Published online Jun 26, 2014. doi: 10.5662/wjm.v4.i2.73
Revised: January 28, 2014
Accepted: April 17, 2014
Published online: June 26, 2014
Processing time: 269 Days and 5 Hours
This review summarizes the therapeutic strategies and the drugs actually in development for the management of myeloma patients. Multiple myeloma is caused by the expansion of monoclonal plasma cells and secretion of M-protein (immunoglobulins, Bence Jones protein and free light chains). Multiple myeloma still remains an incurable disease with a high incidence rate in the elderly, despite the introduction of several new therapeutic agents (bortezomib, lenalidomide and thalidomide) which have changed its natural history. The high heterogeneity of this disease leads to large differences in clinical responses to treatments. Thus, the choice of the best treatment is a difficult issue. However, the introduction of new drugs has made it possible to achieve high response rates and good quality responses with long-term disease control. Interactions between tumor cells and their bone marrow microenvironment play a pivotal role in the development, maintenance, and progression of myeloma, inducing also drug resistance. These knowledges have improved treatment options, leading to the approval of new drugs which not only target the malignant cell itself, but also its microenvironment. These agents are in preclinical/early clinical evaluation and they appear to further improve disease control, but their use is still not approved outside of clinical trials.
Core tip: The aim of this review is to summarize and point out the current therapeutic strategies and the drugs actually in development for the management of multiple myeloma. The rationale of the new treatment strategies is found in their efficacy in targeting tumor cells and their microenvironment. Our understanding of multiple myeloma (MM) pathogenesis including the intracellular mechanisms as well as the interactions between MM cells and their microenvironment has helped the discovery of several targets that have become the focus of drug development. The goal is to improve patient’s survival and to control the disease in a long-term fashion, maintaining the quality of life of our patients.
- Citation: Ria R, Reale A, Vacca A. Novel agents and new therapeutic approaches for treatment of multiple myeloma. World J Methodol 2014; 4(2): 73-90
- URL: https://www.wjgnet.com/2222-0682/full/v4/i2/73.htm
- DOI: https://dx.doi.org/10.5662/wjm.v4.i2.73
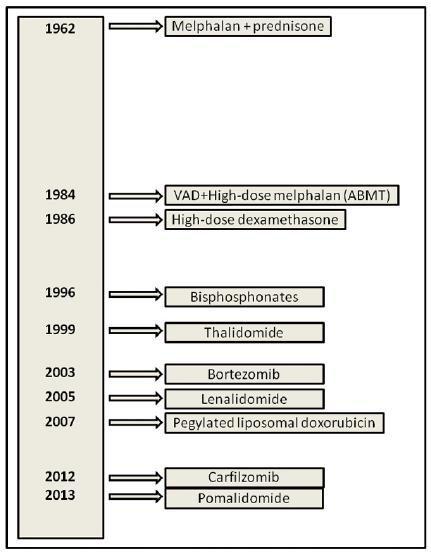
The treatment of multiple myeloma (MM) is rapidly evolving (historical progress in MM treatment options is shown in Figure 1). Near the old active classes of drugs including alkylators (e.g., melphalan and cyclophosphamide), corticosteroids (e.g., prednisone and dexamethasone), and anthracyclines (e.g., doxorubicin), new drug formulations (e.g., liposomal doxorubicin) and new active classes of drugs such as proteasome inhibitors (e.g., bortezomib) and immunomodulatory drugs (e.g., thalidomide and lenalidomide) have been introduced in myeloma therapy[1].
Changes in treatment strategies due to the introduction of novel drugs have been able to significantly improve the quality of responses. In fact, if in the past, complete remission (CR) in MM was rare to achieve, while the introduction of new treatments has increased the rate in younger patients as well as in the non-transplant setting. CR represents a surrogate marker of long survival. It correlates with the long-term progression-free survival (PFS) and overall survival (OS). Achieving CR and sustaining CR within a 3-year landmark from the treatment initiation were associated with highly superior survival. Actually, we agree that “the more profund the remission is, the longer the duration of response is”[2].
In this paper we review the novel agents that could shape future directions for MM management. As far as possible treatment should be individualized. Also, it should be recognized that it is not necessarily best practice to refer particular therapies at specified time points. In the future, it is likely that MM therapy will be “risk-adapted” and the presence or absence of specific prognostic factors may determine the choice of therapy both at diagnosis and relapse.
Improvements in MM biology knowledge have led changes into the rationale of modern therapy[3]. In fact, the role of microenvironment in myeloma pathogenesis and progression has been well established[4] and the new drugs are designed to target myeloma plasma cells and their microenvironment simultaneously. This different approach has changed the natural history of MM, which still remains an incurable disease, but the survival is significantly improved.
Initial therapy for MM depends on the eligibility for autologous stem cell transplant (ASCT) (younger fit patients). Patients who are considered potential candidates for ASCT receive 2-4 cycles of a non-melphalan-containing regimen and then proceed to stem cell harvest[5]. After stem cell harvest, most patients move on to ASCT. However, depending on the response to initial therapy and patient’s preference, initial therapy can be resumed after stem cell harvest, delaying ASCT until first relapse. The role of early vs delayed ASCT is an argument of debate[6]. On the contrary, the second ASCT in patients who do not achieve almost a very good partial response (VGPR) after the first transplant seems to be the best option[7].
In patients who are not candidates for ASCT (elderly or unfit patients), the duration of initial therapy is approximately 9-18 mo for most regimens, although in the case of lenalidomide/low-dose dexamethasone (Rd), therapy is often continued until progression if the patient well tolerates the treatment[8].
The addition of novel agents into the induction regimens significantly improved the outcome of patients with newly diagnosed MM[9].
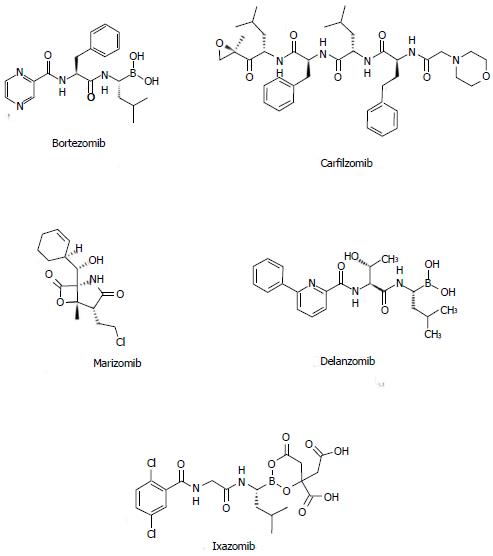
Bortezomib (Figure 2), alone or in combination with dexamethasone (VD), is active in newly diagnosed myeloma patients[10]. VD shows superior response rates when compared with vincristine/doxorubicin/prednisone (VAD), with a VGPR rate of 38% vs 15% after induction therapy in younger patients. The higher VGPR rate was confirmed after transplantation (54% vs 37%), with a PFS improvement (36 mo vs 30 mo)[11]. In spite of these good results, no OS benefit was noticeable.
The three-drug combination bortezomib/thalidomide/dexamethasone (VTD) has been compared with thalidomide/dexamethasone (TD) or VD[12,13]: VTD resulted in better response rates and PFS, but no OS benefit was observed. Nevertheless, these studies evidenced the ability of VTD plus double ASCT followed by bortezomib-based consolidation to overcome the poor prognostic effects of t(4;14) translocation[13]. VTD is particularly useful in patients with acute renal failure since it acts rapidly and can be used without dose modification[13].
Other two combinations can represent excellent choices when considering a bortezomib-containing regimen as frontline therapy in newly diagnosed MM patients: the three-drug combination bortezomib/cyclophosphamide/dexamethasone (CyBorD or VCD) and the four-drug combination bortezomib/cyclophosphamide/lenalidomide/dexamethasone (VCRD)[14]. The EVOLUTION trial[15], a randomized phase 2 trial in newly diagnosed myeloma patients, showed that VCD is well tolerated with similar activity compared with the combination bortezomib/lenalidomide/dexamethasone (VRD), a combination which produces remarkably high overall and complete response rates[16]. In this trial, CR was achieved in 22% and 47% of patients treated with two different schedules of VCD vs 24% of patients treated with VRD. Although highly active, VCRD had similar CR rates compared with either VCD or VRD (Table 1).
| Regimen | Drugs | Ref. |
| Bortezomib-based induction | ||
| VD | Bortezomib/dexamethasone | [12,13] |
| VTD | Bortezomib/thalidomide/dexamethasone | [12,13] |
| CyBorD or VCD | Bortezomib/cyclophosphamide/dexamethasone | [14-16] |
| VCRD | Bortezomib/cyclophosphamide/lenalidomide/dexamethasone | [14-16] |
| VRD | Bortezomib/lenalidomide/dexamethasone | [14-16] |
| Immunomodulatory-based induction | ||
| TD | Thalidomide/dexamethasone | [19,20] |
| VTD | Bortezomib/thalidomide/dexamethasone | [12,13] |
| Rd | Lenalidomide/low-dose dexamethasone | [23] |
| VCRD | Bortezomib/cyclophosphamide/lenalidomide/dexamethasone | [14-16] |
| VRD | Bortezomib/lenalidomide/dexamethasone | [14-16] |
| Bortezomib-based | ||
| VMP | Bortezomib/melphalan/prednisone | [29-31] |
| VMPT | Bortezomib/melphalan/prednisone/thalidomide | [30] |
| Immunomodulatory-based | ||
| TD | Thalidomide/dexamethasone | [32] |
| MPT | Melphalan/prednisone/thalidomide | [40,41] |
| Rd | Lenalidomide/low-dose dexamethasone | [44] |
| RD | Lenalidomide/high-dose dexamethasone | [43] |
| MPR | Melphalan/prednisone/lenalidomide | [45] |
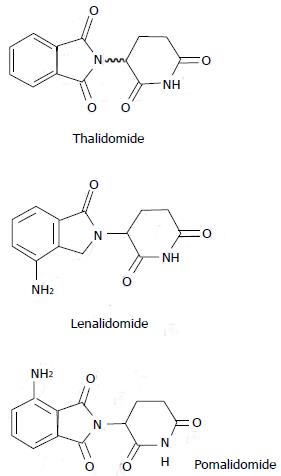
Thalidomide and its derivate lenalidomide are designed as “immunomodulatory” drugs (Figure 3). Recent studies have demonstrated that the mechanism of action of these drugs may be more complex. These drugs target both tumor plasma cells and their microenvironment[17]. Their activity seems to be mediated through cereblon, the putative primary teratogenic target for thalidomide[18].
In newly diagnosed MM, TD produces response rates of 65%-75%[19,20]. Two randomized trials found TD to be superior to dexamethasone alone[21]. Patients receiving thalidomide-based regimens require deep vein thrombosis prophylaxis with aspirin, low-molecular weight heparin or Coumadin[22].
In the transplant setting, there are some trials which aim to clarify the role of lenalidomide as induction therapy[23]. Although its use during induction determines good response rates, it seems to impact on the mobilization of stem cells[24,25]. Therefore, in patients over the age of 65 and those who have received more than 4 cycles of Rd, stem cells must be mobilized with either cyclophosphamide + Granulocyte Colony-Stimulating Factor or with plerixafor[26].
Other combinations (also with bortezomib and alkilators) have been already discussed in the previous paragraph (Table 1).
In the phase 3 VISTA trial, the combination of bortezomib/melphalan/prednisone (VMP) was associated with significantly improved OS compared with melphalan/prednisone (MP). OS persisted with a long-term follow-up[27,28]. In a subsequent randomized trial, there was no significant advantage of bortezomib/thalidomide/prednisone (VTP) over VMP[29]. The principal toxicity of VMP is peripheral neuropathy, which can be reduced using a once-weekly regimen[29,30]. Moreover, subcutaneous administration of bortezomib also reduces the incidence of neurophaty and other toxicities[31].
The combination bortezomib/melphalan/prednisone/thalidomide (VMPT) has been compared with VMP in a randomized phase 3 trial conduct by the Italian group GIMEMA[30]. The 3-year PFS rate was 56% with VMPT compared to 41% with VMP. However, patients in the VMPT arm received maintenance therapy with bortezomib and thalidomide, whereas patients in the VMP arm did not receive any additional therapy beyond 9 mo. Furthermore, no OS differences were observed: the 3-year OS was 89% with VMPT and 87% with VMP. Therefore, it seems that there is no advantage using VMPT as initial therapy (Table 1).
It has been demonstrated that TD is inferior in terms of activity and toxicity when compared to lenalidomide-based regimens. In a Mayo Clinic study of 411 newly diagnosed patients lenalidomide plus dexamethasone was significantly superior to TD in terms of response rates, PFS, and OS[32]. Moreover, in another phase 3 study, the OS with TD was inferior to MP in newly diagnosed elderly patients[33]. This combination can be considered an option for initial therapy only in patients with acute renal failure, and in combination with bortezomib.
MPT has shown better response rates compared with MP in six randomized studies[34-39]. In four of these trials a significant prolongation of PFS with MPT has been observed[34-38], and an OS advantage has been observed in three of them[34,35,38]. The evident superiority of MPT over MP has been confirmed by two meta-analyses of these randomized trials[40,41].
Grade 3/4 adverse events occur in approximately 55% of patients treated with MPT compared with 22% in patients treated with MP and 43% with TD. There is a considerable risk (20%) of deep vein thrombosis with MPT, confirming the necessity of thromboprophylaxis in these patients.
Rd is active in newly diagnosed myeloma patients[42]. In a randomized trial, Rd demonstrated less toxicity than lenalidomide plus high-dose dexamethasone (RD)[43] with better patient’s compliance and OS.
As a result, high-dose dexamethasone is no longer recommended also because its toxicity makes it difficult to incorporate it into combination regimens. Rd is also an attractive option for the treatment of elderly patients with newly diagnosed myeloma because of its excellent tolerability, convenience, and efficacy. The 3-year OS rate with Rd in patients older than 70 who did not receive ASCT is 70%[44], and is comparable to results with MPT and VMP. All patients receiving Rd require antithrombosis prophylaxis with aspirin; low-molecular-weight heparin or Coumadin is mandatory in patients with high risk of DVT[22].
The combination melphalan/prednisone/lenalidomide (MPR) has been recently compared with MP in a randomized trial of patients 65 years of age or older with newly diagnosed MM[45]. The median PFS was similar between MPR and MP, 14 mo vs 13 mo, respectively. The disappointing lack of improvement in PFS with MPR compared with MP may be related to the fact that dose reductions of both melphalan and lenalidomide are often required when the 2 agents are combined (Table 1).
Multiagent combination regimens such as bortezomib/dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide (VDT-PACE) have been tested extensively at the Myeloma Institute for Research and Therapy at Arkansas[46]. VDT-PACE is particularly useful in patients with aggressive disease, such as plasma cell leukemia or multiple extramedullary plasmacytomas.
The EVOLUTION trial, which compared VCD with VCRD and VRD[15,16], evidenced similar activity and CR rates for all the three combinations in newly diagnosed myeloma patients (Table 2).
| Regimen | Drugs | Ref. |
| VDT-PACE | Bortezomib/dexamethasone/thalidomide/cisplatin/doxorubicin/cyclophosphamide/etoposide | [46] |
| VCRD | Bortezomib/cyclophosphamide/lenalidomide/dexamethasone | [15,16] |
| VRD | Bortezomib/lenalidomide/dexamethasone | [15,16] |
MM treatment strategies have largely changed in recent years with numerous new combinations available for initial approaches. A plethora of randomized data enable physicians to choose the best regimen for initial therapy, but most available randomized trial data are comparisons of newer regimens with older alkylator- or anthracycline-based regimens. Only few trials have compared modern regimens between each other, and in these studies surrogate end points, such as response rates and PFS, were used. No randomized data with OS or patient-reported quality-of-life end points are available. PFS cannot represent a good surrogate for clinical benefit. In numerous instances, PFS has proven to be a poor indicator of clinical benefit: the arm with PFS advantage showed[11,14] inferior OS[43]. The choice of therapy is driven by surrogate end points such as CR or PFS that were primary end-points of worldwide randomized trials. OS or patient-reported quality-of-life remain the primary end-points that should drive the rational choice of treatment when a patient with MM needs initial therapy.
Moreover, an optimal treatment strategy for newly diagnosed myeloma patients should be also based on the evaluation of risks and benefits[47]. The actual “cure vs control” debate on whether we should treat myeloma with an aggressive multidrug strategy targeting CR or a sequential disease control approach is concentrated on this aspect[47,48]. The risk-adapted stratification of patients can be a good indicator for personalized treatments[49].
In standard-risk patients VCD seems to be a reasonable option for initial therapy. Higher CR rates, low toxicity, and the lack of any adverse effect on stem cell mobilization are the principal advantages compared to immunomodulator-based regimens. The risk of neurotoxicity early in the disease course represents the major unfavorable factor. However, recent studies show that the neurotoxicity of bortezomib can be greatly diminished by administering bortezomib on a once-weekly schedule[39,40] or by administering the drug subcutaneously[50].
There are no data showing that more expensive regimens (e.g., VRD) are safer or more effective in terms of OS or quality-of-life compared to Rd or VCD (or VTD). Although in elderly patients, melphalan-containing triplet regimens such as VMP and MPT have proven efficacy over MP, it is not clear whether they are superior to non-melphalan-containing regimens.
In intermediate or high-risk patients (particularly t(4;14) translocation), bortezomib-containing regimens followed by double ASCT and eventually mantainance therapy are able to overcome the poor prognosis with good CR rates and sustained CR[13,51]. Therefore, VCD or a similar bortezomib-containing regimen would be the preferred choice in this subset of patients.
VRD is a rather expensive regimen that, although response rates and CR rates are very high, cannot be suggested as standard therapy outside clinical trials because of the lack of data from randomized trials comparing its safety and efficacy. However, it may represent a reasonable treatment option in high-risk myeloma patients since other current options appear inadequate.
At present, no sufficient data are available to recommend any quadruple regimen as initial therapy outside of clinical trials. VDT-PACE may be a good choice in very high-risk patients with extensive extramedullary disease or plasma cell leukemia at the time of initial diagnosis[46].
In patients with acute renal failure due to suspected light-chain cast nephropathy, VCD (or VTD) is of particular value and is preferred as initial therapy[52].
In patients who are not candidates for ASCT, melphalan-containing regimens such as MPT, VMP, and MPR are indicated. Most patients with newly diagnosed MM are > 65 years old with 30% > 75 years.
Finally, elderly patients are more susceptible to side effects and may be unable to tolerate full drug doses[53]. For these patients, lower dose-intensity regimens improve the safety profile and thus optimize treatment outcomes. The occurrence of serious hematological and non-hematological adverse events during treatment should be carefully taken into account to adjust doses and optimize the outcome.
In the relapsed/refractory setting, conventional or high-dose chemotherapy has been a longstanding approach to salvage treatment. The regimens include high-dose melphalan; high-dose methylprednisolone; high-dose dexamethasone; VAD; vincristine/melphalan/cyclophosphamide/prednisone alternating with vincristine/carmustine/doxorubicin/prednisone; doxorubicin/vincristine/dexamethasone/etoposide/cyclophosphamide; cisplatin/doxorubicin/cyclophosphamide/etoposide (DT-PACE); dexamethasone/cyclophosphamide/etoposide/cisplatin (DCEP)[54-61]. Overall response rates for salvage combination chemotherapy are between 25% and 65%, with morbidity and mortality related to the intensity of therapy.
Allogeneic transplant shows limited clinical benefit for the treatment of relapsed/refractory MM with long-term, disease-free survival of 10% to 20% and high toxicity. Few patients, even those with poor-risk disease, are ultimately cured with this approach[62,63].
Second autologous transplantation may be beneficial and safe for some patients with relapsed/refractory disease. Moreover, the overall response rates in recent studies with small sample sizes range from 55% to 69%, with a 100-d mortality rate of 10%[64,65].
Thalidomide was the first novel agent to be evaluated in patients with relapsed/refractory MM. Several studies have demonstrated the effectiveness of thalidomide as a single agent producing partial response or better (> PR) in 30% of patients with relapsed/refractory disease, with a 1-year OS rate of 60% and median OS of 14 mo[66]. In the phase III OPTIMUM study, different doses of thalidomide (100 mg/d, 200 mg/d, or 400 mg/d) demonstrated no difference in response rate and OS[67]. Thalidomide has been successfully combined with multiple conventional cytotoxic agents for the treatment of relapsed/refractory MM. When compared with thalidomide alone, the addition of dexamethasone resulted in higher response rates of about 50%[68]. The addition of cyclophosphamide to thalidomide with or without dexamethasone led to higher responses (> PR: 57%-84%)[69,70]. Evidence also suggests that the efficacy of thalidomide in relapsed/refractory MM may be improved when combined with melphalan (> PR: 59%), MP (> PR: 42%), melphalan/dexamethasone (> PR: 70%), pegylated liposomal doxorubicin/dexamethasone (> PR in 76%), pegylated liposomal doxorubicin/vincristine/dexamethasone (> PR: 75%), DT-PACE (> PR: 32%), or cyclophosphamide/etoposide/dexamethasone (TCED; > PR: 68%)[71-75].
Monotherapy with bortezomib demonstrated response rates of 25% to 35% in patients with relapsed/refractory MM in initial phase I and II studies[76,77]. The randomized phase III APEX study demonstrated a survival benefit with bortezomib compared to high-dose dexamethasone in patients who had received a median of two prior therapies[78]. Bortezomib-based treatment demonstrated superior response rates (43% vs 18%; P < 0.001), TTP (6.2 mo vs 3.5 mo; P < 0.001), and 1-year OS (80% vs 66%; P < 0.003) compared with dexamethasone[78]. The benefit of 6-mo OS for patients who received bortezomib persists despite substantial crossover (> 62%) from dexamethasone to bortezomib[78]. The addition of dexamethasone to bortezomib resulted in response improvement in 18% to 34% of patients[78]. Multiple chemotherapeutic agents have been successfully combined with bortezomib in relapsed/refractory MM, such as the combination with pegylated liposomal doxorubicin (> PR: 44%), low-dose dexamethasone/pegylated liposomal doxorubicin (PAD) (> PR: 67%-85%), oral or intravenous melphalan (> PR: 47%-68%), and low-dose cyclophosphamide/prednisone/dexamethasone (> PR: 68%- 82%)[79-82].
Lenalidomide as a single agent at the maximum dose of 25 mg once daily determined response rates ranging from 29% to 39% in patients who had received a median of three prior therapies[83].
MM-009 and MM-010 trials comparing RD vs dexamethasone alone demonstrated improved overall response (60.6% vs 21.9%; P > 0.001), TTP (13.4 mo vs 4.6 mo; P > 0.001), and OS (38.0 mo vs 31.6 mo; P > 0.045) for patients treated with RD[84-86]. Crossover to RD for patients who previously received dexamethasone alone (41.9%) did not modify the results. Moreover, the RD combination also appears to be effective in very elderly (> 75 years) patients with relapsed MM, demonstrating overall response rates of 62% and median PFS of 14 mo[87]. Currently, all new dexamethasone combination trials use low-dose dexamethasone instead of high-dose dexamethasone.
Lenalidomide has also demonstrated efficacy in combination with doxorubicin/dexamethasone (RAD; > PR: 73%), low-dose cyclophosphamide/prednisone (REP; minimal response or better > MR: 64.3%), cyclophosphamide/dexamethasone (> MR: 75%), and pegylated liposomal doxorubicin/vincristine/dexamethasone (> PR: 75%) in patients with relapsed/refractory MM[88-91].
Numerous studies have evaluated the combination of two established novel agents with conventional and/or cytotoxic drugs in the relapsed/refractory setting[92-97]. Bortezomib has been successely combined with thalidomide and other drugs: VTD; >PR: 63%, PAD > PR: 74%, VCTD; > PR: 88%, VMPT; > PR: 67%, and VMDT; > PR: 66%. Other studies have evaluated the combination of VRD with good response rates (> MR: 61%-86%) even in patients resistant to thalidomide, lenalidomide, or bortezomib[1] (Table 3).
| Regimen | Drugs | Ref. |
| MEL | High-dose melphalan | [54,55] |
| PDN | High-dose methylprednisolone | [56,57] |
| DEX | High-dose dexamethasone | [57] |
| VAD | Vincristine/ doxorubicin/prednisone | [58] |
| VMPC | Vincristine/melphalan/cyclophosphamide/prednisone | [58] |
| VBAP | Vincristine/carmustine/doxorubicin/prednisone | [58] |
| CEVAD | Doxorubicin/vincristine/dexamethasone/etoposide/cyclophosphamide | [59] |
| DCEP | Dexamethasone/cyclophosphamide/etoposide/cisplatin | [60] |
| DT-PACE | Cisplatin/doxorubicin/cyclophosphamide/etoposide | [61] |
| AlloSCT | Allogeneic transplant | [62,63] |
| ASCT | Second autologous transplant | [64,65] |
| THAL | Thalidomide monotherapy | [66,67] |
| TD | Thalidomide/dexamethasone | [68] |
| TC | Thalidomide/cyclophosphamide | [69] |
| TCD | Thalidomide/cyclophosphamide/dexamethasone | [70] |
| MPT | Melphalan/prednisone/thalidomide | [71] |
| MTD | Melphalan/thalidomide/dexamethasone | [72] |
| TAD | Thalidomide/pegylated liposomal doxorubicinxorubicin/dexamethasone | [73] |
| TAVD | Thalidomide/pegylated liposomal doxorubicinoxorubicin/vincristine/dexamethasone | [74] |
| TCED | Thalidomide/cyclophosphamide/etoposide/dexamethasone | [75] |
| BOR | Bortezomib monotherapy | [76-78] |
| VD | Bortezomib/dexametasone | [78] |
| VA | Bortezomib/pegylated liposomal doxorubicin | [79] |
| PAD | Bortezomib/low-dose dexamethasone/pegylated liposomal doxorubicin | [80] |
| VM | Bortezomib/melphalan | [81] |
| VCD | Bortezomib/low-dose cyclophosphamide/prednisone/ | [82] |
| LEN | Lenalidomide monotherapy | [83] |
| RD | Lenalidomide/dexamethasone | [84-87] |
| RAD | Lenalidomide/doxorubicin/dexamethasone | [88,89] |
| REP | Lenalidomide/low-dose cyclophosphamide/prednisone | [90] |
| RCD | Lenalidomide/cyclophosphamide/dexamethasone | [91] |
| RAVD | Lenalidomide/pegylated liposomal doxorubicin/vincristine/dexamethasone | [1] |
| PATD | Bortezomib/ pegylated liposomal doxorubicin/thalidomide/dexamethasone | [92] |
| VMDT | Bortezomib/melphalan/dexamethasone/thalidomide | [93] |
| VMPT | Bortezomib/melphalan/prednisone/thalidomide | [94] |
| VTD | Bortezomib/thalidomide/dexamethasone | [95] |
| PAD | Bortezomib/pegylated liposomal doxorubicin/dexamethasone | [1] |
| VCTD | Bortezomib/cyclophosphamide/thalidomide/dexamethasone | [1] |
| VCRD | Bortezomib/cyclophosphamide/lenalidomide/dexamethasone | [1] |
| VRD | Bortezomib/lenalidomide/dexamethasone | [1] |
Decisions regarding treatment at relapse should be made considering the timing of relapse, the efficacy and toxicity of drugs used in prior therapies, age, bone marrow and renal function, co-morbidities and patient’s preference. Also, it should be recognized that it is not necessarily best practice to refer particular therapies at specified time points. In the future, it is likely that therapy will be ‘risk-adapted’ and the presence or absence of specific prognostic factors may determine the choice of therapy both at diagnosis and relapse. Patients with a long first plateau phase after their initial therapy may be treated with the same first-line regimen because they are likely to respond again at relapse. The use of a second ASCT is a possible choice according to patient’s fitness and if the candidate patient had a good response to the initial transplant procedure (> 18-mo PFS).
For patients presenting with renal failure, strong consideration of treatments with a bortezomib-containing regimen should be given: it determines a rapid reduction in light chain load to the kidneys, and maximizes chances of regaining renal function.
Entry into clinical trials should be considered at each relapse.
Maintenance therapy is actually one of the most controversial debates in the care of patients with MM. Historically, this has been attempted with corticosteroids, interferon, and thalidomide. PFS, but not OS, was often prolonged and was frequently accompanied by significant morbidity and high discontinuation rates[98,99].
Lenalidomide is a more tolerable immunomodulatory drugs (IMiD). Maintenance therapy with this drug has been explored in recent randomized, placebo-controlled trials. Two studies[100,101] evaluated the role of maintenance lenalidomide therapy in patients after ASCT compared with placebo, and a third evaluated extended-use lenalidomide in older patients initially treated with melphalan-based regimens[45]. Only 1 out of the 3 has shown a survival benefit.
In a recent trial comparing PAD with VAD, patients randomized to the PAD arm received maintenance with bortezomib (every 2 wk) after ASCT, and those in the VAD arm received thalidomide as maintenance[102].
Currently available data are not sufficient to recommend routine maintenance with lenalidomide for all patients outside clinical trials, but it can be considered in subgroups of patients in whom the benefits appear to outweigh the risks (e.g., standard-risk patients who are known to be lenalidomide responsive and are not in VGPR or better after completion of initial therapy).
In intermediate- and high-risk myeloma patients, a bortezomib-based maintenance approach may be preferable but needs further studies. Preliminary results are encouraging and suggest improved PFS and OS with bortezomib maintenance, but it is not clear if this can be attributed to differences in induction or maintenance therapy.
The IMiD pomalidomide (Figure 3) exerts its anticancer effects throught the angiogenesis inhibition, immunomodulation, impeding cytokine production, and interaction with the bone marrow and tumor microenvironment dependent on Cereblon expression[103]. Decreased cereblon mRNA expression has been correlated with lenalidomide resistance[18,103]. Interestingly, pomalidomide appears to remain effective in lenalidomide resistant cells[103]. Pomalidomide is the most potent IMiD, having 100 times strength of thalidomide and 10 times that of lenalidomide[104]. Moreover, the proliferation and survival of myeloma cells are largely unaffected by thalidomide, whereas lenalidomide and pomalidomide cause both cell cycle arrest and apoptosis[105]. Specifically, they induce cell cycle arrest by P21 WAF activation independently of P53. This highlights the possibility of using these agents to treat P53 mutated malignancies[106].
Pomalidomide has shown good results in phase I studies with 50% of patients achieving at least a PR and 10% achieving a CR at a maximum tolerated dose of 4 mg for 21 or 28 d [107,108]. In the MM-002 (a randomized phase I/II open-label dose escalation study) heavily treated patients refractory to both lenalidomide and bortezomib were included. Efficacy results in 21% of patients achieving at least a PR and in patients refractory to both lenalidomide and dexamethasone, 25% had a response[109].
Phase II studies demonstrated improvement of the response rate and quality of responses when dexamethasone was associated to pomalidomide in heavily pretreated patients[110]. Responses were observed in > 60% of patients (5% CR, 30% VGPR, and 30% MR) and in 40% of lenalidomide-refractory patients, 40% of thalidomide-refractory patients, and 60% of bortezomib-refractory patients. In high-risk patients (plasma cell labeling index ≥ 3%, deletion 17p, t(4;14) or t(14;16) by fluorescent in situ hybridization, or deletion 13 on conventional cytogenetics) responses were observed in 74% of cases. Also in extramedullary disease in the phase II study conducted at the Mayo Clinic[111] the response rate was 31%, with two patients achieving CR and two patients achieving PR.
Two sequential phase II studies of patients refractory to both bortezomib and lenalidomide conducted at the Mayo Clinic[112], in which two dosing strategies were compared, suggest that 4 mg daily dosing does not yield superior responses than 2 mg daily dosing: the 2 mg cohort had an MR or better (49%) while the 4 mg group had an overall response rate of 43; 26% of patients achieved at least a PR in both cohorts. In contrast, a trend towards a dose-dependent response was observed in the phase I MM-002 study[113]. Toxicities are similar at the 2 and 4 mg dose levels. Neutropenia is the major toxicity described (26%-66% grade 3/4), thrombocytopenia and anemia are also common (13% and 17% grade 3/4, respectively)[112].
Nonhematologic toxicities are seen in 5% of patients: fatigue in 62% of cases (8% grade 3/4), thromboembolic events in 3%, and peripheral neuropathy in 13% (up to 33% in patients with pre-existing neuropathy that worsens). Acute noninfectious pulmonary toxicity has been described in two patients[113].
Carfilzomib (also known as PR-171; Figure 2) is a new stable and irreversible proteasome inhibitor that is potentially more efficacious and less toxic. It inhibits the chymotrypsin-like site of the proteasome, and, at high doses, it shows additional inhibitory effects on the trypsin-like and caspase-like sites[114].
Phase I trials demonstrated sustained proteasome inhibition with two different schedules: escalating doses on 5 consecutive days, followed by a 9-d rest period in a 14-d cycle[115], and on days 1, 2, 8, 9, 15 in a 4-wk cycle[116]. The first schedule evidenced a minimal effective dose at 11 mg/m2 and a maximal tolerated dose at 15 mg/m2. In the second schedule a maximal tolerated dose was not reached and the highest dose administered was 27 mg/m2. The most common side effects were thrombocytopenia and febrile neutropenia (carfilzomib-associated dose-limiting toxicities), low-grade fatigue and nausea, but no significant peripheral neuropathy was observed and no aggravation in patients with preexisting peripheral neuropathy was reported.
In bortezomib-naive patients, carfilzomib showed an impressive single-agent ORR of 52%[117]. Carfilzomib has shown single-agent activity in heavily pretreated MM patients (80% were double refractory) in the twice-weekly regimen with dose-reduction (20 mg/m2) in the first cycle to abrogate potential tumor-lysis syndrome[118]. The dose was escalated to 27 mg/m2 in cycle 2. The response rate was 23.7%, with a median duration of response of 7.8 mo, a PFS of 3.7 mo, and an OS of 15.6 mo. Clinical benefit was observed in one-third of the patients. Outcome was not influenced by adverse cytogenetics, renal impairment, disease stage, or Eastern Cooperative Oncology Group performance score. Drug-related adverse events (AEs) of all grades were most frequently fatigue (37%), nausea (34%), and thrombocythemia. Grade 3/4 hematologic AEs included anemia (24%), thrombocytopenia (29%), lymphopenia (20%), and neutropenia (11%). Grade 3/4 nonhematologic AEs included pneumonia (9%), hyponatremia (8.3%), fatigue (7.5%), and hypophosphatemia (6.0%). Treatment-emergent peripheral neuropathy was uncommon (12.4%) and considered to be carfilzomib related in only 8.3% of patients. Dose adjustment for renal insufficiency was not necessary[119].
The extension of the infusion time to 30 min allowed tolerance of higher doses of carfilzomib with good ORR[120].
Improvement of the response has been obtained with the association of lenalidomide and low-dose dexamethasone to carfilzomib[121,122]. The overall response was 78%-98% with an impressive stringent complete remission rate of 42% after a median of 12 cycles of therapy.
In newly diagnosed elderly patients the combination with melphalan and prednisone (CMP) administered on the usual schedule twice weekly with a 12-d rest in a 42-d cycle was safe and effective with a response rate of 92% at the interim analysis[122].
The cyclophosphamide/carfilzomib/thalidomide/dexamethasone demonstrated a response rate of 100% in newly diagnosed MM patients[123].
Our understanding of the transformation of normal plasma cells into malignant myeloma cells is improving[124]. The interaction between MM cells and their microenvironment is the focus of intense research[125] and several targets have emerged from these studies[126,127]. In addition, drugs that target the cell cycle, membrane receptors, immunomodulators and antiangiogenics are also being investigated in MM.
Delanzomib (CEP18770), Marizomib (NPI-0052) and Ixazomib (MLN9708), (Figure 2) are currently being evaluated in phase I and II studies[128]. Marizomib (NPI-0052) is an orally active proteasome inhibitor and a more potent inhibitor of the NF-kB and other cytokines than bortezomib[128]. It is able to overcome bortezomib resistance both in vitro and in vivo. It interferes with the chymotryptic-like, tryptic-like and caspase-like proteolytic activity of the proteasome. Studies are currently evaluating Marizomib as a single agent as well as in combination with bortezomib since a synergistic effect has been observed[129].
The expression of genes is controlled by the DNA/histone interaction. Excessive deacetylated level of histones has been linked to cancer pathologies by promoting the repression of tumor suppressor genes. Overexpression of histone deacetylase (HDAC) by MM cells results in decreased DNA transcription, including transcription of tumor suppressor genes, and inhibition of HDAC reverses these effects[130]. HDAC inhibitors mediate tumor cell death via caspase-dependent and independent apoptosis and autophagy[131], induce cell cycle arrest via p21 up-regulation, block the aggresome complex and induce cell death via the accumulation of ubiquitinated proteins[132].
Currently several HDAC inhibitors have being studied as single agents or in combination with other agents mainly bortezomib and lenalidomide. Vorinostat (SAHA) is an oral HDAC inhibitor that down-regulates IGF-1 and IL-6 signaling pathways as well as DNA synthesis and repair enzymes[133].
In phase I trials in patients with relapsed/refractory MM disease oral vorinostat was well tolerated. Side effects included fatigue, anorexia, diarrhea and nausea. The combination with bortezomib has shown promising activity[134]. On 23 patients with a median of 7 prior regimens the response rate was 42%, including 3 cases of PR among 9 bortezomib-refractory patients.
A second HDAC inhibitor tested in combination with other therapies for relapsed/refractory MM is Panobinostat (LBH589), a potent pan deacetylase inhibitor that disrupts aggresome and HSP90 function via inhibition of HDAC6 and promotes cytotoxic misfolded/unfolded protein aggregates and MM cell death[132]. In a phase I study response rate was 68% (26/38 pts) in patients across all cohorts and, particularly, 62% (8/13 pts) in bortezomib-refractory patients. Major adverse events included grade 3/4 thrombocytopenia (n = 30), neutropenia (n = 23), diarrhea (n = 23), nausea (n = 18), pyrexia (n = 17) and fatigue (n = 16). No grade 3/4 peripheral neuropathy was observed. Various phase I, II and III studies are ongoing to establish the role of panobinostat and its combinations (i.e., plus bortezomib/dexametasone, lenalidomide/dexamethasone, melphalan/prednisone/thalidomide, everolimus, carfilzomib) in MM treatments.
Other HDAC inhibitors are currently being evaluated in the treatment of MM. Romidepsin, Belinostat, ITF2357 and AR 42 are still under investigation.
The PI3K/AKT pathway is a central signaling pathway in several cellular functions including proliferation, growth, survival and migration. AKT activation induces growth and survival advantage to MM cells through GSK-3β and mTOR phosphorylation and has been shown to be associated with advanced stage and poor prognosis in MM patients and resistance to dexamethasone in MM cells[135].
Perifosine (KRX-0401) is a novel synthetic oral AKT inhibitor[136]. On the basis of a phase I/II study the combination of perifosine and bortezomib with or without dexamethasone showed a 41% response rate (65% in the bortezomib-group vs 32% in the bortezomib-refractory group)[137] with manageable toxicity. A phase III randomized trial evaluating the same combinations in patients with relapsed/refractory MM previously exposed to bortezomib is ongoing.
The Mammalian target of rapamycin (mTOR) is an intracellular serine-threonine kinase that controls cell growth, proliferation, motility, survival and metabolism. mTOR exerts its downstream effects through the formation of protein complexes called mTORC1 and mTORC2 with different functions and target molecules[138]. mTOR acts as a neoplastic switch that is frequently turned on by many mutations found in cancer and, hence, inhibition of mTOR and its complex offers a promising target.
In a phase II study, temsirolimus showed a 38% response rate in 16 patients with relapsed/refractory MM with a TTP of 138 d and a good toxicity profile[139]. The combination of temsirolimus with bortezomib obtains a PR or better in 33% of patients[140]. Everolimus (RAD001) has also been studied in MM as a single agent and in combination with lenalidomide, with no encouraging preliminary results. Other mTOR inhibitors such as ridaforolimus are currently being evaluated for their anti-MM activity in preclinical phase I studies.
Emerging data have shown that rapamycin analogs as well as mTOR inhibitors that have activity against mTORC2 do not appear to be effective as monotherapy (only SD or MR obtained in clinical trials with rare PR). They may represent agents that could be evaluated in combination with other anti-myeloma agents in the future.
Heat shock protein 90 (HSP90) is a molecular chaperone that facilitates the folding and stability of numerous signaling molecules that control the growth and survival of cancer cells[141]. The client proteins for HSP90 include transcription factors, oncogenic kinases and receptors that are associated functionally with cell cycle control and signaling, and it has been shown to be a key molecular chaperone for signal transduction proteins critical to MM cell growth, survival and drug resistance.
Preclinical studies with tanespimycin (KOS-953) (a small molecule inhibitor of HSP90) have shown a rationale for combining HSP90 inhibitors with bortezomib because of the HSP90 induction by bortezomib along with up-regulation of stress response gene transcripts. HSP90 inhibition increases bortezomib-induced apoptosis in MM cells by blocking the HSP90 stress response. Moreover, tanespimycin may also be protective against peripheral neuropathy associated with bortezomib. The combination tanespimycin/bortezomib in relapsed/refractory MM patients obtained a response rate of 41% in bortezomib-naïve, 20% in bortezomib-pretreated and 14% in bortezomib-refractory patients. Adverse events included diarrhea (60%), fatigue (49%), nausea (49%), thrombocytopenia (40%), and aspartate transaminase elevation (29%). Grade 1-2 peripheral neuropathy was seen in 21% patients, but no grade 3/4 peripheral neuropathy was observed, which is consistent with tanespimycin’s neuroprotective effect[142]. Other HSP90 inhibitors are currently in early phase trials in MM.
Monoclonal antibodies (MoAb) represent a new anticancer strategy[143] for patients refractory to new drugs. These patients were found to have a median OS of 9 mo[144]. Ideally, targets for therapeutic MoAbs should be specifically expressed on cancer cells but not on normal cells as demonstrated in other hematologic diseases[145].
A large variety of antigen targets have been studied in MM, which can be expressed either on myeloma cells or on components of the bone marrow microenvironment (bone marrow stromal cells and signaling molecules), but the majority of them are in preclinical phase/early clinical trials[143,146].
When employed as monotherapy, MoAbs have generally not produced impressive levels of response in patients with MM. However, preclinical and preliminary clinical results in patients with relapsed/refractory MM suggest that MAbs are likely to act synergistically with dexamethasone, immunomodulators, and bortezomib, showing the ability to overcome resistance to these drugs[147]. Moreover, substantial efforts are underway to develop antibodies conjugated to cytotoxic agents, such as calicheamicin, doxorubicin, taxanes, maytansinoids, dolastatins, and CC-1065 analogs[148].
The near future will see a novel interest in developing novel targets for antibody-based therapies for MM. BM angiogenesis has an important role in the initiation and progression of MM. Berardi et al[127] looked at novel mechanisms of vessel formation in patients with MM through a comparative proteomic analysis between BM endothelial cells (ECs) of patients with active MM (MMECs) and ECs of patients with monoclonal gammopathy of undetermined significance (MGECs) and of subjects with benign anemia (normal ECs). Four proteins were found to be overexpressed in MMECs: filamin A, vimentin, a-crystallin B, and 14-3-3f/d protein. Berardi et al[127] investigated the differences in MMEC vs MGEC proteome to identify new targets for MM anti-angiogenic management. They found that FLNA (filamin A), VIM (vimentin), CRYAB (a-crystallin B), and YWHAZ (14-3-3f/d protein) are constantly overexpressed in MMECs and enhanced by vascular endothelial growth factor, fibroblast growth factor 2, hepatocyte growth factor, and MM plasma cell CM. These proteins are critically involved in MMEC overangiogenic phenotype, and indeed, their silencing is anti-angiogenic.
To date we cannot establish when and in which patients the newest drugs are useful. Their use should be limited to patients enrolled in clinical trials until their conclusion and until the role of these newest drugs in myeloma therapy is clarified. Finally, the regulatory agencies (FDA for United States and EMA for Europe) should approve the regular use of these drugs before their clinical use outside of clinical trials (Table 4).
| Monoclonal antibodies targeting tumor cells specific antigens | |
| Antigen | MoAb |
| CD20 | Rituximab, Tositumomab, 20-C2-2b Veltuzumab |
| CS1 | Elotuzumab |
| CD138 | B-B4, BC/B-B4, DL-101, 1D4, 1.BB.210, MI15, 2Q1484, 5F7, 104-9, 281-2, nBT062-SMCL-DM1, BT062-SPDM4, nBT062-SPP-DM1 |
| CD38 | Doratumumab, MOR202 |
| CD40 | Lucatuzumab, Lorvotuzumab |
| IGF-1 | AVE1642, AMG479 IMCA12, R15507, Figitumumab, Dalotuzumab |
| CD317 | AHM, Defucosylated AHM, XmAb 5592 |
| CD48 | Anti-CD48 MoAb |
| b2 m | IgG anti-b2m, IgM anti-b2m |
| CD70 | SGN-70 |
| CD74 | Milatuzumab |
| HLADR | ID09C3, 2D7-DB |
| CD229 | Anti-CD229 |
| GM2 ganglioside | BIW-8962 |
| ICAM-1 | BI-505 |
| Ku | 5E2 |
| Monoclonal antibodies targeting components of bone marrow microenvironment and plasma cellr–bone marrow stromalcell interaction | |
| IL-6 | Siltuximab, Tocilizumab, NRI, Elsilimomab, Azintrel, SANT-7 |
| VEGF | Bevacizumab |
| EGFR | Cetuximab |
| FGFR-3 | MFGR1877A |
| RANKL | Denosumab |
| Dickkopf | Anti-DKK1, BrlQ880 |
| Activin | RAP-011, ACE-011 |
| BAFF | Atacicept, SG1 |
| Other potential targets | |
| TRAIL-R1 | Mapatuzumab |
| TRAIL-R2 | Lexatuzumab |
| PD-L1 | CD-011 |
| VLA-4 | Natalizumab |
| Kininogen | C11C1 |
The therapy and treatment strategies for MM have largely changed in the past decade. The goal is to improve patient’s survival. The evaluation of treatments must include patient’s stratification and personalized therapies. At the same time, it is becoming more important to control the disease in a long-term fashion, maintaining the quality of life of the patient since it is still difficult to cure this disease.
Our understanding of MM pathogenesis including the intracellular mechanisms as well as the interactions between MM cells and the microenvironment has helped the discovery of several targets that have become the focus of drug development. Studies on the biology of MM have highlighted the need for agents which not only target the tumor cells themselves but also disrupt their supportive microenvironment in the bone marrow.
Future studies will focus on the use of these targeted agents in multidrug-combinations: this will maximize their synergism while minimizing toxicities. Several agents and combinations are currently in different phases of clinical studies and likely they will change the natural history of MM in the near future.
The ability to modify the biology of MM using such new therapies raises the question whether a change in the treatment paradigm towards continuous therapy, providing both tumor reduction and tumor suppression, is warranted.
The authors would like to thank Professor Perillo C for editing the English version of this manuscript. The sponsors of this study are public or non-profit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data; the authors are fully responsible for the content and editorial decisions for this manuscript.
P- Reviewer: Arafa ASM, Chandra P, Iyngkaran P S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 2. | Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Borrello I. Can we change the disease biology of multiple myeloma? Leuk Res. 2012;36 Suppl 1:S3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. 2012;2012:157496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Gertz MA, Ansell SM, Dingli D, Dispenzieri A, Buadi FK, Elliott MA, Gastineau DA, Hayman SR, Hogan WJ, Inwards DJ. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Moreau P, Rajkumar SV. “Should all eligible patients with multiple myeloma receive autologous stem-cell transplant as part of initial treatment?”. Leuk Res. 2012;36:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, Di Raimondo F, Volpe E, Ronconi S, Cangini D. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434-2441. [PubMed] |
| 8. | Palumbo A, Niesvizky R. Sustained disease control in transplant-ineligible patients: the role of continuous therapy. Leuk Res. 2012;36 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-2520. [PubMed] |
| 10. | Dispenzieri A, Jacobus S, Vesole DH, Callandar N, Fonseca R, Greipp PR. Primary therapy with single agent bortezomib as induction, maintenance and re-induction in patients with high-risk myeloma: results of the ECOG E2A02 trial. Leukemia. 2010;24:1406-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus P. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621-4629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 416] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 12. | Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo A. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 662] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 13. | Moreau P, Avet-Loiseau H, Facon T, Attal M, Tiab M, Hulin C, Doyen C, Garderet L, Randriamalala E, Araujo C. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752-5758; quiz 5982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, Noble B, Pirooz NA, Spong JE, Piza JG. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 15. | Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, Stewart AK, Turturro F, Rifkin R, Wolf J. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375-4382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 349] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, Avigan DE, Xie W, Ghobrial IM, Schlossman RL. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 717] [Cited by in RCA: 685] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 17. | Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565-1571. [PubMed] |
| 18. | Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771-4779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 19. | Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16-19. [PubMed] |
| 20. | Cavo M, Zamagni E, Tosi P, Cellini C, Cangini D, Tacchetti P, Testoni N, Tonelli M, de Vivo A, Palareti G. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica. 2004;89:826-831. [PubMed] |
| 21. | Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431-436. [PubMed] |
| 22. | Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, Harousseau J, Zonder JA, Cavo M, Zangari M. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414-423. [PubMed] |
| 23. | Zou Y, Sheng Z, Niu S, Wang H, Yu J, Xu J. Lenalidomide versus thalidomide based regimens as first-line therapy for patients with multiple myeloma. Leuk Lymphoma. 2013;54:2219-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, Litzow MR, Fonseca R, Roy V, Rajkumar SV. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035-2042. [PubMed] |
| 25. | Ria R, Reale A, Solimando AG, Mangialardi G, Moschetta M, Gelao L, Iodice G, Vacca A. Induction therapy and stem cell mobilization in patients with newly diagnosed multiple myeloma. Stem Cells Int. 2012;2012:607260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Kumar S, Munshi NC, Dispenzieri A, Kyle R. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia. 2009;23:1904-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 1441] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 28. | Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 29. | Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, de Paz R, García-Laraña J, Bengoechea E, Martín A, Mediavilla JD. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 361] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 30. | Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, Offidani M, Patriarca F, Nozzoli C, Guglielmelli T. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101-5109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 31. | Mateos MV, San Miguel JF. Safety and efficacy of subcutaneous formulation of bortezomib versus the conventional intravenous formulation in multiple myeloma. Ther Adv Hematol. 2012;3:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Gay F, Hayman SR, Lacy MQ, Buadi F, Gertz MA, Kumar S, Dispenzieri A, Mikhael JR, Bergsagel PL, Dingli D. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Ludwig H, Hajek R, Tóthová E, Drach J, Adam Z, Labar B, Egyed M, Spicka I, Gisslinger H, Greil R. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113:3435-3442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209-1218. [PubMed] |
| 35. | Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, Dib M, Guillerm G, Salles B, Eschard JP. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, Cangialosi C, Grasso M, Rossini F, Galli M. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825-831. [PubMed] |
| 37. | Waage A, Gimsing P, Fayers P, Abildgaard N, Ahlberg L, Björkstrand B, Carlson K, Dahl IM, Forsberg K, Gulbrandsen N. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 38. | Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, Zweegman S, van Marwijk Kooy M, van der Griend R, Lokhorst H. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28:3160-3166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 39. | Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, Sucak G, Kaygusuz I, Karakus S, Kaya E. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Kapoor P, Rajkumar SV, Dispenzieri A, Gertz MA, Lacy MQ, Dingli D, Mikhael JR, Roy V, Kyle RA, Greipp PR. Melphalan and prednisone versus melphalan, prednisone and thalidomide for elderly and/or transplant ineligible patients with multiple myeloma: a meta-analysis. Leukemia. 2011;25:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksaç M, Bringhen S, Mary JY, Gimsing P, Termorshuizen F. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 42. | Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF, Whittenberger BF, Abidi MH, Durie BG, Barlogie B. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood. 2010;116:5838-5841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 743] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 44. | Jacobus S, Callander N, Siegel D, Abonour R, David D, Fonseca R, Williams M, Katz M, Greipp P, Rajkumar V. Outcome of elderly patients 70 years and older with newly diagnosed myeloma in the ECOG randomized trial of lenalidomide/high-dose dexamethasone (RD) versus lenalidomide/low-dose dexamethasone (Rd) [abstract]. Haematologica. 2010;95:370. |
| 45. | Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, Gisslinger H, Wiktor-Jędrzejczak W, Zodelava M, Weisel K. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 46. | Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, Cottler-Fox M, Mohiuddin A, Alsayed Y, Tricot G. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176-185. [PubMed] |
| 47. | Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Rajkumar SV. Treatment of myeloma: cure vs control. Mayo Clin Proc. 2008;83:1142-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, Dispenzieri A, Fonseca R, Sher T, Kyle RA. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88:360-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 395] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 50. | Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 723] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 51. | Nair B, van Rhee F, Shaughnessy JD, Anaissie E, Szymonifka J, Hoering A, Alsayed Y, Waheed S, Crowley J, Barlogie B. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood. 2010;115:4168-4173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 52. | San-Miguel J, Harousseau JL, Joshua D, Anderson KC. Individualizing treatment of patients with myeloma in the era of novel agents. J Clin Oncol. 2008;26:2761-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Bladé J, Mateos MV, Rosiñol L, Boccadoro M, Cavo M, Lokhorst H. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118:4519-4529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 54. | Barlogie B, Alexanian R, Dicke KA, Zagars G, Spitzer G, Jagannath S, Horwitz L. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood. 1987;70:869-872. [PubMed] |
| 55. | Barlogie B, Hall R, Zander A, Dicke K, Alexanian R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67:1298-1301. [PubMed] |
| 56. | Alexanian R, Barlogie B, Dixon D. High-dose glucocorticoid treatment of resistant myeloma. Ann Intern Med. 1986;105:8-11. [PubMed] |
| 57. | Gertz MA, Garton JP, Greipp PR, Witzig TE, Kyle RA. A phase II study of high-dose methylprednisolone in refractory or relapsed multiple myeloma. Leukemia. 1995;9:2115-2118. [PubMed] |
| 58. | Durie BG, Dixon DO, Carter S, Stephens R, Rivkin S, Bonnet J, Salmon SE, Dabich L, Files JC, Costanzi JJ. Improved survival duration with combination chemotherapy induction for multiple myeloma: a Southwest Oncology Group Study. J Clin Oncol. 1986;4:1227-1237. [PubMed] |
| 59. | Giles FJ, Wickham NR, Rapoport BL, Somlo G, Lim SW, Shan J, Lynott AM. Cyclophosphamide, etoposide, vincristine, adriamycin, and dexamethasone (CEVAD) regimen in refractory multiple myeloma: an International Oncology Study Group (IOSG) phase II protocol. Am J Hematol. 2000;63:125-130. [PubMed] |
| 60. | Dadacaridou M, Papanicolaou X, Maltesas D, Megalakaki C, Patos P, Panteli K, Repousis P, Mitsouli-Mentzikof C. Dexamethasone, cyclophosphamide, etoposide and cisplatin (DCEP) for relapsed or refractory multiple myeloma patients. J BUON. 2007;12:41-44. [PubMed] |
| 61. | Lee CK, Barlogie B, Munshi N, Zangari M, Fassas A, Jacobson J, van Rhee F, Cottler-Fox M, Muwalla F, Tricot G. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21:2732-2739. [PubMed] |
| 62. | Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, Lamy T, Marit G, Maloisel F, Berthou C. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474-3480. [PubMed] |
| 63. | Kröger N, Perez-Simon JA, Myint H, Klingemann H, Shimoni A, Nagler A, Martino R, Alegre A, Tomas JF, Schwerdtfeger R. Relapse to prior autograft and chronic graft-versus-host disease are the strongest prognostic factors for outcome of melphalan/fludarabine-based dose-reduced allogeneic stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2004;10:698-708. [PubMed] |
| 64. | Elice F, Raimondi R, Tosetto A, D’Emilio A, Di Bona E, Piccin A, Rodeghiero F. Prolonged overall survival with second on-demand autologous transplant in multiple myeloma. Am J Hematol. 2006;81:426-431. [PubMed] |
| 65. | Olin RL, Vogl DT, Porter DL, Luger SM, Schuster SJ, Tsai DE, Siegel DL, Cook RJ, Mangan PA, Cunningham K. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Glasmacher A, Hahn C, Hoffmann F, Naumann R, Goldschmidt H, von Lilienfeld-Toal M, Orlopp K, Schmidt-Wolf I, Gorschlüter M. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584-593. [PubMed] |
| 67. | Kropff M, Baylon HG, Hillengass J, Robak T, Hajek R, Liebisch P, Goranov S, Hulin C, Bladé J, Caravita T. Thalidomide versus dexamethasone for the treatment of relapsed and/or refractory multiple myeloma: results from OPTIMUM, a randomized trial. Haematologica. 2012;97:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Anagnostopoulos A, Weber D, Rankin K, Delasalle K, Alexanian R. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768-771. [PubMed] |
| 69. | Kropff MH, Lang N, Bisping G, Dominé N, Innig G, Hentrich M, Mitterer M, Südhoff T, Fenk R, Straka C. Hyperfractionated cyclophosphamide in combination with pulsed dexamethasone and thalidomide (HyperCDT) in primary refractory or relapsed multiple myeloma. Br J Haematol. 2003;122:607-616. [PubMed] |
| 70. | Sidra G, Williams CD, Russell NH, Zaman S, Myers B, Byrne JL. Combination chemotherapy with cyclophosphamide, thalidomide and dexamethasone for patients with refractory, newly diagnosed or relapsed myeloma. Haematologica. 2006;91:862-863. [PubMed] |
| 71. | Offidani M, Marconi M, Corvatta L, Olivieri A, Catarini M, Leoni P. Thalidomide plus oral melphalan for advanced multiple myeloma: a phase II study. Haematologica. 2003;88:1432-1433. [PubMed] |
| 72. | Srkalovic G, Elson P, Trebisky B, Karam MA, Hussein MA. Use of melphalan, thalidomide, and dexamethasone in treatment of refractory and relapsed multiple myeloma. Med Oncol. 2002;19:219-226. [PubMed] |
| 73. | Offidani M, Corvatta L, Marconi M, Visani G, Alesiani F, Brunori M, Galieni P, Catarini M, Burattini M, Centurioni R. Low-dose thalidomide with pegylated liposomal doxorubicin and high-dose dexamethasone for relapsed/refractory multiple myeloma: a prospective, multicenter, phase II study. Haematologica. 2006;91:133-136. [PubMed] |
| 74. | Hussein MA, Baz R, Srkalovic G, Agrawal N, Suppiah R, Hsi E, Andresen S, Karam MA, Reed J, Faiman B. Phase 2 study of pegylated liposomal doxorubicin, vincristine, decreased-frequency dexamethasone, and thalidomide in newly diagnosed and relapsed-refractory multiple myeloma. Mayo Clin Proc. 2006;81:889-895. [PubMed] |
| 75. | Moehler TM, Neben K, Benner A, Egerer G, Krasniqi F, Ho AD, Goldschmidt H. Salvage therapy for multiple myeloma with thalidomide and CED chemotherapy. Blood. 2001;98:3846-3848. [PubMed] |
| 76. | Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420-4427. [PubMed] |
| 77. | Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609-2617. [PubMed] |
| 78. | Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557-3560. [PubMed] |
| 79. | Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892-3901. [PubMed] |
| 80. | Jakubowiak AJ, Kendall T, Al-Zoubi A, Khaled Y, Mineishi S, Ahmed A, Campagnaro E, Brozo C, Braun T, Talpaz M. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol. 2009;27:5015-5022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Berenson JR, Yang HH, Sadler K, Jarutirasarn SG, Vescio RA, Mapes R, Purner M, Lee SP, Wilson J, Morrison B. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24:937-944. [PubMed] |
| 82. | Reece DE, Rodriguez GP, Chen C, Trudel S, Kukreti V, Mikhael J, Pantoja M, Xu W, Stewart AK. Phase I-II trial of bortezomib plus oral cyclophosphamide and prednisone in relapsed and refractory multiple myeloma. J Clin Oncol. 2008;26:4777-4783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Richardson P, Jagannath S, Hussein M, Berenson J, Singhal S, Irwin D, Williams SF, Bensinger W, Badros AZ, Vescio R. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 84. | Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133-2142. [PubMed] |
| 85. | Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foà R. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123-2132. [PubMed] |
| 86. | Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, Petrucci MT, Yu Z, Olesnyckyj M, Zeldis JB. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 87. | Touzeau C, Blin N, Clavert A, Roland V, Loirat M, Tessoulin B, Le Gouill S, Planche L, Pennetier M, Mahe B. Efficacy of lenalidomide plus dexamethasone in patients older than 75 years with relapsed multiple myeloma. Leuk Lymphoma. 2012;53:1318-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Baz R, Walker E, Karam MA, Choueiri TK, Jawde RA, Bruening K, Reed J, Faiman B, Ellis Y, Brand C. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: safety and efficacy. Ann Oncol. 2006;17:1766-1771. [PubMed] |
| 89. | Knop S, Gerecke C, Liebisch P, Topp MS, Platzbecker U, Sezer O, Vollmuth C, Falk K, Glasmacher A, Maeder U. Lenalidomide, adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: a report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom). Blood. 2009;113:4137-4143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | van de Donk NW, Wittebol S, Minnema MC, Lokhorst HM. Lenalidomide (Revlimid) combined with continuous oral cyclophosphamide (endoxan) and prednisone (REP) is effective in lenalidomide/dexamethasone-refractory myeloma. Br J Haematol. 2010;148:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Morgan GJ, Schey SA, Wu P, Srikanth M, Phekoo KJ, Jenner M, Davies FE. Lenalidomide (Revlimid), in combination with cyclophosphamide and dexamethasone (RCD), is an effective and tolerated regimen for myeloma patients. Br J Haematol. 2007;137:268-269. [PubMed] |
| 92. | Ciolli S, Leoni F, Casini C, Breschi C, Santini V, Bosi A. The addition of liposomal doxorubicin to bortezomib, thalidomide and dexamethasone significantly improves clinical outcome of advanced multiple myeloma. Br J Haematol. 2008;141:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Terpos E, Kastritis E, Roussou M, Heath D, Christoulas D, Anagnostopoulos N, Eleftherakis-Papaiakovou E, Tsionos K, Croucher P, Dimopoulos MA. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22:2247-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, Cangialosi C, Caravita T, Morabito F, Musto P. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767-2772. [PubMed] |
| 95. | Pineda-Roman M, Zangari M, van Rhee F, Anaissie E, Szymonifka J, Hoering A, Petty N, Crowley J, Shaughnessy J, Epstein J. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, Mazumder A, Munshi NC, Ghobrial IM, Doss D. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27:5713-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 97. | Palumbo A, Larocca A, Falco P, Sanpaolo G, Falcone AP, Federico V, Canepa L, Crugnola M, Genuardi M, Magarotto V. Lenalidomide, melphalan, prednisone and thalidomide (RMPT) for relapsed/refractory multiple myeloma. Leukemia. 2010;24:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Cunningham D, Powles R, Malpas J, Raje N, Milan S, Viner C, Montes A, Hickish T, Nicolson M, Johnson P. A randomized trial of maintenance interferon following high-dose chemotherapy in multiple myeloma: long-term follow-up results. Br J Haematol. 1998;102:495-502. [PubMed] |
| 99. | Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, Yakoub Agha I, Bourhis JH, Garderet L, Pegourie B. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289-3294. [PubMed] |
| 100. | McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, Giralt S, Stadtmauer EA, Weisdorf DJ, Vij R. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 889] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 101. | Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, Stoppa AM, Hulin C, Benboubker L, Garderet L. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 890] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 102. | Scheid C, Sonneveld P, Schmidt-Wolf IG, van der Holt B, el Jarari L, Bertsch U, Salwender H, Zweegman S, Blau IW, Vellenga E. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99:148-154. [PubMed] |
| 103. | Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326-2335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 517] [Cited by in RCA: 639] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 104. | Gertz MA. Pomalidomide and myeloma meningitis. Leuk Lymphoma. 2013;54:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 106. | Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, Brady HA, Gandhi AK, Schafer PH, Muller GW, Worland PJ, Chan KW, Verhelle D. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69:7347-7356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 107. | Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22:3269-3276. [PubMed] |
| 108. | Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA. Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br J Haematol. 2008;141:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 109. | Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, Sullivan D, Alsina M, Schlossman R, Ghobrial IM. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 110. | Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, Mathiot C, Petillon MO, Macro M, Roussel M. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myélome 2009-02. Blood. 2013;121:1968-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 111. | Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, Gertz M, Kumar S, Mikhael J, Roy V. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 112. | Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, Dispenzieri A, Kumar S, Greipp PR, Lust JA. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970-2975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 113. | Geyer HL, Viggiano RW, Lacy MQ, Witzig TE, Leslie KO, Mikhael JR, Stewart K. Acute lung toxicity related to pomalidomide. Chest. 2011;140:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 114. | Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, Jiang J, Laidig GJ, Lewis ER, Parlati F. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383-6391. [PubMed] |
| 115. | O’Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, Orlowski RZ. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085-7091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 116. | Alsina M, Trudel S, Furman RR, Rosen PJ, O’Connor OA, Comenzo RL, Wong A, Kunkel LA, Molineaux CJ, Goy A. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18:4830-4840. [PubMed] |
| 117. | Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, Kukreti V, Jagannath S, McDonagh KT, Alsina M. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661-5670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 118. | Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, Trudel S, Kukreti V, Bahlis N, Alsina M. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 119. | Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, Badros AZ, Jagannath S, McCulloch L, Rajangam K. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 120. | Papadopoulos KP, Burris HA, Gordon M, Lee P, Sausville EA, Rosen PJ, Patnaik A, Cutler RE, Wang Z, Lee S. A phase I/II study of carfilzomib 2-10-min infusion in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;72:861-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 121. | Niesvizky R, Martin TG, Bensinger WI, Alsina M, Siegel DS, Kunkel LA, Wong AF, Lee S, Orlowski RZ, Wang M. Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. 2013;19:2248-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 122. | Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, Al-Zoubi A, Anderson T, Nordgren B, Detweiler-Short K. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 123. | Mikhael JR, Reeder CB, Libby EN III, Costa LJ, Bergsagel PL, Buadi F, Mayo A, Gano K, Wolf C, Dueck AC. A phase I/II trial of cyclophosphamide, carfilzomib, thalidomide, and dexamethasone (CYCLONE) in patients with newly diagnosed multiple myeloma. ASCO Annual Meeting Abstracts. 2012;30 Suppl 15:8010. |
| 124. | Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 125. | Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585-598. [PubMed] |
| 126. | Ria R, Reale A, De Luisi A, Ferrucci A, Moschetta M, Vacca A. Bone marrow angiogenesis and progression in multiple myeloma. Am J Blood Res. 2011;1:76-89. [PubMed] |
| 127. | Berardi S, Caivano A, Ria R, Nico B, Savino R, Terracciano R, De Tullio G, Ferrucci A, De Luisi A, Moschetta M. Four proteins governing overangiogenic endothelial cell phenotype in patients with multiple myeloma are plausible therapeutic targets. Oncogene. 2012;31:2258-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407-419. [PubMed] |
| 129. | Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654-1664. [PubMed] |
| 130. | Ocio EM, Mateos MV, Maiso P, Pandiella A, San-Miguel JF. New drugs in multiple myeloma: mechanisms of action and phase I/II clinical findings. Lancet Oncol. 2008;9:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 131. | Mitsiades N, Mitsiades CS, Richardson PG, McMullan C, Poulaki V, Fanourakis G, Schlossman R, Chauhan D, Munshi NC, Hideshima T. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood. 2003;101:4055-4062. [PubMed] |
| 132. | Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981-989. [PubMed] |
| 133. | Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA. 2004;101:540-545. [PubMed] |
| 134. | Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, Harris C, Zwiebel J, Wright JJ, Espinoza-Delgado I. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250-5257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 135. | Kawauchi K, Ogasawara T, Yasuyama M, Otsuka K, Yamada O. The PI3K/Akt pathway as a target in the treatment of hematologic malignancies. Anticancer Agents Med Chem. 2009;9:550-559. [PubMed] |
| 136. | Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053-4062. [PubMed] |
| 137. | Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, Densmore J, Krishnan A, Raje N, Bar M. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011;29:4243-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 139. | Farag SS, Zhang S, Jansak BS, Wang X, Kraut E, Chan K, Dancey JE, Grever MR. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leuk Res. 2009;33:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 140. | Ghobrial IM, Weller E, Vij R, Munshi NC, Banwait R, Bagshaw M, Schlossman R, Leduc R, Chuma S, Kunsman J. Weekly bortezomib in combination with temsirolimus in relapsed or relapsed and refractory multiple myeloma: a multicentre, phase 1/2, open-label, dose-escalation study. Lancet Oncol. 2011;12:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 141. | Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761-772. [PubMed] |
| 142. | Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, Albitar M, Berman D, Messina M, Anderson KC. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 143. | Di Bernardo A, Macor P, Guarnotta C, Franco G, Florena AM, Tedesco F, Tripodo C. Humoral immunotherapy of multiple myeloma: perspectives and perplexities. Expert Opin Biol Ther. 2010;10:863-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 144. | Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau P. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 616] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 145. | Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J, Moran M, Lucas M, Lin T, Hackbarth ML. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;103:3278-3281. [PubMed] |
| 146. | Bladé J, de Larrea CF, Rosiñol L. Incorporating monoclonal antibodies into the therapy of multiple myeloma. J Clin Oncol. 2012;30:1904-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Richardson PG, Lonial S, Jakubowiak AJ, Harousseau JL, Anderson KC. Monoclonal antibodies in the treatment of multiple myeloma. Br J Haematol. 2011;Jul 21; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 148. | Lambert JM. Drug-conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol. 2013;76:248-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |