Published online Jun 26, 2013. doi: 10.5662/wjm.v3.i2.19
Revised: June 4, 2013
Accepted: June 12, 2013
Published online: June 26, 2013
AIM: To validate the protocol described here to be used in future clinical trials related to the effect of laser therapy on dental pulp.
METHODS: Histologically treated samples from eight human healthy premolar teeth obtained from the middle root level were distributed in four groups: group 1 (G1) absolute control; group 2 (G2) only laser irradiation; group 3 (G3) exposed only to orthodontics; and group 4 (G4) treated with orthodontics and laser. Laser treatment was performed at 830 nm wavelength, 100 mW (energy 80 J/cm2, 2.2 J), for 22 s in the vestibular surface and 22 s in the palatal surface, 1 mm away from the dental root mucosa. Three staining methods were performed: hematoxylin-eosin (HE), Masson’s Trichrome method and Gomori’s method.
RESULTS: The pulp histology parameters were evaluated and the results classified in to 3 parts: an inflammatory response, soft tissue response (dental pulp) and hard tissue response (dentin and predentin). There was no inflammation (chronic or acute) in any of the evaluated groups. The zones of pulp necrosis were found in one premolar of G3 and in one of G4; in groups G2 and G4 there was higher angiogenesis than in the other two groups. G4 group presented the highest level of vascularization. A reduced nerve density was observed in G3. A G2 specimen showed increased nerve density. A higher rate of calcification was observed in G1 compared to G2. Denticles, either real or false, were observed in G1, G2 and G3. Sclerosis of dentin and focal dentin loss was observed among all the groups. Secondary dentin was present in one sample in G1 and G2. A necrosis zone was found in one sample of G3 and G4. No differences between groups were observed in the odontoblast irregularity layer but the layer was wider in the group treated with laser only. A notable difference was detected in reduction of the cell-free layer between the groups G1 and G4. The findings in pulp tissue favor its adaptative response against dental movement induced by orthodontics. No definitive conclusions may be derived as this is a pilot study.
CONCLUSION: The protocol described here was shown to be an effective method to evaluate changes in dental pulp submitted to low level laser in teeth under orthodontic movement.
- Citation: Domínguez &, Ballesteros RE, Viáfara JH, Tamayo OM. Effect of low level laser therapy on dental pulp during orthodontic movement. World J Methodol 2013; 3(2): 19-26
- URL: https://www.wjgnet.com/2222-0682/full/v3/i2/19.htm
- DOI: https://dx.doi.org/10.5662/wjm.v3.i2.19
Low level laser therapy (LLLT) causes positive effects to physiological bone remodeling[1-6] and its effects are considered positive and not cytotoxic for cells participating in induced dental movement, such as fibroblasts[7-12] osteoblasts[13-18], osteoclasts and pre osteoclasts[19,20]. Laser application during orthodontic treatment accelerates orthodontic induced movement[21-25] and reduces pain symptoms during the different treatment stages[26-30].
The orthodontic dental movement has an inflammatory-like effect on pulp tissue, initially causing changes in blood flow[31-33], increasing the level of angiogenic growth factors[34], central and peripheral angiogenesis[35] and generating changes in the odontoblastic layer[36]. Early biochemical changes include a reduction in the activity of alkaline phosphatase[37] and increased activity of aspartate aminotransferase[38].
At the neuronal level, the expression of substance P (SP) in response to orthodontic movement in pulp has been described as well as its potent action in neurogenic inflammation which is directly related to the pain sensation during orthodontic treatment[39].
Some studies in orthodontically treated teeth showed injuries such as root canal calcification[40] and degeneration of the odontoblastic layer due to blood flow alteration, accompanied by edema with pulp vessel congestion and fibrotic changes in pulp tissue, including necrosis[41-43].
In vitro and animal model studies suggest that laser application during orthodontic movement may be able to accelerate pulp damage repair. Miyata et al[44] analyzed the effect of low level laser on pulp tissue from an extracted third molar, finding that this irradiation activated the phosphorylation of mitogen activated protein kinase (MAPK) and increased extracellular signals regulated by protein kinase (ERK). This MAPK/ERK activation is indicative of cell proliferation, differentiation and survival.
Abi-Ramia et al[45] published a study on the effect of LLLT on Wistar rats. They applied a 0.4 N force and laser (Ga-Al-As of 830 nm, 100 mW, 18 J) on the control group. In this group, the authors found increased pulp vascularization and concluded that laser application during orthodontic movement may help to repair pulp tissue.
The effect of low level laser therapy used to accelerate dental movement and reduce pain during orthodontic treatments on human dental pulp is not clear. The purpose of this pilot study is to first validate the modified protocol described here to be used in future clinical trials. Secondly, to describe the histological changes in human pulp tissue related to the effect of low level laser therapy as used during the alignment and leveling stage of orthodontic treatment.
This study protocol number 124-010 was approved by the Ethics Committee of the Universidad del Valle (Cali, Colombia), conforms to the principles of the declaration of Helsinki and all the patients signed informed consent before any intervention.
According to the Colombian Ministry of Health Resolution 8430 from 1993, this research study is classified in the category of minimum risk for the patients.
Two patients treated at the orthodontics department of dentistry of the Universidad del Valle (23 and 25 years old) provided the sample of eight first premolar teeth programmed to be extracted as part of the orthodontic treatment. Histological sections taken from the middle root zone were obtained from the freshly extracted premolar teeth.
The selected teeth should be completely healthy, without previous endodontic, orthodontic, whitening or restorative treatment, periodontally healthy and free of any trauma or fracture.
The premolar teeth were randomly assigned to four groups, as follows: (1) Control group (G1): Two healthy premolar teeth, without laser therapy and orthodontic treatment; (2) Laser treated group (G2): Two healthy premolar teeth treated with laser without orthodontic treatment; (3) Orthodontic group (G3): Two healthy premolar teeth submitted to orthodontic alignment with standard brackets but not laser treated. The extractions were carried out one month after initiating orthodontic treatment; and (4) Orthodontic and laser group (G4): Two healthy premolar teeth were submitted to the same orthodontic treatment as in G3. The laser treatment was performed after bracket bonding, following the protocol for therapeutic laser use. The extractions were carried out one month later and a second laser dose was applied before the procedure.
The patients were treated using a straight-wire technique with Synthesis® brackets (Ormco, S.A. de C.V. Mexico). All the brackets were bonded with Transbond XT® resin (Unitek, Monrovia, CA). The initial arch wire was Cu-NiTi 0.014 inch.
The healthy premolars in the corresponding groups to be laser treated were irradiated using a Photon II Laser (Equipamentos DMC, Sao Carlos, Brazil) at a wavelength of 830 nm, 100 mW (energy: 80 J/cm2, 2.2 J) at a distance of 1mm away from the mucosa in the vestibular and palatal surface each one for 22 s. This protocol has been shown to be effective for therapeutic purposes in previous studies[24,30].
All the extracted teeth were treated following the same histological protocol by the same operator and read by a previously standardized pathologist.
The histological protocol includes: (1) Immediately after extraction, the premolar is cross-sectioned in the middle part using a high speed hand piece and diamond tronco-cone bur; (2) The two fragments obtained are immediately submerged in a recipient with a mixture of glutaraldehyde 2.5% and paraformaldehyde 3% in PBS (phosphate buffer saline) at 4 °C. Each sample was fixed in a recipient with a key indicating the group number, specimen number, tooth number according to the international dental nomenclature and the histological treatment date; (3) After the first week of fixation, the specimens are decalcified with a mixture of citrate, EDTA and formate following a technique developed by Dr. Oscar Tamayo from the histology laboratory of the Universidad del Valle (patent under process); (4) The specimens are washed in PBS 0.01 M during two days (4 wash outs per day); (5) The specimens are dehydrated in ethanol (70%, 95%, 100%, plus Xylol) and then included in paraffin molds to be sectioned with microtome at the level of the cement-enamel junction in 5 micrometer slices; (6) The slices are extended in a water bath at 40 °C and dried out in a furnace for one hour at 56 °C. The slide sheet are immersed in xylol and absolute ethanol (100%, 95%, 70% and water); (7) Staining for general cell contents and angiogenesis evaluation is performed using hematoxylin-eosin (HE); (8) The Masson’s Trichrome method staining is used to observe type I collagen fibers; and (9) The Gomori’s method for reticulum staining is used to visualize the reticulum fibers (Type III collagen fibers).
Each descriptive parameter was classified in an ordinal scale as 0: absent, 1: low, 2: moderate, 3: severe, NC: not changed.
The pulp histology parameters were evaluated and the results are summarized in Table 1, classified in to 3 parts: an inflammatory response, soft tissue response (dental pulp) and hard tissue response (dentin and predentin), as recommended by Sübay et al[46].
| Histological findings | G1 | G2 | G3 | G4 |
| Necrosis | 0 | 0 | 1 | 1 |
| 0 | 0 | 0 | 0 | |
| Central angiogenesis | 1 | _ | 1 | _ |
| Peripheral angiogenesis | 1 | 1 | _ | 1 |
| Central and peripheral | _ | 3 | 1 | 2 |
| Vascular density | 1 | 1 | 1 | 2 |
| 1 | 1 | 2 | 2 | |
| Nerve density | 1 | 1 | NC | 1 |
| 1 | 2 | NC | NC | |
| Calcifications | 2 | 0 | 1 | 1 |
| 2 | 1 | 3 | 3 | |
| False denticles | 1 | 1 | 0 | 0 |
| Real denticles | 1 | 1 | 1 | 0 |
Inflammatory response: There was no inflammation (chronic or acute) in any of the evaluated groups, G1, G2, G3 and G4.
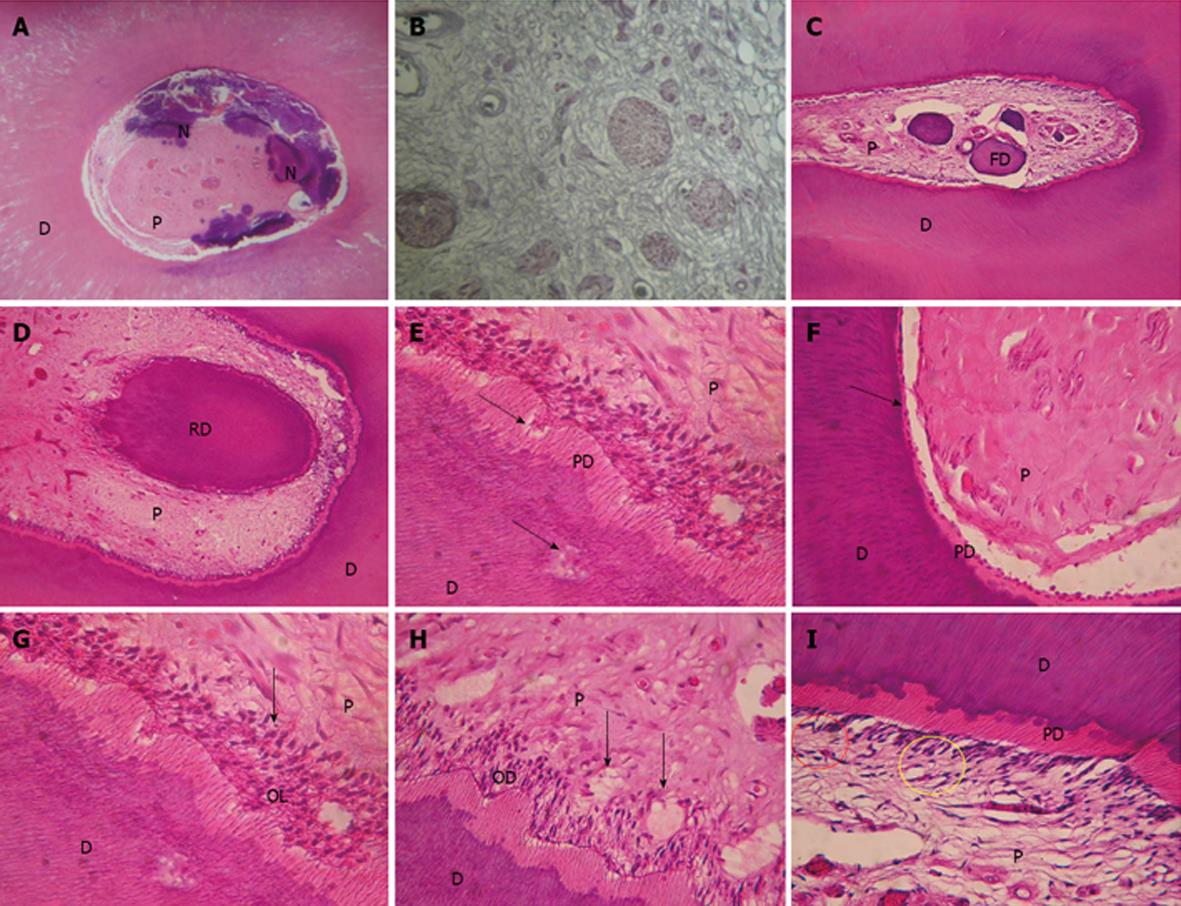
Soft tissue response: Connective tissue histological findings: The observations made under this three stain techniques applied to evaluate connective tissue fibrosis are summarized in Table 2. Figure 1A clearly shows pulp fibrosis. Zones of pulp necrosis: Zones of pulp necrosis were found in one premolar of G3 and G4 (Figure 1B). Vascularization: There was more angiogenesis in the G2 and G4 groups than in the other two groups. The group receiving orthodontic treatment plus laser presented with the highest level of vascularization. Angiogenesis is observed under HE staining where the vascular endothelium shows no muscular layer, a clear indication that it is only developing. Nerves: A reduced nerve density was observed in the orthodontic positive group (laser negative). A G2 specimen (laser treated) showed increased nerve density (Figure 1C). Presence of calcification and denticles: A higher calcification was observed in G1 than in G2. No difference was detected in the orthodontic positive groups. Denticles, either real or false, were observed in G1, G2 and G3 (Figure 1D, E). Fibroblast morphology: No descriptive alterations in histological findings were observed between groups in the morphology of fibroblasts.
| Staining | G1 | G2 | G3 | G4 |
| Hematoxylin-eosin | 1 | 1 | 1 | 2 |
| 1 | 2 | 3 | 2 | |
| Masson’s Trichrome method | 2 | 1 | 1 | 2 |
| 2 | 1 | 2 | 2 | |
| Gomori’s method for reticulum | 2 | 1 | 1 | 2 |
| 2 | 2 | 2 | 2 |
Hard tissue response (dentin and predentin): Histological findings in hard tissues are summarized in Table 3. Sclerosis of dentin and focal dentin lost was observed in all the groups (Figure 1F, G). Secondary dentin was present in one sample of G1 and G2. A necrosis zone was found in one sample of G3 and G4. Odontoblast layer: No relevant differences between groups were observed in odontoblast irregularities but the layer was wider in the group treated with laser only. No differences were detected regarding odontoblast vacuolization (Figure 1H, I). Acellular zone (Weil zone): A notable difference was detected in a reduction of the cell-free layer between the groups G1 and G4. Cell-rich zone: Fibroblast proliferation was minimal in G1 group.
| Histological findings | G1 | G2 | G3 | G4 |
| Sclerosis of dentin | 2 | 1 | 1 | 1 |
| 0 | 0 | 0 | 0 | |
| Secondary dentin | 2 | 2 | 0 | 0 |
| 0 | 0 | 0 | 0 | |
| Necrosis in dentin | 0 | 0 | 1 | 1 |
| 0 | 0 | 0 | 0 | |
| Focal dentin lost | 2 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | |
| Focal predentin lost | 2 | 2 | 1 | 1 |
| 0 | 0 | 0 | 0 | |
| Irregularities in odontoblast layer | 1 | 2 | 1 | 1 |
| 1 | 3 | 1 | 1 | |
| Thickening of odontoblasts | 1 | 2 | 3 | 2 |
| 1 | 3 | 1 | 0 | |
| Odontoblast vacuolization | 1 | 2 | 1 | 1 |
| 2 | 2 | 2 | 2 | |
| Reduction of Weil zone | 1 | 2 | 1 | 3 |
| 1 | 2 | 3 | 3 | |
| Cell-rich zone | 1 | 2 | 1 | 2 |
| 1 | 2 | 3 | 2 |
The present study performed in human premolar healthy teeth explored possible histological changes in the pulp, related to orthodontics and/or LLLT during the initial phases or orthodontic treatment.
In their study, Villa et al[34] evaluated pulp-dentinal reactions after the application of a 4 ounce intrusive orthodontic force to human maxillary first premolars in patients given the NSAID nabumetone. The root surface histological slides were stained with hematoxylin-eosin, Masson’s Trichrome method and Gomori’s method for reticulum. Masson’s Trichrome method was used to visualize fibrin, fibrosis and type I collagen fibers. Gomori’s method for reticulum was used to visualize reticulum fibers (Type III collagen fibers). Even although there was this difference, similar findings are described for the pulp tissue.
The most relevant results are those related to pulp vascularization; the scientific literature indicates that orthodontic dental movement affects pulp vascularization. Taking into account that the pulp is surrounded by rigid structures and the blood supply comes from the apical foramen, any change in blood flow or tissue pressure may affect the pulp integrity[39,47,48].
Vascular alterations have a direct impact upon pulp metabolism, especially changes in blood supply and angiogenesis, as the research line of Derringer has proved[49-51].
Angiogenesis is the formation of new capillary structures through neovascularization. Angiogenesis stages include a breakdown of vascular membrane, endothelial cell mitosis and migration to form new capillaries, as well as cell folding to preserve the vessel lumen[35].
The importance of the increased vascularization found in the present study is that it accelerates the pulp repair, as described by Abi-Ramia et al[45] in 2010 in a report about the effect of LLLT in an animal model. The study was performed in 45 Wistar rats. The control group (n = 20 rats) received a 0.4 N stress for mesial movement and laser irradiation from Ga-Al-As laser at 830 nm, 100 mW, 18 J/cm2 4 s per point in the vestibular, mesial and palatal surfaces, perpendicular to the molar axis. The authors found transitory hyperemia in the pulp in this group and suggested that the application of laser during orthodontic movement may accelerate pulp injury repair.
The same hypothesis is supported by the study of McDonald and Pitt Ford[52] made in maxillary permanent canine teeth; it suggests that the increment of pulp blood flow is a consequence of the inflammatory process triggered by the force applied for dental movement. During this process, the increased blood supply and the presence of inflammatory cells in the zone aim to repair the tissue.
The results of the present study show necrosis zones in G3 and G4. These zones can be the result of the orthodontic induced movement, as suggested by Woloshyn et al[42], Perinetti et al[37] and Bauss et al[53]. It should also be considered that the result is a consequence of the use of a high speed cutting bur when the teeth were cut into 2 fragments. The possibilities of a previous pulp necrosis related to dental trauma or leveling an alignment are ruled out due to a close verification process during experimentation time. The alterations observed in the odontoblastic layer are consistent with those described in previous studies. Santamaria et al[54], after applying 0.4 N to produce mesial tipping in maxillary molar teeth of rats, found hypertrophy of odontoblasts, especially at the mesial area of the coronal pulp.
There are also previous reports about the proliferative effect of LLLT on different cell lines, including odontoblasts[55-57]. In the present study, an irregular distribution of the odontoblast layer in all groups was observed, compared to the absolute control group. The widening of the odontoblast layer appears under LLLT stimulation compared to the control group.
Reports presented by Stenvik et al[58] in teeth with a closed apex submitted to dental movement indicate few or no vacuolization of the odontoblastic layer, indicating that the inflammatory changes generated by the orthodontic force are made without causing important degenerative breakdown in the odontoblasts. However, in the present study, vacuolization of odontoblasts in all the groups studied was observed, including the control group.
The reduction of the cell-free zone (Weil) was notorious when the control group G1 was compared to G4, especially in areas where more alterations were found in the odontoblast layer, agreeing with findings in previous reports[46,55,58].
In teeth under orthodontic movement with or without LLLT, it is usual to observe a cell-rich zone inside the cell-free layer that is differentiated from the central pulp portion by the high number of cells per area unit, mainly fibroblasts and undifferentiated mesenchymal cells, due to its proliferative effects on fibroblasts and collagen fibers. It is also known that this cell-rich zone is more abundant on irradiated teeth than in the control group[45]. In the present study, the difference was not relevant when the control group was compared to the experimental groups or among the experimental groups, probably due to the sample size.
It is not possible to compare the present study results with other human studies as there are only reports from animal models[45] that show some evidence that orthodontic movement associated with LLLT enhances vascularization and therefore could accelerate pulp tissue repair. On the other hand, there are reports in human subjects indicating that LLLT has deleterious effects when the energy released is high enough to increase the temperature in the pulp camera above the threshold of 5.5 °C[59]. The present investigation suggests that the pulp tissue reacts in a way that tends to favor repair from the initial injury produced by the orthodontic force.
The most relevant contribution of the present study is the number of histological findings not previously shown in high quality slices in human pulp after application of LLLT in teeth under orthodontic movement. It is also relevant that this design and protocol might be applied to further studies using a sample of at least 12 dental specimens per group.
In conclusion, the protocol described here was shown to be an effective method to evaluate changes in human dental pulp tissue submitted to low level laser therapy on teeth under orthodontic movement. The findings made in pulp tissue favor its adaptative response against dental movement injury induced by orthodontics and this data should be validated in future randomized clinical trials.
The authors wish to thank the following methodological and technical advisors: Dr. Adriana Jaramillo, Magister in Microbiology, Universidad del Valle, Dr. Herney Rengifo, Magister in Public Health and Epidemiology, Universidad del Valle and Dr Roberto Jaramillo, pathologist, Universidad del Valle. We also thank Dr. Luis Rogelio Hernández, Master of Science from the University of Southampton, and Dr. Sergio Velasquez, orthodontist, Universidad del Valle, for their assistance in the writing and translation of this article.
Laser application during orthodontic treatment accelerates orthodontic movement and reduces pain symptoms during the different treatment stages; however, the effect of low level laser therapy on human dental pulp is not clear.
Some studies in orthodontically treated teeth showed injuries such as root canal calcification and degeneration of the odontoblastic layer due to blood flow alteration, accompanied by edema with pulp vessel congestion and fibrotic changes in pulp tissue, including necrosis. In vitro and animal model studies suggest that laser application during orthodontic movement may be able to accelerate pulp damage repair.
It is not possible to compare the present study results with other human studies as there are only reports from animal models that show some evidence that orthodontic movement associated with low level laser therapy (LLLT) enhances vascularization and therefore could accelerate pulp tissue repair. The present investigation suggests that the pulp tissue reacts in a way that tends to favor repair from the initial injury produced by the orthodontic force.
The most relevant contribution of the present study is the number of histological findings not previously shown in high quality slices in human pulp after application of LLLT in teeth under orthodontic movement. It is also relevant that this design and protocol might be applied to further studies with a higher number of specimens.
LLLT has been advocated as a collateral-free therapy and its application has shown considerable reduction in pain caused by orthodontic appliance placement. Furthermore, LLLT has also shown biostimulatory effects; the energy output of the device is low enough not to exceed an irradiated tissue temperature of 36.5 °C.
This is an interesting study revealing the histological response of human dental pulp to the application of LLLT in teeth under orthodontic tooth movement.
P- Reviewers Ajcharanukul O, do Prado M L- Editor Roemmele A S- Editor Wen LL E- Editor Lu YJ
| 1. | Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Habib FA, Gama SK, Ramalho LM, Cangussú MC, Santos Neto FP, Lacerda JA, Araújo TM, Pinheiro AL. Laser-induced alveolar bone changes during orthodontic movement: a histological study on rodents. Photomed Laser Surg. 2010;28:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Fujita S, Yamaguchi M, Utsunomiya T, Yamamoto H, Kasai K. Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod Craniofac Res. 2008;11:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Yamaguchi M, Hayashi M, Fujita S, Yoshida T, Utsunomiya T, Yamamoto H, Kasai K. Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha(v) beta(3) integrin in rats. Eur J Orthod. 2010;32:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Almeida-Lopes L, Rigau J, Zângaro RA, Guidugli-Neto J, Jaeger MM. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med. 2001;29:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 247] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med. 2002;31:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Kreisler M, Christoffers AB, Willershausen B, d’Hoedt B. Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J Clin Periodontol. 2003;30:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Choi EJ, Yim JY, Koo KT, Seol YJ, Lee YM, Ku Y, Rhyu IC, Chung CP, Kim TI. Biological effects of a semiconductor diode laser on human periodontal ligament fibroblasts. J Periodontal Implant Sci. 2010;40:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med Sci. 2008;23:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | DomØnguez A, Clarkson A, LŦpez R. An in vitro study of the reaction of periodontal and gingival fibroblasts to low- level laser irradiation: A pilot study. J Oral Laser Applications. 2008;8:235-244. |
| 12. | Ozawa Y, Shimizu N, Kariya G, Abiko Y. Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone. 1998;22:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001;4:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Fujihara NA, Hiraki KR, Marques MM. Irradiation at 780 nm increases proliferation rate of osteoblasts independently of dexamethasone presence. Lasers Surg Med. 2006;38:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts--an in vitro study. Lasers Med Sci. 2012;27:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Dominguez A, Castro P, Morales M. An In Vitro Study of the Reaction of Human Osteoblasts to Low-level Laser Irradiation. J Oral Laser Appl. 2009;9:21-28. |
| 17. | Dominguez AC, Castro PZ, Morales MC. Cellular Effects related to the clinical uses of laser in orthodontics. J Oral Laser Appl. 2009;9:199-203. |
| 18. | Aihara N, Yamaguchi M, Kasai K. Low-energy irradiation stimulates formation of osteoclast-like cells via RANK expression in vitro. Lasers Med Sci. 2006;21:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | DomØnguez A, Bayona G, Casas A. In vitro response of Human Pre-Osteoclasts to low intensity Laser irradiation. J Res Biol. 2012;2:733-741. |
| 20. | Cruz DR, Kohara EK, Ribeiro MS, Wetter NU. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med. 2004;35:117-120. [PubMed] |
| 21. | Limpanichkul W, Godfrey K, Srisuk N, Rattanayatikul C. Effects of low-level laser therapy on the rate of orthodontic tooth movement. Orthod Craniofac Res. 2006;9:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Youssef M, Ashkar S, Hamade E, Gutknecht N, Lampert F, Mir M. The effect of low-level laser therapy during orthodontic movement: a preliminary study. Lasers Med Sci. 2008;23:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | DomØnguez A, VelÐsquez SA. Acceleration Effect of Orthodontic Movement by Application of Low-intensity Laser. J Oral Laser Appl. 2010;10:99-105. |
| 24. | Sousa MV, Scanavini MA, Sannomiya EK, Velasco LG, Angelieri F. Influence of low-level laser on the speed of orthodontic movement. Photomed Laser Surg. 2011;29:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Lim HM, Lew KK, Tay DK. A clinical investigation of the efficacy of low level laser therapy in reducing orthodontic postadjustment pain. Am J Orthod Dentofacial Orthop. 1995;108:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Harazaki M, Takahashi H, Ito A, Isshiki Y. Soft laser irradiation induced pain reduction in orthodontic treatment. Bull Tokyo Dent Coll. 1998;39:95-101. [PubMed] |
| 27. | Tortamano A, Lenzi DC, Haddad AC, Bottino MC, Dominguez GC, Vigorito JW. Low-level laser therapy for pain caused by placement of the first orthodontic archwire: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 2009;136:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Xiaoting L, Yin T, Yangxi C. Interventions for pain during fixed orthodontic appliance therapy. A systematic review. Angle Orthod. 2010;80:925-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Domínguez A, Velásquez SA. Effect of low-level laser therapy on pain following activation of orthodontic final archwires: a randomized controlled clinical trial. Photomed Laser Surg. 2013;31:36-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Barwick PJ, Ramsay DS. Effect of brief intrusive force on human pulpal blood flow. Am J Orthod Dentofacial Orthop. 1996;110:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Ikawa M, Fujiwara M, Horiuchi H, Shimauchi H. The effect of short-term tooth intrusion on human pulpal blood flow measured by laser Doppler flowmetry. Arch Oral Biol. 2001;46:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Sano Y, Ikawa M, Sugawara J, Horiuchi H, Mitani H. The effect of continuous intrusive force on human pulpal blood flow. Eur J Orthod. 2002;24:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Derringer KA, Jaggers DC, Linden RW. Angiogenesis in human dental pulp following orthodontic tooth movement. J Dent Res. 1996;75:1761-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Villa PA, Oberti G, Moncada CA, Vasseur O, Jaramillo A, Tobón D, Agudelo JA. Pulp-dentine complex changes and root resorption during intrusive orthodontic tooth movement in patients prescribed nabumetone. J Endod. 2005;31:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Hamilton RS, Gutmann JL. Endodontic-orthodontic relationships: a review of integrated treatment planning challenges. Int Endod J. 1999;32:343-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Perinetti G, Varvara G, Salini L, Tetè S. Alkaline phosphatase activity in dental pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2005;128:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Perinetti G, Varvara G, Festa F, Esposito P. Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2004;125:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endod. 1990;16:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Parris WG, Tanzer FS, Fridland GH, Harris EF, Killmar J, Desiderio DM. Effects of orthodontic force on methionine enkephalin and substance P concentrations in human pulpal tissue. Am J Orthod Dentofacial Orthop. 1989;95:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Delivanis HP, Sauer GJ. Incidence of canal calcification in the orthodontic patient. Am J Orthod. 1982;82:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Mostafa YA, Iskander KG, El-Mangoury NH. Iatrogenic pulpal reactions to orthodontic extrusion. Am J Orthod Dentofacial Orthop. 1991;99:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Woloshyn H, Artun J, Kennedy DB, Joondeph DR. Pulpal and periodontal reactions to orthodontic alignment of palatally impacted canines. Angle Orthod. 1994;64:257-264. [PubMed] |
| 43. | Yamaguchi M, Kasai K. The Effects of Orthodontic Mechanics on the Dental Pulp. Seminars in orthodontics. 2007;13:272-280. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Miyata H, Genma T, Ohshima M, Yamaguchi Y, Hayashi M, Takeichi O, Ogiso B, Otsuka K. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation of cultured human dental pulp cells by low-power gallium-aluminium-arsenic laser irradiation. Int Endod J. 2006;39:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Abi-Ramia LB, Stuani AS, Stuani AS, Stuani MB, Mendes Ade M. Effects of low-level laser therapy and orthodontic tooth movement on dental pulps in rats. Angle Orthod. 2010;80:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Sübay RK, Kaya H, Tarim B, Sübay A, Cox CF. Response of human pulpal tissue to orthodontic extrusive applications. J Endod. 2001;27:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Hamersky PA, Weimer AD, Taintor JF. The effect of orthodontic force application on the pulpal tissue respiration rate in the human premolar. Am J Orthod. 1980;77:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Kim S, Dörscher-Kim J. Hemodynamic regulation of the dental pulp in a low compliance environment. J Endod. 1989;15:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Derringer KA, Linden RW. Enhanced angiogenesis induced by diffusible angiogenic growth factors released from human dental pulp explants of orthodontically moved teeth. Eur J Orthod. 1998;20:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Derringer KA, Linden RW. Angiogenic growth factors released in human dental pulp following orthodontic force. Arch Oral Biol. 2003;48:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Derringer KA, Linden RW. Vascular endothelial growth factor, fibroblast growth factor 2, platelet derived growth factor and transforming growth factor beta released in human dental pulp following orthodontic force. Arch Oral Biol. 2004;49:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | McDonald F, Pitt Ford TR. Blood flow changes in permanent maxillary canines during retraction. Eur J Orthod. 1994;16:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Bauss O, Schäfer W, Sadat-Khonsari R, Knösel M. Influence of orthodontic extrusion on pulpal vitality of traumatized maxillary incisors. J Endod. 2010;36:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Santamaria M, Milagres D, Iyomasa MM, Stuani MB, Ruellas AC. Initial pulp changes during orthodontic movement: histomorphological evaluation. Braz Dent J. 2007;18:34-39. [PubMed] |
| 55. | Shigetani Y, Sasa N, Suzuki H, Okiji T, Ohshima H. GaAlAs laser irradiation induces active tertiary dentin formation after pulpal apoptosis and cell proliferation in rat molars. J Endod. 2011;37:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Pereira LB, Chimello DT, Ferreira MR, Bachmann L, Rosa AL, Bombonato-Prado KF. Low-level laser therapy influences mouse odontoblast-like cell response in vitro. Photomed Laser Surg. 2012;30:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Santamaria M, Milagres D, Stuani AS, Stuani MB, Ruellas AC. Initial changes in pulpal microvasculature during orthodontic tooth movement: a stereological study. Eur J Orthod. 2006;28:217-220. [PubMed] |
| 58. | Stenvik A, Mjör IA. Pulp and dentine reactions to experimental tooth intrusion. A histologic study of the initial changes. Am J Orthod. 1970;57:370-385. [PubMed] |
| 59. | de Alencar Mollo M, Frigo L, Favero GM, Lopes-Martins RA, Brugnera Junior A. In vitro analysis of human tooth pulp chamber temperature after low-intensity laser therapy at different power outputs. Lasers Med Sci. 2011;26:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |