in IL-13/IL-4-STAT6 signaling pathway), significantly inhibits eosinophilic inflammation and goblet cell metaplasia and mucus hyper-production in FOXA2∆/∆ mice. These results indicates that IL-4Rα-STAT6 pathway mediated the Th2 pulmonary inflammation and goblet cell hyperplasia in FOXA2∆/∆ mice during lung development[21]. Wan et al[15] found that intratracheal administration IL-4 resulted in the decrease expression of FOXA2 and this effect is STAT6-depended. However, over-expression of FOXA2 in airway epithelium of adult mice inhibis goblet cell metaplasia and mucus hyper-production caused by OVA, but not Th2-mediated pulmonary inflammation[21]. These results indicate that the interaction between FOXA2 and IL-13/IL-4-STAT6 signaling pathway may be reciprocal in Th2-mediated inflammation and goblet cell hyperplasia in the lung.
FOXA2 in airway epithelial cells plays important role in lung development and Th2-mediated pulmonary inflammation, as well as in goblet cell hyperplasia. Therefore, regulation of FOXA2 expression in airway epithelial cells may have potential role in the pathogenesis and treatment of lung diseases, such as asthma and chronic obstructive pulmonary disease (COPD). Unfortunately, No medicine up-regulating FOXA2 expression has been investigated in animal model or patients with COPD and asthma. Recent study indicated that Tetrapeptide Ala-Asp-Glu-Leu, a peptide which is effective on models of acute bacterial lung inflammation, fibrosis, and toxic lung damage, could increase the expression of FOXA2 and decrease expression of MUC5ac in cultured bronchial epithelium[58]. Whether this peptide has the same effect in vivo has not been tested yet.
Thioredoxin-interacting protein (TXNIP) increases the expression of human islet amyloid polypeptide (IAPP) in beta-cell. TXNIP-induced FOXA2 transcription factor expression is conferring this effect by promoting FOXA2 enrichment at the proximal FOXA2 site in the IAPP promoter[59]. TXNIP can down-regulate miR-124a expression, which can directly target FOXA2. Indeed, miR-124a overexpression led to decreased FOXA2 expression and also can be effectively inhibited by TXNIP[59]. Thus, this study identifies a novel TXNIP/miR-124a/FOXA2/IAPP signaling cascade linking the critical beta-cell signaling pathway. However, whether this pathway also plays a role in airway epithelial cells and thus regulates the goblet cell hyperplasia and mucus production remains unknown.
Recent study demonstrates that over expression of NK2 homeobox 1 (NKX2-1, also known as TTF-1), inhibits allergen-induced goblet cell hyperplasia and airway inflammation[60]. Further study indicates that loss of FOXA2 in airway epithelial cell is prevented by over expression of NKX2-1 at the same time[60]. All these results suggest that NKX2-1 may regulate the FOXA2 expression in airway epithelial cell.
In conclusion, as a member of the FOX superfamily, FOXA2 participates in the formation and development of organs. Meanwhile, FOXA2 plays a very important role in lung development, Th2-mediated pulmonary inflammation and goblet cell hyperplasia. Lose of FOXA2 in the early stage of lung development will result in abnormal morphology of the lung and Th2-mediated pulmonary inflammation. FOXA2 regulates the goblet cell differentiation during lung development and in pulmonary diseases such as asthma and COPD. LTs pathway and STAT6 pathway which are regulated by FOXA2 mediate the Th2 pulmonary inflammation and goblet cell hyperplasia. Moreover, other transcription factors, such as NXK-2-1, may cooperate with FOXA2 in lung development, Th2-mediated pulmonary inflammation, and also in lung diseases with goblet cell hyperplasia.
P- Reviewer: Ciccone MM, Iyngkaran P, Peng Y S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Lai E, Prezioso VR, Smith E, Litvin O, Costa RH, Darnell JE. HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev. 1990;4:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 386] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Lai E, Clark KL, Burley SK, Darnell JE. Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: a family of transcription factors of diverse biologic function. Proc Natl Acad Sci USA. 1993;90:10421-10423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 255] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1005] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Maruyama R, Grevengoed E, Stempniewicz P, Andrew DJ. Genome-wide analysis reveals a major role in cell fate maintenance and an unexpected role in endoreduplication for the Drosophila FoxA gene Fork head. PLoS One. 2011;6:e20901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 613] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 452] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Ruiz i Altaba A, Prezioso VR, Darnell JE, Jessell TM. Sequential expression of HNF-3 beta and HNF-3 alpha by embryonic organizing centers: the dorsal lip/node, notochord and floor plate. Mech Dev. 1993;44:91-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 767] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 10. | Stott SR, Metzakopian E, Lin W, Kaestner KH, Hen R, Ang SL. Foxa1 and foxa2 are required for the maintenance of dopaminergic properties in ventral midbrain neurons at late embryonic stages. J Neurosci. 2013;33:8022-8034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Clevidence DE, Overdier DG, Tao W, Qian X, Pani L, Lai E, Costa RH. Identification of nine tissue-specific transcription factors of the hepatocyte nuclear factor 3/forkhead DNA-binding-domain family. Proc Natl Acad Sci USA. 1993;90:3948-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 190] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Xu C, Lu X, Chen EZ, He Z, Uyunbilig B, Li G, Ma Y, Hui L, Xie B, Gao Y. Genome-wide roles of Foxa2 in directing liver specification. J Mol Cell Biol. 2012;4:420-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb Symp Quant Biol. 2008;73:119-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Porter JL, Bukey BR, Geyer AJ, Willnauer CP, Reynolds PR. Immunohistochemical detection and regulation of α5 nicotinic acetylcholine receptor (nAChR) subunits by FoxA2 during mouse lung organogenesis. Respir Res. 2011;12:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Pandey AK, Bhardwaj V, Datta M. Tumour necrosis factor-alpha attenuates insulin action on phosphoenolpyruvate carboxykinase gene expression and gluconeogenesis by altering the cellular localization of Foxa2 in HepG2 cells. FEBS J. 2009;276:3757-3769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | von Meyenn F, Porstmann T, Gasser E, Selevsek N, Schmidt A, Aebersold R, Stoffel M. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab. 2013;17:436-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Tang Y, Shu G, Yuan X, Jing N, Song J. FOXA2 functions as a suppressor of tumor metastasis by inhibition of epithelial-to-mesenchymal transition in human lung cancers. Cell Res. 2011;21:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Birnbaum DJ, Adélaïde J, Mamessier E, Finetti P, Lagarde A, Monges G, Viret F, Gonçalvès A, Turrini O, Delpero JR. Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 2011;50:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/β-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30:1868-1879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Chen G, Wan H, Luo F, Zhang L, Xu Y, Lewkowich I, Wills-Karp M, Whitsett JA. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol. 2010;184:6133-6141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Tang X, Liu XJ, Tian C, Su Q, Lei Y, Wu Q, He Y, Whitsett JA, Luo F. Foxa2 regulates leukotrienes to inhibit Th2-mediated pulmonary inflammation. Am J Respir Cell Mol Biol. 2013;49:960-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 676] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 24. | Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: key players in health and disease. Trends Genet. 2011;27:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1182] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 26. | Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes Dev. 2009;23:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 395] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 28. | Romanelli MG, Tato’ L, Lorenzi P, Morandi C. Nuclear localization domains in human thyroid transcription factor 2. Biochim Biophys Acta. 2003;1643:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Wang DY, Kumar S, Hedges SB. Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc Biol Sci. 1999;266:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 250] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Monaghan AP, Kaestner KH, Grau E, Schütz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567-578. [PubMed] |
| 31. | Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47-59. [PubMed] |
| 32. | Filosa S, Rivera-Pérez JA, Gómez AP, Gansmuller A, Sasaki H, Behringer RR, Ang SL. Goosecoid and HNF-3beta genetically interact to regulate neural tube patterning during mouse embryogenesis. Development. 1997;124:2843-2854. [PubMed] |
| 33. | Hwang JT, Kelly GM. GATA6 and FOXA2 regulate Wnt6 expression during extraembryonic endoderm formation. Stem Cells Dev. 2012;21:3220-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Sasaki H, Hogan BL. Enhancer analysis of the mouse HNF-3 beta gene: regulatory elements for node/notochord and floor plate are independent and consist of multiple sub-elements. Genes Cells. 1996;1:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Yasui K, Sasaki H, Arakaki R, Uemura M. Distribution pattern of HNF-3beta proteins in developing embryos of two mammalian species, the house shrew and the mouse. Dev Growth Differ. 1997;39:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809-13816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci USA. 2004;101:14449-14454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Ikeda K, Shaw-White JR, Wert SE, Whitsett JA. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol Cell Biol. 1996;16:3626-3636. [PubMed] |
| 40. | Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 41. | Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277-8290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Ye H, Kelly TF, Samadani U, Lim L, Rubio S, Overdier DG, Roebuck KA, Costa RH. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol Cell Biol. 1997;17:1626-1641. [PubMed] |
| 44. | Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev Biol. 2009;333:238-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 45. | Stahlman MT, Gray ME, Whitsett JA. Temporal-spatial distribution of hepatocyte nuclear factor-3beta in developing human lung and other foregut derivatives. J Histochem Cytochem. 1998;46:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 294] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 47. | Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 49. | Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, Huang X, Woodruff PG, Fahy JV, Erle DJ. Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am J Respir Crit Care Med. 2009;180:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176:4440-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789-798; quiz 799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Andersson CK, Claesson HE, Rydell-Törmänen K, Swedmark S, Hällgren A, Erjefält JS. Mice lacking 12/15-lipoxygenase have attenuated airway allergic inflammation and remodeling. Am J Respir Cell Mol Biol. 2008;39:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Rådmark O, Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. J Lipid Res. 2009;50 Suppl:S40-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Profita M, Sala A, Bonanno A, Siena L, Ferraro M, Di Giorgi R, Montalbano AM, Albano GD, Gagliardo R, Gjomarkaj M. Cysteinyl leukotriene-1 receptor activation in a human bronchial epithelial cell line leads to signal transducer and activator of transcription 1-mediated eosinophil adhesion. J Pharmacol Exp Ther. 2008;325:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Meliton AY, Munoz NM, Leff AR. Blockade of avidity and focal clustering of beta 2-integrin by cysteinyl leukotriene antagonism attenuates eosinophil adhesion. J Allergy Clin Immunol. 2007;120:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Hacha J, Tomlinson K, Maertens L, Paulissen G, Rocks N, Foidart JM, Noel A, Palframan R, Gueders M, Cataldo DD. Nebulized anti-IL-13 monoclonal antibody Fab’ fragment reduces allergen-induced asthma. Am J Respir Cell Mol Biol. 2012;47:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev. 2010;19:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 58. | Khavinson VKh, Tendler SM, Vanyushin BF, Kasyanenko NA, Kvetnoy IM, Linkova NS, Ashapkin VV, Polyakova VO, Basharina VS, Bernadotte A. Peptide regulation of gene expression and protein synthesis in bronchial epithelium. Lung. 2014;192:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J Biol Chem. 2014;289:11807-11815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR, Howarth PH, Davies DE, Holgate ST, Whitsett JA. Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation. Am J Respir Crit Care Med. 2011;184:421-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |