Published online Mar 20, 2025. doi: 10.5662/wjm.v15.i1.98482
Revised: August 1, 2024
Accepted: August 13, 2024
Published online: March 20, 2025
Processing time: 94 Days and 3.5 Hours
Atypical optic neuritis, consisting of neuromyelitis optica spectrum disorders (NMOSD) or myelin oligodendrocyte glycoprotein antibody disease (MOGAD), has a very similar presentation but different prognostic implications and long-term management strategies. Vascular and metabolic factors are being thought to play a role in such autoimmune neuro-inflammatory disorders, apart from the obvious immune mediated damage. With the advent of optical coherence tomo
To study the macular microvascular and structural changes on OCTA in atypical optic neuritis.
This observational cross-sectional study involved 8 NMOSD and 17 MOGAD patients, diagnosed serologically, as well as 10 healthy controls. Macular vascular density (MVD) and ganglion cell + inner plexiform layer thickness (GCIPL) were studied using OCTA.
There was a significant reduction in MVD in NMOSD and MOGAD affected as well as unaffected eyes when compared with healthy controls. NMOSD and MOGAD affected eyes had significant GCIPL thinning compared with healthy controls. NMOSD unaffected eyes did not show significant GCIPL thinning compared to healthy controls in contrast to MOGAD unaffected eyes. On comparing NMOSD with MOGAD, there was no significant difference in terms of MVD or GCIPL in the affected or unaffected eyes.
Although significant microvascular and structural changes are present on OCTA between atypical optic neuritis and normal patients, they could not help in differentiating between NMOSD and MOGAD cases.
Core Tip: Our observational study shows that although there are microvascular and structural changes seen on optical coherence tomography angiography in atypical optic neuritis, these changes could not help in differentiating between neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody disorder cases.
- Citation: Mahatme C, Kaushik M, Saravanan VR, Kumar K, Shah VM. Macular microvascular and structural changes on optical coherence tomography angiography in atypical optic neuritis. World J Methodol 2025; 15(1): 98482
- URL: https://www.wjgnet.com/2222-0682/full/v15/i1/98482.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i1.98482
Neuromyelitis optica spectrum disorders (NMOSD) and myelin oligodendrocyte glycoprotein antibody disorder (MOGAD) are both autoimmune demyelinating disorders of the central nervous system[1]. The retina and optic nerve being a part of the central nervous system are affected in these disorders, leading to ocular manifestations in these diseases, typically optic neuritis which can lead to visual impairment or even blindness[2].
There is a significant overlap between the clinical presentation of NMOSD and MOGAD in the acute clinical setting; however, it is essential to establish an accurate diagnosis as this may affect treatment strategies and outcomes. Confirmatory lab tests like testing for aquaporin-4 (AQP4) antibody or MOG antibody might be unavailable, expensive, and time-consuming in certain settings[3,4].
Besides the well-established immune mediated mechanisms that play a role in autoimmune neuroinflammatory disorders, vascular and metabolic factors are being recognized to play an important role as well[4]. Optical coherence tomography angiography (OCTA) is a new non-invasive and rapid investigative modality that has already been used to observe changes in the retinal nerve fiber layer, ganglion cell inner plexiform complex, and retinal microvascular density in AQP4 antibody seropositive NMOSD[5].
In this observational cross-sectional study, we used OCTA to delineate structural and microvascular changes in NMOSD and MOGAD to better understand the pathophysiological changes in these disorders and aid in their rapid diagnosis and apt management.
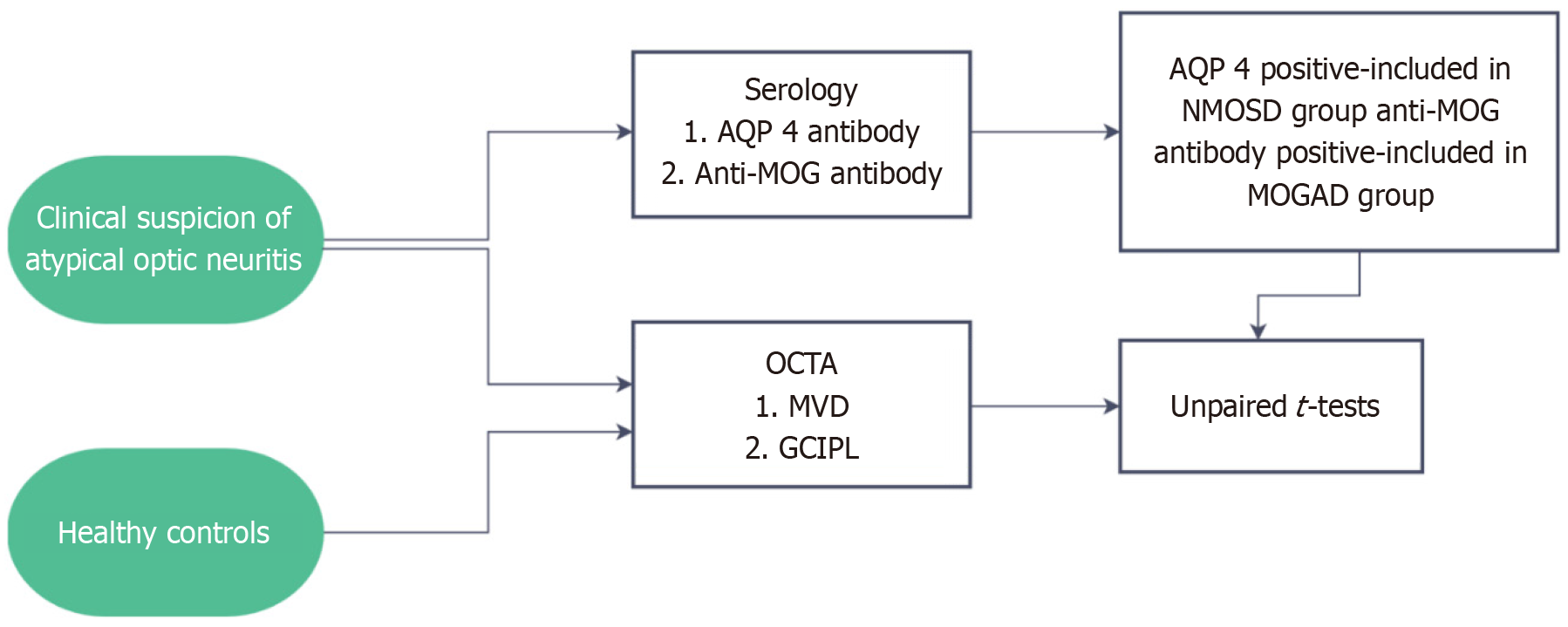
This was an observational cross-sectional study in which 8 NMOSD patients (8 affected eyes), 17 MOGAD patients (25 affected eyes), and 10 healthy controls were enrolled. The study was carried out in the neuro-ophthalmology department of a tertiary eye care center in southern India, and data was collected over a period of 6 months.
Patients presenting to the neuro-ophthalmology department with clinical features suggestive of autoimmune neuroinflammatory disorders were subjected to OCTA imaging for both eyes and AQP4 as well as MOG antibody testing after providing informed consent for participation in the study.
The diagnosis and grouping of patients were based on serology, AQP4 positivity confirming the diagnosis of NMOSD, and MOG antibody positivity confirming the diagnosis of MOGAD. For comparison, 12 healthy age and gender matched controls who underwent OCTA imaging only for both eyes were enrolled in the study (Figure 1).
The key parameters measured and compared were ganglion cell and inner plexiform layer thickness (μm) and macular vessel density (mm/mm2) in a 3 mm × 3 mm area around the fovea.
Patients with clinical suspicion of NMO or MOGAD (atypical optic neuritis) were subjected to OCTA and serology for AQP4 as well as MOG antibody. AQP 4 positivity was a criterion for inclusion in the NMOSD group (8 patients), those with MOG positive test results were included in the MOGAD group (17 patients). Patients with co-existing posterior segment pathologies were excluded from the study. Healthy controls were subjected to OCTA imaging only. Parameters measured for statistical analysis were GCIPL thickness (μm) and macular vascular density (MVD) (mm/mm2).
The patients and controls were subjected to OCTA imaging using the Zeiss Cirrus 6000 with AngioPlex OCTA machine. The MVD was measured in a 3 mm × 3 mm area around the fovea and the GCIPL thickness was measured as well in all quadrants around the fovea and an average value was obtained. The values from the MVD and GCIPL readouts were then entered into an Excel sheet. Then, an unpaired t-test was carried out for all groups and affected as well as unaffected eyes within each group and results analyzed.
NMO showed a strong female predisposition and had a unilateral presentation in contrast to MOGAD which had an equal distribution in terms of sex, while being bilateral in most cases. Visual deficit was more severe in NMO compared to MOGAD, and NMO also had a poorer visual recovery compared to MOGAD as seen in Table 1.
| Parameter | NMOSD group | MOGAD group | Control group | |
| Average age (years) | 39.6 | 36.9 | 36.9 | |
| Sex | Male | 1 (12.5) | 6 (35) | 3 (30) |
| Female | 7 (87.5) | 11 (65) | 7 (70) | |
| Laterality | Unilateral | 8 (100) | 9 (53) | NA |
| Bilateral | 0 (0) | 8 (47) | ||
| Average visual acuity of affected eye at presentation (LogMAR) | 2.42 ± 0.56 | 1.13 ± 0.86 | NA | |
| Average final visual acuity of affected eye (LogMAR) | 0.77 ± 0.95 | 0.15 ± 0.25 | NA | |
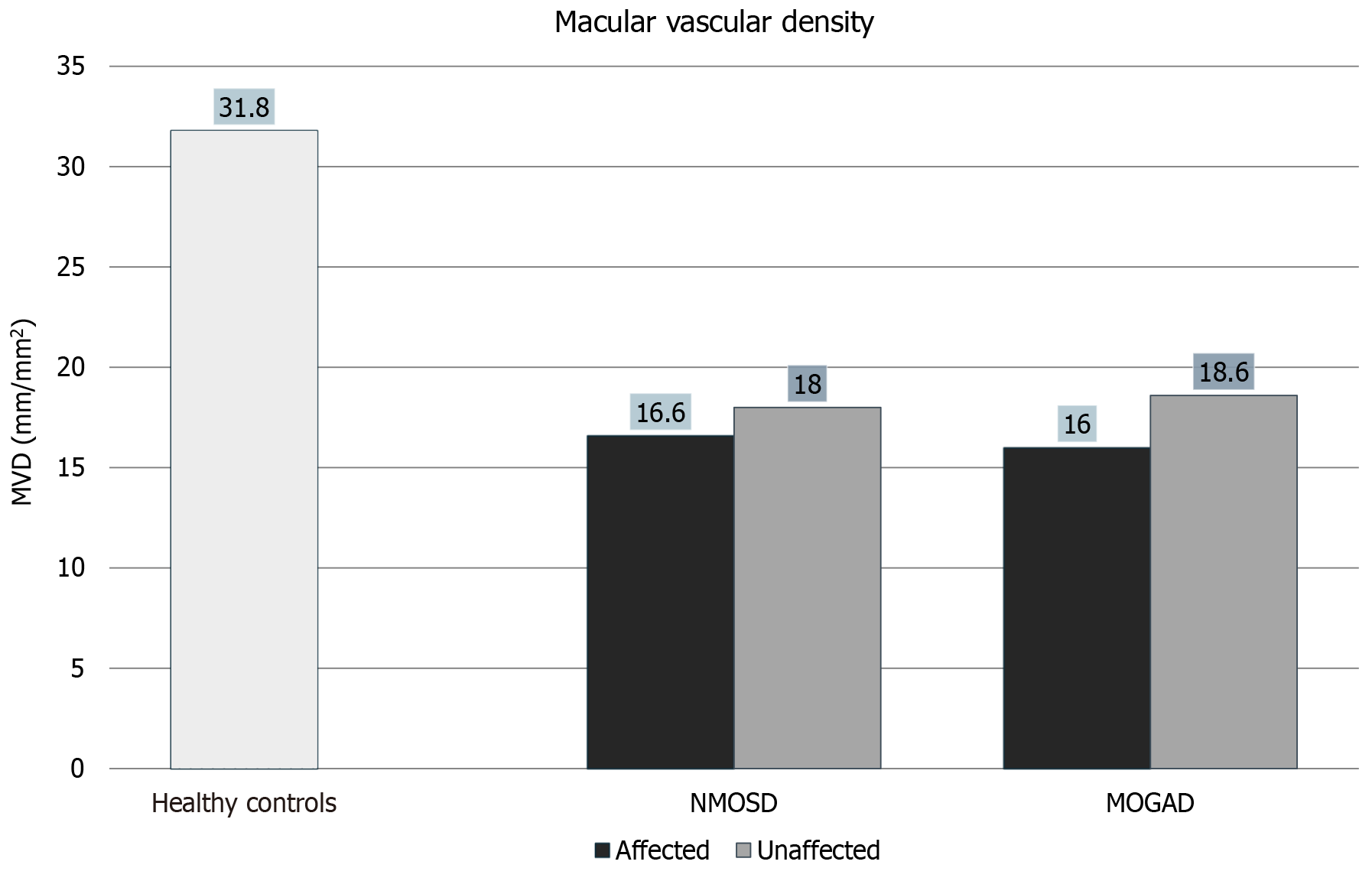
There was a significant reduction in MVD in NMO and MOGAD affected eyes compared to healthy controls as seen in Figure 2. What is interesting, however, is that even NMO and MOGAD unaffected eyes showed a significant reduction in MVD compared to healthy controls. NMO and MOGAD affected eyes did not show a significant difference in MVD compared to NMO and MOGAD unaffected eyes as can be seen in Figure 2, Figure 3, Table 2 and Table 3.
| Variable measured | NMOSD group | MOGAD group | Control group | ||
| Affected eyes | Unaffected eyes | Affected eyes | Unaffected eyes | Healthy eyes | |
| Average macular vascular density (mm/mm2) | 16.6 ± 2.4 | 18.6 ± 3.3 | 16.0 ± 3.8 | 18.6 ± 1.4 | 31.8 ± 5.8 |
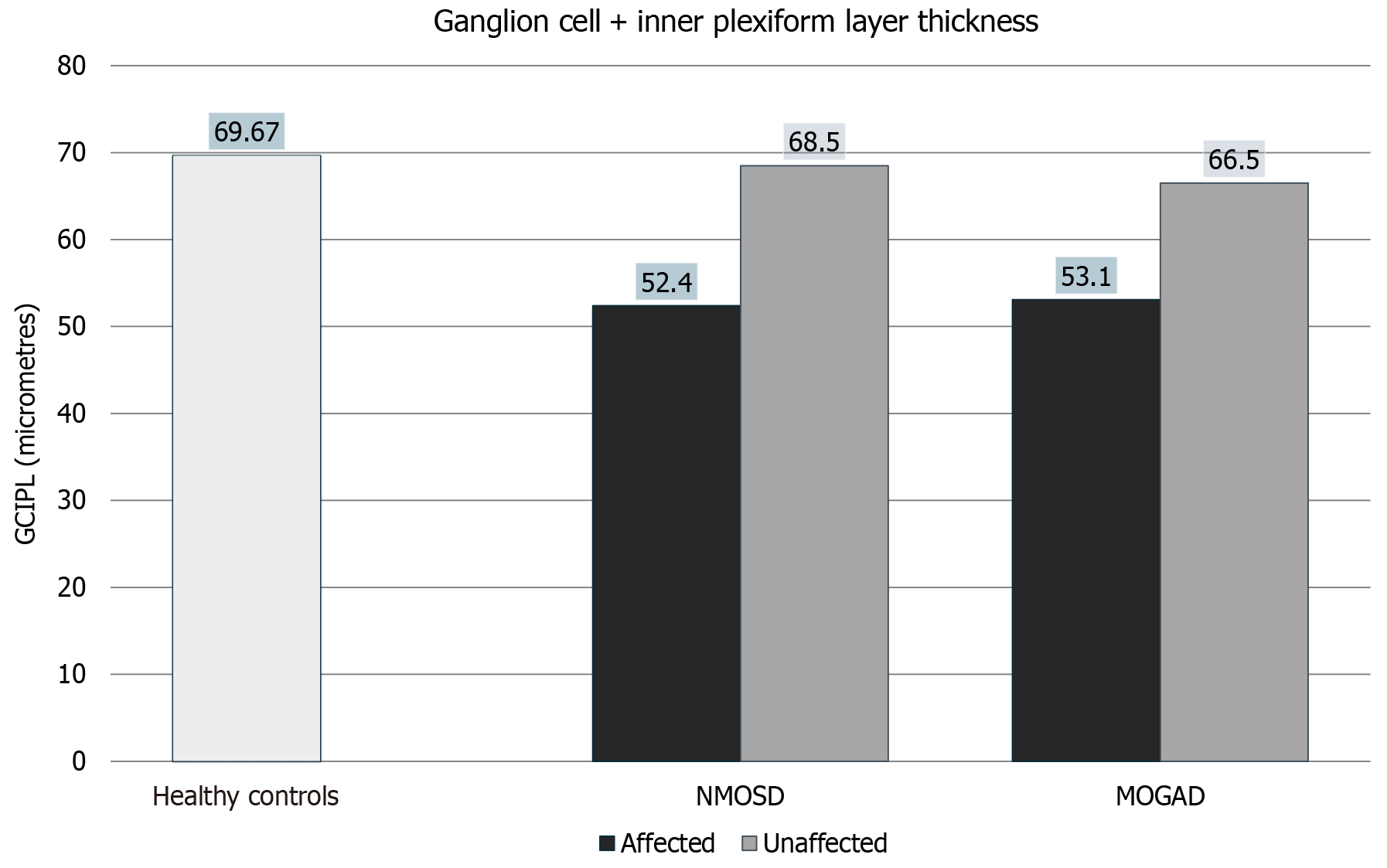
| Average ganglion cell + inner plexiform layer thickness (micrometers) | 52.4 ± 10.0 | 68.5 ± 7.9 | 53.1 ± 11.3 | 66.5 ± 5.4 | 69.67 ± 7.6 |
| Variable compared | P values | |||||||
| NMOSD affected eyes compared to controls | NMOSD unaffected eyes compared to controls | NMOSD affected eyes compared to NMO unaffected eyes | MOGAD affected eyes compared to controls | MOGAD unaffected eyes compared to controls | MOGAD affected eyes compared to MOGAD unaffected eyes | NMO affected eyes compared to MOGAD affected eyes | NMO unaffected eyes compared to MOGAD unaffected eyes | |
| Macular vascular density | 0.0002 | 0.0048 | 0.1228 | 0.0001 | 0.0012 | 0.3207 | 0.7814 | 0.6321 |
| Average ganglion cell + inner plexiform layer thickness | 0.0016 | 0.5398 | 0.0091 | 0.0003 | 0.0009 | 0.1472 | 0.8876 | 0.5622 |
NMO and MOGAD affected eyes had statistically significant thinning of ganglion cell + inner plexiform layer (GCIPL) compared to healthy controls as seen in Figure 3. NMO unaffected eyes did not have significant thinning compared to controls; however, MOGAD unaffected eyes did show significant thinning compared to controls. NMO affected eyes had significant thinning of GCIPL compared to their unaffected counterparts; however, MOGAD affected and unaffected eyes did show a statistically significant difference in GCIPL thickness as can be seen in Figure 2, Figure 3, Table 2 and Table 3.
The findings of this study resonated well with the pre-existing evidence about NMO and MOGAD in terms of gender wise distribution, laterality, and effect on visual acuity[6,7].
The fact that decreased MVD was universally present in patients of both these diseases irrespective of whether the eye was affected or not gives an insight into the systemic nature of these diseases and demands need for further research into the vascular and metabolic features of these disorders. It has already been demonstrated that hyalinization of small vessels and perivascular inflammatory infiltrates are one of the earliest pathological changes in NMO[8]. AQP4 antibodies in NMO target AQP4 channels which are abundant in perivascular astrocytic foot processes, justifying the changes in vasculature as well[9]. Vascular changes in MOGAD still need to be studied in greater detail and no pathophysiologic evidence is present regarding the same currently.
As far as subclinical disease is concerned, this study has not found any subclinical OCT/OCTA changes that culminated into a subsequent symptomatic illness in either NMO or MOGAD patients; however, a longer follow up is required to be able to comment about the same.
Microvascular changes were universal in NMO as well as MOGAD be it affected or unaffected eyes; however, GCIPL thinning in the healthy eye was noticed more with MOGAD. This could provide an insight into the more common bilateral presentation of the disease, and the molecular mechanisms of the same need to be better understood.
When we compare our findings to the recently published study by Lang et al[10], the findings of reduction in MVD in both affected and unaffected eyes are common to both studies. However, they found a significant difference in GCIPL thinning between affected and unaffected eyes in both NMO and MOGAD, in contrast to our study where this difference was seen only in the MOGAD group.
It is still to be determined if OCTA can pick up pre-clinical changes in NMO and MOGAD and longer and closer follow-ups will be required to determine the same in future, but looking at the theoretical pathophysiology of the disease and the changes seen after the disease has set in. We believe that this investigative modality can prove to be of great significance in the early diagnosis and management of these disorders.
In terms of limitations of the study, there was a mismatch of the number of samples in the NMO and MOGAD study groups and more patients need to be evaluated for changes in MVD and GCIPL thickness. A better control of the duration after which the investigations are being performed on the patients, after the onset of symptoms, can give a better frame of reference to the changes observed. In our study, the average duration of symptoms after which the scans were done was around 3 mo, with a high variability, however, owing to poor follow-up of these patients in our setting. In our study, only the superficial vascular plexus was studied, as it is closest to where the pathophysiology of these disorders is occurring; however, a multivariate analysis of superficial, intermediate, and deep capillary plexus could lead to a more holistic insight into the vascular changes occurring in NMO and MOGAD.
In conclusion, our study highlights that decreased MVD is a consistent feature in both NMO and MOGAD, irrespective of whether the eye is clinically affected, indicating a systemic vascular involvement in these diseases. The universal presence of microvascular changes and the notable GCIPL thinning in unaffected eyes of MOGAD patients suggest potential bilateral disease processes that warrant further molecular investigation. Despite the limitations, such as sample size disparity and variability in the timing of scans post-symptom onset, our findings align with existing research and underscore the potential of OCTA as a significant tool for early diagnosis and management. Future studies with larger cohorts and comprehensive vascular analyses are necessary to validate these findings and explore the preclinical diagnostic capabilities of OCTA in NMO and MOGAD.
| 1. | Lana-Peixoto MA, Talim N. Neuromyelitis Optica Spectrum Disorder and Anti-MOG Syndromes. Biomedicines. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hümmert MW, Ringelstein M, Trebst C, Winkelmann A, Schwarz A, Buttmann M, Zimmermann H, Kuchling J, Franciotta D, Capobianco M, Siebert E, Lukas C, Korporal-Kuhnke M, Haas J, Fechner K, Brandt AU, Schanda K, Aktas O, Paul F, Reindl M, Wildemann B; in cooperation with the Neuromyelitis Optica Study Group (NEMOS). MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 667] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 3. | Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015;2:e62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | Kleerekooper I, Houston S, Dubis AM, Trip SA, Petzold A. Optical Coherence Tomography Angiography (OCTA) in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder. Front Neurol. 2020;11:604049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Chen Y, Shi C, Zhou L, Huang S, Shen M, He Z. The Detection of Retina Microvascular Density in Subclinical Aquaporin-4 Antibody Seropositive Neuromyelitis Optica Spectrum Disorders. Front Neurol. 2020;11:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Dauby S, Dive D, Lutteri L, Andris C, Hansen I, Maquet P, Lommers E. Comparative study of AQP4-NMOSD, MOGAD and seronegative NMOSD: a single-center Belgian cohort. Acta Neurol Belg. 2022;122:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Li Y, Liu X, Wang J, Pan C, Tang Z. Clinical Features and Imaging Findings of Myelin Oligodendrocyte Glycoprotein-IgG-Associated Disorder (MOGAD). Front Aging Neurosci. 2022;14:850743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Bukhari W, Barnett MH, Prain K, Broadley SA. Molecular pathogenesis of neuromyelitis optica. Int J Mol Sci. 2012;13:12970-12993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Lucchinetti CF, Guo Y, Popescu BF, Fujihara K, Itoyama Y, Misu T. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. 2014;24:83-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Lang Y, Kwapong WR, Kong L, Shi Z, Wang X, Du Q, Wu B, Zhou H. Retinal structural and microvascular changes in myelin oligodendrocyte glycoprotein antibody disease and neuromyelitis optica spectrum disorder: An OCT/OCTA study. Front Immunol. 2023;14:1029124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |