Published online Jun 26, 2015. doi: 10.5662/wjm.v5.i2.68
Peer-review started: November 6, 2014
First decision: January 20, 2015
Revised: February 20, 2015
Accepted: May 26, 2015
Article in press: May 28, 2015
Published online: June 26, 2015
Processing time: 243 Days and 23.6 Hours
Although myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS) are considered to be synonymous, the definitional criteria for ME and CFS define two distinct, partially overlapping, clinical entities. ME, whether defined by the original criteria or by the recently proposed criteria, is not equivalent to CFS, let alone a severe variant of incapacitating chronic fatigue. Distinctive features of ME are: muscle weakness and easy muscle fatigability, cognitive impairment, circulatory deficits, a marked variability of the symptoms in presence and severity, but above all, post-exertional “malaise”: a (delayed) prolonged aggravation of symptoms after a minor exertion. In contrast, CFS is primarily defined by (unexplained) chronic fatigue, which should be accompanied by four out of a list of 8 symptoms, e.g., headaches. Due to the subjective nature of several symptoms of ME and CFS, researchers and clinicians have questioned the physiological origin of these symptoms and qualified ME and CFS as functional somatic syndromes. However, various characteristic symptoms, e.g., post-exertional “malaise” and muscle weakness, can be assessed objectively using well-accepted methods, e.g., cardiopulmonary exercise tests and cognitive tests. The objective measures acquired by these methods should be used to accurately diagnose patients, to evaluate the severity and impact of the illness objectively and to assess the positive and negative effects of proposed therapies impartially.
Core tip: The diagnostic criteria for myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS) define two distinct clinical entities. Cognitive impairment and post-exertional “malaise” (a long-lasting aggravation of typical symptoms, e.g., muscle weakness and cognitive “brain fog”, after minor exertion) are obligatory for the diagnosis ME, while chronic fatigue is the only mandatory symptom for the diagnosis CFS. There is debate about the nature and severity of the symptoms in ME and CFS. For clinical and research purposes it is essential to accurately diagnose patients using objective tests for characteristic symptoms if possible. This article reviews accepted methods to assess various distinctive symptoms of ME and CFS.
- Citation: Twisk FN. Accurate diagnosis of myalgic encephalomyelitis and chronic fatigue syndrome based upon objective test methods for characteristic symptoms. World J Methodol 2015; 5(2): 68-87
- URL: https://www.wjgnet.com/2222-0682/full/v5/i2/68.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i2.68
There is debate about various aspects of myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS), including the nature of the symptoms, the etiology, the pathophysiology and presumed effective interventions, e.g., cognitive behavioural therapy (CBT) and graded exercise therapies (GET)[1].
In light of the dispute about the origin of the symptoms, it is essential to assess the presence and severity of characteristic symptoms, and the impact and the disability in ME and CFS impartially as much as possible[2]. In the context of disability, it is important to establish physiological limitations in a specific patient objectively[3], independently of ones view on the etiology and the pathophysiology of ME and CFS.
To date diagnosis, symptom assessment and patient selection criteria of research studies of ME and CFS are often based upon self-report, questionnaires and subjective measures, e.g., fatigue severity and impact. However, well-accepted methods can provide objective measures which can be used to diagnose patients more accurately. This article reviews relevant methods in this context.
ME/CFS is often initiated by an infection or another immunological insult[4]. Full recovery from ME/CFS seems rare (5%[5], 12%[6]). A long-term follow-up study[7] found that people who remitted from ME/CFS had non-significant differences in impairment on 17 out of 23 outcomes compared to those who maintained a CFS diagnosis. So, even patients who don’t meet a CFS diagnosis anymore will not return to their premorbid level of functioning. ME/CFS has a greater negative impact on functional status and well-being than other chronic diseases, e.g., cancer or lung diseases[8], and is associated with a drastic decrement in physical functioning[9]. In a comparison study[10] ME/CFS patients scored significantly lower than patients with hypertension, congestive heart failure, acute myocardial infarction, and multiple sclerosis (MS), on all of the eight Short Form Health Survey (SF-36)[11] subscales. As compared to patients with depression, ME/CFS patients scored significantly lower on all the scales, except for scales measuring mental health and role disability due to emotional problems, on which they scored significantly higher. Looking at several studies[12-16] the financial consequences of ME/CFS for the individual patient and the economic impact on society are often very profound.
This article aims to: (1) compares the diagnostic criteria for ME and CFS; and (2) reviews well-accepted methods to assess characteristic symptoms of ME and CFS objectively.
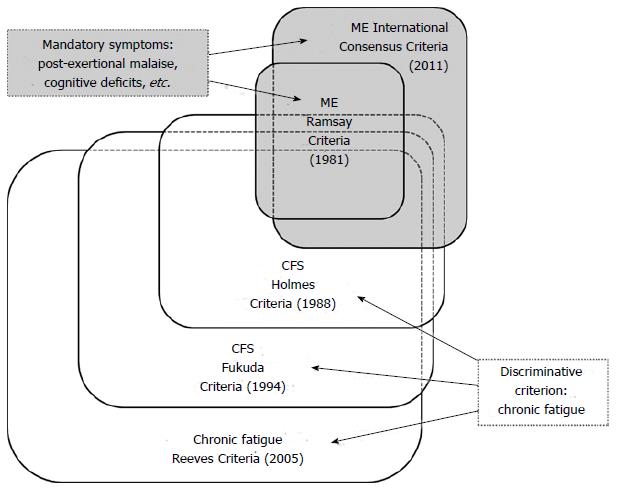
Although ME, CFS and post-viral fatigue syndrome are used interchangeably[17], the case criteria for ME[18] and CFS[19] define two distinctive clinical entities[1], delineating partially overlapping and partially disjoint patient populations (Figure 1).
ME, a neurological disease[20,21], has been described in the medical literature since 1934 under various names[22], e.g., epidemic neuromyasthenia and atypical poliomyelitis, often on account of outbreaks[23-25]. Characteristic symptoms of ME, classified as a disease of the nervous system by the WHO since 1969[26], are: muscle weakness, neurological dysfunction, especially of cognitive, autonomic and neurosensory functions; variable involvement of the cardiac and other systems; a prolonged relapsing course; but above all general or local muscular fatigue after minimal exertion with prolonged recovery times (post-exertional “malaise”)[20].
The clinical entity CFS was introduced in 1988[27] and redefined in 1994[19]. The diagnosis CFS is primarily based upon the ambiguous notion “chronic fatigue”[28,29]. According to commonly used criteria for CFS[19] “chronic fatigue” must be accompanied by at least 4 out of 8 symptoms, e.g., tender lymph nodes and muscle and joint pain. However, 5 of the 8 “minor” symptoms, i.e., headaches, lymph node pain, sore throat, joint pain, and muscle pain, do not differentiate people with melancholic depression group from healthy controls[30]. The CFS criteria[19] by definition select a heterogeneous population of people with “chronic fatigue”[31-34].
The diagnostic criteria for ME[18] and CFS[19] define distinctly nosological entities, since cognitive impairment and post-exertional “malaise”, obligatory for the diagnosis ME, are not mandatory for the diagnosis CFS, and the diagnosis ME doesn’t require “chronic fatigue”. The distinction between ME and CFS is illustrated by a study[35] which found that 60% of the “less severe CFS” patents reported post-exertional “malaise” and 45% reported cognitive impairment. This implies that many “less severe” CFS patients don’t fulfil the original[20] or new[18] criteria for ME. Looking at relevant studies[36-39] ± 30%-50% of subjects meeting the CFS criteria[19] seem to fulfil the more stringent International Consensus Criteria (ICC) for ME[18]. How many ME/ICC[18] patients don’t meet the CFS[19] criteria is unknown, since almost all studies until now applied case definitions sequentially, i.e., used other diagnostic criteria on a patient population preselected by chronic fatigue or CFS criteria[40]. In a recent study[41] ME/ICC[18] patients reported significantly more severe disability across all domains of the World Health Organisation Disability Adjustment Schedule 2.0[42] (P < 0.05), when compared to patients only fulfilling the criteria for CFS[19]. Another study[43] supports the notion that the ICC criteria for ME[18] identify patients with greater functional impairment and move severe physical, mental, and cognitive symptoms than patients who only meet the Fukuda criteria for CFS[19].
Diagnostic criteria applied (Figure 1) are crucial, not only because of the sensitivity and specificity[44-46] of the criteria[19,47], e.g., resulting into the inclusion of people with psychiatric disorders[48], but also for a judgment about the effects of proposed effective interventions in specific patient populations[49-53].
Clinical assessment has shown to be essential for an accurate diagnosis and establishing prevalence rates. A recent study[54] for example observed that the pooled prevalence of CFS[19] was substantially higher for self-reporting assessment (3.28%, 95%CI: 2.24-4.33) than for clinical assessment (0.76%, 95%CI: 0.23-1.29).
In conclusion, ME[18] (Table 1) is not equivalent to CFS[19] (Table 2) or incapacitating chronic fatigue[55] (Table 3). While chronic fatigue is a common complaint, CFS[19] is a relatively rare condition (prevalence rate: 0.19%[38], 0.20%[58]). The prevalence of ME (CFS), as defined by more strict criteria[59], is even lower: 0.11%[38].
| Post-exertional neuro-immune exhaustion: |
| A pathological inability to produce sufficient energy on demand with prominent symptoms primarily in the neuroimmune regions |
| Neurological impairments |
| At least one symptom from three of the following four symptom categories: Cognitive impairments (information processing and short-term memory) |
| Pain (e.g., headache, muscle, joint, abdominal and/or chest pain) |
| Sleep disturbance (disturbed sleep patterns and unrefreshing sleep) |
| Neurosensory, perceptual and motor disturbances |
| Immune, gastro-intestinal and genitourinary impairments |
| At least one symptom from three of the following five symptom categories: |
| Flu-like symptoms, e.g., sore throat and tender lymph nodes |
| Susceptibility to viral infections with prolonged recovery periods |
| Gastro-intestinal tract complaints, e.g., irritable bowel syndrome |
| Genitourinary complaints: e.g., nocturia |
| Sensitivities to food, medications, odours or chemicals |
| Energy production and - transportation impairments |
| At least one of the following four symptoms: |
| Cardiovascular symptoms, e.g., (delayed) orthostatic intolerance |
| Respiratory problems, e.g., air hunger and fatigue of chest wall muscles |
| Loss of thermostatic stability, e.g., sweating episodes or feverish feeling |
| Intolerance of extremes of temperature |
| Primary symptom: |
| Clinically evaluated, unexplained, persistent or relapsing chronic fatigue |
| That is of new or definite onset; is not the result of ongoing exertion |
| That is not substantially alleviated by rest; and |
| That results in substantial reduction in previous levels of occupational, educational, social, or personal activities |
| Secondary symptoms: |
| The concurrent occurrence of four or more of the following symptoms, all of which must have persisted or recurred during 6 or more consecutive months of illness and must not have predated the fatigue: |
| Self-reported impairment in short-term memory or concentration severe enough to cause substantial reduction in previous levels of occupational, educational, social, or personal activities |
| Sore throat |
| Tender cervical or axillary lymph nodes |
| Muscle pain |
| Multi-joint pain without joint swelling or redness |
| Headaches of a new type, pattern, or severity |
| Unrefreshing sleep |
| Post-exertional malaise lasting more than 24 h |
| Fatigue: |
| A score |
| ≥ 13 (out of 20) on the general fatigue or |
| ≥ 10 (out of 20) on the reduced activity |
| subscales of the multidimensional fatigue inventory[56] |
| Functional impairment: |
| A score |
| ≤ 70 (out of 100) on the physical function, or |
| ≤ 50 (out of 100) on role physical, or |
| ≤ 75 (out of 100) on the social function, or |
| ≤ 66.7 (out of 100) on the role emotional |
| subscales of the medical outcomes survey short form-36 (SF-36)[11] |
| Secondary symptoms: |
| ≥ 4 of the following 8 symptoms: |
| Impaired memory or concentration |
| Unrefreshing sleep |
| Headaches |
| Muscle pain |
| Joint pain |
| Sore throat |
| Tender cervical nodes and |
| Unusual post exertional fatigue |
| And A score of ≥ 25 (out of 128) |
| On the Symptom Inventory Case Definition subscale[57] |
ME/CFS patients often report a plethora of symptoms, which can vary in number and severity among individual patients and fluctuate within an individual over time, possibly as a result of daily activity[60]. Symptoms often reported by ME/CFS patients are post-exertional “malaise”, cognitive deficits (“brain fog”), “fatigue” (lack of energy), muscle weakness, (muscle and/or joint) pain, impaired sleep, a new type of headaches, stress intolerance, orthostatic intolerance and visual symptoms[61].
Various typical symptoms, e.g., post-exertional “malaise” and muscle weakness, can be quantified objectively using accepted, reproducible methods[1] (Table 4), while others symptoms, e.g., fatigue, cannot be evaluated objectively due to their nature.
| Symptoms | Tests | Ref. |
| Lack of energy: physical weakness and ”fatigue” | CPET 1: workload and oxygen uptake at exhaustion and at the anaerobic threshold | [62,63] |
| Cognitive impairment | Specific neuropsychological tests | [64-67] |
| Post-exertional "malaise” | ||
| Physical effects | Repeated CPETs 1, 24 h apart | |
| Cognitive effects | Specific neuropsychological tests | |
| before and after a CPET or | ||
| before and during a tilt table test | ||
| Repeated neuropsychological tests | ||
| Muscle weakness | Examination of the muscles (power, endurance, recovery) | [68-71] |
| Orthostatic intolerance | Tilt-table test | [72-74] |
| Defective stress response | Hormonal investigation (HPA axis, thyroid) in rest, at specific moments, e.g., at wakening, and during the day, after provocation, e.g., by adrenocorticotropic hormone and insulin, and in response to an exercise test or psychological stress test | [75-78] |
| Sleep impairment | Polysomnographic investigation (EEG) | [79-81] |
| Maintenance of wakefulness test | [79,82,83] | |
| Multiple sleep latency test | [79,82,83] | |
| Visual symptoms | Useful field of view tests | [84,85] |
| Eye movement tests | [86,87] |
Due to the multi-systemic nature of ME/CFS, objective assessment of symptoms and disability in ME/CFS involves various medical specialists, e.g., cardiologists, neuropsychologists, exercise physiologists, and endocrinologists. The exclusion of psychiatric diseases[18,19] and assessment of comorbid psychological disorders requires the input of psychologists/psychiatrists.
Table 4 denominates tests that have demonstrated to yield aberrant results in ME/CFS. However, Table 4 should not consider to be exhaustive. Due to the heterogeneity of the ME/CFS patients population[1,34,88], not all patients will experience all symptoms, i.e., not all tests will show deviant results in all ME/CFS patients. Nevertheless, to assess the clinical status, the severity of the illness, and the disability of an individual patient impartially, patients should be subjected to the abovementioned and other objective tests as much is feasible.
ME/CFS is often incorrectly considered to be equivalent to chronic fatigue. “Fatigue” in ME/CFS is a multi-dimensional entity that is distinct from the generalized form of fatigue experienced by the general population[28]. Fatigue in ME/CFS encompasses at least five dimensions: a lack of energy resources needed for basic daily functioning, “brain fog”, post-exertional “malaise”, a “wired feeling” when very tired, and a flu-like feeling[28]. While these latter two aspects of “fatigue” are subjective due to their nature, the first three dimensions can be assessed more objectively. This paragraph focuses on “lack of energy”, while ”brain fog” (neurocognitive deficits) and post-exertional “malaise” will be discussed in the next paragraphs.
Cardiopulmonary exercise testing (CPET) is regarded to be an accurate method for assessing functional capacity[62,89]. The (maximum) oxygen uptake (O2) measured at a CPET is associated with the concept of Metabolic Equivalent of Task (MET): the energy cost of a physical activity compared to the energy produced by an average person seated at rest. By definition, 1 MET is equivalent to an oxygen utilization of 3.5 mL O2/kg per minute. The functional capacity established by a CPET can be set against the metabolic requirements[90] of self-care tasks essential for fundamental functioning (Basic Activities of Daily Living, ADLs) and activities crucial to live independently from others (Instrumental Activities of Daily Living, IADLs)[91].
Although contradicted by some studies, e.g.,[92,93], various studies, e.g.,[94-98], implicate that the “lack of energy” experienced by ME/CFS patients is reflected in the performance levels at a CPET: a low maximum workload (Wmax) and oxygen uptake (VO2max) and a reduced anaerobic threshold (W AT) and corresponding oxygen uptake (VO2 AT), when compared to sedentary controls. When looking at the “high” mean performance levels of patients in some studies, e.g.,[92,99], contradictory findings are likely due to heterogeneity of the patient samples, e.g., participation rates of “severe”vs“less severe cases”.
Whether or not the exercise capacity is decreased or not in an individual patient should be assessed impartially by a CPET. Objective measures should be employed to establish the degree of effort during exercise, e.g., the respiratory exchange ratio at peak exertion (RERmax). According to well-accepted criteria[62,63] a RERmax > 1.10 indicates excellent effort, while a RERmax < 1.0 reflects submaximal effort. Although (some) patients seem to be able to perform at a level comparable to that of sedentary controls once-off, a CPET often has profound negative effects on the exercise capacity 24 h later at a second CPET (see Post-exertional “malaise”).
A “lack of energy” seems to be accompanied by hypovolemia (low blood volume)[98,100], low cardiac mass[101-103] and reduced cardiac function[100,104]. Some studies implicate interrelations between hypovolemia and low cardiac output[100] and between hypovolemia and (maximum) oxygen uptake[105]. A reduction of the exercise capacity seems to be associated with typical immunological abnormalities in ME/CFS, including immune activation and immune dysfunction[106-108].
In addition to a reduced exercise capacity, the “lack of energy” of ME/CFS patients seems to manifest itself in post-exertional malaise[109], muscle weakness[110] and orthostatic intolerance[111], which will be discussed in separate paragraphs.
A second characteristic symptom of ME/CFS is cognitive impairment (“brain fog”)[39]. Several studies, e.g.,[112-118], have established a wide range of neurocognitive deficits in ME/CFS. In addition, various studies have observed neurological aberrations[119-121], e.g., reduced white[122-124] and grey[123,125,126] matter volume, electroencephalogra-phy (EEG) abnormalities[127], hypoperfusion of the brain[128-130], hypometabolism[131,132], neuro-inflammation of widespread brain regions[133], increased fractional anisotropy in the right arcuate fasciculus and, in right-handed patients, of the right inferior longitudinal fasciculus[124], and spinal fluid abnormalities[134,135]. A relationship between neurological anomalies and cognitive symptoms has also been observed[136-138]. Some findings indicate that the neurocognitive problems are induced or intensified by exercise[97,139] and an upright (orthostatic) position[140]. Cognitive impairment seems to be more severe in sudden onset-ME/CFS[141,142].
ME/CFS patients can present with moderate to large deficits in simple and complex information processing speed (attention, memory and reaction time)[143], in tasks which require working memory over a sustained period of time[143,144], in tasks which necessitate (simultaneous) processing of complex information[116,117] and in conflict-monitoring tasks (interference control)[145]. Specific cognitive deficits, reduced exercise capacity, decreased muscle power (strength and endurance) and immunological aberrations, e.g., inflammation, seem to be interrelated[146,147].
Cognitive impairments can be identified, but only if the appropriate measures are used[114]. This important observation is confirmed by a meta review of 50 studies and 79 tests[143]. All tests for assessing attention, including attention span and working memory, showed significant deficits in ME/CFS. The effect sizes for most word list learning and recall tests were significant, but some tests seem more sensitive to memory deficits in ME/CFS than others. Reaction time is substantially impaired for responses to both simple and complex (choice) stimuli. Only two of the five tests used to assess movement times revealed significant group differences. Most tests for visuospatial ability, verbal abilities and language, cognitive reasoning and flexibility, and global functioning didn’t yield significant group differences. In order to determine cognitive impairment objectively, ME/CFS patients should be subjected to neuropsychological tests[64-67] aimed at the abnormalities found in ME/CFS patients, e.g., attention and memory[112,116,143].
Cognitive deficits don’t seem to be related to “fatigue” or comorbid depression[148,149]. Goedendorp et al[150] have suggested that low cognitive test scores are due to underperformance, but this view is based upon the subjective premise that ME/CFS has not proven to be a cognitive disorder[151]. Objective measures indicate high levels of effort and an intention to do well during neurocognitive testing[152].
Post-exertional “malaise” has been defined as “a pathological inability to produce sufficient energy on demand”[18], resulting into a (delayed) increase of typical symptoms, e.g., weakness, muscular and/or joint pain, cognitive deficits, after a minor physical or mental exertion, with prolonged “recovery” times[109,153].
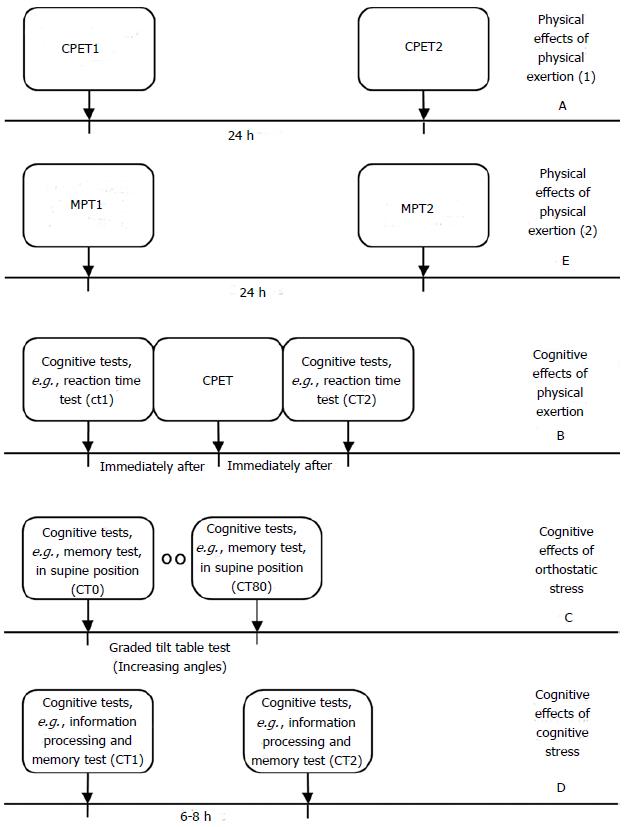
Looking at the research, post-exertional malaise in ME/CFS can present itself in several forms, including a decline in physiological exercise capacity at a second exercise test 24 h later[94,154], cognitive impairment induced or intensified by exercise[97,139] and orthostatic stress[140], and cognitive deficits due to mental exertion, e.g., cognitive testing[155]. These various dimensions of post-exertional “malaise” can be assessed by combining CPETs, tilt-table and specific cognitive tests (Figure 2). The (long-lasting) physical effects of physical exertion (Figure 2A) can be evaluated objectively by subjecting a patient to two CPETs until exhaustion separated by 24 h[156]. The cognitive effects of physical exertion (Figure 2B) can be assessed impartially by comparing the cognitive performance, e.g., simple and choice reaction times, before and immediately after a CPET, the cognitive effects of orthostatic stress (Figure 2C) can be established by subjecting patients to cognitive tests at various stages of a graded tilt table test, while the mental effects of cognitive exertion (Figure 2D) can be assessed objectively by exposing a patient twice to the same cognitive tests with several hours rest in-between. The prolonged negative effect of muscle contractions on muscular strength (Figure 2E), another aspect of the physical dimension of post-exertional “malaise”, will be discussed in the paragraph Muscle weakness.
Physical effects of physical exertion: A profound decrease in the exercise capacity at a second CPET 24 h after the first CPET seems typical for ME/CFS and is neither observed in sedentary healthy controls nor in patients with other diseases[154,157]. The “exercise intolerance” in ME/CFS can be reflected in significantly lower oxygen uptake and performance levels at exhaustion (VO2max and Wmax) or at the anaerobic threshold (VO2 AT and W AT) at the second CPET[154]. In contrast, the first CPET appears to have a positive effect on the anaerobic threshold in sedentary controls at the second CPET[94]. Due to the first CPET the anaerobic threshold can decrease to a level below 5 METS; a level at or below that which is required by many job-related activities and IADLs[158]. Since many daily activities fall into the 3-5 MET energy range, persons with ME/CFS will exacerbate symptoms simply by completing normal daily activities[158]. A recent study[157] observed that VO2max at the first exercise test was reduced in ME/CFS (mean ± SD: 21.9 ± 4.75 mL/k per min), that all patients showed clinically significant decreases in either VO2max and/or oxygen uptake at the ventilatory threshold (VO2 VT) at the second CPET, and that a classification of impairment[159] based on the VO2max or VO2 VT of the first CPET would result in overestimation of functional ability for 50% of the patients.
A real-life example of the effect of a CPET on the performance levels at a second CPET 24 h is summarized in Table 5 (male patient, 45 years, 65 kg).
| CPET Day 1 | CPET Day 2 | |
| Rest | ||
| Heart rate | 88 | 80 |
| Oxygen uptake (VO2min) | 6 | 6 |
| Anaerobic threshold | ||
| Heart rate (HR AT) | 105 | 89 |
| Oxygen uptake (VO2 AT) | 11 | 9 |
| Workload (W AT) | 54 | 35 |
| Exhaustion | ||
| Heart rate (HRmax) | 151 | 131 |
| Oxygen uptake (VO2max) | 23 | 22 |
| Workload (Wmax) | 159 | 133 |
The VO2max at day 1 was 23 mL/min per kilogram, while the anaerobic threshold was reached at a workload level of 54 Watt (W). The corresponding oxygen uptake (VO2 AT) was 11 mL/min per kilogram. Washing the floor requires 58 W and walking at a speed of 5 km/h 56 W[160], which implies that the anaerobic threshold (AT) is reached just by doing light household activities or low-speed walking. On the second CPET the AT declined to a workload of 35 W, which is equivalent to the energy cost of ironing (35 W) and walking at 3 km/h (32 W)[160]. The oxygen uptake at the anaerobic threshold (VO2 AT) has decreased to 9 mL/min per kilogram on day 2. The difference between the heart rate at the anaerobic threshold and the heart rate at rest is only 9 bpm at day 2. This example perfectly illustrates the (prolonged) negative effects of exertion in ME/CFS.
Mental (cognitive) effects of physical exertion: Although research studies into the effects of physical exercise on cognitive performance are scarce, there are several indications that exercise has a (durable) negative effects on cognitive functioning, e.g., focused and sustained attention[161], simple reaction time and choice reaction times[97] and accuracy at the Symbol Digit Modalities Test, Stroop Word Test and Stroop Color Test[139]. This negative effect seems to be the opposite of the effect of exercise on cognitive performance in sedentary controls[139]. The negative impact of physical exertion on cognitive functioning could be mediated by reduced prefrontal cortex oxygenation during and after exercise[98] and/or diminished cardiovascular response to cognitive stress[162]. The effects of physical exertion on cognitive impairment can be assessed by subjecting a patient to specific cognitive tests[114,143] 0-24 h after a CPET and comparing the results on these tests with the pre-exercise test scores.
Mental (cognitive) effects of orthostatic stress: A subgroup of patients present with (delayed) orthostatic intolerance[163], as implicated by a substantially increased heart rate and/or reduced blood pressure in an upright position (POTS: postural tachycardia syndrome, respectively postural hypotension)[74]. Orthostatic symptoms are independently associated with functional impairment[164]. Orthostatic stress seems to impair working memory and information processing, as indicated by a deterioration of scores and reaction times on the N-back test as orthostasis progresses[140,165]. This phenomenon could be related to reduced neuronal activated cerebral blood flow velocity during orthostatic stress[165]. To assess the effects of orthostatic stress objectively, the patient should be subjected to cognitive tests at various angles during tilt-table testing. The N-back test could be a suitable cognitive test, since studies[140,165] have found that while N-back outcome in controls decreased with the value of N, the score was independent of tilt angle, while N-back outcome in ME/CFS patients also decreased with the value of N, but deteriorates as the tilt angle increases. A recent study[166] found that upright tilting caused a significant increase in the N-back normalized response time and a profound drop in cerebral blood flow velocity.
Mental (cognitive) effects of cognitive stress: Although scarcely investigated, there are indications that cognitive stress induces long-lasting mental/cognitive effect in ME/CFS. As mentioned in the paragraph Cognitive Impairment, “recovery” from a 3-h lasting cognitive test to pre-test levels of mental energy seems to take much longer in ME/CFS patients[155]. This phenomenon could be associated to a greater mental and neurological effort to process information as effectively as healthy controls[136-138,167]. A recent study[168] observed significant differences between self-reported levels of general, mental, and physical fatigue[58] and depression[169] before and two days after a cognitively fatiguing task[67]. Whether the effects of cognitive stress encompasses a mental dimension in a specific patient, could be assessed objectively by subjecting a ME/CFS patient to cognitive tasks 6-8 h after a fatiguing cognitive test battery and comparing the first and second scores on specific cognitive tests[114,143].
Muscle weakness: Many patients report muscle weakness[30,35,44]. According to a recent study[170] muscle recovery is closely related to cognitive deficits (see cognitive impairment). Several studies indicate reduced muscle strength and endurance and prolonged recovery from muscle contractions in ME/CFS. One study[171] for example found that the hand grip strength of patients was significantly (26%) less than sedentary controls and that the maximum voluntary contraction (MVC) force in the patient group significantly reduced to 83% of the low baseline strength after 50 contractions with 10 s and 50 contractions with 5 s rest between trials. This study also observed deviant EEG-recorded brain signals in controlling voluntary muscle activities, especially when the activities induce fatigue. Another study[172] observed that ME/CFS patients were able to sustain a 10 kg handgrip contraction for less time than healthy controls and that the mean of handgrip contraction strength was substantially lower in ME/CFS patients when compared to healthy controls. A third study[110] found that the maximum twitch interpolated voluntary isometric contraction force of the quadriceps muscle of the dominant leg was significantly lower in ME/CFS patients (interquartile range, IQR: 234N-386N) than in sedentary controls (IQR: 364N-518N). In addition to reduced muscle strength and endurance, recovery of voluntary muscle contractions seem to be prolonged. This is illustrated by a study[173] in which patients and sedentary controls were subjected to an experiment involving 18 MVCs with a 50% duty cycle (10 s contraction, 10 s rest), followed by a recovery phase, lasting 200 min, in which the strength of the quadriceps muscle group was assessed, and a follow-up session 24 h post-exercise, involving three 10 s MVCs. The MVC forces in the control group were significantly higher than those of the ME/CFS patients, with a decline in force over the 18 contractions in both groups. Recovery was prolonged in the patient group, with a significant decline in the MVCs during the recovery phase and also at 24 h-post exercise (73% ± 9% of the initial force levels in patients vs 91% ± 7% in controls). In a recent study[174] patients exhibited lower isometric MVC levels for handgrip strength and slower and incomplete recovery in the 45 min after a fatiguing exercise (18 maximum contractions using a 50% duty cycle, 5 s contraction, 5 s rest), compared to both MS patients and sedentary healthy controls.
All in all, muscle weakness in ME/CFS seems to manifest itself into reduced muscle power, declining dynamic muscular endurance and the long-lasting recovery from repeated voluntary muscle contractions (Figure 2E). In order to assess muscle weakness and recovery from muscle exercise objectively and reliably the use of isokinetic and isometric dynamometers is essential[68-71,175-178].
Various studies have observed impaired skeletal muscle metabolism in ME/CFS, e.g., decreased basal values of PCr/(PCr + Pi), increased pH levels during exercise, and low intracellular concentrations of ATP at exhaustion of a graded exercise of the right gastrocnemius muscle[179,180]. In addition to a significant prolongation (almost 4-fold) of the time taken by pH to recover to baseline after exercise, Jones et al[181] revealed the existence of two CFS[19] subgroups: patients with normal PCr depletion in response to a low-level voluntary contraction exercise, but with substantially increased intramuscular acidosis, and patients with low PCr depletion during exertion, generating abnormally low muscle power. An impaired cardiovascular response to standing, orthostatic intolerance, cardiac bioenergetic abnormalities, as implicated by low PCr/ATP values, and reduced muscle metabolism (longer PCr and ADP recovery times) seem to be interrelated[101].
Orthostatic intolerance: Orthostatic intolerance is accompanied by symptoms that arise or aggravate while standing, e.g., light-headedness, blurred vision, fainting and syncope. Several studies[182-185] indicate orthostatic intolerance in ME/CFS patients or subgroups. Altered cardiovascular autonomic control and responses to orthostatic stress are associated with other typical symptoms, e.g., cognitive deficits, and disability[186]. Since orthostatic intolerance is already present in the early stages of the disease[182,187] and CFS patients with POTS were have found to be significantly younger and to have a shorter length of illness than CFS patients without POTS[188], it seems unlikely that prolonged inactivity accounts for the orthostatic symptoms.
Orthostatic intolerance (in ME/CFS) seems associated with specific cardiovascular abnormalities in an upright position[111,189-191], e.g., POTS and neurally mediated hypotension (NMH). These cardiovascular aberrations can be assessed using a tilt table test. The head-up tilt testing is considered a clinically useful diagnostic tool to assess susceptibility to orthostatic intolerance in patients with syncope, allowing reproduction of the patient’s symptoms in a safe environment, under medical control[72,192]. With regard to deviant cardiovascular responses to orthostatic stress five types of abnormalities[74] can be distinguished (Table 6).
| Abnormality | Definition |
| Orthostatic systolic hypotension | A fall in the systolic blood pressure of 20 mmHg or more[74,193] |
| Orthostatic diastolic hypotension | A fall in the diastolic blood pressure of 10 mmHg or more[74,193] |
| Orthostatic diastolic hypertension | A rise in dBP to 98 mmHg or more[74] |
| Orthostatic postural tachycardia | An increase in heart rate of 28[74]/30[194] beats per minute (bpm) or a pulse of more than 110[74]/120[194] bpm |
| Orthostatic narrowing of pulse pressure | A fall in the pulse pressure to 18 mmHg or less[74] |
In a tilt table test a patient has to lie on a special table/bed, which gradually moves in posture from lying to an “upright position”, e.g., 70 degrees. Heart rate and blood pressures are monitored at various angles in order to establish hemodynamic abnormalities in a specific subject. It is relevant to note that the abovementioned aberrations seem to occur delayed and suddenly in ME/CFS[163,195]. So, in order to assess potential orthostatic abnormalities in a patient, the patient should remain in an “upright” position as long as possible, preferably longer than 15 min.
Defective stress response: Not only physical but psychological stress as well seems to intensify the symptoms in ME/CFS[196]. This phenomenon seems to be associated with hypothalamic-pituitary-adrenal (HPA) axis dysfunction[197,198], including hypocortisolism and deviant physiological responses to stress. HPA dysfunction in ME/CFS can manifest itself in reduced levels of stress hormones, e.g., cortisol, at specific moments of the day[199,200] and aberrant diurnal production of specific hormones, e.g., cortisol, cortisone and adrenocorticotropic hormone (ACTH)[201,202]; a blunted response to provocation, e.g., by insulin[203], ACTH[204] or CRH[78]; a (long-lasting) deviant response to psychological[205] or physical stress[205,206], and an enhanced sensitivity of the cellular immune system to glucocorticoids[207,208] and increased negative feedback of glucocorticoids to the HPA axis[209,210]. HPA axis dysfunction is not likely to be the primary cause of the illness, since HPA axis hypofunction, e.g., hypocortisolism, is only present in a subgroup of patients[200,211], HPA axis abnormalities manifest themselves at a later stage of the illness[212-214] and hydrocortisone/fludrocortisone seem to have limited[215] or adverse[216] effects.
HPA axis dysfunction in ME/CFS can result into (1) low basal levels of ACTH; (free and total) cortisol (according to gas chromatography-mass spectrometry and high-performance liquid chromatography are considered to be the golden standard for assessing cortisol levels[78]), DHEA/DHEAS and noradrenalin at specific moments of the day, e.g., at awakening; (2) reduced synthesis of ACTH and cortisol during the day; and (3) blunted HPA axis responses to “provocation”, exercise or psychological stress. Tests[75,217] to assess HPA axis dysfunction objectively should be aimed at these aberrations.
ME/CFS has also been associated with thyroid dysfunction[218]. This finding is in line with inflammationmediated loss of thyroid function[219-221]. Thyroid dysfunction can present itself in (1) low (free) thyroxine (T4) levels[222], due to decreased levels of thyroid stimulating hormone (TSH) secreted by the pituitary or a blunted response of the pituitary to TSH; (2) by reduced uptake of triiodothyronine (T3) and T4 by the cell[223,224]; (3) by diminished T4-T3-conversion, resulting into increased levels of reverse triiodothyronine (rT3)[225,226]; (4) by a diminished production of TSH and free T3 and T4 after administration of thyrotropin-releasing hormone (TRH)[227]; and/or (5) antithyroid microsomal antibodies[228]. Thyroid tests[229] could reveal if these aberrations are present in a particular patient.
Sleep impairment: Many patients report sleep disturbances[30,35,230,231], e.g., insomnia, frequent awakenings, vivid dreams/nightmares and day/night reversal. Non-restorative sleep is the most specific and sensitive “minor” symptom[232] of CFS[19]. Sleep seems to be disturbed differently patterns in ME/CFS patients with and without comorbid fibromyalgia[233]. Abnormalities have been observed in reduced theta, sigma, and beta spectral power during the various sleep stages and shorter duration and higher frequency of transitions between the sleep stages[234-239].
Some methods to establish sleep dysfunction in ME/CFS objectively are: polysomnographic sleep investigation (EEG), aimed at the frequency of transitions between and the duration of sleep phases[233,237] and spectral power analysis[235,239], the maintenance of wakefulness and the multiple sleep latency test, although the latter two could be considered subjective, and not objective tests.
Visual symptoms: Patients often report visual symptoms, e.g., problems with focusing, blurred vision and light insensitivity[240-242]. While various visual symptoms can be qualified as subjective, some aspects of the visual function can be assessed objectively. Abnormal visual attention, e.g., conjunctive search (with divided and selective attention) and spatial cueing (selective attention with distraction)[243], can be assessed with the Useful Field of View test[84,85]. According to a recent study[244] dysfunctional eye movements in ME/CFS can present itself in reduced antisaccade focus accuracy and less precision and speed at smooth pursuing a target.
While several symptoms can be assessed objectively, other characteristic symptoms[18,30] can’t be quantified easily due to their nature. These symptoms include pain (muscle and joint pain, headaches, etc.), abdominal pain and other gastro-intestinal symptoms, “sickness behavior” (flu-like feeling, depression, etc.), intolerance of light (photophobia), sound (phonophobia) and odors (osmophobia), food and chemicals, and disturbed thermoregulation[18,30]. However, several of these symptoms could logically be explained by aberrations observed in ME/CFS patients or subgroups. Pain e.g., could be the result of (1) inflammation[245,246]; (2) reduced oxidative metabolism[180,247], mitochondrial dysfunction[248,249] or damage[250,251]; (3) low cardiac output[101,185] and reduced blood and oxygen supply to the brain[98,129] and muscles[252,253], possibly leading to acidosis[181,254], accelerated glycolysis[180] and elevated lactate levels[132,255]; (4) central sensitisation[256], as a potential sequel of inflammation[257,258] and oxidative and nitrosative stress[259,260]; and (5) elevated pain receptors[261,262].
When assessing patients it is crucial to keep in mind that while various symptoms are obligatory for the diagnosis ME[18], they are not obligatory for to fulfil the CFS[19] diagnostic criteria. As argued, objective tests are to be preferred when possible. Nevertheless, when questionnaires and subjective measures are used to assess the clinical status of a patient, applying minimum thresholds for the frequency and the severity of symptoms (e.g., moderate severe about half of the time) can reduce the likelihood of possible misclassification of healthy persons and ME/CFS patients[61].
ME/CFS has a profound effect on the functional status[10,263] and life[264,265] of patients. As argued in this chapter, an objective assessment of the clinical status can quantify the severity of characteristic symptoms, e.g., cognitive impairment, low physiological exercised capacity and the detrimental effects of exertion. However, next to the illness burden, patients experience serious medical, financial, social and psychological consequences of their illness, which can have a profound impact.
An objective assessment of symptoms could help to resolve the controversy around the nature and impact of ME/CFS within the medical profession. Patients for example often report negative experiences with health care workers[266]. Some medical professionals don’t consider ME/CFS to be a legitimate illness[267]. Despite the neurological classification of ME/CFS[17,26] and various neuro-immunological abnormalities in ME/CFS observed repetitively[18,268], 84% of respondents in a survey of members of the Association of British Neurologists answered they did not consider ME/CFS to be a neurological condition[269]. According to a study[270] only half of the general practitioners (GPs) believed that ME/CFS actually exists. In a survey[271] patients reported that they felt that the doctors psychologized too much or trivialized the symptoms. Increasing physical activity had been recommend by doctors, but most of the respondents reported that this made them worse[271].
ME/CFS often has a huge impact on the occupational status[272] and income of patients[16], school attendance[273] and performance[265] of young patients, and the income of parents of children with ME/CFS[14]. A substantial proportion of ME/CFS patients, 50.1% according to[16], has to discontinue their employment due to their illness. Looking at the data of various studies[12,13,14] the average annual direct cost (medical costs, etc.) vary from $ 2342 to $ 8675, and the indirect cost (work productivity losses, disability reimbursements, services provided by family members, friends and others, etc.) vary from $ 8554 to $ 20000, for men: $ 23124[12,15,16]. Based upon the prevalence rates of[38] the direct and indirect cost of ME/CFS to the US society could be estimated at $ 8.5-$11 billion annually.
The prevalence of ME/CFS in children based upon a cross-sectional national sample among GPs was 0.111%[274], which is comparable with other prevalence figures[38]. The impact of ME/CFS on school attendance of children and adolescent is profound. On study for example found that 62% of children and young people aged under 18 years with ME/CFS, attended 40% of school or less[275]. Another study[274] observed that 45% of young patients with ME/CFS (aged 10 to 18 years) reported > 50% school absence during the previous 6 mo. A substantial subgroup of young patients, 29% of patients aged 12-18 years according to Bould et al[276], reports symptoms of comorbid depression, which seems to be associated with the degree of disability. The prognosis of children with CFS seems better in adolescents, e.g., in CFS induced by infectious mononucleosis[277,278], but in both adults and adolescents the severity of the acute phase seems to be the sole predictor of the outcome[187,278].
ME/CFS also can have serious social and emotional consequences, e.g., marginalisation, social isolation, stigmatisation and transformation of identity. Many patients with ME/CFS feel that their illness is not acknowledged as a legitimate illness within the social and medical context[271], and patients often report marginalization from family, friends, and medical professionals[279]. Not being able to be with friends or to attend school, makes adolescents with ME/CFS feel isolated, different and forgotten[280]. ME/CFS can also result into a transformation in identity[272,279] and values, expectations and life priorities[281]. In addition to destroying relationships and careers[272], ME/CFS also can disrupt self-perceptions[282]. Much of the stigma experienced by ME/CFS patients seems to originate from the associations with the name CFS[283], the lack of diagnostic biomarkers[284] and the absence of clear-cut etiologic models for ME and CFS[284]. Questioning the veracity of ME/CFS might represent a potent stressor in ME/CFS, and even coping methods thought to be useful in other conditions, are not associated with a reduction of distress among those with ME/CFS[284]. Doctors’ beliefs can result into negative stereotyping of ME/CFS patients[285]. However, there don’t seem to be major differences between the personalities of ME/CFS patients and patients with rheumatoid arthritis and the stereotype of ME/CFS patients as being “perfectionists with negative attitudes toward psychiatry” doesn’t seem to be applicable[286].
ME/CFS is a serious disorder, which can have profound consequences on a patients’ life and health status. In addition to the impact of the symptoms on everyday life, patients often disbelief, e.g., when claiming disability related benefits[156]. Due to the fact that “chronic fatigue” is an ambiguous and subjective notion[28], that patients often report a plethora of symptoms which can fluctuate over time very rapidly, and that there are (yet) no clear-cut etiological models for ME and CFS, patients frequently encounter difficulties in proving their level of disability, which can have substantial financial consequences.
An objective assessment of core symptoms could not only impartially confirm the patients’ self-reported disability[287], but could also contribute to reversal of other problems experienced by patients, e.g., stigmatization and the attitude of medical professionals towards the illness(es) and patients. In this context and in light of the controversy surrounding ME and CFS, it is essential to establish the functional (dis)abilities of a patient (output or functional consequences) objectively without an a priori judgment about the causes (the “black box”: etiology and pathophysiology). However, establishing the functional impact objectively using well-accepted tests, e.g., (repeated) exercise tests (CPETs), neurocognitive tests and tilt table tests, also could point towards the physiological origin of various symptoms, e.g., post-exertional “malaise”.
One very relevant limitation with regard to the objective assessment of symptoms relates to practical and ethical perspectives, since moderate and severe cases of ME/CFS may not be able to perform specific tests, e.g., CPETs and tilt table testing, and the ethics of requiring patients to undertake a test likely to intensify pain and other symptoms could be questioned[154].
Assessing symptoms objectively, if possible, instead of using questionnaires and subjective measures, could also largely improve scientific progress. For example, the controversy about the claim that CBT and GET are effective interventions[49,288] without detrimental effects[289], which is challenged by others[50,290], could be resolved by subjecting the patients to objective tests, e.g., CPETs and cognitive tests, before during and after CBT/GET. Especially since studies have shown that reduction in “fatigue” after behavioural interventions is not reflected by a clinical improvement in objective terms, e.g., activity levels[291], distance walked in 6 min[49] or oxygen uptake[292]. Future trials into proposed effective pharmaceutical[52,53] and behavioural therapies[49,288] should be using objective measures to establish positive and negative effects in clear-defined patient populations impartially[293].
Although the labels ME and CFS are often used interchangeably, the diagnostic criteria for ME and CFS define two distinct, partially overlapping, clinical entities. ME, whether defined by the original[20] or the new consensus criteria[18], is not equivalent to CFS[19]. Muscle weakness, cognitive impairment, and above all, post-exertional “malaise”, obligatory for the diagnosis ME, is not mandatory for the diagnosis CFS, while “chronic fatigue”, the core feature of the diagnosis CFS, is not mandatory for ME.
Partly due to the subjective nature of the symptom-based definitions of ME and CFS and the use of self-report, questionnaires and subjective measures, some researchers and clinicians have questioned the physiological origin of the symptoms and qualified ME and CFS as functional somatic syndromes. The use of objective tests and measures to assess symptoms and functional limitations, e.g., CPETs and cognitive tests, could resolve the controversy with regard to the nature of ME and CFS and the consequences for patients’ lives and professional abilities. Looking at the medical, financial, social and emotional impact of ME and CFS on patients and society, and the future perspective of patients, an objective assessment of the symptoms and disability is a crucial step.
To explore the etiology and pathophysiology in well-defined ME and CFS patient subgroups research should employ objective test and biomarkers[1]. Therapies proposed to be effective for ME and/or CFS, should be evaluated by employing an objective assessment of the clinical status and biomarkers before, during and after the intervention in well-defined patient (sub)groups.
P- Reviewer: Mentes O, Murdaca G, Weng CF S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Twisk FN. The status of and future research into Myalgic Encephalomyelitis and Chronic Fatigue Syndrome: the need of accurate diagnosis, objective assessment, and acknowledging biological and clinical subgroups. Front Physiol. 2014;5:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Twisk FN. A definition of recovery in myalgic encephalomyelitis and chronic fatigue syndrome should be based upon objective measures. Qual Life Res. 2014;23:2417-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Klimas N, Patarca R. Disability and chronic fatigue syndrome: clinical, legal and patient perspectives. Binghamton NY: Haworth Medical Press 1997; . |
| 4. | De Becker P, McGregor N, de Meirleir K. Possible triggers and mode of onset of chronic fatigue syndrome. J Chronic Fatigue Syndr. 2002;10:3-18. [DOI] [Full Text] |
| 5. | Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med (Lond). 2005;55:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Pheley AM, Melby D, Schenck C, Mandel J, Peterson PK. Can we predict recovery in chronic fatigue syndrome? Minn Med. 1999;82:52-56. [PubMed] |
| 7. | Brown MM, Bell DS, Jason LA, Christos C, Bell DE. Understanding long-term outcomes of chronic fatigue syndrome. J Clin Psychol. 2012;68:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Nacul LC, Lacerda EM, Campion P, Pheby D, Drachler Mde L, Leite JC, Poland F, Howe A, Fayyaz S, Molokhia M. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health. 2011;11:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Herrell R, Goldberg J, Hartman S, Belcourt M, Schmaling K, Buchwald D. Chronic fatigue and chronic fatigue syndrome: a co-twin control study of functional status. Qual Life Res. 2002;11:463-471. [PubMed] |
| 10. | Komaroff AL, Fagioli LR, Doolittle TH, Gandek B, Gleit MA, Guerriero RT, Kornish RJ, Ware NC, Ware JE, Bates DW. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. 1996;101:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23352] [Cited by in RCA: 23948] [Article Influence: 725.7] [Reference Citation Analysis (0)] |
| 12. | Lin JM, Resch SC, Brimmer DJ, Johnson A, Kennedy S, Burstein N, Simon CJ. The economic impact of chronic fatigue syndrome in Georgia: direct and indirect costs. Cost Eff Resour Alloc. 2011;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Jason LA, Benton MC, Valentine L, Johnson A, Torres-Harding S. The economic impact of ME/CFS: individual and societal costs. Dyn Med. 2008;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Missen A, Hollingworth W, Eaton N, Crawley E. The financial and psychological impacts on mothers of children with chronic fatigue syndrome (CFS/ME). Child Care Health Dev. 2012;38:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Cost Eff Resour Alloc. 2004;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Collin SM, Crawley E, May MT, Sterne JA, Hollingworth W. The impact of CFS/ME on employment and productivity in the UK: a cross-sectional study based on the CFS/ME national outcomes database. BMC Health Serv Res. 2011;11:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | World Health Organization. International Classification of Diseases, Tenth Revision (ICD-10), Code G93.1. Geneva: Switzerland 1992; . |
| 18. | Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R. Myalgic Encephalomyelitis International Consensus Criteria. J Intern Med. 2011;270:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 789] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 19. | Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3442] [Cited by in RCA: 3335] [Article Influence: 107.6] [Reference Citation Analysis (1)] |
| 20. | Dowsett EG, Ramsay AM, McCartney RA, Bell EJ. Myalgic encephalomyelitis - a persistent enteroviral infection? Postgrad Med J. 1990;66:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Parish JG. Early outbreaks of ‘epidemic neuromyasthenia’. Postgrad Med J. 1978;54:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Acheson ED. The clinical syndrome variously called benign myalgic encephalomyelitis, Iceland disease and epidemic neuromyasthenia. Am J Med. 1959;26:569-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 114] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Gilliam AG. Epidemiological study on an epidemic, diagnosed as poliomyelitis, occurring among the personnel of Los Angeles County General Hospital during the summer of 1934. Washington, DC: United States Treasury Department Public Health Service Public Health Bulletin 1938; 1-90. |
| 24. | Crowley N, Nelson M, Stovin S. Epidemiological aspects of an outbreak of encephalomyelitis at the Royal Free Hospital, London, in the summer of 1955. J Hyg (Lond). 1957;55:102-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Strickland PS, Levine PH, Peterson DL, O’Brien K, Fears T. Neuromyasthenia and chronic fatigue syndrome (CFS) in Northern Nevada/California: a ten-year follow-up of an outbreak. J Chronic Fatigue Syndr. 2001;9:3-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | World Health Organization. International Classification of Diseases, Eighth Revision (ICD-8): I (Code 323): 158. Geneva: Switzerland 1967; . |
| 27. | Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, Jones JF, Dubois RE, Cunningham-Rundles C, Pahwa S. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 991] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 28. | Jason LA, Jessen T, Porter N, Boulton A, Njoku MG, Friedberg F. Examining types of fatigue among individuals with ME/CFS (DSQ 2009; 29). Available from: http://www.dsq-sds.org/article/view/938/1113. |
| 29. | Jason LA, Boulton A, Porter NS, Jessen T, Njoku MG, Friedberg F. Classification of myalgic encephalomyelitis/chronic fatigue syndrome by types of fatigue. Behav Med. 2010;36:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Jason LA, Torres-Harding SR, Carrico AW, Taylor RR. Symptom occurrence in persons with chronic fatigue syndrome. Biol Psychol. 2002;59:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Wessely S. Chronic fatigue syndrome. Summary of a report of a joint committee of the Royal Colleges of Physicians, Psychiatrists and General Practitioners. J R Coll Physicians Lond. 1996;30:497-504. [PubMed] |
| 32. | Lane RJ, Barrett MC, Taylor DJ, Kemp GJ, Lodi R. Heterogeneity in chronic fatigue syndrome: evidence from magnetic resonance spectroscopy of muscle. Neuromuscul Disord. 1998;8:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Aslakson E, Vollmer-Conna U, Reeves WC, White PD. Replication of an empirical approach to delineate the heterogeneity of chronic unexplained fatigue. Popul Health Metr. 2009;7:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Wilson A, Hickie I, Hadzi-Pavlovic D, Wakefield D, Parker G, Straus SE, Dale J, McCluskey D, Hinds G, Brickman A. What is chronic fatigue syndrome? Heterogeneity within an international multicentre study. Aust N Z J Psychiatry. 2001;35:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Peckerman A, LaManca JJ, Dahl KA, Chemitiganti R, Qureishi B, Natelson BH. Abnormal impedance cardiography predicts symptom severity in chronic fatigue syndrome. Am J Med Sci. 2003;326:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Maes M, Twisk FN, Johnson C. Myalgic Encephalomyelitis (ME), Chronic Fatigue Syndrome (CFS), and Chronic Fatigue (CF) are distinguished accurately: results of supervised learning techniques applied on clinical and inflammatory data. Psychiatry Res. 2012;200:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Brenu EW, Johnston S, Hardcastle SL, Huth TK, Fuller K, Ramos SB, Ramos TK, Huth DR, Staines KF. Immune abnormalities in patients meeting new diagnostic criteria for chronic fatigue syndrome/Myalgic Encephalomyelitis. J Mol Biomark Diagn. 2013;4:152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Nacul LC, Lacerda EM, Pheby D, Campion P, Molokhia M, Fayyaz S, Leite JC, Poland F, Howe A, Drachler ML. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med. 2011;9:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Jason LA, Brown A, Clyne E, Bartgis L, Evans M, Brown M. Contrasting case definitions for chronic fatigue syndrome, Myalgic Encephalomyelitis/chronic fatigue syndrome and Myalgic Encephalomyelitis. Eval Health Prof. 2012;35:280-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Brurberg KG, Fønhus MS, Larun L, Flottorp S, Malterud K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review. BMJ Open. 2014;4:e003973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Johnston SC, Brenu EW, Hardcastle SL, Huth TK, Staines DR, Marshall-Gradisnik SM. A comparison of health status in patients meeting alternative definitions for chronic fatigue syndrome/myalgic encephalomyelitis. Health Qual Life Outcomes. 2014;12:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | World Health Organization. Disability Adjustment Schedule II (WHODAS II) (updated 2014 Nov 6). Available from: http://www.who.int/classifications/icf/whodasii/en/. |
| 43. | Jason LA, Sunnquist M, Brown A, Evans M, Newton JL. Are Myalgic Encephalomyelitis and chronic fatigue syndrome different illnesses? A preliminary analysis. J Health Psychol. 2014;Feb 7; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Kennedy G, Abbot NC, Spence V, Underwood C, Belch JJ. The specificity of the CDC-1994 criteria for chronic fatigue syndrome: comparison of health status in three groups of patients who fulfill the criteria. Ann Epidemiol. 2004;14:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Jason LA, King CP, Richman JA, Taylor RR, Torres SR, Song S. U.S. case definition of chronic fatigue syndrome: diagnostic and theoretical issues. J Chronic Fatigue Syndr. 1998;5:3-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Jason LA, Evans M, Brown A, Brown M, Porter N, Hunnell J, Anderson V, Lerch A. Sensitivity and specificity of the CDC empirical chronic fatigue syndrome case definition. Psychology. 2010;1:9-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Reeves WC, Wagner D, Nisenbaum R, Jones JF, Gurbaxani B, Solomon L, Papanicolaou DA, Unger ER, Vernon SD, Heim C. Chronic fatigue syndrome--a clinically empirical approach to its definition and study. BMC Med. 2005;3:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Jason LA, Najar N, Porter N, Reh C. Evaluating the Centers for Disease Control’s empirical chronic fatigue syndrome case definition. J Disabil Policy Stud. 2009;20:93-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, Baber HL, Burgess M, Clark LV, Cox DL. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377:823-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 619] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 50. | Núñez M, Fernández-Solà J, Nuñez E, Fernández-Huerta JM, Godás-Sieso T, Gomez-Gil E. Health-related quality of life in patients with chronic fatigue syndrome: group cognitive behavioural therapy and graded exercise versus usual treatment. A randomised controlled trial with 1 year of follow-up. Clin Rheumatol. 2011;30:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Twisk FN, Maes M. A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME) / chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuro Endocrinol Lett. 2009;30:284-299. [PubMed] |
| 52. | Fluge Ø, Bruland O, Risa K, Storstein A, Kristoffersen EK, Sapkota D, Næss H, Dahl O, Nyland H, Mella O. Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLoS One. 2011;6:e26358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Watt T, Oberfoell S, Balise R, Lunn MR, Kar AK, Merrihew L, Bhangoo MS, Montoya JG. Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein-Barr virus IgG antibody titers. J Med Virol. 2012;84:1967-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Johnston S, Brenu EW, Staines D, Marshall-Gradisnik S. The prevalence of chronic fatigue syndrome/ myalgic encephalomyelitis: a meta-analysis. Clin Epidemiol. 2013;5:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 55. | Reeves WC, Jones JF, Maloney E, Heim C, Hoaglin DC, Boneva RS, Morrissey M, Devlin R. Prevalence of chronic fatigue syndrome in metropolitan, urban, and rural Georgia. Popul Health Metr. 2007;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2188] [Cited by in RCA: 2425] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 57. | Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC. Psychometric properties of the CDC Symptom Inventory for assessment of chronic fatigue syndrome. Popul Health Metr. 2005;3:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Steele L, Dobbins JG, Fukuda K, Reyes M, Randall B, Koppelman M, Reeves WC. The epidemiology of chronic fatigue in San Francisco. Am J Med. 1998;105:83S-90S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Carruthers BM, Jain AK, de Meirleir K, Peterson DL, Klimas NG, Lerner AM, Bested AC, Flor-Henry P, Joshi P, Powles ACP. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. 2003;11:7-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 595] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 60. | Meeus M, van Eupen I, van Baarle E, De Boeck V, Luyckx A, Kos D, Nijs J. Symptom fluctuations and daily physical activity in patients with chronic fatigue syndrome: a case-control study. Arch Phys Med Rehabil. 2011;92:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Jason LA, Sunnquist M, Brown A, Evans M, Vernon SD, Furst J, Simonis V. Examining case definition criteria for chronic fatigue syndrome and myalgic encephalomyelitis. Fatigue. 2014;2:40-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 8th ed. Ambler, PA: Lippincott Williams and Wilkins 2009; . |
| 63. | Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1437] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 64. | Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests. 3rd ed. New York: Oxford University Press 2006; . |
| 65. | Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York: Oxford University Press 2004; . |
| 66. | Wechsler D. Wechsler adult intelligence scale-revised San Antonio. Texas: The Psychological Corporation 1981; . |
| 67. | Cambridge Cognition. Cambridge Neuropsychological Test Automated Battery (CANTAB). Cambridge: Cambridge Cognition Limited 2006; . |
| 68. | Stark T, Walker B, Phillips JK, Fejer R, Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. 2011;3:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 69. | Wang CY, Olson SL, Protas EJ. Test-retest strength reliability: hand-held dynamometry in community-dwelling elderly fallers. Arch Phys Med Rehabil. 2002;83:811-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248-259. [PubMed] |
| 71. | van der Ploeg RJ, Fidler V, Oosterhuis HJ. Hand-held myometry: reference values. J Neurol Neurosurg Psychiatry. 1991;54:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1224] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 73. | Benditt DG, Ferguson DW, Grubb BP, Kapoor WN, Kugler J, Lerman BB, Maloney JD, Raviele A, Ross B, Sutton R. Tilt table testing for assessing syncope. American College of Cardiology. J Am Coll Cardiol. 1996;28:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 330] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 74. | Streeten DH. Orthostatic disorders of the circulation: mechanisms, manifestations and treatment. New York: Plenum Medical Book Publishin 1987; . [DOI] [Full Text] |
| 75. | Melmed S, Polonsky KS, Larsen RMD, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia: Elsevier/Saunders 2011; . |
| 76. | Kovacs WJ, Ojeda SR. Textbook of endocrine physiology. 6th ed. Oxford, Oxfordshire: Oxford University Press 2011; . |
| 77. | Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3809] [Cited by in RCA: 3894] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 78. | Holtorf K. Diagnosis and treatment of hypothalamic-pituitary-adrenal (HPA) axis dysfunction in patients with chronic fatigue syndrome (CFS) and fibromyalgia (FM). J Chronic Fatigue Syndr. 2008;14:59-88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine 2007; . |
| 80. | Dumermuth G, Lange B, Lehmann D, Meier CA, Dinkelmann R, Molinari L. Spectral analysis of all-night sleep EEG in healthy adults. Eur Neurol. 1983;22:322-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Lo CC, Nunes Amaral LA, Havlin S, Ivanov C, Penzel T, Peter JH, Stanley HE. Dynamics of sleep-wake transitions during sleep. Europhys Lett. 2002;57:625-631. [DOI] [Full Text] |
| 82. | Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, Hirshkowitz M, Daniel LL, Bailey D, Berry RB. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113-121. [PubMed] |
| 83. | Wise MS. Objective measures of sleepiness and wakefulness: application to the real world? J Clin Neurophysiol. 2006;23:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110-3123. [PubMed] |
| 85. | Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64:71-79. [PubMed] |
| 86. | Rommelse NN, Van der Stigchel S, Sergeant JA. A review on eye movement studies in childhood and adolescent psychiatry. Brain Cogn. 2008;68:391-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 87. | Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 88. | Fischer DB, William AH, Strauss AC, Unger ER, Jason L, Marshall GD Jr, Dimitrakoff JD. Chronic fatigue syndrome: the current status and future potentials of emerging biomarkers. Fatigue. 2014;2:93-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Forman DE, Myers J, Lavie CJ, Guazzi M, Celli B, Arena R. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med. 2010;122:68-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3482] [Cited by in RCA: 4200] [Article Influence: 300.0] [Reference Citation Analysis (2)] |
| 91. | Roley SS, DeLany JV, Barrows CJ, Brownrigg S, Honaker D, Sava DI, Talley V, Voelkerding K, Amini DA, Smith E. Occupational therapy practice framework: domain & practice, 2nd edition. Am J Occup Ther. 2008;62:625-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 92. | Sargent C, Scroop GC, Nemeth PM, Burnet RB, Buckley JD. Maximal oxygen uptake and lactate metabolism are normal in chronic fatigue syndrome. Med Sci Sports Exerc. 2002;34:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Bazelmans E, Bleijenberg G, Van Der Meer JW, Folgering H. Is physical deconditioning a perpetuating factor in chronic fatigue syndrome? A controlled study on maximal exercise performance and relations with fatigue, impairment and physical activity. Psychol Med. 2001;31:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Vermeulen RC, Kurk RM, Visser FC, Sluiter W, Scholte HR. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med. 2010;8:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | De Becker P, Roeykens J, Reynders M, McGregor N, De Meirleir K. Exercise capacity in chronic fatigue syndrome. Arch Intern Med. 2000;160:3270-3277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Sisto SA, LaManca J, Cordero DL, Bergen MT, Ellis SP, Drastal S, Boda WL, Tapp WN, Natelson BH. Metabolic and cardiovascular effects of a progressive exercise test in patients with chronic fatigue syndrome. Am J Med. 1996;100:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 97. | VanNess JM, Snell CR, Stevens SR, Stiles TL. Metabolic and neurocognitive responses to an exercise challenge in chronic fatigue syndrome (CFS). Med Sci Sports Exerc. 2007;39:S445. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 98. | Patrick Neary J, Roberts AD, Leavins N, Harrison MF, Croll JC, Sexsmith JR. Prefrontal cortex oxygenation during incremental exercise in chronic fatigue syndrome. Clin Physiol Funct Imaging. 2008;28:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | LaManca JJ, Sisto SA, Zhou XD, Ottenweller JE, Cook S, Peckerman A, Zhang Q, Denny TN, Gause WC, Natelson BH. Immunological response in chronic fatigue syndrome following a graded exercise test to exhaustion. J Clin Immunol. 1999;19:135-142. [PubMed] |
| 100. | Hurwitz BE, Coryell VT, Parker M, Martin P, Laperriere A, Klimas NG, Sfakianakis GN, Bilsker MS. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond). 2010;118:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Hollingsworth KG, Hodgson T, Macgowan GA, Blamire AM, Newton JL. Impaired cardiac function in chronic fatigue syndrome measured using magnetic resonance cardiac tagging. J Intern Med. 2012;271:264-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Miwa K, Fujita M. Cardiovascular dysfunction with low cardiac output due to a small heart in patients with chronic fatigue syndrome. Intern Med. 2009;48:1849-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | De Lorenzo F, Xiao H, Mukherjee M, Harcup J, Suleiman S, Kadziola Z, Kakkar VV. Chronic fatigue syndrome: physical and cardiovascular deconditioning. QJM. 1998;91:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Hollingsworth KG, Jones DE, Taylor R, Blamire AM, Newton JL. Impaired cardiovascular response to standing in chronic fatigue syndrome. Eur J Clin Invest. 2010;40:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Farquhar WB, Hunt BE, Taylor JA, Darling SE, Freeman R. Blood volume and its relation to peak O(2) consumption and physical activity in patients with chronic fatigue. Am J Physiol Heart Circ Physiol. 2002;282:H66-H71. [PubMed] |
| 106. | Nijs J, Meeus M, McGregor NR, Meeusen R, de Schutter G, van Hoof E, de Meirleir K. Chronic fatigue syndrome: exercise performance related to immune dysfunction. Med Sci Sports Exerc. 2005;37:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Snell CR, Vanness JM, Strayer DR, Stevens SR. Physical performance and prediction of 2-5A synthetase/RNase L antiviral pathway activity in patients with chronic fatigue syndrome. In Vivo. 2002;16:107-109. [PubMed] |
| 108. | Nijs J, De Meirleir K, Meeus M, McGregor NR, Englebienne P. Chronic fatigue syndrome: intracellular immune deregulations as a possible etiology for abnormal exercise response. Med Hypotheses. 2004;62:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | VanNess JM, Stevens SR, Bateman L, Stiles TL, Snell CR. Postexertional malaise in women with chronic fatigue syndrome. J Womens Health (Larchmt). 2010;19:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 110. | Fulcher KY, White PD. Strength and physiological response to exercise in patients with chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 2000;69:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Stewart JM, Gewitz MH, Weldon A, Arlievsky N, Li K, Munoz J. Orthostatic intolerance in adolescent chronic fatigue syndrome. Pediatrics. 1999;103:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Dimitrov M, Grafman J. Neuropsychological assessment of chronic fatigue syndrome. Disability and chronic fatigue syndrome: clinical, legal and patient perspectives. Binghamton NY: Haworth Medical Press 1997; 31-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Dickson A, Toft A, O’Carroll RE. Neuropsychological functioning, illness perception, mood and quality of life in chronic fatigue syndrome, autoimmune thyroid disease and healthy participants. Psychol Med. 2009;39:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Thomas M, Smith A. An investigation into the cognitive deficits associated with chronic fatigue syndrome. Open Neurol J. 2009;3:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Constant EL, Adam S, Gillain B, Lambert M, Masquelier E, Seron X. Cognitive deficits in patients with chronic fatigue syndrome compared to those with major depressive disorder and healthy controls. Clin Neurol Neurosurg. 2011;113:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Tiersky LA, Johnson SK, Lange G, Natelson BH, DeLuca J. Neuropsychology of chronic fatigue syndrome: a critical review. J Clin Exp Neuropsychol. 1997;19:560-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 117. | DeLuca J, Johnson SK, Natelson BH. Information processing efficiency in chronic fatigue syndrome and multiple sclerosis. Arch Neurol. 1993;50:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 118. | Deluca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Chen R, Liang FX, Moriya J, Yamakawa J, Sumino H, Kanda T, Takahashi T. Chronic fatigue syndrome and the central nervous system. J Int Med Res. 2008;36:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Komaroff AL, Cho TA. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol. 2011;31:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | Gonzalez MB, Cousins JC, Doraiswamy PM. Neurobiology of chronic fatigue syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 122. | Barnden LR, Crouch B, Kwiatek R, Burnet R, Mernone A, Chryssidis S, Scroop G, Del Fante P. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. 2011;24:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 123. | Puri BK, Jakeman PM, Agour M, Gunatilake KD, Fernando KA, Gurusinghe AI, Treasaden IH, Waldman AD, Gishen P. Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3 T MRI study. Br J Radiol. 2012;85:e270-e273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 124. | Zeineh MM, Kang J, Atlas SW, Raman MM, Reiss AL, Norris JL, Valencia I, Montoya JG. Right arcuate fasciculus abnormality in chronic fatigue syndrome. Radiology. 2015;274:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 125. | de Lange FP, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JW, Toni I. Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage. 2005;26:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 126. | Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004;4:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 127. | Duffy FH, McAnulty GB, McCreary MC, Cuchural GJ, Komaroff AL. EEG spectral coherence data distinguish chronic fatigue syndrome patients from healthy controls and depressed patients--a case control study. BMC Neurol. 2011;11:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 128. | Costa DC, Tannock C, Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM. 1995;88:767-773. [PubMed] |
| 129. | Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. 2011;301:9-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 130. | Yoshiuchi K, Farkas J, Natelson BH. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. 2006;26:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Tirelli U, Chierichetti F, Tavio M, Simonelli C, Bianchin G, Zanco P, Ferlin G. Brain positron emission tomography (PET) in chronic fatigue syndrome: preliminary data. Am J Med. 1998;105:54S-58S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Murrough JW, Mao X, Collins KA, Kelly C, Andrade G, Nestadt P, Levine SM, Mathew SJ, Shungu DC. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR Biomed. 2010;23:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, Onoe K, Fukuda S, Kawabe J, Takahashi K. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET Study. J Nucl Med. 2014;55:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 134. | Natelson BH, Weaver SA, Tseng CL, Ottenweller JE. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clin Diagn Lab Immunol. 2005;12:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 135. | Schutzer SE, Angel TE, Liu T, Schepmoes AA, Clauss TR, Adkins JN, Camp DG, Holland BK, Bergquist J, Coyle PK. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLoS One. 2011;6:e17287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 136. | Lange G, Steffener J, Cook DB, Bly BM, Christodoulou C, Liu WC, Deluca J, Natelson BH. Objective evidence of cognitive complaints in Chronic Fatigue Syndrome: a BOLD fMRI study of verbal working memory. Neuroimage. 2005;26:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 137. | Cook DB, O’Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36:108-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 138. | Schmaling KB, Lewis DH, Fiedelak JI, Mahurin R, Buchwald DS. Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome. Psychosom Med. 2003;65:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | LaManca JJ, Sisto SA, DeLuca J, Johnson SK, Lange G, Pareja J, Cook S, Natelson BH. Influence of exhaustive treadmill exercise on cognitive functioning in chronic fatigue syndrome. Am J Med. 1998;105:59S-65S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond). 2012;122:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 141. | Claypoole KH, Noonan C, Mahurin RK, Goldberg J, Erickson T, Buchwald D. A twin study of cognitive function in chronic fatigue syndrome: the effects of sudden illness onset. Neuropsychology. 2007;21:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 142. | DeLuca J, Johnson SK, Ellis SP, Natelson BH. Sudden vs gradual onset of chronic fatigue syndrome differentiates individuals on cognitive and psychiatric measures. J Psychiatr Res. 1997;31:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 143. | Cockshell SJ, Mathias JL. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40:1253-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Capuron L, Welberg L, Heim C, Wagner D, Solomon L, Papanicolaou DA, Craddock RC, Miller AH, Reeves WC. Cognitive dysfunction relates to subjective report of mental fatigue in patients with chronic fatigue syndrome. Neuropsychopharmacology. 2006;31:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 145. | van de Putte EM, Böcker KB, Buitelaar J, Kenemans JL, Engelbert RH, Kuis W, Kimpen JL, Uiterwaal CS. Deficits of interference control in adolescents with chronic fatigue syndrome. Arch Pediatr Adolesc Med. 2008;162:1196-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 146. | Ickmans K, Clarys P, Nijs J, Meeus M, Aerenhouts D, Zinzen E, Aelbrecht S, Meersdom G, Lambrecht L, Pattyn N. Association between cognitive performance, physical fitness, and physical activity level in women with chronic fatigue syndrome. J Rehabil Res Dev. 2013;50:795-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 147. | Neu D, Mairesse O, Montana X, Gilson M, Corazza F, Lefevre N, Linkowski P, Le Bon O, Verbanck P. Dimensions of pure chronic fatigue: psychophysical, cognitive and biological correlates in the chronic fatigue syndrome. Eur J Appl Physiol. 2014;114:1841-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 148. | Togo F, Lange G, Natelson BH, Quigley KS. Attention network test: assessment of cognitive function in chronic fatigue syndrome. J Neuropsychol. 2015;9:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 149. | Cockshell SJ, Mathias JL. Cognitive deficits in chronic fatigue syndrome and their relationship to psychological status, symptomatology, and everyday functioning. Neuropsychology. 2013;27:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 150. | Goedendorp MM, van der Werf SP, Bleijenberg G, Tummers M, Knoop H. Does neuropsychological test performance predict outcome of cognitive behavior therapy for Chronic Fatigue Syndrome and what is the role of underperformance? J Psychosom Res. 2013;75:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 151. | Twisk FN. Underperformance of myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) patients at neurocognitive tests should be assessed objectively without an a priori judgment about the etiology. J Psychosom Res. 2014;76:339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 152. | Cockshell SJ, Mathias JL. Test effort in persons with Chronic Fatigue Syndrome when assessed using the Validity Indicator Profile. J Clin Exp Neuropsychol. 2012;34:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 153. | Stiles TL, Snell CR, Stevens SR, Moran M, VanNess JM. Post-exertional symptomology in chronic fatigue syndrome (CFS). Med Sci Sports Exerc. 2007;39:S445. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 154. | Snell CR, Stevens SR, Davenport TE, Van Ness JM. Discriminative validity of metabolic and workload measurements for identifying people with chronic fatigue syndrome. Phys Ther. 2013;93:1484-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 155. | Cockshell SJ, Mathias JL. Cognitive functioning in people with chronic fatigue syndrome: a comparison between subjective and objective measures. Neuropsychology. 2014;28:394-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 156. | Ciccolella ME, Boone T, Davenport T. Part II - Legal aspects of aerobic capacity: objective evidence of the ability to work (Prof Exerc Physiol Online 2011; 14). Available from: http://www.asep.org/asep/asep/PartIILegalMARGARET CICCOLELLA.pdf. |
| 157. | Keller BA, Pryor JL, Giloteaux L. Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO₂peak indicates functional impairment. J Transl Med. 2014;12:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 158. | Keller BA, Micale FG. Exercise testing to quantify effects of fatigue on functional capacity in patients with CFS. IACFS/ME biennial conference: Translating evidence into practice. Ottawa (Ontario) Canada 2011; 123-124. |
| 159. | Weber KT, Janicki JS. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am J Cardiol. 1985;55:22A-31A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 267] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 160. | Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 832] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 161. | Blackwood SK, MacHale SM, Power MJ, Goodwin GM, Lawrie SM. Effects of exercise on cognitive and motor function in chronic fatigue syndrome and depression. J Neurol Neurosurg Psychiatry. 1998;65:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 162. | LaManca JJ, Peckerman A, Sisto SA, DeLuca J, Cook S, Natelson BH. Cardiovascular responses of women with chronic fatigue syndrome to stressful cognitive testing before and after strenuous exercise. Psychosom Med. 2001;63:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 163. | Streeten DH, Anderson GH. The role of delayed orthostatic hypotension in the pathogenesis of chronic fatigue. Clin Auton Res. 1998;8:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 164. | Costigan A, Elliott C, McDonald C, Newton JL. Orthostatic symptoms predict functional capacity in chronic fatigue syndrome: implications for management. QJM. 2010;103:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 165. | Stewart JM, Medow MS, Messer ZR, Baugham IL, Terilli C, Ocon AJ. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1185-H1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 166. | Medow MS, Sood S, Messer Z, Dzogbeta S, Terilli C, Stewart JM. Phenylephrine alteration of cerebral blood flow during orthostasis: effect on n-back performance in chronic fatigue syndrome. J Appl Physiol (1985). 2014;117:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Caseras X, Mataix-Cols D, Giampietro V, Rimes KA, Brammer M, Zelaya F, Chalder T, Godfrey EL. Probing the working memory system in chronic fatigue syndrome: a functional magnetic resonance imaging study using the n-back task. Psychosom Med. 2006;68:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 168. | Arroll MA, Attree EA, O’Leary JM, Dancey CP. The delayed fatigue effect in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Fatigue. 2014;2:57-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 169. | Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28548] [Cited by in RCA: 31728] [Article Influence: 755.4] [Reference Citation Analysis (0)] |
| 170. | Ickmans K, Meeus M, De Kooning M, Lambrecht L, Pattyn N, Nijs J. Can recovery of peripheral muscle function predict cognitive task performance in chronic fatigue syndrome with and without fibromyalgia? Phys Ther. 2014;94:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 171. | Siemionow V, Fang Y, Calabrese L, Sahgal V, Yue GH. Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clin Neurophysiol. 2004;115:2372-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | Lawrie SM, MacHale SM, Cavanagh JT, O’Carroll RE, Goodwin GM. The difference in patterns of motor and cognitive function in chronic fatigue syndrome and severe depressive illness. Psychol Med. 2000;30:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 173. | Paul L, Wood L, Behan WM, Maclaren WM. Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. Eur J Neurol. 1999;6:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 174. | Meeus M, Ickmans K, Struyf F, Kos D, Lambrecht L, Willekens B, Cras P, Nijs J. What is in a name? Comparing diagnostic criteria for chronic fatigue syndrome with or without fibromyalgia. Clin Rheumatol. 2014;Oct 14; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 175. | van der Ploeg RJ, Oosterhuis HJ. Physical examination--measurement of muscle strength. Ned Tijdschr Geneeskd. 2001;145:19-23. [PubMed] |
| 176. | Schwartz S, Cohen ME, Herbison GJ, Shah A. Relationship between two measures of upper extremity strength: manual muscle test compared to hand-held myometry. Arch Phys Med Rehabil. 1992;73:1063-1068. [PubMed] |
| 177. | Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 463] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 178. | Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiotherapy. 2006;92:11-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 179. | Block W, Träber F, Kuhl CK, Keller E, Lamerichs R, Karitzky J, Rink H, Schild HH. 31P-mr spectroscopy of peripheral skeletal musculature under load: demonstration of normal energy metabolites compared with metabolic muscle diseases. Rofo. 1998;168:250-257. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 180. | Wong R, Lopaschuk G, Zhu G, Walker D, Catellier D, Burton D, Teo K, Collins-Nakai R, Montague T. Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest. 1992;102:1716-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 181. | Jones DE, Hollingsworth KG, Jakovljevic DG, Fattakhova G, Pairman J, Blamire AM, Trenell MI, Newton JL. Loss of capacity to recover from acidosis on repeat exercise in chronic fatigue syndrome: a case-control study. Eur J Clin Invest. 2012;42:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 182. | Katz BZ, Stewart JM, Shiraishi Y, Mears CJ, Taylor R. Autonomic symptoms at baseline and following infectious mononucleosis in a prospective cohort of adolescents. Arch Pediatr Adolesc Med. 2011;165:765-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 183. | Newton JL, Okonkwo O, Sutcliffe K, Seth A, Shin J, Jones DE. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM. 2007;100:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 184. | Rowe PC, Bou-Holaigah I, Kan JS, Calkins H. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet. 1995;345:623-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 164] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 185. | Miwa K. Cardiac dysfunction and orthostatic intolerance in patients with myalgic encephalomyelitis and a small left ventricle. Heart Vessels. 2014;Apr 16; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 186. | Wyller VB, Helland IB. Relationship between autonomic cardiovascular control, case definition, clinical symptoms, and functional disability in adolescent chronic fatigue syndrome: an exploratory study. Biopsychosoc Med. 2013;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 187. | Jason LA, Katz BZ, Shiraishi Y, Mears CJ, Im Y, Taylor R. Predictors of Post-Infectious Chronic Fatigue Syndrome in Adolescents. Health Psychol Behav Med. 2014;2:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 188. | Reynolds GK, Lewis DP, Richardson AM, Lidbury BA. Comorbidity of postural orthostatic tachycardia syndrome and chronic fatigue syndrome in an Australian cohort. J Intern Med. 2014;275:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 189. | Galland BC, Jackson PM, Sayers RM, Taylor BJ. A matched case control study of orthostatic intolerance in children/adolescents with chronic fatigue syndrome. Pediatr Res. 2008;63:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 190. | Bou-Holaigah I, Rowe PC, Kan J, Calkins H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA. 1995;274:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 193] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 191. | Hoad A, Spickett G, Elliott J, Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. QJM. 2008;101:961-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 192. | Forleo C, Guida P, Iacoviello M, Resta M, Monitillo F, Sorrentino S, Favale S. Head-up tilt testing for diagnosing vasovagal syncope: a meta-analysis. Int J Cardiol. 2013;168:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 193. | Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 790] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 194. | Karas B, Grubb BP, Boehm K, Kip K. The postural orthostatic tachycardia syndrome: a potentially treatable cause of chronic fatigue, exercise intolerance, and cognitive impairment in adolescents. Pacing Clin Electrophysiol. 2000;23:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 195. | De Lorenzo F, Hargreaves J, Kakkar VV. Pathogenesis and management of delayed orthostatic hypotension in patients with chronic fatigue syndrome. Clin Auton Res. 1997;7:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 196. | Lutgendorf SK, Antoni MH, Ironson G, Fletcher MA, Penedo F, Baum A, Schneiderman N, Klimas N. Physical symptoms of chronic fatigue syndrome are exacerbated by the stress of Hurricane Andrew. Psychosom Med. 1995;57:310-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 197. | Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol. 2012;8:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 198. | Tomas C, Newton J, Watson S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. 2013;2013:784520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 199. | Torres-Harding S, Sorenson M, Jason L, Maher K, Fletcher MA, Reynolds N, Brown M. The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. J Appl Biobehav Res. 2008;13:157-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 200. | Tak LM, Cleare AJ, Ormel J, Manoharan A, Kok IC, Wessely S, Rosmalen JG. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 201. | Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. 2005;87:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 202. | MacHale SM, Cavanagh JT, Bennie J, Carroll S, Goodwin GM, Lawrie SM. Diurnal variation of adrenocortical activity in chronic fatigue syndrome. Neuropsychobiology. 1998;38:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Gaab J, Engert V, Heitz V, Schad T, Schürmeyer TH, Ehlert U. Associations between neuroendocrine responses to the Insulin Tolerance Test and patient characteristics in chronic fatigue syndrome. J Psychosom Res. 2004;56:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 204. | De Becker P, De Meirleir K, Joos E, Campine I, Van Steenberge E, Smitz J, Velkeniers B. Dehydroepiandrosterone (DHEA) response to i.v. ACTH in patients with chronic fatigue syndrome. Horm Metab Res. 1999;31:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 205. | Gaab J, Hüster D, Peisen R, Engert V, Heitz V, Schad T, Schürmeyer TH, Ehlert U. Hypothalamic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosom Med. 2008;64:951-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 206. | Habib KE, Negrao ABYMR, Deuster P, Gold PW. Altered interrelation among plasma stress hormones during treadmill exercise in females with chronic fatigue syndrome. The 84th Annual Meeting of The Endocrine Society;. IACFS/ME biennial conference: Translating evidence into practice. Ottawa (Ontario) Canada 2002; California, 2002. |
| 207. | Visser J, van Boxel-Dezaire A, Methorst D, Brunt T, de Kloet ER, Nagelkerken L. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 1998;91:4255-4264. [PubMed] |
| 208. | Visser JT, De Kloet ER, Nagelkerken L. Altered glucocorticoid regulation of the immune response in the chronic fatigue syndrome. Ann N Y Acad Sci. 2000;917:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 209. | Jerjes WK, Taylor NF, Wood PJ, Cleare AJ. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. 2007;32:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 210. | Van Den Eede F, Moorkens G, Hulstijn W, Van Houdenhove B, Cosyns P, Sabbe BG, Claes SJ. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. Psychol Med. 2008;38:963-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 211. | Parker AJ, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med. 2001;31:1331-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 212. | Cleare AJ. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol Metab. 2004;15:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 213. | Candy B, Chalder T, Cleare AJ, Peakman A, Skowera A, Wessely S, Weinman J, Zuckerman M, Hotopf M. Predictors of fatigue following the onset of infectious mononucleosis. Psychol Med. 2003;33:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 214. | Katz BZ, Zimmerman D, Gorman MRG, Mears CJ, Shiraishi Y, Taylor R. Normal salivary cortisol and NK cell function in adolescents with chronic fatigue syndrome following infectious mononucleosis. Arch Pediatr Infect Dis. 2013;1:211-216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 215. | Whiting P, Bagnall AM, Sowden AJ, Cornell JE, Mulrow CD, Ramírez G. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA. 2001;286:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 320] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 216. | Bagnall A, Hempel S, Chambers D, Orton V, Forbes C. The treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis in adults and children. Centre for Reviews and Dissemination (CRD), University of York. [accessed from 2007]. Available from: http://www.york.ac.uk/inst/crd/CRD_Reports/crdreport35.pdf. |
| 217. | Pagana KD, Pagana TJ. Mosby’s diagnostic and laboratory test reference. 10th ed. St. Louis: Elsevier Mosby 2011; . |
| 218. | Fuite J, Vernon SD, Broderick G. Neuroendocrine and immune network re-modeling in chronic fatigue syndrome: an exploratory analysis. Genomics. 2008;92:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 219. | Nagaya T, Fujieda M, Otsuka G, Yang JP, Okamoto T, Seo H. A potential role of activated NF-kappa B in the pathogenesis of euthyroid sick syndrome. J Clin Invest. 2000;106:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 220. | Boelen A, Kwakkel J, Wiersinga WM, Fliers E. Chronic local inflammation in mice results in decreased TRH and type 3 deiodinase mRNA expression in the hypothalamic paraventricular nucleus independently of diminished food intake. J Endocrinol. 2006;191:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 221. | Bartalena L, Bogazzi F, Brogioni S, Grasso L, Martino E. Role of cytokines in the pathogenesis of the euthyroid sick syndrome. Eur J Endocrinol. 1998;138:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 222. | Broderick G, Craddock RC, Whistler T, Taylor R, Klimas N, Unger ER. Identifying illness parameters in fatiguing syndromes using classical projection methods. Pharmacogenomics. 2006;7:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 223. | Englebienne P, Verhas M, Herst CV, De Meirleir K. Type I interferons induce proteins susceptible to act as thyroid receptor (TR) corepressors and to signal the TR for destruction by the proteasome: possible etiology for unexplained chronic fatigue. Med Hypotheses. 2003;60:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 224. | Tjørve E, Tjørve KM, Olsen JO, Senum R, Oftebro H. On commonness and rarity of thyroid hormone resistance: a discussion based on mechanisms of reduced sensitivity in peripheral tissues. Med Hypotheses. 2007;69:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 225. | Chopra IJ. An assessment of daily production and significance of thyroidal secretion of 3, 3’, 5’-triiodothyronine (reverse T3) in man. J Clin Invest. 1976;58:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 164] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 226. | Wiersinga WM, Fliers E. Determining the thyroid hormones T3 and T4 in the urine: an unreliable test for hypothyroidism. Ned Tijdschr Geneeskd. 2007;151:2813-2815. [PubMed] |
| 227. | Moncayo H, Dapunt O, Moncayo R. Diagnostic accuracy of basal TSH determinations based on the intravenous TRH stimulation test: an evaluation of 2570 tests and comparison with the literature. BMC Endocr Disord. 2007;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 228. | Hilgers A, Frank J. Chronic fatigue syndrome: evaluation of a 30-criteria score and correlation with immune activation. J Chronic Fatigue Syndr. 1996;2:35-47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 229. | Dunlap DB. Thyroid function tests. The history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths 1990; . |
| 230. | Unger ER, Nisenbaum R, Moldofsky H, Cesta A, Sammut C, Reyes M, Reeves WC. Sleep assessment in a population-based study of chronic fatigue syndrome. BMC Neurol. 2004;4:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 231. | Jackson ML, Bruck D. Sleep abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: a review. J Clin Sleep Med. 2012;8:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 232. | Hamaguchi M, Kawahito Y, Takeda N, Kato T, Kojima T. Characteristics of chronic fatigue syndrome in a Japanese community population : chronic fatigue syndrome in Japan. Clin Rheumatol. 2011;30:895-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 233. | Kishi A, Natelson BH, Togo F, Struzik ZR, Rapoport DM, Yamamoto Y. Sleep-stage dynamics in patients with chronic fatigue syndrome with or without fibromyalgia. Sleep. 2011;34:1551-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 234. | Spitzer AR, Broadman M. A retrospective review of the sleep characteristics in patients with chronic fatigue syndrome and fibromyalgia. Pain Pract. 2010;10:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 235. | Decker MJ, Tabassum H, Lin JM, Reeves WC. Electroencephalographic correlates of Chronic Fatigue Syndrome. Behav Brain Funct. 2009;5:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 236. | Togo F, Natelson BH, Cherniack NS, FitzGibbons J, Garcon C, Rapoport DM. Sleep structure and sleepiness in chronic fatigue syndrome with or without coexisting fibromyalgia. Arthritis Res Ther. 2008;10:R56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 237. | Kishi A, Struzik ZR, Natelson BH, Togo F, Yamamoto Y. Dynamics of sleep stage transitions in healthy humans and patients with chronic fatigue syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1980-R1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 238. | Krupp LB, Jandorf L, Coyle PK, Mendelson WB. Sleep disturbance in chronic fatigue syndrome. J Psychosom Res. 1993;37:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 239. | Neu D, Mairesse O, Verbanck P, Linkowski P, Le Bon O. Non-REM sleep EEG power distribution in fatigue and sleepiness. J Psychosom Res. 2014;76:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 240. | Leslie SB. Chronic fatigue syndrome: optometric clinical presentation and management. J Behav Optom. 1997;8:155-161. |
| 241. | Vedelago LJ. Visual dysfunction in chronic fatigue syndrome: behavioural optometric assessment and management. J Behav Optom. 1997;8:149-154. |
| 242. | Hutchinson CV, Maltby J, Badham SP, Jason LA. Vision-related symptoms as a clinical feature of chronic fatigue syndrome/myalgic encephalomyelitis? Evidence from the DePaul symptom questionnaire. Br J Ophthalmol. 2014;98:144-145. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 243. | Hutchinson CV, Badham SP. Patterns of abnormal visual attention in myalgic encephalomyelitis. Optom Vis Sci. 2013;90:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 244. | Badham SP, Hutchinson CV. Characterising eye movement dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. Graefes Arch Clin Exp Ophthalmol. 2013;251:2769-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 245. | Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 246. | Klimas NG, Koneru AO. Chronic fatigue syndrome: inflammation, immune function, and neuroendocrine interactions. Curr Rheumatol Rep. 2007;9:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 247. | McCully KK, Natelson BH, Iotti S, Sisto S, Leigh JS. Reduced oxidative muscle metabolism in chronic fatigue syndrome. Muscle Nerve. 1996;19:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 248. | Vernon SD, Whistler T, Cameron B, Hickie IB, Reeves WC, Lloyd A. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect Dis. 2006;6:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 249. | Booth NE, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int J Clin Exp Med. 2012;5:208-220. [PubMed] |
| 250. | Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 251. | Hokama Y, Campora CE, Hara C, Kuribayashi T, Le Huynh D, Yabusaki K. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J Clin Lab Anal. 2009;23:210-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 252. | Javierre C, Alegre J, Ventura JL, Garcia-Quintana A, Segura R, Suarez A, Morales A, Comella A, De Meirleir K. Physiological responses to arm and leg exercise in women patients with chronic fatigue syndrome. J Chronic Fatigue Syndr. 2007;1:43-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 253. | McCully KK, Natelson BH. Impaired oxygen delivery to muscle in chronic fatigue syndrome. Clin Sci (Lond). 1999;97:603-608; discussion 611-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 254. | Arnold DL, Bore PJ, Radda GK, Styles P, Taylor DJ. Excessive intracellular acidosis of skeletal muscle on exercise in a patient with a post-viral exhaustion/fatigue syndrome. A 31P nuclear magnetic resonance study. Lancet. 1984;1:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 255. | Lane RJ, Barrett MC, Woodrow D, Moss J, Fletcher R, Archard LC. Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1998;64:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 256. | Nijs J, Meeus M, Van Oosterwijck J, Ickmans K, Moorkens G, Hans G, De Clerck LS. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur J Clin Invest. 2012;42:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 257. | Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 258. | Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 639] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 259. | Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 260. | Little JW, Doyle T, Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids. 2012;42:75-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 261. | Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, Light KC. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J Intern Med. 2012;271:64-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 262. | Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10:1099-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 263. | Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med. 1996;101:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 264. | Anderson VR, Jason LA, Hlavaty LE. A qualitative natural history study of ME/CFS in the community. Health Care Women Int. 2014;35:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 265. | Taylor RR, O’Brien J, Kielhofner G, Lee SW, Katz B, Mears C. The occupational and quality of life consequences of chronic fatigue syndrome/myalgic encephalomyelitis in young people. Br J Occup Ther. 2010;73:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 266. | Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J Nerv Ment Dis. 1997;185:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 267. | Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130:910-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 547] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 268. | Maes M, Twisk FN. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med. 2010;8:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 269. | Wojcik W, Armstrong D, Kanaan R. Chronic fatigue syndrome: labels, meanings and consequences. J Psychosom Res. 2011;70:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 270. | Thomas MA, Smith AP. Primary healthcare provision and Chronic Fatigue Syndrome: a survey of patients’ and General Practitioners’ beliefs. BMC Fam Pract. 2005;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 271. | Gilje AM, Söderlund A, Malterud K. Obstructions for quality care experienced by patients with chronic fatigue syndrome (CFS)--a case study. Patient Educ Couns. 2008;73:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 272. | Asbring P. Chronic illness -- a disruption in life: identity-transformation among women with chronic fatigue syndrome and fibromyalgia. J Adv Nurs. 2001;34:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 273. | Garralda ME, Rangel L. Impairment and coping in children and adolescents with chronic fatigue syndrome: a comparative study with other paediatric disorders. J Child Psychol Psychiatry. 2004;45:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 274. | Nijhof SL, Maijer K, Bleijenberg G, Uiterwaal CS, Kimpen JL, van de Putte EM. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. 2011;127:e1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 275. | Crawley E, Sterne JA. Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child. 2009;94:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 276. | Bould H, Collin SM, Lewis G, Rimes K, Crawley E. Depression in paediatric chronic fatigue syndrome. Arch Dis Child. 2013;98:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 277. | Katz BZ, Jason LA. Chronic fatigue syndrome following infections in adolescents. Curr Opin Pediatr. 2013;25:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 278. | Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 546] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 279. | Donalek JG. When a parent is chronically ill: chronic fatigue syndrome. Nurs Res. 2009;58:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 280. | Winger A, Ekstedt M, Wyller VB, Helseth S. ‘Sometimes it feels as if the world goes on without me’: adolescents’ experiences of living with chronic fatigue syndrome. J Clin Nurs. 2014;23:2649-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 281. | Whitehead LC. Quest, chaos and restitution: living with chronic fatigue syndrome/myalgic encephalomyelitis. Soc Sci Med. 2006;62:2236-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 282. | Tuck I, Wallace D. Chronic fatigue syndrome: a woman’s dilemma. Health Care Women Int. 2000;21:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 283. | Jason LA, Taylor RR, Stepanek Z, Plioplys S. Attitudes regarding chronic fatigue syndrome: the importance of a name. J Health Psychol. 2001;6:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 284. | McInnis OA, Matheson K, Anisman H. Living with the unexplained: coping, distress, and depression among women with chronic fatigue syndrome and/or fibromyalgia compared to an autoimmune disorder. Anxiety Stress Coping. 2014;27:601-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 285. | Raine R, Carter S, Sensky T, Black N. General practitioners’ perceptions of chronic fatigue syndrome and beliefs about its management, compared with irritable bowel syndrome: qualitative study. BMJ. 2004;328:1354-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 286. | Wood B, Wessely S. Personality and social attitudes in chronic fatigue syndrome. J Psychosom Res. 1999;47:385-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 287. | Ciccolella M, Stevens SR, Snell CR, Mark VanNess J. Legal and scientific considerations of the exercise stress test. Journ of Chr Fatigue Syndr. 2007;14:61-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 288. | Knoop H, Bleijenberg G, Gielissen MF, van der Meer JW, White PD. Is a full recovery possible after cognitive behavioural therapy for chronic fatigue syndrome? Psychother Psychosom. 2007;76:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 289. | Heins MJ, Knoop H, Prins JB, Stulemeijer M, van der Meer JW, Bleijenberg G. Possible detrimental effects of cognitive behaviour therapy for chronic fatigue syndrome. Psychother Psychosom. 2010;79:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 290. | Twisk FN, Arnoldus RJ. Graded exercise therapy (GET)/cognitive behavioural therapy (CBT) is often counterproductive in myalgic encephalomyelitis (ME) and chronic fatigue syndrome (CFS). Eur J Clin Invest. 2012;42:1255-1256; author reply 1257-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 291. | Wiborg JF, Knoop H, Stulemeijer M, Prins JB, Bleijenberg G. How does cognitive behaviour therapy reduce fatigue in patients with chronic fatigue syndrome? The role of physical activity. Psychol Med. 2010;40:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 292. | [Evaluation Report 2002-2004 with respect to rehabilitation contracts between the RIZIV and the CFS Reference Centers] [Dutch] Evaluation Report (2002- 2004) with respect to Rehabilitation Contracts between the RIZIV and the CFS Reference Centers. 2006, July. . |
| 293. | Kindlon T. Objective compliance and outcome measures should be used in trials of exercise interventions for Chronic Fatigue Syndrome. Eur J Clin Invest. 2012;42:1360-1361; author reply 1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |