Published online Jun 26, 2014. doi: 10.5662/wjm.v4.i2.91
Revised: February 10, 2014
Accepted: April 11, 2014
Published online: June 26, 2014
Processing time: 267 Days and 15 Hours
Autoimmune inner ear disease (AIED) represents a very fertile research field and the advancements in the understanding of this disease have a direct application not only in patients affected with this condition but also in other inner ear disorders that share the same injury mechanism, damage to the inner ear hair cells. AIED also presents many challenges that have still to be overcome. Firstly, access to the inner ear is limited, as many interventions such as biopsies can result in great irreversible damage. Secondly, there are no completely specific markers for AIED. Lack of a definitive diagnosis can result in the treatment of patients not affected with the disease and, therefore, no response. Finally, some patients become refractory to glucocorticoids and new therapies are needed. This review offers an overview of the animal models that have contributed to the understanding of AIED pathophysiology, the value of currently available diagnostic tests, and therapeutic options, with a special focus on new therapies for non responders or patients refractory to glucocorticoids. Among these new options for therapy, biological agents have been tested recently, whereas gene and stem cell therapy may have a role in the future. The intratympanic route of administration avoids the systemic side effects associated with currently used drugs, and may become a more frequent approach in the future.
Core tip: Readers interested in inner ear pathology will find in this review a brief summary of autoimmune inner ear disease, with special focus on the major advances achieved in the knowledge of its etiology and pathophysiology, and the diagnostic and therapeutic challenges that remain and may guide research in the next few years and beyond.
- Citation: Lobo DR, García-Berrocal JR, Ramírez-Camacho R. New prospects in the diagnosis and treatment of immune-mediated inner ear disease. World J Methodol 2014; 4(2): 91-98
- URL: https://www.wjgnet.com/2222-0682/full/v4/i2/91.htm
- DOI: https://dx.doi.org/10.5662/wjm.v4.i2.91
In 1979, Brian McCabe proposed a new clinical entity which he called autoimmune sensorineural hearing loss on the basis of a clinical and diagnostic study of a series of 18 patients and the experience acquired in their treatment[1]. He defined the disease as a bilateral, generally asymmetric hearing loss that progresses in the course of weeks or months and responds to immunosuppressive therapy. This last characteristic is essential because this disease is one of the few forms of sensorineural hearing loss potentially reversible with medical treatment.
The concept of autoimmunity was not fully accepted by the scientific community until the late fifties and early sixties. Autoimmunity is defined as an immune reaction against the body’s own antigens. Although the etiology of autoimmune inner ear disease (AIED) is not well known, several etiopathogenic mechanisms that could explain this autoimmune reaction are similar to those giving rise to other autoimmune diseases. These mechanisms involve autoreactive CD4+ T cells. However, there is another group of diseases, called autoinflammatory diseases, in which the chronic inflammatory reaction is not mediated by T cells. This group includes Muckle-Wells syndrome, which presents clinical features similar to those of other autoimmune diseases, and may cause sensorineural hearing loss, but this does not respond to corticosteroids and a genetic origin has been proposed[2]. The involvement of CD4+ T cells is because not all autoreactive T cells are eliminated in the thymus. While this elimination process is efficient with most antigens expressed in the human body, this is not the case with the less frequent antigens, such as those expressed in the inner ear. Fortunately there are other regulatory mechanisms that prevent the activation of these T lymphocytes.
The autoimmune reaction in AIED could be initiated by an autoimmune attack when the immune system tries to protect the inner ear against infection or external insult[3]. Viruses or pathogenic bacteria can reach the inner ear from the bloodstream, cerebrospinal fluid or middle ear and could contribute to the autoimmune response by altering host molecules so that they become self-antigens[4]. Lesions caused by surgery, trauma or drugs can expose inner ear antigens to the immune system, thereby inducing an immune response against both ears. Moreover, these insults can result in permanent tissue damage which can trigger an immune response in the future.
According to the revised Witebsky postulates proposed by Rose et al[5], there are three levels of evidence of an autoimmune disease: direct, indirect and circumstantial. Direct evidence requires the transmission of the characteristic lesions from human to human or from human to animal. This could occur, for instance, if the clinical features are reproduced in newborns from mothers with AIED, or in animals after injecting them with antibodies detected in AIED patients. However, in AIED most of the evidence is indirect or circumstantial. Indirect evidence is based on the re-creation of the human disease in an animal model by transferring antibodies or autoimmune T cells, or on the use of animal models of multisystem autoimmune disease. Circumstantial evidence found in AIED includes a family history of autoimmune disease, coexistence with other autoimmune diseases (such as systemic lupus erythematosus, Behçet’s disease, Wegener granulomatosis, relapsing polychondritis among others), predominance of certain major histocompatibility complex alleles (DR4-, cw7+, cw4+, B35), raised immunoglobulin G antibodies titers, and clinical response to immunosuppressive therapy. In AIED patients it is impossible to obtain other circumstantial evidence such as the presence of mononuclear cell infiltrate or of antigen-antibody complex deposits in the inner ear because the inner ear cannot be biopsied, although this evidence can be detected in animal models.
The main animal models that have contributed to the understanding of the pathophysiology of AIED are as follows.
This was the first animal model ever employed to study inner ear autoimmunity[6]. The results obtained have been variable, ranging from non-histopathological changes to the development of endolymphatic hydrops, edema, hemorrhage, or perivascular inflammatory infiltrates. This model employs an inner ear homogenate and cannot, therefore, characterize specific autoantigens involved in the development of AIED[7].
The activation of T lymphocytes is achieved by employing both inner ear homogenates and specific peptides such as cochlin, which is highly expressed in the inner ear, or beta-tectorin[8-10].
Monoclonal autoantibodies against inner ear cells have been generated by immunizing mice with guinea pig or chicken inner ear extracts. KHRI-3 binds to the supporting cells in the organ of Corti causing hearing loss in guinea pigs[11]. The lack of infiltrates suggests that the lesions of the stria vascularis, spiral ligament or supporting cells are mediated by antibodies or immune complexes[12].
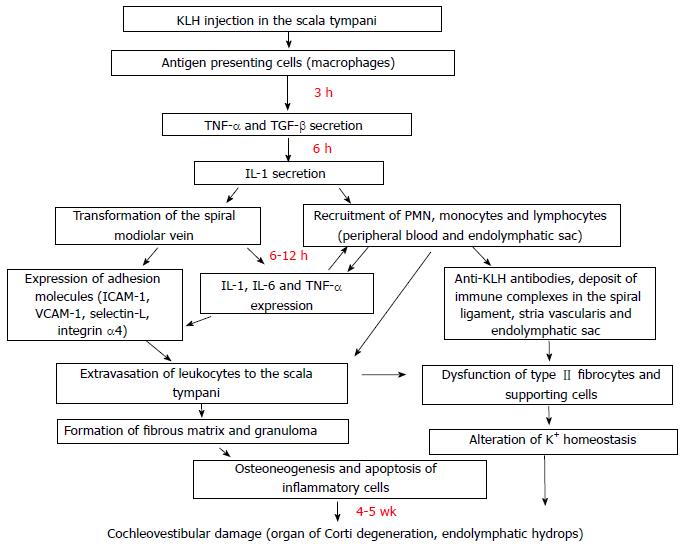
This model was developed by Harris and coworkers and has contributed greatly to the knowledge of AIED pathophysiology. The endolymphatic sac contains immune system cells capable of inducing or reinforcing an immune response[13]. The spiral vein of modiolus is the entryway for immunologic elements (T cells, B cells, natural killer (NK) cells, polymorphonuclear cells, macrophages) that can induce a labyrinthitis that results in functional impairment with loss of sensorial cells and ultimately leads to cochlear fibrosis and osteoneogenesis (Figure 1)[14,15].
The incidence of AIED is not well-known because there is no definitive diagnostic test. Nevertheless, it is considered to be less frequent than sudden deafness, with 1 case out of 5000-10000 people per year. Like other autoimmune diseases, it appears to be more frequent in women. It generally presents between the ages of 20 to 50 years, and it is uncommon in childhood[16].
AIED can present alone or associated with other autoimmune systemic disease (secondary AIED). Generally, three forms of clinical presentation are recognized: sudden deafness, rapidly progressive hearing loss, and fluctuating hearing loss. Vertigo appears in almost 50% of cases, making AIED difficult to distinguish from Meniere’s disease[17]. Eventually, AIED can affect both ears and progress to deafness unless a correct diagnosis is made and prompt treatment is established. Since this clinical picture is not specific, and could cover almost all inner ear disorders, diagnostic criteria are needed to orientate the introduction of medical treatment.
A prompt diagnosis and treatment has a great impact on the hearing prognosis of patients with AIED and thus has stimulated the search for specific markers of inner ear inflammation. The presence of autoantibodies is usually the first step in recognizing the autoimmune nature of a disease, but this is not enough as autoantibodies are also common in people without an autoimmune disease. In fact, autoantibodies against specific cochlear antigens have a low specificity for rapidly progressive sensorineural hearing loss. Although other localized autoimmune diseases such as pemphigus or cutaneous vasculitis are diagnosed by immunofluorescence in biopsied tissues, this is not possible in the case of AIED because an inner ear biopsy represents the destruction of the organ and its function.
The diagnosis of AIED is based fundamentally on clinical evaluation, the demonstration of a progressive sensorineural hearing loss in periodic audiological tests and a response to immunomodulatory drugs such as corticosteroids.
Once other causes of sensorineural hearing loss have been ruled out, patients undergo a battery of non-specific test common to other autoimmune diseases: blood tests, biochemistry, erythrocyte sedimentation rate, etc. In certain cases, serological studies can detect some specific autoantibodies, but there are no immunological or serological tests that are specific or sensitive enough to establish a definitive diagnosis[18]. A summary of the main specific and non-specific autoantibodies found in AIED patients and experimental models is given in Table 1. Most studies deal with one or several autoantibodies and no single study has examined them all in the same population.
| Specific autoantibodies |
| Collagen type II |
| Collagen type IX |
| Cochlin |
| DEP-1/CD 148 |
| KHRI-3 |
| Myelin protein P0 |
| Raf-1 |
| Beta-tectorin |
| Beta-actin |
| Connexin 26 |
| Non-specific autoantibodies |
| Antinuclear antibodies |
| Anti-neutrophil cytoplasmic antibodies |
| Anti-endothelial cell antibodies |
| Rheumatoid factor |
| Heat shock protein of 70 kDa |
| Anti-phospholipids/anticardiolipin antibodies |
| Antithyroid antibodies |
Numerous tests have been proposed, antibodies against collagen type II, endothelial cells, sulfoglucoronosyl glycolipids, major peripheral protein P0, etc (Table 1). The role of immune complexes and changes in blood lymphocytic populations has also been studied[19].
Harris and Sharp proposed this technique to identify specific autoantibodies against inner ear antigens in immunized animals and in patients with sensorineural hearing loss[20]. Among these autoantibodies, the most studied is an antibody that binds to a 68 kD antigen derived from a bovine temporal bone extract and the inducible form of heat shock protein 70 (HSP-70)[21]. HSP-70 is expressed in a variety of pathological inner ear conditions, as a marker of early cell damage, but is not specific. In AIED patients, the frequency of antibodies against HSP-70 is not different in patients and controls and is not useful in the diagnosis of AIED[22]. Mice immunized with HSP-70 produce anti HSP-70 antibodies without presenting a hearing loss, which indicates that these antibodies are not directly involved in the pathogenesis of AIED. However, these antibodies could have a role as markers of disease activity and treatment response.
Neither magnetic resonance imaging (MRI) nor positron emission tomography (PET) has demonstrated their utility in the diagnosis of AIED in spite of early promising results.
Although specific tests have an unquestionable value, there is no currently available test that has proved to be effective. For this reason, the development of diagnostic profiles can contribute to cost saving by restricting the diagnostic tests to those which are really cost-effective[23]. A proposed diagnostic profile for AIED is shown in Table 2. An AIED is suspected when three major criteria or two major and two minor criteria are met.
| Major criteria |
| Bilateral hearing loss |
| Systemic autoimmune disease |
| ANA > 1:80 |
| Decrease of native T cells (CD4CD45RA) |
| Hearing recovery rate > 80% |
| Minor criteria |
| Unilateral hearing loss |
| Young or middle aged |
| Woman |
| Hearing recovery rate < 80% |
The treatment most widely used for AIED is corticosteroids therapy. The initial dosage regimen is 60 mg or 1 mg/kg per day of prednisone or 6-methylprednisolone for a month. Shorter courses or lower doses have proved to be ineffective and increase the risk of relapse[24]. In rapidly progressive forms 1 mg/kg per day is maintained for 4 wk until the audiogram is stable and the dose is then tapered over 8 wk to 10-20 mg per day, which is maintained for another 6 wk. In cases of sudden hearing loss, 1 mg/kg per day of 6-methylprednisolone is administered for four weeks. In severe hearing loss (over 70 dB) three pulses of 500 mg are administered, and then the above-mentioned dosage regimen is applied. When patients receive high doses of corticosteroids, active tuberculosis must be ruled out, and glycemia, potassium and blood pressure must be monitored. Tapering must be gradual, slower if glucocorticoids have been given at higher doses or for a longer time.
In AIED patients, severe adverse reactions have rarely been reported (0%-0.9%) though they may be more frequent when high dose intravenous pulse corticosteroids are employed. The overall rate of side effects is not greater than 7.8%[25].
Some patients do not respond to corticoids or require high doses to control the disease, and other immunosuppressants such as methotrexate or cyclophosphamide have been tried. The empirical basis for using these drugs is the observation that in certain cases their effect enhances that of the corticosteroids, thus obtaining remission of one or more symptoms that is not achieved with corticosteroids alone, or allowing reduction of the required dose of corticosteroids to maintain the patient symptom-free.
Methotrexate: A meta-analysis showed that there was no benefit with methotrexate compared with corticosteroids alone[26]. However, vertigo or instability can improve with long treatments.
The most frequently employed regimen is 7.5 mg weekly administered in one single dose. Once the response is achieved, the drug is given orally (15 mg weekly) for 12 mo. Methotrexate is associated with blood toxicity (leukopenia, thrombocytopenia), liver toxicity (elevated liver enzymes, periportal fibrosis, cirrhosis) and gastrointestinal toxicity (nausea, vomiting, mucositis). Folic acid supplements reduce the adverse effects, preserve its efficacy and are, therefore, recommended.
Cyclophosphamide: This drug was used by McCabe[1], who advocated its use as the treatment of choice, in his original series of cases. However, because of its adverse effect profile (gonadal, bladder and bone marrow toxicity) it is not frequently used and is limited to those patients who do not respond to corticosteroids or do not maintain their response after dose tapering. The oral dose is 1-2 mg/kg per day for 4-6 wk. Intravenously, the starting dose is 0.75 g/m2 or 0.5 g/m2 if the glomerular filtration rate is lower than a third of the normal value, and this is repeated every 1-3 mo. The white cell count should not be lower than 2000/mm3 and neutrophils should remain over 1000/mm3. When both cyclophosphamide and high doses of corticosteroids are employed trimethoprim/sulphamethoxazole or dapsone is administered to prevent Pneumocystis carinii pneumonia.
This procedure allows the blood to be separated into its two components - blood cells and plasma - and allows some components such as antibodies to be removed before the cells and plasma are transfused back to the patient. In a long term study performed in AIED, 50% of the patients achieved an improvement or stabilization of hearing loss after this therapy[27].
The overall response rate to corticosteroids is 60%, but the response rate varies considerably. In most responders the dose can be lowered or corticosteroids can be withdrawn without relapse, but some patients can present a corticosteroid-dependant hearing loss. Hearing loss may become refractory to corticosteroids, and other immunosuppressants should be considered in these cases. Finally, treatment can result in unacceptable adverse reactions (gastritis, peptic ulcer, fluid retention, glucose intolerance, avascular necrosis of the femoral head, psychiatric problems, sleep disorders, cataracts, osteoporosis, cushingoid habitus) and this has prompted the search for new drugs or different modes of administration such as the intratympanic route.
The use of intratympanic corticosteroids is an attractive therapeutic approach because it is minimally invasive and, since the drug is applied directly to the affected ear, side effects are minimized. However, there is no consensus regarding the doses and length of treatment. Moreover, it is not easy to control the dose that actually enters the inner ear (part of it is absorbed in the middle ear and part is eliminated through the Eustachian tube); as a result, its efficacy has so far not been fully determined[28].
Biological therapy agents are fusion proteins (made from a fusion gene, which is created by joining parts of two or more genes) or monoclonal antibodies designed to block specific components of the inflammatory cascade. Tumor necrosis factor α inhibitors and lymphocyte CD20 receptor antagonists have recently been tested on AIED patients (Table 3).
| Drug | Anti | Dosage | Licensed indications | EMA approval | FDA approval |
| Etanercept | TNF-α | 25 mg × 2/wk or 50 mg/wk sc | RA, JRA, PsA, AS, Ps | 2000 | 1998 |
| Infliximab | TNF-α | 3 mg/kg at 0, 2 and 6 wk followed by 3.5-7.5 mg/8 wk iv | RA, PsA, AS, Ps, UC, CD | 1999 | 1998 |
| Adalimumab | TNF-α | 40 mg/1-2 wk sc | RA, JRA, PsA, AS, Ps, CD | 2003 | 2002 |
| Anakinra | IL-1 | 100 mg/d sc | NHL, CLL, RA | 2002 | 2001 |
| Rituximab | B-cell CD20R | 1 g/wk × 2 iv | RA | 1998 | 1997 |
Among the biological therapy agents the most frequently used are tumor necrosis factor alpha blockers. Tumor necrosis factor (TNF) is a proinflammatory cytokine produced by multiple cells, especially macrophages, that stimulates the maturation and migration of dendritic cells, activates neutrophils and NK cells, and increases vascular permeability. It was isolated by Carswell et al[29] in 1975 when they were seeking to identify the factors responsible for Meth A sarcoma necrosis. It is expressed early in the inflammatory response in different inner ear structures. Of the different TNF-α blockers that have been developed, etanercept, infliximab and adalimumab have been tested on AIED patients. X-ray or Mantoux screening is recommended before initiating treatment with TNF-α blockers because TNF-α is a key component in the body’s defense against M. tuberculosis and other granulomatous diseases.
Etanercept: The results obtained so far are promising but not conclusive, as very few studies have been performed[30]. Anecdotically, it has been used together with methotrexate with good results, allowing corticosteroid therapy to be withdrawn[31]. The usual dose is 25 mg administered by subcutaneous injection twice a week or 50 mg once a week for an indefinite period of time. Side effects that have been a concern are infections including tuberculosis and sepsis, tumors such as lymphomas, anemia and pancytopenia, demyelinating diseases, congestive heart failure and hypersensitivity. However, a meta-analysis that examined the adverse reactions with etanercept and other biologic therapies in 163 randomized controlled studies with 50010 participants and 46 extension studies with 11954 participants reported that the severe adverse reactions rate for the biological products was not different from that of the control therapy (e.g., corticosteroids)[32].
Adalimumab: It is administered by a subcutaneous injection of 40 mg every two weeks for an indefinite period of time. The dose can be increased to 40 mg weekly if a decrease in the response is observed. It has been employed successfully in one patient with autoimmune sensorineural hearing loss and rheumatoid arthritis[33].
Infliximab: The usual regimen is slow intravenous infusion (2 h) of 3 mg/kg at the start of treatment, and at 2 and 6 wk, followed by maintenance therapy every 8 wk indefinitely. Intratympanic administration of infliximab can help to reduce corticosteroids doses in patients with AIED[34].
B lymphocyte CD20 receptor antagonist: Apart from TNF-α blockers other biological therapy agents such as rituximab have been recently tested on patients with AIED. Rituximab is a chimeric monoclonal antibody that binds to the CD20 receptor of B lymphocytes, thereby inducing apoptosis and reducing their number. The few studies that have used rituximab in AIED patients have yielded encouraging results[35,36]. However, more studies are needed for reliable conclusions to be reached. The recommended dose is 1000 mg in intravenous injection, followed by a second injection perfusion of 1000 mg 2 wk later. The most common side effect associated with rituximab is a reaction to the injection (low blood pressure, nausea, eruption, fever, itching, urticaria, throat irritation, tachycardia, peripheral edema). Infections of the upper airway and urinary tract have also been reported (but not in AIED patients).
Anakinra is an IL-1 inhibitor that has been successfully used in chronic infantile neurological cutaneous and articular (CINCA) syndrome and Muckle-Wells syndrome, which can present with hearing loss and belong to a group of autoinflammatory febrile syndromes caused by mutations in the CIAS/NALP3 gene on chromosome 1q4432[37]. These mutations seem to interrupt apoptosis mechanisms and lead to overexpression of IL-1 with devastating proinflammatory effects. The role that anakinra could have in the management of AIED has yet to be elucidated (Table 3).
Other lines of AIED therapy not available at present are represented by gene therapy and stem cell therapy, which would attempt to repair established damage to the inner ear. These therapeutic strategies are based on the knowledge of cell signaling routes involved in the development of the cochlear sensorial epithelium during embryogenesis. This sensorial epithelium derives from a group of cells that after several divisions start to differentiate into hair cells and supporting cells. Adult mammals have lost the capacity to regenerate damaged hair cells[38]. Gene and stem cell therapy attempt to revert this situation[39]. However, both approaches present the same hazards and difficulties: access to the whole cochlea, integration and maturation of hair cells in the correct position within the cochlea and not in ectopic locations, and risk of tumor development[40]. At present, these difficulties need to be overcome before clinical trials can be started.
Different animal models of experimental labyrinthitis have contributed to the understanding of AIED pathophysiology. In particular, the model of experimental labyrinthitis by KLH has allowed a chronological sequence of inner ear damage to be established and has, therefore, provided a rational basis for testing new therapies.
In spite of all the efforts to find a good marker for the disease, the available tests are not specific or sensitive enough to establish a definitive diagnosis. However, the search for specific autoantibodies for AIED remains a valid approach, because the diagnostic value of autoantibodies depends on a statistical and epidemiological association with disease more than on a cause-effect relation. It would be very useful to study how these autoantibody titers vary with time and with response to therapy. Moreover, some autoantibodies could provide information on group of patients with different prognoses or different clinical responses.
Finally, new treatments have been tested recently. Biologics, a new family of immunomodulatory agents, could play a role in the treatment of AIED in the future, and the first studies conducted with these drugs have produced promising results. They could be indicated in patients who do not respond to, or who have become refractory to, glucocorticoids. However, more clinical studies are necessary to evaluate their real value. Intratympanic therapy avoids many of the adverse reactions associated with currently used drugs, but this approach has not been sufficiently evaluated yet.
P- Reviewer: Hong YR, Schuurman HJ S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88:585-589. [PubMed] |
| 2. | Biswas D, Stafford N. Otolaryngological manifestations of ‘Muckle-Wells syndrome’. Int J Pediatr Otorhinolaryngol. 2010;74:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J Clin Invest. 2004;113:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Fukuda S, Keithley EM, Harris JP. Experimental cytomegalovirus infection: viremic spread to the inner ear. Am J Otolaryngol. 1988;9:135-141. [PubMed] |
| 5. | Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol Today. 1993;14:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 505] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Beickert P. On the problem of perception deafness and autoallergy. Z Laryngol Rhinol Otol. 1961;40:837-842. [PubMed] |
| 7. | Yamanobe S, Harris JP. Spontaneous remission in experimental autoimmune labyrinthitis. Ann Otol Rhinol Laryngol. 1992;101:1007-1014. [PubMed] |
| 8. | Gloddek B, Arnold W. Clinical and experimental studies of autoimmune inner ear disease. Acta Otolaryngol Suppl. 2002;10-14. [PubMed] |
| 9. | Solares CA, Edling AE, Johnson JM, Baek MJ, Hirose K, Hughes GB, Tuohy VK. Murine autoimmune hearing loss mediated by CD4+ T cells specific for inner ear peptides. J Clin Invest. 2004;113:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Billings P. Experimental autoimmune hearing loss. J Clin Invest. 2004;113:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Nair TS, Prieskorn DM, Miller JM, Mori A, Gray J, Carey TE. In vivo binding and hearing loss after intracochlear infusion of KHRI-3 antibody. Hear Res. 1997;107:93-101. [PubMed] |
| 12. | García-Berrocal JR, Ramírez-Camacho R, Trinidad A, Zurita M, de la Fuente R, Lobo D. Controversies and criticisms on designs for experimental autoimmune labyrinthitis. Ann Otol Rhinol Laryngol. 2004;113:404-410. [PubMed] |
| 13. | Tomiyama S, Harris JP. The endolymphatic sac: its importance in inner ear immune responses. Laryngoscope. 1986;96:685-691. [PubMed] |
| 14. | Harris JP, Fukuda S, Keithley EM. Spiral modiolar vein: its importance in inner ear inflammation. Acta Otolaryngol. 1990;110:357-365. [PubMed] |
| 15. | Ma C, Billings P, Harris JP, Keithley EM. Characterization of an experimentally induced inner ear immune response. Laryngoscope. 2000;110:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Huang NC, Sataloff RT. Autoimmune inner ear disease in children. Otol Neurotol. 2011;32:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Hughes GB, Barna BP, Kinney SE, Calabrese LH, Nalepa NJ. Clinical diagnosis of immune inner-ear disease. Laryngoscope. 1988;98:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Lobo D, López FG, García-Berrocal JR, Ramírez-Camacho R. Diagnostic tests for immunomediated hearing loss: a systematic review. J Laryngol Otol. 2008;122:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Veldman JE, Roord JJ, O’Connor AF, Shea JJ. Autoimmunity and inner ear disorders: an immune-complex mediated sensorineural hearing loss. Laryngoscope. 1984;94:501-507. [PubMed] |
| 20. | Harris JP, Sharp PA. Inner ear autoantibodies in patients with rapidly progressive sensorineural hearing loss. Laryngoscope. 1990;100:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 183] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Bloch DB, San Martin JE, Rauch SD, Moscicki RA, Bloch KJ. Serum antibodies to heat shock protein 70 in sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 1995;121:1167-1171. [PubMed] |
| 22. | Yeom K, Gray J, Nair TS, Arts HA, Telian SA, Disher MJ, El-Kashlan H, Sataloff RT, Fisher SG, Carey TE. Antibodies to HSP-70 in normal donors and autoimmune hearing loss patients. Laryngoscope. 2003;113:1770-1776. [PubMed] |
| 23. | García-Berrocal JR, Trinidad A, Ramírez-Camacho R, Lobo D, Verdaguer M, Ibáñez A. Immunologic work-up study for inner ear disorders: looking for a rational strategy. Acta Otolaryngol. 2005;125:814-818. [PubMed] |
| 24. | Ryan AF, Harris JP, Keithley EM. Immune-mediated hearing loss: basic mechanisms and options for therapy. Acta Otolaryngol Suppl. 2002;38-43. [PubMed] |
| 25. | García-Berrocal JR, Ramírez-Camacho R, Lobo D, Trinidad A, Verdaguer JM. Adverse effects of glucocorticoid therapy for inner ear disorders. ORL J Otorhinolaryngol Relat Spec. 2008;70:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Harris JP, Weisman MH, Derebery JM, Espeland MA, Gantz BJ, Gulya AJ, Hammerschlag PE, Hannley M, Hughes GB, Moscicki R. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA. 2003;290:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Luetje CM, Berliner KI. Plasmapheresis in autoimmune inner ear disease: long-term follow-up. Am J Otol. 1997;18:572-576. [PubMed] |
| 28. | Rivera T, Sanz L, Camarero G, Varela-Nieto I. Drug delivery to the inner ear: strategies and their therapeutic implications for sensorineural hearing loss. Curr Drug Deliv. 2012;9:231-242. [PubMed] |
| 29. | Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666-3670. [PubMed] |
| 30. | Lobo D, García-Berrocal JR, Trinidad A, Verdaguer JM, Ramírez-Camacho R. Review of the biologic agents used for immune-mediated inner ear disease. Acta Otorrinolaringol Esp. 2013;64:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Street I, Jobanputra P, Proops DW. Etanercept, a tumour necrosis factor alpha receptor antagonist, and methotrexate in acute sensorineural hearing loss. J Laryngol Otol. 2006;120:1064-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Singh J, Well G, Christensen R, Tanjong Ghogomu E, Maxwell L, MacDonald J. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;16:CD008794. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Morovic Vergles J, Radic M, Kovacic J, Salamon L. Successful use of adalimumab for treating rheumatoid arthritis with autoimmune sensorineural hearing loss: two birds with one stone. J Rheumatol. 2010;37:1080-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Van Wijk F, Staecker H, Keithley E, Lefebvre PP. Local perfusion of the tumor necrosis factor alpha blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiol Neurootol. 2006;11:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Cohen S, Roland P, Shoup A, Lowenstein M, Silverstein H, Kavanaugh A, Harris J. A pilot study of rituximab in immune-mediated inner ear disease. Audiol Neurootol. 2011;16:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Orsoni JG, Laganà B, Rubino P, Zavota L, Bacciu S, Mora P. Rituximab ameliorated severe hearing loss in Cogan’s syndrome: a case report. Orphanet J Rare Dis. 2010;5:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, Lachmann HJ, Bybee A, Gaudet R, Woo P. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Kesser BW, Hashisaki GT, Fletcher K, Eppard H, Holt JR. An in vitro model system to study gene therapy in the human inner ear. Gene Ther. 2007;14:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Sugahara K, Shimogori H, Okuda T, Takemoto T, Yamashita H. Novel method for homogeneous gene transfer to the inner ear. Acta Otolaryngol Suppl. 2004;19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |