Published online Sep 20, 2025. doi: 10.5662/wjm.v15.i3.99080
Revised: November 9, 2024
Accepted: December 5, 2024
Published online: September 20, 2025
Processing time: 236 Days and 6.1 Hours
Historically intraoperative drains were employed after pancreatic surgery but over the last decade, there has been debate over the routine usage of drains.
To assess the necessity of intra-abdominal drain placement, identify the most effective drain type, and determine the optimal timing for drain removal.
A systematic review of electronic databases, including PubMed, MEDLINE, PubMed Central, and Google Scholar, was conducted using Medical Subject Hea
Routine use of drains is associated with a statistically significant increase in the risk of CR-POPF and DGE. Conversely, patients who did not have drains placed experienced a significant reduction in morbidity, readmission rates, and reoperations. No significant differences were observed between active and passive drain types. Early drain removal (< 3 days) yielded favorable outcomes compared to delayed removal.
Analysis of randomized controlled trials and cohort studies did not demonstrate an advantage of routine drain placement following pancreatic resection, potentially contributing to increased morbidity and mortality. The decision to use drains should be left to the discretion of the operating surgeon. However, early drain removal can substantially reduce morbidity.
Core Tip: Routine intraoperative drain placement after pancreatic surgery increases the risk of clinically relevant postoperative pancreatic fistula (CR-POPF) and delayed gastric emptying. Patients without drains had lower morbidity, readmission rates, and reoperations. The necessity of routine drain placement is questionable. No clear recommendation can be made between active suction and passive gravity drainage methods. Early drain removal is supported to reduce the occurrence of CR-POPF and associated morbidity and mortality.
- Citation: Kodali R, Parasar K, Anand U, Singh BN, Kant K, Arora A, Karthikeyan V, Anwar S, Saha B, Wadaskar S. Evidence-based approach for intraabdominal drainage in pancreatic surgery: A systematic review and meta-analysis. World J Methodol 2025; 15(3): 99080
- URL: https://www.wjgnet.com/2222-0682/full/v15/i3/99080.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i3.99080
Pancreatic resection, encompassing procedures like pancreaticoduodenectomy (PD) and distal pancreatectomy (DP), has evolved to become safer especially with advancements in surgical techniques and perioperative care[1,2]. Despite a mortality rate of less than 5%, pancreatic surgery still presents a considerable challenge due to high morbidity rates ranging from 30% to 60%, primarily attributed to complications such as postoperative pancreatic fistula (POPF)[3]. Given the significant impact on patient’s quality of life and healthcare costs, there is a need to focus on strategies to mitigate POPF occurrence and improve postoperative outcomes[4,5].
The routine placement and management of intra-abdominal drains after pancreatic resection have garnered significant attention and discussion in recent years[6-10]. The practice of inserting prophylactic intra-abdominal drains has roots dating back to the 19th century, driven by the belief that these drains could evacuate fluids such as blood, bile, and pancreatic juice that might accumulate post-surgery[11]. In modern pancreatic surgery, the management of intra-abdominal drains has become a vital consideration, as indicated by earlier research highlighting its substantial impact on the frequency of postoperative complications[12]. Additionally, drains play a role in mitigating complications related to POPF and aid in the detection of other intra-abdominal hemorrhage. However, the contrarian view of routine use of abdominal drains can lead to retrograde infection, discomfort, foreign body reactions, and prolonged hospital stays[13]. Furthermore drains, which generate substantial negative pressure may contribute to the development of POPFs.
One of the pivotal questions concerning the necessity of placing drains after pancreatic resection was addressed by Conlon et al[7] which marked the initial evidence-based approach, reinforcing the idea that intra-abdominal drains should not be deemed mandatory. However, subsequent randomized controlled trials (RCTs) yielded conflicting results on the efficacy of drains[6,10]. Several systematic reviews and meta-analyses have since been conducted on the role of routine drainage after pancreatic resection. Unfortunately, the available evidence remains limited, underscoring the need for establishing evidence-based guidelines for prophylactic intraabdominal drainage in pancreatic surgery[13,14]. Thus, this systematic review and meta-analysis were aimed to analyse the most recent evidence based data and to answer the following questions: (1) Is the drainage at the operative site more beneficial after pancreatectomy compared to no drain; and (2) If drainage is used, how long should it remain, and what type of drainage should be preferred.
A comprehensive literature search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[15]. PubMed, MEDLINE, PubMed Central, and Google Scholar databases were systematically reviewed using Medical Subject Headings terms and keywords up to December 2023. The search strategy outlined in (Table 1) aimed to identify the relevant studies. After applying inclusion criteria and removing duplicates, abstracts of shortlisted articles were screened, followed by independent full-text review by two authors.
| Search strategy | Database |
| (("Drainage" [Mesh] OR "Drainage, Surgical" [Mesh] OR "Suction" [Mesh] OR "Drainage, Closed" [Mesh]) AND ("Pancreatectomy" [Mesh] OR "Pancreatic resection" [Mesh] OR "Distal pancreatectomy" [Mesh] OR "Pancreatic tail resection" [Mesh] OR "Pancreaticoduodenectomy" [Mesh] OR "Whipple procedure" [Mesh] OR "Duodenopancreatectomy" [Mesh]) | PubMed, PubMed Central, and MEDLINE |
| “Intraabdominal drainage”, “Pancreatic resection”, “Distal pancreatectomy”, “Pancreaticoduodenectomy” separately and in combination | Google Scholar |
Studies comparing the following parameters were included: (1) Intrabdominal drainage vs no drainage; (2) Active (suction) vs passive (gravity) drainage; (3) Early vs late (traditional) drain removal; and (4) Selective drain usage. Studies in which the full text is available in English, and studies that were conducted on humans.
Review articles, letters to the editor, case reports, case series, systematic reviews, meta-analyses, and animal studies were excluded.
Data extraction was carried out by two researchers independently using standardized forms. The quality of the RCTs was evaluated using the Cochrane risk of bias tool while the quality of the non-RCTs was evaluated using the Newcastle-Ottawa scale.
The main outcomes considered were the clinically relevant POPF (CR-POPF), delayed gastric emptying (DGE), and overall morbidity rate. The outcome was measured in terms of the reoperation, readmission, length of hospital stay, and overall mortality rate.
For continuous variables, we utilized the inverse variance method to determine the standardized mean differences and 95%CI. Dichotomous outcomes were assessed using risk ratios and 95%CI were calculated through the Mantel-Haenszel model. The results were visually displayed in forest plots, and a random effects model was applied to estimate pooled odds ratios (OR) for postoperative complications. Heterogeneity was assessed using the χ2 test and I2 statistic, while publication bias was examined through Egger’s test and funnel plots. The analysis was conducted using R programming version 4.2.
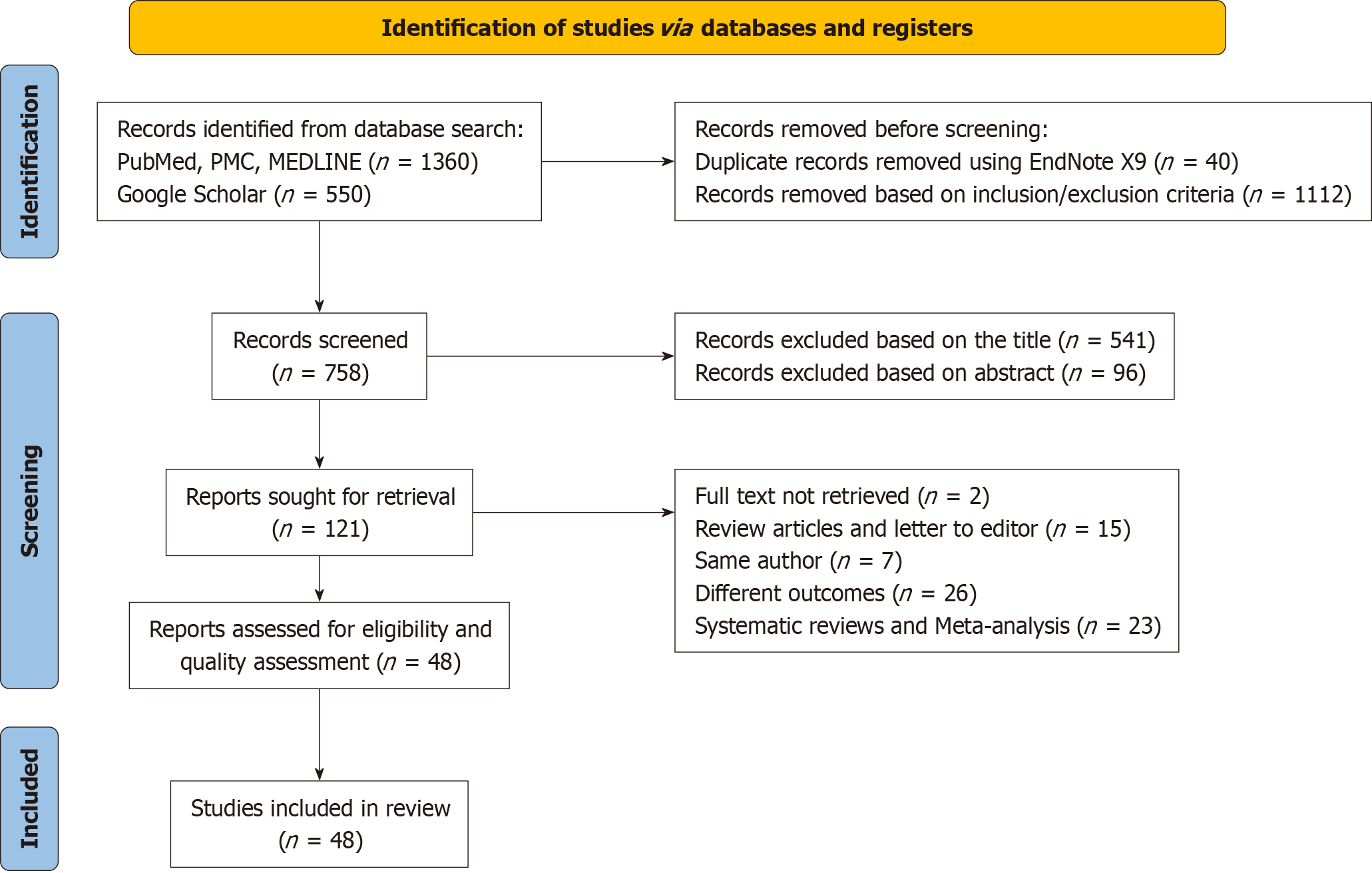
Initially, 1910 studies were identified from major databases (Figure 1). After removing 40 duplicates and excluding 1749 studies not meeting inclusion criteria, full texts of 121 remaining studies were retrieved. Following further screening, 73 studies were eliminated for inconsistency with inclusion criteria, and two inaccessible full-text studies were removed. Ultimately, 48 articles were included, focusing on comparisons between presence or absence of drainage, active (closed suction) vs passive (gravity) drainage, and early vs traditional removal of drainage.
In the current systematic review and meta-analysis, we meticulously examined a total of 22 studies, comprising five RCTs, and the remaining were retrospective, or cohort studies as shown in Supplementary Table 1[4,6-10,16-31]. These studies collectively encompassed a broad population, involving a total of 30029 participants.
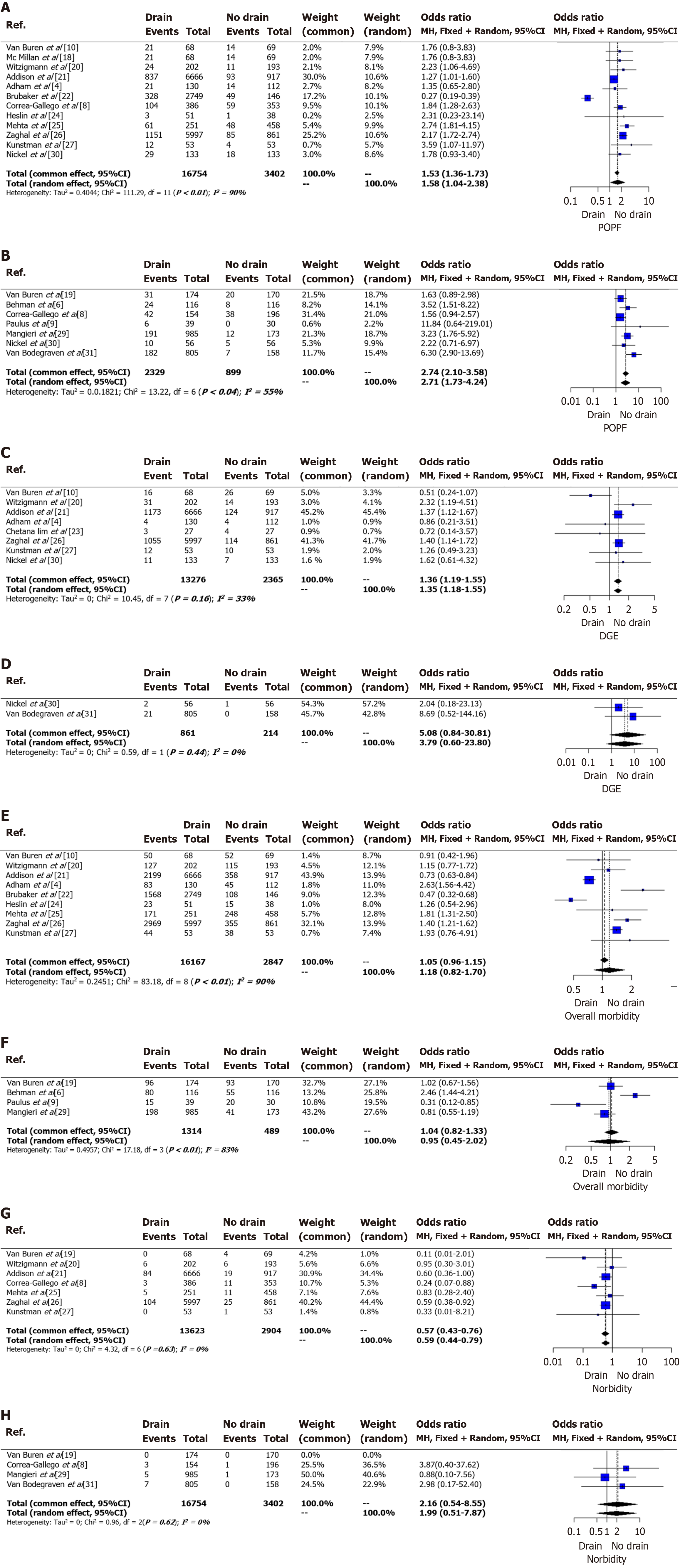
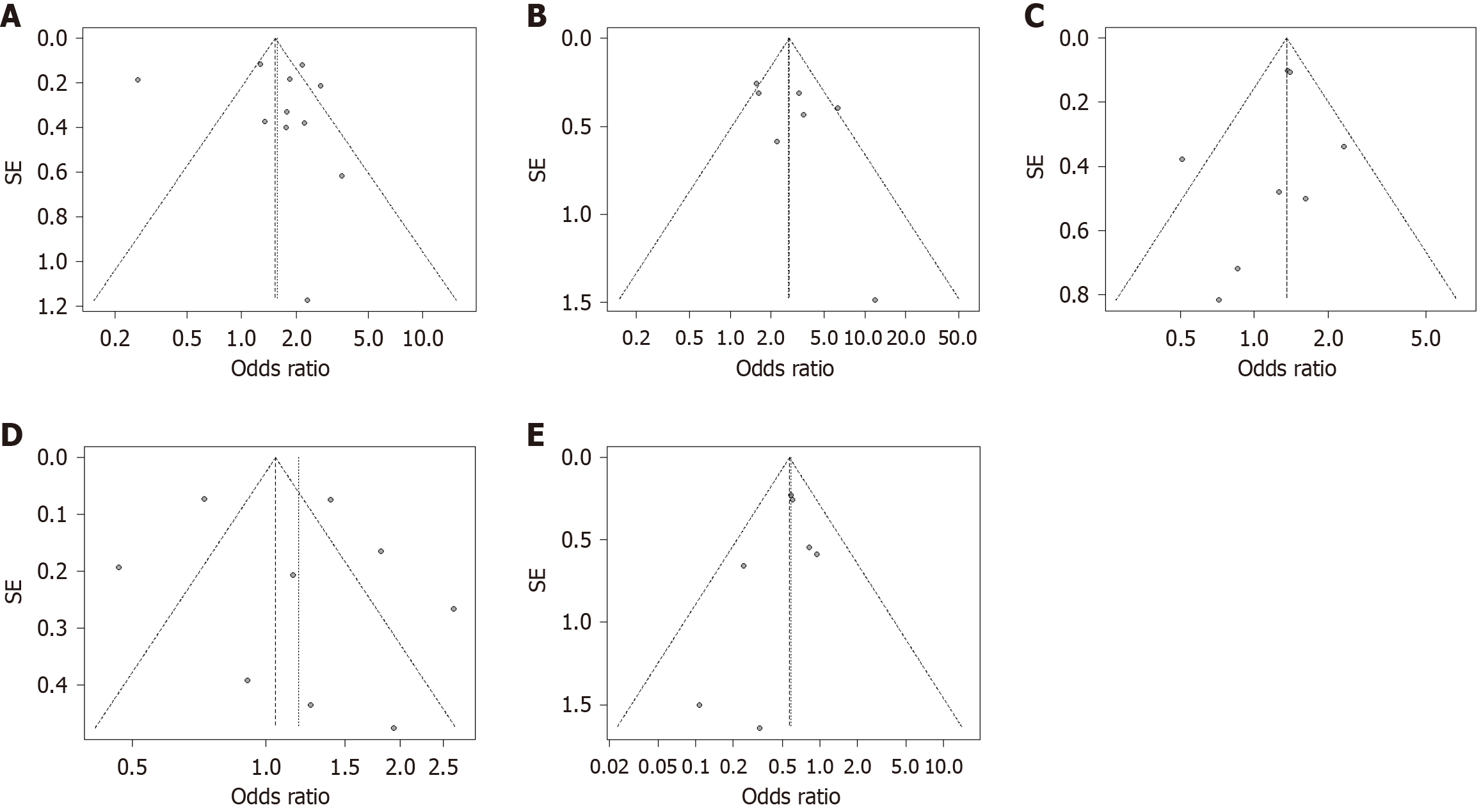
Twelve studies, comprising 16754 patients in the drain group and 3402 patients in the no-drain group, examined CR-POPF outcomes in PD[4,6,8-10,18-27,29-31]. Substantial heterogeneity was observed (I2 = 90%, P < 0.01) (Figure 2A)[4,8,10,18,20-22,24-27,30]. The pooled analysis revealed a significantly higher CR-POPF rate in the drain group (OR = 1.58, 95%CI: 1.04-2.38), supported by a symmetrical funnel plot and non-significant Egger test (P = 0.7613) (Figure 3A). Additionally, seven studies involving 2329 patients in the drain group and 899 in the no-drain group examined CR-POPF outcomes in DP[6,8,9,19,29-31]. Significant heterogeneity was noted (I2 = 55%, P = 0.04), with the drain group exhibiting a significantly higher CR-POPF rate (OR = 2.71, 95%CI: 1.73-4.24), supported by a symmetrical funnel plot (Figure 2B, Figure 3B).
In the pooled analysis of eight studies focusing on DGE post-PD, the drain group (n = 13276) exhibited a significantly higher incidence compared to the no-drain group (n = 2365), with OR of 1.36 (95%CI: 1.18-1.55)[4,10,20,21,23,26,27,30]. Moderate heterogeneity was observed (I2 = 33%, P = 0.16), and the funnel plot was symmetrical (Figure 2C, Figure 3C). Conversely, pooled analysis of two studies on DGE post-DP showed no significant correlation between the drain (n = 861) and no-drain (n = 214) groups, with a pooled OR of 3.79 (95%CI: 0.6–23.8). No heterogeneity was present among the included studies (I2 = 0%, P = 0.44) (Figure 2D)[30,31].
Pooled analysis of nine studies reporting the outcome of overall morbidity in PD showed no significant difference between the drain group (n = 16167) and the no-drain group (n = 2847) with OR of 1.18 (95%CI: 0.82-1.7) (Figure 2E)[4,10,20-22,24-27]. There was considerable heterogeneity present among the included studies (I2 = 90%, P < 0.01). The funnel plot was symmetrical (Figure 3D).
Pooled analysis of four studies reporting the outcome of overall morbidity in DP showed no significant difference between the drain group (n = 1314) and the no-drain group (n = 489) with OR of 0.95 (95%CI: 0.45-2.02) (Figure 2H)[6,9,19,29]. There was substantial heterogeneity present among the included studies (I2 = 83%, P < 0.01) (Figure 2F).
Pooled analysis with seven studies reporting mortality post-PD showed a significantly lower incidence of mortality among the drain group (n = 13623) than in the no-drain group (n = 2904), with an OR of 0.59 (95%CI: 0.44–0.79) (Figure 2G)[8,19-21,25-27]. No heterogeneity was present among the included studies (I2 = 0%, P = 0.63). The funnel plot was symmetrical (Figure 3E).
Pooled analysis of four studies reporting the outcome of mortality in DP showed no significant difference between the drain group (n = 2118) and the no-drain group (n = 697) with OR of 1.99 (95%CI: 0.51-7.87) (Figure 2H)[8,19,29,31]. No heterogeneity was present among the included studies (I2 = 0%, P = 0.62).
A comprehensive analysis of ten studies, including three RCTs and seven retrospective or cohort studies, involving 24475 patients as shown in Supplementary Table 2[32–41]. While four studies reported a higher incidence of CR-POPF with closed suction drainage[35,37,39,41], others employing gravity drainage showed no significant difference in complication rates. Secondary outcomes indicated slightly more adverse events with active closed suction drainage but no notable impact on 30-day postoperative mortality, overall morbidity, or re-intervention rates. These findings suggest that the choice of drainage method has limited effect on specific outcomes in pancreatic surgery.
A review of sixteen studies, including five RCTs and eleven retrospective cohort studies, focused on the timing of drain removal following pancreatic surgery as shown in Supplementary Table 3[13,42–56]. Definitions of "early removal" varied, with most studies considering removal within 3 days. Late removal (> 5 days) was associated with higher surgical complication rates, though not statistically significant. Delayed removal correlated with increased rates of CR-POPF, bile leak, Clavien Dindo grade ≥ 3 complications, reoperations, readmissions, and 30-day mortality. These findings highlight the importance of timely drain removal for better postoperative outcomes in pancreatic surgery.
The historical tradition of employing prophylactic peritoneal drainage following gastrointestinal surgery, epitomized by the adage "when in doubt, drain", lacks compelling contemporary data validating its efficacy[57]. While drains placed near anastomoses serve to remove pancreatic juice or bile and can indicate complications, they come with associated risks such as infections, abdominal pain, and prolonged hospital stays. Despite their routine use for preventing POPFs, the clinical impact can still be significant, and timely identification is crucial for mitigating severe complications[33]. Thus, the ongoing debate within the surgical community questions the necessity of prophylactic drain placement and emphasizes the need for evidence-based practices in this regard.
In our comprehensive review, comparing outcomes between patients with and without drainage after pancreatic resection revealed significant differences. The drainage cohort exhibited higher rates of CR-POPFs and DGE, along with prolonged hospital stays and increased intervention requirements. Despite higher rates of CR-POPFs and DGE in the drainage cohort, this group exhibited a lower mortality rate. The presence of a drain allows for early detection and effective management of complications, preventing severe outcomes like sepsis and organ failure. Consequently, the benefits of early intervention in the drainage group have likely contributed to the observed reduction in mortality. Subgroup analysis of PD and DP revealed consistent findings indicating increased risks of CR-POPFs in both PD and DP groups, and increased DGE specifically in the PD group. These findings underscore the potential disadvantages of routine drainage in pancreatic surgery.
A meta-analysis of 15290 patients from ten studies found a higher incidence of CR-POPF in the drainage group compared to the no-drainage group[58]. However, there was no substantial correlation between drainage and DGE. Subgroup analyses for PD and DP showed comparable outcomes. Additionally, comprehensive analysis of eleven studies on postoperative complications, including PPH, intra-abdominal abscess, wound infection, and reoperation, revealed no notable differences between the groups[4,7,8,16,19,20,24,27,29,31,32]. However, the group with drains exhibited a significantly higher rate of readmission compared to the group without drain[58].
Conflicting findings from recent studies suggest varied impacts of drainage in pancreatic surgery. Brubaker et al[22] found higher rates of POPF, reoperation, and serious morbidity in patients without drainage, while Fisher et al[16] reported increased incidences of POPF, DGE, and readmission with drain usage. Other studies, such as those by Paulus et al[9] and Van Buren et al[10], found no significant differences in outcomes with or without drainage. Behrman et al[6] and Mangieri et al[29] observed increased morbidity and readmission rates associated with drain placement, and Liu et al[58] suggested a potential elevation in POPF incidence with use of drainage. These findings suggest caution in routine drainage employment during pancreatic resection.
Patients undergoing surgical drain placement commonly experience two main drainage methods: (1) Closed-suction drains (CSDs); and (2) Passive gravity drains. CSDs function by generating a negative pressure gradient, ensuring continuous suction within the abdominal cavity irrespective of the patient's position. In contrast, gravity drainage devices establish a route for fluid extraction by leveraging the pressure difference between intra-abdominal and atmospheric pressure. In our review, we found significant differences between CSDs, and passive gravity drains for patients undergoing pancreatic resection. CSDs were associated with higher rates of CR-POPF and DGE, while passive gravity drains led to longer hospital stays and a greater need for intervention but lower mortality. Čečka et al[34] found no significant differences in rates of CR-POPF or overall morbidity between groups using different drainage methods after PD. Additionally, there were no notable variations in reoperation rate, readmission rate, length of hospital stay, or incidence of post-pancreatectomy hemorrhage. Lee et al[32] demonstrated that utilizing a closed-suction drainage system with an external pancreatic duct stent led to a significant reduction in CR-POPF following PD. The system's negative pressure effectively diverted pancreatic juice away from the anastomotic site, potentially enhancing healing and long-term patency of the pancreatic duct.
Veziant et al[3] found no significant differences in outcomes between active and passive drainage methods, including rates of CR-POPF, overall morbidity, and length of hospital stay. Similarly, Aumont et al[35] reported that gravity drainage was independently associated with lower rates of CR-POPF, DGE , and readmission following PD. Overall, the literature suggests that the choice of drainage method does not significantly impact postoperative outcomes, particularly after PD, and may be left to surgeon preference.
The absence of a universally agreed-upon definition for early removal of abdominal drainage following pancreatic surgery has led to a typical interval ranging from 3 days to 5 days. Late removal is generally considered when drains are in place for at least five days. In our systematic review, we found significant differences between early and late removal of abdominal drainage following pancreatic surgery. Late removal (≥ 5 days) was associated with higher rates of complications such as CR-POPF, DGE, severe morbidity, prolonged hospital stays, and increased need for intervention and readmission. However, mortality rates did not differ notably. Therefore, if drainage is necessary, early removal within three days seems to offer more benefits.
Xourafas et al[50] found that early drain removal in patients undergoing PD, especially those with POD1 amylase levels of 5000, was associated with improved perioperative outcomes regardless of the Fistula Risk Score (FRS). Both high-risk and low-risk modified FRS patients showed reduced rates of CR-POPF, shorter hospital stays, and overall morbidity with early drain removal. In the RCT by Bassi et al[42] which included both PD and DP, the results posed some interpretative challenges, despite the notable reduction in POPF and overall morbidity associated with early drain removal. Conversely, Dembinski et al[44] focusing on PD patients did not show statistically significant differences between groups, although there was a trend toward reduced rates of complications and shorter hospital stays with early drain removal, potentially limited by statistical power of the study. Overall, while the definitive benefits of early drain removal remain uncertain, evidence suggests it may facilitate earlier recovery without increasing complications.
The meta-analysis faced limitations due to predominantly nonrandomized studies, potentially biasing results. Several studies lacked comprehensive data on secondary outcomes. High heterogeneity, indicated by I² statistic and low P-value, urges cautious interpretation. Further well-designed randomized trials with robust data collection are crucial to enhance understanding of intra-abdominal drains in pancreatic surgery.
After a comprehensive review of 48 articles encompassing patients with PD and DP, our study refrained from making specific recommendations regarding the routine use of drainage. However, an increasing body of evidence suggests that the routine placement of primary drains is not mandatory. Our systematic review does not yield a definitive recommendation concerning the choice between active suction or passive gravity drainage following pancreatic resection. The choice of the drainage method can be at the discretion of the surgeon. Furthermore, our findings support the safe practice of early drain removal for patients undergoing pancreatic surgery which may decrease the occurrence of post operative pancreatic fistula and mitigate the associated morbidity and mortality. Recognizing the potential contribution of intra-abdominal drainage to increased morbidity, further research should be done in this area. This approach will aid in refining the guidelines to optimize drainage practices, balancing the benefits and risks, and promoting evidence-based decision-making in pancreatic surgery.
We extend our sincere gratitude to Naga KG, John AG, Anto JV and Yadav S for their invaluable contributions and support in this study. Their expertise and dedication have been instrumental in shaping our research, and we are deeply appreciative of their guidance and collaboration throughout the project.
| 1. | Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017;96:e6858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310-1314; discussion 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Veziant J, Selvy M, Buc E, Slim K. Evidence-based evaluation of abdominal drainage in pancreatic surgery. J Visc Surg. 2021;158:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Adham M, Chopin-Laly X, Lepilliez V, Gincul R, Valette PJ, Ponchon T. Pancreatic resection: drain or no drain? Surgery. 2013;154:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Čečka F, Loveček M, Jon B, Skalický P, Šubrt Z, Neoral Č, Ferko A. Intra-abdominal drainage following pancreatic resection: A systematic review. World J Gastroenterol. 2015;21:11458-11468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 6. | Behrman SW, Zarzaur BL, Parmar A, Riall TS, Hall BL, Pitt HA. Routine drainage of the operative bed following elective distal pancreatectomy does not reduce the occurrence of complications. J Gastrointest Surg. 2015;19:72-79; discussion 79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, Merchant N, Brennan MF. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487-493; discussion 493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Correa-Gallego C, Brennan MF, Dʼangelica M, Fong Y, Dematteo RP, Kingham TP, Jarnagin WR, Allen PJ. Operative drainage following pancreatic resection: analysis of 1122 patients resected over 5 years at a single institution. Ann Surg. 2013;258:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Paulus EM, Zarzaur BL, Behrman SW. Routine peritoneal drainage of the surgical bed after elective distal pancreatectomy: is it necessary? Am J Surg. 2012;204:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Van Buren G 2nd, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Vollmer C, Velanovich V, Riall T, Muscarella P, Trevino J, Nakeeb A, Schmidt CM, Behrns K, Ellison EC, Barakat O, Perry KA, Drebin J, House M, Abdel-Misih S, Silberfein EJ, Goldin S, Brown K, Mohammed S, Hodges SE, McElhany A, Issazadeh M, Jo E, Mo Q, Fisher WE. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 11. | Nitsche U, Müller TC, Späth C, Cresswell L, Wilhelm D, Friess H, Michalski CW, Kleeff J. The evidence based dilemma of intraperitoneal drainage for pancreatic resection - a systematic review and meta-analysis. BMC Surg. 2014;14:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Cyr DP, Truong JL, Lam-McCulloch J, Cleary SP, Karanicolas PJ. Canadian practice patterns for pancreaticoduodenectomy. Can J Surg. 2015;58:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Villafane-Ferriol N, Baugh KA, McElhany AL, Van Buren G 2nd, Fang A, Tashakori EK, Reyes JEM, Cao HST, Silberfein EJ, Massarweh N, Hsu C, Barakat O, Schmidt C, Zyromski NJ, Dillhoff M, Villarreal JA, Fisher WE. Evidence Versus Practice in Early Drain Removal After Pancreatectomy. J Surg Res. 2019;236:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Zhang W, He S, Cheng Y, Xia J, Lai M, Cheng N, Liu Z. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database Syst Rev. 2018;6:CD010583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40131] [Article Influence: 10032.8] [Reference Citation Analysis (2)] |
| 16. | Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, Brunicardi FC. Pancreatic resection without routine intraperitoneal drainage. HPB (Oxford). 2011;13:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Jeekel J. No abdominal drainage after Whipple's procedure. Br J Surg. 1992;79:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | McMillan MT, Fisher WE, Van Buren G 2nd, McElhany A, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, Velanovich V, Brown K, Morgan KA, Vollmer C. The value of drains as a fistula mitigation strategy for pancreatoduodenectomy: something for everyone? Results of a randomized prospective multi-institutional study. J Gastrointest Surg. 2015;19:21-30; discussion 30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Van Buren G 2nd, Bloomston M, Schmidt CR, Behrman SW, Zyromski NJ, Ball CG, Morgan KA, Hughes SJ, Karanicolas PJ, Allendorf JD, Vollmer CM Jr, Ly Q, Brown KM, Velanovich V, Winter JM, McElhany AL, Muscarella P 2nd, Schmidt CM, House MG, Dixon E, Dillhoff ME, Trevino JG, Hallet J, Coburn NSG, Nakeeb A, Behrns KE, Sasson AR, Ceppa EP, Abdel-Misih SRZ, Riall TS, Silberfein EJ, Ellison EC, Adams DB, Hsu C, Tran Cao HS, Mohammed S, Villafañe-Ferriol N, Barakat O, Massarweh NN, Chai C, Mendez-Reyes JE, Fang A, Jo E, Mo Q, Fisher WE. A Prospective Randomized Multicenter Trial of Distal Pancreatectomy With and Without Routine Intraperitoneal Drainage. Ann Surg. 2017;266:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Witzigmann H, Diener MK, Kienkötter S, Rossion I, Bruckner T, Bärbel Werner, Pridöhl O, Radulova-Mauersberger O, Lauer H, Knebel P, Ulrich A, Strobel O, Hackert T, Büchler MW. No Need for Routine Drainage After Pancreatic Head Resection: The Dual-Center, Randomized, Controlled PANDRA Trial (ISRCTN04937707). Ann Surg. 2016;264:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 21. | Addison P, Nauka PC, Fatakhova K, Amodu L, Kohn N, Rodriguez Rilo HL. Impact of Drain Placement and Duration on Outcomes After Pancreaticoduodenectomy: A National Surgical Quality Improvement Program Analysis. J Surg Res. 2019;243:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Brubaker LS, Casciani F, Fisher WE, Wood AL, Cagigas MN, Trudeau MT, Parikh VJ, Baugh KA, Asbun HJ, Ball CG, Behrman SW, Berger AC, Bloomston MP, Callery MP, Christein JD, Fernandez-Del Castillo C, Dillhoff ME, Dixon E, House MG, Hughes SJ, Kent TS, Kunstman JW, Wolfgang CL, Zureikat AH, Vollmer CM Jr, Van Buren G 2nd. A risk-adjusted analysis of drain use in pancreaticoduodenectomy: Some is good, but more may not be better. Surgery. 2022;171:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lim C, Dokmak S, Cauchy F, Aussilhou B, Belghiti J, Sauvanet A. Selective policy of no drain after pancreaticoduodenectomy is a valid option in patients at low risk of pancreatic fistula: a case-control analysis. World J Surg. 2013;37:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Heslin MJ, Harrison LE, Brooks AD, Hochwald SN, Coit DG, Brennan MF. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg. 1998;2:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA. Is it time to abandon routine operative drain use? A single institution assessment of 709 consecutive pancreaticoduodenectomies. J Am Coll Surg. 2013;216:635-642; discussion 642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Zaghal A, Tamim H, Habib S, Jaafar R, Mukherji D, Khalife M, Mailhac A, Faraj W. Drain or No Drain Following Pancreaticoduodenectomy: The Unsolved Dilemma. Scand J Surg. 2020;109:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kunstman JW, Starker LF, Healy JM, Salem RR. Pancreaticoduodenectomy Can Be Performed Safely with Rare Employment of Surgical Drains. Am Surg. 2017;83:265-273. [PubMed] |
| 28. | El Khoury R, Kabir C, Maker VK, Banulescu M, Wasserman M, Maker AV. Do Drains Contribute to Pancreatic Fistulae? Analysis of over 5000 Pancreatectomy Patients. J Gastrointest Surg. 2018;22:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Mangieri CW, Kuncewitch M, Fowler B, Erali RA, Moaven O, Shen P, Clark CJ. Surgical drain placement in distal pancreatectomy is associated with an increased incidence of postoperative pancreatic fistula and higher readmission rates. J Surg Oncol. 2020;122:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Nickel F, Lang F, Kowalewski KF, Haney CM, Menrath M, Berchtold C, Hoffmann K, Loos M, Mehrabi A, Probst P, Schmidt T, Schneider M, Diener MK, Strobel O, Müller-Stich BP, Hackert T. Pancreatic surgery with or without drainage: propensity score-matched study. Br J Surg. 2022;109:739-745. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | van Bodegraven EA, De Pastena M, Vissers FL, Balduzzi A, Stauffer J, Esposito A, Malleo G, Marchegiani G, Busch OR, Salvia R, van Hilst J, Bassi C, Besselink MG, Asbun HJ. Routine prophylactic abdominal drainage versus no-drain strategy after distal pancreatectomy: A multicenter propensity score matched analysis. Pancreatology. 2022;22:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Lee SE, Ahn YJ, Jang JY, Kim SW. Prospective randomized pilot trial comparing closed suction drainage and gravity drainage of the pancreatic duct in pancreaticojejunostomy. J Hepatobiliary Pancreat Surg. 2009;16:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Jiang H, Liu N, Zhang M, Lu L, Dou R, Qu L. A Randomized Trial on the Efficacy of Prophylactic Active Drainage in Prevention of Complications after Pancreaticoduodenectomy. Scand J Surg. 2016;105:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Čečka F, Jon B, Skalický P, Čermáková E, Neoral Č, Loveček M. Results of a randomized controlled trial comparing closed-suction drains versus passive gravity drains after pancreatic resection. Surgery. 2018;164:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Aumont O, Dupré A, Abjean A, Pereira B, Veziant J, Le Roy B, Pezet D, Buc E, Gagnière J. Does intraoperative closed-suction drainage influence the rate of pancreatic fistula after pancreaticoduodenectomy? BMC Surg. 2017;17:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Marchegiani G, Perri G, Pulvirenti A, Sereni E, Azzini AM, Malleo G, Salvia R, Bassi C. Non-inferiority of open passive drains compared with closed suction drains in pancreatic surgery outcomes: A prospective observational study. Surgery. 2018;164:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Lemke M, Park L, Balaa FK, Martel G, Khalil JA, Bertens KA. Passive Versus Active Intra-Abdominal Drainage Following Pancreaticoduodenectomy: A Retrospective Study Using The American College of Surgeons NSQIP Database. World J Surg. 2021;45:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | O'Grady J, Sutton TL, Potter KC, Gilbert E, Pommier R, Mayo SC, Sheppard BC. The power of suction: Theory and practice in closed suction vs gravity drains and postoperative pancreatic fistulas. Am J Surg. 2022;224:737-741. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Hall BR, Egr ZH, Krell RW, Padussis JC, Shostrom VK, Are C, Reames BN. Association of gravity drainage and complications following Whipple: an analysis of the ACS-NSQIP targeted database. World J Surg Oncol. 2021;19:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Kone LB, Maker VK, Banulescu M, Maker AV. Should Drains Suck? A Propensity Score Analysis of Closed-Suction Versus Closed-Gravity Drainage After Pancreatectomy. J Gastrointest Surg. 2021;25:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Schmidt CM, Choi J, Powell ES, Yiannoutsos CT, Zyromski NJ, Nakeeb A, Pitt HA, Wiebke EA, Madura JA, Lillemoe KD. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg. 2009;2009:404520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 43. | McMillan MT, Malleo G, Bassi C, Butturini G, Salvia R, Roses RE, Lee MK, Fraker DL, Drebin JA, Vollmer CM Jr. Drain Management after Pancreatoduodenectomy: Reappraisal of a Prospective Randomized Trial Using Risk Stratification. J Am Coll Surg. 2015;221:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Dembinski J, Mariette C, Tuech JJ, Mauvais F, Piessen G, Fuks D, Schwarz L, Truant S, Cosse C, Pruvot FR, Regimbeau JM. Early removal of intraperitoneal drainage after pancreatoduodenectomy in patients without postoperative fistula at POD3: Results of a randomized clinical trial. J Visc Surg. 2019;156:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Dai M, Liu Q, Xing C, Kleeff J, Liao Q, Guo J, Han X, Xu Q, Wang S. [Early drain removal after major pancreatectomy reduces postoperative complications: a single-center, randomized, controlled trial]. Yixianbingxue Zazhi. 2020;3:93-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Dai M, Liu Q, Xing C, Tian X, Cao F, Tang W, Lv S, Ma Y, Zhang D, Kleeff J, Yang Y, Liu R, He Q, Li F, Li G, Guo J, Liao Q, Zhao Y. Early Drain Removal is Safe in Patients With Low or Intermediate Risk of Pancreatic Fistula After Pancreaticoduodenectomy: A Multicenter, Randomized Controlled Trial. Ann Surg. 2022;275:e307-e314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Beane JD, House MG, Ceppa EP, Dolejs SC, Pitt HA. Variation in Drain Management After Pancreatoduodenectomy: Early Versus Delayed Removal. Ann Surg. 2019;269:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Ven Fong Z, Correa-Gallego C, Ferrone CR, Veillette GR, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Early Drain Removal--The Middle Ground Between the Drain Versus No Drain Debate in Patients Undergoing Pancreaticoduodenectomy: A Prospective Validation Study. Ann Surg. 2015;262:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Xourafas D, Ejaz A, Tsung A, Dillhoff M, Pawlik TM, Cloyd JM. Population-Based Assessment of Selective Drain Placement During Pancreatoduodenectomy Using the Modified Fistula Risk Score. J Am Coll Surg. 2019;228:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, Miyazawa M, Uchiyama K, Yamaue H. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 52. | Seykora TF, Liu JB, Maggino L, Pitt HA, Vollmer CM Jr. Drain Management Following Distal Pancreatectomy: Characterization of Contemporary Practice and Impact of Early Removal. Ann Surg. 2020;272:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Adachi T, Kuroki T, Kitasato A, Hirabaru M, Matsushima H, Soyama A, Hidaka M, Takatsuki M, Eguchi S. Safety and efficacy of early drain removal and triple-drug therapy to prevent pancreatic fistula after distal pancreatectomy. Pancreatology. 2015;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Linnemann RJA, Patijn GA, van Rijssen LB, Besselink MG, Mungroop TH, de Hingh IH, Kazemier G, Festen S, de Jong KP, van Eijck CHJ, Scheepers JJG, van der Kolk M, Dulk MD, Bosscha K, Busch OR, Boerma D, van der Harst E, Nieuwenhuijs VB; Dutch Pancreatic Cancer Group. The role of abdominal drainage in pancreatic resection - A multicenter validation study for early drain removal. Pancreatology. 2019;19:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Sakamoto T, Yagyu Y, Uchinaka EI, Hanaki T, Miyatani K, Kihara K, Yamamoto M, Matsunaga T, Tokuyasu N, Honjo S, Fujiwara Y. Surgical Outcomes Following Early Drain Removal After Distal Pancreatectomy in Elderly Patients. In Vivo. 2020;34:2837-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Yoon SJ, Yoon SK, Jung JH, Han IW, Choi DW, Heo JS, Shin SH. Realistic Advantages of Early Surgical Drain Removal after Pancreatoduodenectomy: A Single-Institution Retrospective Study. J Clin Med. 2021;10:2716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 57. | Pai D, Sharma A, Kanungo R, Jagdish S, Gupta A. Role of abdominal drains in perforated duodenal ulcer patients: a prospective controlled study. Aust N Z J Surg. 1999;69:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Liu X, Chen K, Chu X, Liu G, Yang Y, Tian X. Prophylactic Intra-Peritoneal Drainage After Pancreatic Resection: An Updated Meta-Analysis. Front Oncol. 2021;11:658829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |