Published online Mar 20, 2025. doi: 10.5662/wjm.v15.i1.97512
Revised: July 25, 2024
Accepted: July 29, 2024
Published online: March 20, 2025
Processing time: 120 Days and 8.2 Hours
Barrett's esophagus (BE) is a known premalignant precursor to esophageal adenocarcinoma (EAC). The prevalence rates continue to rise in the United States, but many patients who are at risk of EAC are not screened. Current practice guidelines include male gender as a predisposing factor for BE and EAC. The population-based clinical evidence regarding female gender remains limited.
To study comparative trends of gender disparities in patients with BE in the United States.
A nationwide retrospective study was conducted using the 2009-2019 National Inpatient Sample (NIS) database. Patients with a primary or secondary diagnosis code of BE were identified. The major outcome of interest was determining the gender disparities in patients with BE. Trend analysis for respective outcomes for females was also reported to ascertain any time-based shifts.
We identified 1204190 patients with BE for the study period. Among the included patients, 717439 (59.6%) were men and 486751 (40.4%) were women. The mean age was higher in women than in men (67.1 ± 0.4 vs 66.6 ± 0.3 years, P < 0.001). The rate of BE per 100000 total NIS hospitalizations for males increased from 144.6 in 2009 to 213.4 in 2019 (P < 0.001). The rate for females increased from 96.8 in 2009 to 148.7 in 2019 (P < 0.001). There was a higher frequency of obesity among women compared to men (17.4% vs 12.6%, P < 0.001). Obesity prevalence among females increased from 12.3% in 2009 to 21.9% in 2019 (P < 0.001). A lower prevalence of smoking was noted in women than in men (20.8% vs 35.7%, P < 0.001). However, trend analysis showed an increasing prevalence of smoking among women, from 12.9% in 2009 to 30.7% in 2019 (P < 0.001). Additionally, there was a lower prevalence of alcohol abuse, Helicobacter pylori (H. pylori), and diabetes mellitus among females than males (P < 0.001). Trend analysis showed an increasing prevalence of alcohol use disorder and a decreasing prevalence of H. pylori and diabetes mellitus among women (P < 0.001).
The prevalence of BE among women has steadily increased from 2009 to 2019. The existing knowledge concerning BE development has historically focused on men, but our findings show that the risk in women is not insignificant.
Core Tip: Currently, large epidemiologic data evaluating the gender-based differences in Barrett’s esophagus (BE) remain limited. Using the 2009-2019 National Inpatient Sample database, we conducted this retrospective study to assess the gender disparities and trends in patients with BE. According to our data, the prevalence of BE in women increased steadily during the course of the study. Trend analysis among females revealed an increasing prevalence of obesity, smoking, and alcohol abuse and a declining prevalence of Helicobacter pylori and diabetes. These observations indicate that the risk of BE in women is not insignificant. Further research is required to carefully screen for risk factors and determine the underlying mechanisms of BE and esophageal adenocarcinoma in female patients.
- Citation: Fatakhova K, Inayat F, Ali H, Patel P, Rehman AU, Afzal A, Sarfraz M, Sarfraz S, Nawaz G, Chaudhry A, Dhillon R, Dilibe A, Glazebnik B, Jones L, Glazer E. Gender disparities and woman-specific trends in Barrett’s esophagus in the United States: An 11-year nationwide population-based study. World J Methodol 2025; 15(1): 97512
- URL: https://www.wjgnet.com/2222-0682/full/v15/i1/97512.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i1.97512
Barrett’s esophagus (BE), a complication of chronic gastroesophageal reflux disease (GERD), is a precursor lesion of esophageal adenocarcinoma (EAC)[1,2]. The pathological diagnosis of BE is based on the presence of distinctive metaplastic changes in the esophageal squamous epithelium, which transforms into a specialized columnar epithelium[3,4]. In 1950, the Australian-born surgeon Norman Rupert Barrett first used the term "chronic peptic ulcer of the eso
A number of organizations and expert consensus panels have developed clinical practice guidelines for BE[12-15]. These recommendations designate the male gender as a risk factor for developing BE and EAC. However, it is still an ongoing debate whether women should be entitled to screening endoscopies based on specific risk factors. Previous research shows that few studies have evaluated the prevalence of BE in females[16-18]. A meta-analysis based on pooled data from 19337 patients showed that females had a remarkably lower prevalence of BE and a reduced probability of developing high-grade dysplasia and EAC[19]. However, the majority of the gender estimations were from studies with limited sample sizes based on patient groups with institutional or geographical restrictions[19]. This highlights the dearth of large population-based data to adequately address the gender-based differences. The gender gap translates into a lack of robust evidence to tailor the guidelines for surveillance intervals for at-risk women. Therefore, more data are required to provide current trends in the gender distribution of BE. The global increase in incidence rates of EAC may be attributed to the rising prevalence rates of BE risk factors such as GERD, obesity, and smoking[20]. The American Gastroenterological Association Clinical Practice Update has now recommended endoscopic screening for BE and EAC in patients with three or more known risk factors for these conditions[21]. Therefore, gender variations in risk factors also require an epidemiological evaluation.
Our study aims to address the literature gap by providing a comprehensive analysis of the prevalence of BE among nationwide inpatient hospitalizations over a decade in the United States. We have used substantial epidemiological trends to assess risk factors for BE among women.
The National Inpatient Sample (NIS) is the largest inpatient database in the country[22]. The Agency for Healthcare Research and Quality designed it for the Healthcare Cost and Utilization Project[22]. This database is structured to approximate a stratified 20% sample of medical centers; sampling weights are applied to generate national estimates[22]. The data in NIS are provided using the International Classification of Diseases (ICD), Ninth Revision (ICD-9) (before September 2015), and Tenth Revision (ICD-10) (after October 2015) codes. Our research used the NIS registry to study adult patients (≥ 18 years of age) with a primary or secondary diagnosis code of BE between January 01, 2009 and December 31, 2019. The ICD-9 code "53085" and the ICD-10 codes "K2270", "K22710", "K22711", and "K22719" were used for identification of these patients.
The major outcome of interest was determining the gender disparities in patients with BE. A number of variables were compared, including age, race, Charlson Comorbidity Index (CCI) score, hospital region, teaching status, primary insurance, and socioeconomic status. In addition, we also compared the prevalence of obesity, smoking, alcohol abuse, Helicobacter pylori (H. pylori) infection, diabetes mellitus, and EAC. Trend analysis for respective outcomes for females was also reported to ascertain any time-based shifts.
The Statistical Software for Data Science, version 16.0 (StataCorp LLC, College Station, TX, United States), was used in this work. The analysis used two-sided P values, with a value of 0.05 as the statistically significant threshold. Bivariate analysis was carried out using the independent-samples t-test for continuous variables and the chi-square (χ2) test for categorical data. Frequency (N) and percentage (%) were used to present categorical variables. The mean and standard deviation represented continuous data, as appropriate. A similar analysis has been widely used in NIS-based studies[23]. The adjusted Wald test was utilized to compare the slopes of time-based linear regression outcomes and margin plots to generate figures[24,25].
The NIS comprises de-identified data. It has effective protocols designed to protect the privacy of patients, healthcare providers, and hospitals. Therefore, the institutional review board approval was exempted for this research. Patient consent was not required because the study only used anonymized data from hospitalization records. According to the Healthcare Cost and Utilization Project Data Use Agreement, any individual table cell counts of less than or equal to 10 have been suppressed to protect privacy and compliance.
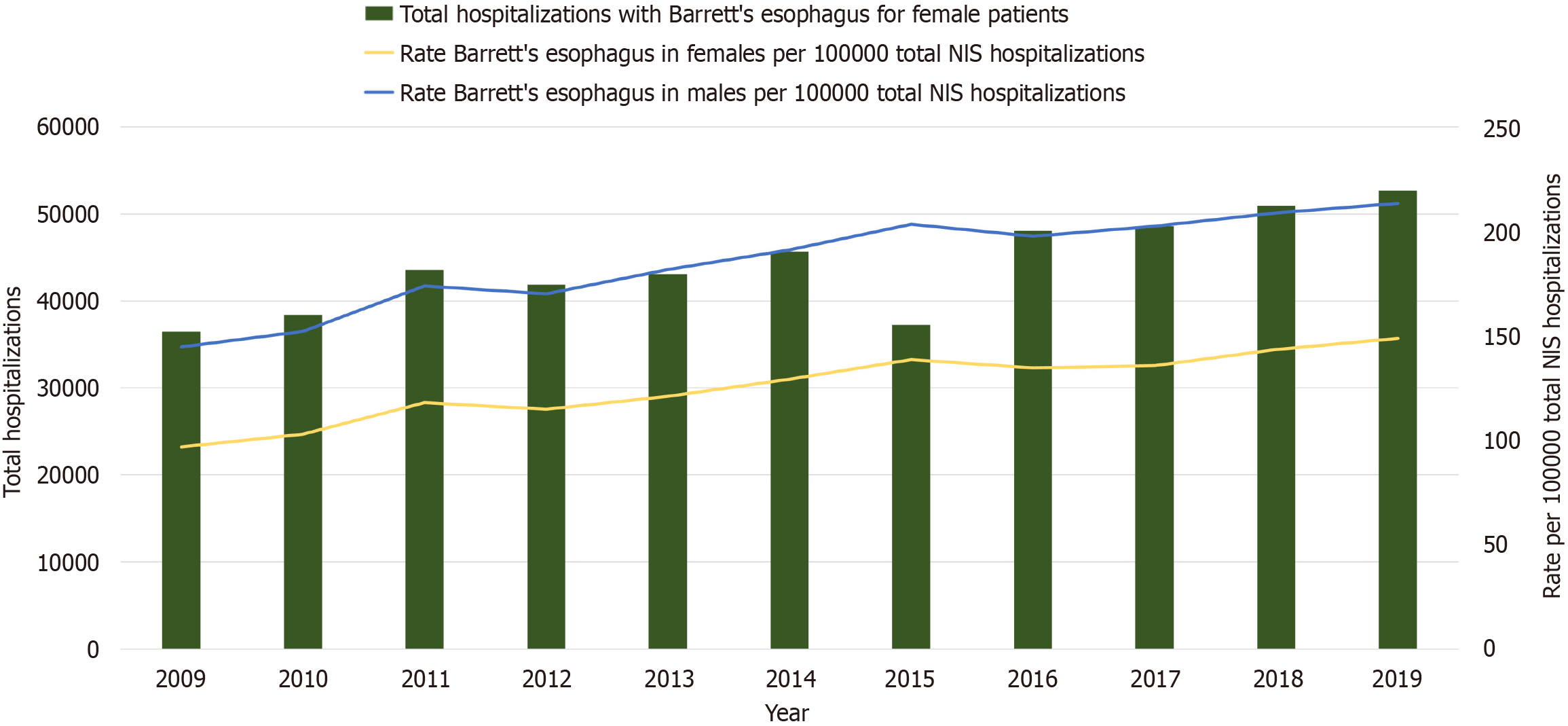
Of the 1204190 patients with BE, 717439 (59.6%) were men and 486751 (40.4%) were women. Trend analysis revealed that the number of women with a diagnosis of BE increased from 36518 in 2009 to 52665 in 2019 (P < 0.001). The rate of BE per 100000 total NIS hospitalizations for males increased from 144.6 in 2009 to 213.4 in 2019, while the rate for females increased from 96.8 in 2009 to 148.7 in 2019 (P < 0.001) (Figure 1).
The mean age was higher in women compared to men (67.1 ± 0.4 vs 66.6 ± 0.3 years, P < 0.001) (Table 1). The mean age for females significantly increased from 66.4 ± 0.7 years in 2009 to 68.0 ± 0.2 years in 2019 (P < 0.001). The age group that was most affected was 65-79 years for both genders. This accounted for 46.8% of females and 46.9% of males. Trend analysis among females in this age group revealed an increasing prevalence, from 15629 (42.8%) in 2009 to 27072 (51.4%) in 2019 (P < 0.001) (Table 2). There was a downward trend in the prevalence of BE in females younger than 65 years (P < 0.001). Male and female patients with BE had a White race predominance (P < 0.001). There was no significant racial trend for females during the course of the study (P = 0.61).
| Males | Females | P value | |
| Total patients | 717439 (59.6) | 486751 (40.4) | |
| Mean age (years) | 66.6 ± 0.3 | 67.1 ± 0.4 | < 0.001 |
| Age groups (years) | < 0.001 | ||
| 18-34 | 14504 (2.0) | 12191 (2.5) | |
| 35-49 | 77463 (10.8) | 56421 (11.6) | |
| 50-64 | 262752 (36.6) | 167052 (34.3) | |
| 65-79 | 336496 (46.9) | 227662 (46.8) | |
| ≥ 80 | 26524 (3.7) | 23425 (4.8) | |
| Race | < 0.001 | ||
| White | 649806 (90.6) | 440110 (90.4) | |
| Black | 24510 (3.4) | 18551 (3.8) | |
| Hispanic | 35231 (4.9) | 22276 (4.6) | |
| Asian/Native American | 7892 (1.1) | 5815 (1.2) | |
| Charlson comorbidity index score | < 0.001 | ||
| 0 | 169348 (23.6) | 129794 (26.7) | |
| 1 | 151256 (21.1) | 128760 (26.4) | |
| 2 | 132933 (18.5) | 85975 (17.7) | |
| ≥ 3 | 263902 (36.8) | 142222 (29.2) | |
| Hospital region | < 0.001 | ||
| Northeast | 159271 (22.2) | 119644 (24.6) | |
| Midwest | 207339 (28.9) | 140049 (28.8) | |
| South | 224560 (31.3) | 151918 (31.2) | |
| West | 126269 (17.6) | 75140 (15.4) | |
| Hospital location and teaching status | < 0.001 | ||
| Rural | 67439 (9.4) | 47346 (9.7) | |
| Urban nonteaching | 220254 (30.7) | 157188 (32.3) | |
| Urban teaching | 429746 (59.9) | 282217 (58.0) | |
| Primary payer | 0.27 | ||
| Medicare | 459878 (64.1) | 329593 (67.7) | |
| Medicaid | 60264 (8.4) | 39804 (8.2) | |
| Private | 177210 (24.7) | 106659 (21.9) | |
| Other | 20087 (2.8) | 10695 (2.2) | |
| Median household income (quartile) | 0.075 | ||
| 1st (0-25th) | 158557 (22.1) | 107916 (22.2) | |
| 2nd (26th -50th) | 189403 (26.4) | 130652 (26.8) | |
| 3rd (51st -75th) | 188685 (26.3) | 128664 (26.4) | |
| 4th (76th -100th) | 180794 (25.2) | 119519 (24.6) | |
| Obesity | 90172 (12.6) | 84844 (17.4) | < 0.001 |
| Smoking | 256152 (35.7) | 100630 (20.8) | < 0.001 |
| Alcohol abuse | 81405 (11.3) | 20609 (4.2) | < 0.001 |
| Helicobacter pylori | 3944 (0.5) | 2124 (0.4) | 0.0002 |
| Diabetes | 132826 (18.5) | 83322 (17.1) | < 0.001 |
| Esophageal adenocarcinoma | 11733 (1.6) | 2544 (0.5) | < 0.001 |
| Variables | Years | P values | ||||||||||
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||
| Mean age (years) | 66.4 ± 0.7 | 66.5 ± 0.5 | 66.9 ± 0.4 | 67.1 ± 0.2 | 66.9 ± 0.4 | 67.0 ± 0.8 | 66.9 ± 0.9 | 67.3 ± 0.8 | 67.2 ± 0.9 | 67.8 ± 1.9 | 68.0 ± 0.2 | < 0.001 |
| Age groups (years) | < 0.001 | |||||||||||
| 18-34 | 1168 (3.2) | 1114 (2.9) | 1351 (3.1) | 1174 (2.8) | 948 (2.2) | 1462 (3.2) | 709 (1.9) | 865 (1.8) | 1021 (2.1) | 1223 (2.4) | 1156 (2.2) | |
| 35-49 | 5514 (15.1) | 5685 (14.8) | 5360 (12.3) | 5069 (12.1) | 4695 (10.9) | 5301 (11.6) | 4175 (11.2) | 4710 (9.8) | 5297 (10.9) | 5148 (10.1) | 5467 (10.4) | |
| 50-64 | 12745 (34.9) | 13292 (34.6) | 15779 (36.2) | 14956 (35.7) | 15547 (36.1) | 15766 (34.5) | 13382 (35.9) | 16438 (34.2) | 15989 (32.9) | 16463 (32.3) | 16695 (31.7) | |
| 65-79 | 15629 (42.8) | 16288 (42.4) | 18872 (43.3) | 18516 (44.2) | 19726 (45.8) | 20976 (45.9) | 17185 (46.1) | 23599 (49.1) | 23958 (49.3) | 25841 (50.7) | 27072 (51.4) | |
| ≥ 80 | 1462 (4.0) | 2036 (5.3) | 2223 (5.1) | 2178 (5.2) | 2153 (5.0) | 2194 (4.8) | 1827 (4.9) | 2451 (5.1) | 2333 (4.8) | 2293 (4.5) | 2275 (4.3) | |
| Race | 0.61 | |||||||||||
| White | 33194 (90.9) | 34689 (90.3) | 39009 (89.5) | 37787 (90.2) | 39020 (90.6) | 40992 (89.7) | 33699 (90.4) | 43785 (91.1) | 43884 (90.3) | 46126 (90.5) | 47925 (91.0) | |
| Black | 1023 (2.8) | 1613 (4.2) | 1700 (3.9) | 1718 (4.1) | 1852 (4.3) | 1828 (4.0) | 1417 (3.8) | 1730 (3.6) | 1993 (4.1) | 1886 (3.7) | 1791 (3.4) | |
| Hispanic | 1899 (5.2) | 1652 (4.3) | 2397 (5.5) | 1843 (4.4) | 1766 (4.1) | 2376 (5.2) | 1715 (4.6) | 1923 (4.0) | 2041 (4.2) | 2294 (4.5) | 2370 (4.5) | |
| Asian/Native American | 402 (1.1) | 461 (1.2) | 479 (1.1) | 545 (1.3) | 431 (1.0) | 503 (1.1) | 447 (1.2) | 625 (1.3) | 680 (1.4) | 663 (1.3) | 579 (1.1) | |
| Charlson comorbidity index | < 0.001 | |||||||||||
| 0 | 12562 (34.4) | 11947 (31.1) | 12771 (29.3) | 12526 (29.9) | 12146 (28.2) | 13390 (29.3) | 10028 (26.9) | 11103 (23.1) | 10983 (22.6) | 10857 (21.3) | 11481 (21.8) | |
| 1 | 10078 (27.6) | 10833 (28.2) | 11942 (27.4) | 11353 (27.1) | 12016 (27.9) | 11470 (25.1) | 9804 (26.3) | 12689 (26.4) | 12198 (25.1) | 12895 (25.3) | 13482 (25.6) | |
| 2 | 6318 (17.3) | 7107 (18.5) | 7932 (18.2) | 7164 (17.1) | 7795 (18.1) | 7952 (17.4) | 6598 (17.7) | 8603 (17.9) | 8797 (18.1) | 9072 (17.8) | 8637 (16.4) | |
| ≥ 3 | 7560 (20.7) | 8528 (22.2) | 10940 (25.1) | 10850 (25.9) | 11112 (25.8) | 12887 (28.2) | 10848 (29.1) | 15668 (32.6) | 16620 (34.2) | 18144 (35.6) | 19065 (36.2) | |
| Hospital region | 0.99 | |||||||||||
| Northeast | 8691 (23.8) | 8490 (22.1) | 10591 (24.3) | 10415 (24.9) | 10810 (25.1) | 11289 (24.7) | 9394 (25.2) | 12256 (25.5) | 12101 (24.9) | 12283 (24.1) | 13324 (25.3) | |
| Midwest | 11174 (30.6) | 11448 (29.8) | 12727 (29.2) | 11968 (28.6) | 11931 (27.7) | 12841 (28.1) | 10587 (28.4) | 13362 (27.8) | 13802 (28.4) | 14883 (29.2) | 15326 (29.1) | |
| South | 11466 (31.4) | 12407 (32.3) | 14339 (32.9) | 13060 (31.2) | 13265 (30.8) | 14349 (31.4) | 11370 (30.5) | 14995 (31.2) | 15114 (31.1) | 15596 (30.6) | 15957 (30.3) | |
| West | 5187 (14.2) | 6070 (15.8) | 5928 (13.6) | 6450 (15.4) | 7063 (16.4) | 7220 (15.8) | 5927 (15.9) | 7450 (15.5) | 7581 (15.6) | 8206 (16.1) | 8058 (15.3) | |
| Hospital location and teaching status | < 0.001 | |||||||||||
| Rural | 3944 (10.8) | 4302 (11.2) | 5187 (11.9) | 5069 (12.1) | 4479 (10.4) | 4250 (9.3) | 3020 (8.1) | 4614 (9.6) | 3985 (8.2) | 4230 (8.3) | 4266 (8.1) | |
| Urban nonteaching | 17090 (46.8) | 18324 (47.7) | 19308 (44.3) | 16925 (40.4) | 17874 (41.5) | 12476 (27.3) | 10885 (29.2) | 12593 (26.2) | 11518 (23.7) | 10347 (20.3) | 9848 (18.7) | |
| Urban teaching | 15484 (42.4) | 15789 (41.1) | 19090 (43.8) | 19899 (47.5) | 20716 (48.1) | 28973 (63.4) | 23373 (62.7) | 30856 (64.2) | 33095 (68.1) | 36391 (71.4) | 38551 (73.2) | |
| Primary payer | < 0.001 | |||||||||||
| Medicare | 22897 (62.7) | 24278 (63.2) | 28809 (66.1) | 28026 (66.9) | 29416 (68.3) | 30846 (67.5) | 25424 (68.2) | 33356 (69.4) | 33581 (69.1) | 35779 (70.2) | 37181 (70.6) | |
| Medicaid | 2301 (6.3) | 3112 (8.1) | 3574 (8.2) | 3142 (7.5) | 3187 (7.4) | 3747 (8.2) | 3131 (8.4) | 4566 (9.5) | 4179 (8.6) | 4230 (8.3) | 4635 (8.8) | |
| Private | 10444 (28.6) | 10180 (26.5) | 10068 (23.1) | 9677 (23.1) | 9303 (21.6) | 10054 (22.0) | 7940 (21.3) | 9516 (19.8) | 10108 (20.8) | 9837 (19.3) | 9532 (18.1) | |
| Other | 876 (2.4) | 845 (2.2) | 1134 (2.6) | 1048 (2.5) | 1163 (2.7) | 1052 (2.3) | 782 (2.1) | 625 (1.3) | 730 (1.5) | 1121 (2.2) | 1317 (2.5) | |
| Median household income | 0.51 | |||||||||||
| 1st (0-25th) | 7486 (20.5) | 8605 (22.4) | 10068 (23.1) | 9300 (22.2) | 9475 (22.0) | 10374 (22.7) | 8648 (23.2) | 10718 (22.3) | 10740 (22.1) | 10652 (20.9) | 11850 (22.5) | |
| 2nd (26th -50th) | 9130 (25.0) | 9911 (25.8) | 11288 (25.9) | 11227 (26.8) | 11672 (27.1) | 12933 (28.3) | 9879 (26.5) | 12593 (26.2) | 13267 (27.3) | 14322 (28.1) | 14430 (27.4) | |
| 3rd (51st -75th) | 9969 (27.3) | 10410 (27.1) | 11681 (26.8) | 10851 (25.9) | 11801 (27.4) | 11379 (24.9) | 9320 (25.0) | 13025 (27.1) | 12684 (26.1) | 14220 (27.9) | 13324 (25.3) | |
| 4th (76th -100th) | 9933 (27.2) | 9489 (24.7) | 10548 (24.2) | 10515 (25.1) | 10121 (23.5) | 11013 (24.1) | 9431 (25.3) | 11727 (24.4) | 11907 (24.5) | 11774 (23.1) | 13061 (24.8) | |
| Obesity (BMI ≥ 30 kg/m2) | 4477 (12.3) | 4728 (12.3) | 5625 (12.9) | 6030 (14.4) | 7210 (16.7) | 7995 (17.5) | 6874 (18.4) | 9320 (19.4) | 9975 (20.5) | 11055 (21.7) | 11555 (21.9) | < 0.001 |
| Smoking | 4704 (12.9) | 5464 (14.2) | 6308 (14.5) | 6105 (14.6) | 6045 (14.0) | 6625 (14.5) | 5875 (15.8) | 13240 (27.5) | 14645 (30.1) | 15474 (30.4) | 16145 (30.7) | < 0.001 |
| Alcohol abuse | 1063 (2.9) | 1243 (3.2) | 1599 (3.7) | 1410 (3.4) | 1570 (3.6) | 1630 (3.6) | 1590 (4.2) | 2600 (5.4) | 2484 (5.1) | 2560 (5.0) | 2860 (5.4) | < 0.001 |
| Helicobacter pylori | 242 (0.7) | 244 (0.6) | 293 (0.7) | 290 (0.7) | 195 (0.5) | 200 (0.4) | 135 (0.4) | 115 (0.2) | 150 (0.3) | 140 (0.3) | 120 (0.2) | < 0.001 |
| Diabetes | 6541 (17.9) | 7014 (18.3) | 8284 (19.0) | 8195 (19.6) | 8445 (19.6) | 8540 (18.7) | 7315 (19.6) | 8554 (17.8) | 6829 (14.1) | 6715 (13.2) | 6890 (13.1) | < 0.001 |
| Esophageal adenocarcinoma | 341 (0.9) | 250 (0.7) | 348 (0.8) | 240 (0.6) | 330 (0.8) | 320 (0.7) | 300 (0.8) | 115 (0.2) | 115 (0.2) | 110 (0.2) | 75 (0.1) | 0.1 |
Females had a lower CCI score of ≥ 3 than males (29.2% vs 36.8%, P < 0.001). Trend analysis among females revealed an increasing CCI score of ≥ 3, from 20.7% in 2009 to 36.2% in 2019 (P < 0.001). A higher frequency of obesity was noted in females compared to males (17.4% vs 12.6%, P < 0.001). Female BE patients had a rising obesity prevalence from 12.3% in 2009 to 21.9% in 2019 (P < 0.001). A lower prevalence of smoking was noted in females than males (20.8% vs 35.7%, P < 0.001). However, trend analysis among females revealed an increasing prevalence of smoking, from 12.9% in 2009 to 30.7% in 2019 (P < 0.001). Additionally, there was a lower prevalence of alcohol abuse, H. pylori, and diabetes mellitus among females than males during the study period (P < 0.001). Trend analysis showed an increasing prevalence of alcohol use disorder and a decreasing prevalence of H. pylori and diabetes mellitus among women (P < 0.001). The prevalence of EAC also showed a decline from 0.9% in 2009 to 0.1% in 2019 (P = 0.1).
This is the first nationwide study to evaluate gender disparities in patients with BE and associated risk factors using a multicenter database. It shows that the prevalence of BE among women has steadily increased from 2009 to 2019 in the United States. The rate of BE for females in 2019 was comparable to the rate for males in 2009. Our findings also highlight the feasibility and potential of screening a larger population based on gender differences in BE risk factors.
A male predominance has been historically observed in patients with BE[26]. The results of our study indicate that women also have a considerable risk of developing this condition. The available clinical evidence shows that there are several risk factors associated with the development of BE[27-30]. It is important to note that patients may also exhibit gender-specific variations in these risk factors[31-33]. Our findings show several correlations that may explain the increasing prevalence of BE among females. We found a higher frequency of obesity among women compared to men (17.4% vs 12.6%). Obesity in females increased from 12.3% in 2009 to 21.9% in 2019. Clinical data show that a high body mass index (BMI) has been strongly associated with the development of frequent reflux symptoms[34,35]. A prospective cohort study from the Netherlands based on 120852 participants revealed that increased BMI was a significant risk factor for BE development in females but not in males[36]. A study from the United States surveyed 10545 women to under
Gender-related differences in body fat distribution may potentially have an impact on BE development. Females usually have fat located in subcutaneous tissues compared to males, who have centralized, visceral fat[39]. Adipokines, such as leptin and adiponectin, play an active role in the pathogenesis of BE[40]. Therefore, the higher overall prevalence of BE among men could be attributed to the higher production of these adipokines by visceral fat[40]. In our analysis, the prevalence of BE showed a significant increase from 42.4% in 2009 to 73.2% in 2019 in urban teaching hospitals. Certain urban layouts may impact physical activity and promote a sedentary lifestyle, which can result in weight gain. These factors increase the risk of GERD, leading to a higher probability of BE. Moreover, obese patients with nondysplastic BE have a significantly higher risk of dysplasia development in three to five years compared to nonobese patients[41]. These findings highlight the critical need to curtail the ongoing obesity epidemic in order to reduce the burden of BE and EAC.
In our data, women aged 65-79 years showed a significant increase in BE prevalence from 42.8% in 2009 to 51.4% in 2019. Contrarily, females under the age of 65 years showed a downtrend over the study period. The National Ambulatory Medical Care Survey-based study analyzed the trend in physician visits for GERD from 1995 to 2006[42]. It found that ambulatory visits for GERD were strongly associated with older age, female gender, and use of calcium channel blockers[42]. Therefore, the trend of BE seen in women older than 65 in our data may be explained by worsening acid reflux with age. Our analysis showed that the smoking prevalence among females also increased from 12.9% in 2009 to 30.7% in 2019. The biological pathway of smoking in BE is unclear. However, a study based on patient data from five case-control studies included in the international Barrett's and Esophageal Adenocarcinoma Consortium suggested cigarette smoking as a risk factor for BE[43]. An Australian case-control study reported that people with both acid reflux and smoking had a higher risk of BE than those with either reflux or smoking alone (odds ratio: 51.4)[44]. However, even in the absence of acid reflux, the metabolic syndrome alone may also increase the risk of BE. A population-based study from the United States suggested that metabolic syndrome-related systemic inflammation could increase BE risk in individuals without GERD[45].
We found the highest prevalence of BE among White Americans. The American College of Gastroenterology guidelines included Caucasian race among the risk factors for BE[46]. A retrospective case-control study from the United Kingdom also revealed White Caucasian ethnicity as an independent risk factor for BE[47]. Similar observations have also been made in other population-based studies, predicting that the prevalence of EAC is three to five times higher among Caucasians compared to African Americans[48,49]. An observational study based on the United States Surveillance, Epidemiology, and End Results Program also showed that the incidence of EAC remains higher in non-Hispanic Whites[50]. In our data, trend analysis among females revealed an increasing CCI score of ≥ 3, from 20.7% in 2009 to 36.2% in 2019. Compared to men, women had lower rates of diabetes, H. pylori, and alcohol abuse during the study period. A trend analysis of female patients revealed a rise in the prevalence of alcohol usage and a decrease in the prevalence of H. pylori and diabetes. Therefore, it is plausible that the interaction of the risk factors may contribute to the rise of BE among females. Future research should investigate the causal link between BE and the concurrent occurrence of various risk variables.
Patients with BE may have gender-based differences in presentation patterns. A comparative study from the United States reported that women with reflux have less esophageal acid exposure than men[51]. In addition, the female gender does not provide the same protection against the development of BE as males in patients with advanced GERD[51]. A population-based study conducted in Italy found that 46.2% of BE patients may develop an asymptomatic disease[52]. Therefore, a diagnosis for females with asymptomatic BE might not be made until the disease has progressed. It complicates clinical management, directly impacting healthcare costs. In our study, the prevalence of EAC showed a decline for the study period, but the downtrend was not significant (P = 0.1). A multicenter prospective cohort study from the Netherlands revealed that males had a higher probability and time to cancer progression, but females were proportionally more frequently diagnosed with advanced stages of EAC[53]. Therefore, new prediction models for BE patients should focus on gender-based EAC risk stratification[53].
The American College of Gastroenterology and the European Society of Gastrointestinal Endoscopy recommend screening for a nondysplastic BE every three to five years, based on the length of the Barrett’s segment[54,55]. However, there is an ongoing debate about how to shorten the surveillance intervals using molecular and individualized risk stratification[56]. It would not be financially feasible to rule out BE based only on heartburn complaints, given the frequency of these symptoms in large communities[57]. Therefore, the initial use of nonendoscopic screening devices may help in this regard[58,59]. A careful evaluation of the risk factor profiles of suspected patients could aid in improving noninvasive screening for BE and EAC[60,61]. In addition, novel artificial intelligence-driven risk assessment models can also be combined with nonendoscopic screening techniques[62-65]. These artificial intelligence tools can also improve the diagnostic yield of advanced endoscopic modalities[66]. Our study indicates that there has been a significant increase in BE diagnoses among females, especially in older age groups. This trend could be linked to long-term metaplasia caused by GERD, obesity, smoking, and other concurrent comorbidities. Therefore, we suggest that healthcare departments should also consider suspected female patient populations while identifying the high-risk groups for BE. The selected patients may then undergo upper endoscopy, with proper follow-up surveillance to monitor the disease progression. Asymptomatic individuals who progress to EAC may have worse clinical outcomes. Therefore, early detection of dysplasia may also help guide effective management to halt cancer progression in BE[67].
Our retrospective study is one of the largest comparative analyses conducted to examine the prevalence of BE among women. It implies that women may have BE at a higher rate than previously believed and that this condition is not exclusive to men. We also assessed the risk of BE among females through variables such as obesity, smoking, alcohol use disorder, H. pylori, and diabetes mellitus. The 11-year study period is one of the major strengths of this research. The nationwide sample population broadens the applicability of our findings by providing greater generalizability and enhancing the understanding of the gender distribution patterns. Clinical outcomes can be improved by a tailored surveillance approach for BE patients who may be at risk for EAC. Our findings carry pertinent clinical implications and underscore the importance of screening and surveillance considering gender-based risk variables. Public health campaigns can be carried out among communities to educate individuals about risk factors. Our data could also prove to be a forerunner for future comprehensive studies to further refine the surveillance recommendations for BE among women.
This study has certain limitations. The use of coding systems may introduce inaccuracies, potentially skewing the analysis when utilizing a large database. The ICD coding system for BE may not be sufficiently reliable for epidemiological studies[68,69]. However, these codes have been previously used to investigate inpatient outcomes of atrial fibrillation among BE patients and the prevalence of BE among Asian Americans[70,71]. Since BE is not typically an inpatient diagnosis and does not usually lead to hospitalization, we used these inpatient diagnosis codes as a surrogate to measure changes in the overall prevalence of BE among females. The NIS database does not provide information on the progression of BE to EAC. In addition, data on the severity of the disease and treatment course are also not reported. Therefore, patients classified as BE in our data might already have progressed to EAC due to a lack of endoscopic screening. BE was not the primary diagnosis in these patients hospitalized for any cause. Therefore, we did not report hospital stays, charges, or procedures. Moreover, the data collected from NIS might only include those hospitals participating in the Healthcare Cost and Utilization Project[72]. Despite these limitations, this is the first national research study to compare gender-based differences in patients with BE. It will add to the current clinical understanding of the prevalence of BE in women and the related risk factors.
This study has thoroughly analyzed the prevalence and trends of BE and its risk factors among women. According to our findings, there was a noticeable increase in the prevalence of BE in females from 2009 to 2019. The prevalence was significantly higher in the older age groups. Women had a higher frequency of obesity compared to men. A trend analysis of female BE patients revealed a rise in the prevalence of obesity, smoking, and alcohol usage and a decline in the prevalence of H. pylori and diabetes. These results underscore the clinical importance of recognizing gender differences in BE risk factors. Endoscopic evaluation tailored to woman-specific risk factors could also help refine surveillance intervals. Such actions may facilitate the detection of EAC at less advanced stages among women. It could potentially improve clinical outcomes by decreasing morbidity and mortality in at-risk patient populations.
The preliminary form of these data was presented as an abstract at the Annual Scientific Meeting of the American College of Gastroenterology, 20-25 October 2023, in Vancouver, BC, Canada.
| 1. | Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 2. | Sharma P. Barrett Esophagus: A Review. JAMA. 2022;328:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Shaheen NJ, Richter JE. Barrett's oesophagus. Lancet. 2009;373:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Peters Y, Al-Kaabi A, Shaheen NJ, Chak A, Blum A, Souza RF, Di Pietro M, Iyer PG, Pech O, Fitzgerald RC, Siersema PD. Barrett oesophagus. Nat Rev Dis Primers. 2019;5:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Barrett NR. Chronic peptic ulcer of the oesophagus and 'oesophagitis'. Br J Surg. 1950;38:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 545] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 6. | Martinez-Uribe O, Becker TC, Garman KS. Promises and Limitations of Current Models for Understanding Barrett's Esophagus and Esophageal Adenocarcinoma. Cell Mol Gastroenterol Hepatol. 2024;17:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Eusebi LH, Cirota GG, Zagari RM, Ford AC. Global prevalence of Barrett's oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: a systematic review and meta-analysis. Gut. 2021;70:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Saha B, Vantanasiri K, Mohan BP, Goyal R, Garg N, Gerberi D, Kisiel JB, Singh S, Iyer PG. Prevalence of Barrett's Esophagus and Esophageal Adenocarcinoma With and Without Gastroesophageal Reflux: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2024;22:1381-1394.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Crowe BR, Krigel A, Li T, Haile R, Al-Ani F, Lebwohl B, Abrams JA, Araujo JL. Veterans with multiple risk factors for Barrett's esophagus are infrequently evaluated with upper endoscopy. Dis Esophagus. 2023;36:doad007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Sharma P, Falk GW, Bhor M, Ozbay AB, Latremouille-Viau D, Guerin A, Shi S, Elvekrog MM, Limburg P. Healthcare Resource Utilization and Costs Among Patients With Gastroesophageal Reflux Disease, Barrett's Esophagus, and Barrett's Esophagus-Related Neoplasia in the United States. J Health Econ Outcomes Res. 2023;10:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Holmberg D, Lagergren J. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Foregut: The Journal of the American Foregut Society. 2023;3:52-59. [DOI] [Full Text] |
| 12. | Thrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig Dis Sci. 2018;63:1988-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | ASGE Standards Of Practice Committee; Qumseya B, Sultan S, Bain P, Jamil L, Jacobson B, Anandasabapathy S, Agrawal D, Buxbaum JL, Fishman DS, Gurudu SR, Jue TL, Kripalani S, Lee JK, Khashab MA, Naveed M, Thosani NC, Yang J, DeWitt J, Wani S; ASGE Standards of Practice Committee Chair. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc. 2019;90:335-359.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 14. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O'Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J; British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 874] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 15. | Wani S, Yadlapati R, Singh S, Sawas T, Katzka DA; Post-Endoscopy Esophageal Neoplasia Expert Consensus Panel. Post-endoscopy Esophageal Neoplasia in Barrett's Esophagus: Consensus Statements From an International Expert Panel. Gastroenterology. 2022;162:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Allen JE, Desai M, Roumans CAM, Vennalaganti S, Vennalaganti P, Bansal A, Falk G, Lieberman D, Sampliner R, Thota P, Vargo J, Gupta N, Moawad F, Bruno M, Kennedy KF, Gaddam S, Young P, Mathur S, Cash B, Spaander M, Sharma P. Low Risk of Progression of Barrett's Esophagus to Neoplasia in Women. J Clin Gastroenterol. 2021;55:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Falk GW, Thota PN, Richter JE, Connor JT, Wachsberger DM. Barrett's esophagus in women: demographic features and progression to high-grade dysplasia and cancer. Clin Gastroenterol Hepatol. 2005;3:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Stephanie M, Nour H, de Sá Inês M, Shanker K, Kevin K, Mario DR, Prateek S. Gender differences in Barrett's esophagus and progression of disease: a systematic review and meta-analysis. Dis Esophagus. 2022;35:doab075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Beydoun AS, Stabenau KA, Altman KW, Johnston N. Cancer Risk in Barrett's Esophagus: A Clinical Review. Int J Mol Sci. 2023;24:6018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Muthusamy VR, Wani S, Gyawali CP, Komanduri S; CGIT Barrett’s Esophagus Consensus Conference Participants. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review. Clin Gastroenterol Hepatol. 2022;20:2696-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 22. | Nationwide Inpatient Sample. Rockville, MD: Agency for Healthcare Research and Quality. [cited 15 May 2024]. Available from: http://hcup-us.ahrq.gov/nisoverview.jsp. |
| 23. | Patel P, Ali H, Inayat F, Pamarthy R, Giammarino A, Ilyas F, Smith-Martinez LA, Satapathy SK. Racial and gender-based disparities and trends in common psychiatric conditions in liver cirrhosis hospitalizations: A ten-year United States study. World J Hepatol. 2023;15:289-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Mize TD, Doan L, Long JS. A General Framework for Comparing Predictions and Marginal Effects across Models. SM. 2019;49:152-189. [DOI] [Full Text] |
| 25. | Martín N, Li Y. Multiple comparison of trends in cancer rates taking into account overlapping cases. Underst Complex Syst. 2011;72:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 196] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 27. | Kubota D, Takahashi Y, Yamamichi N, Matsui M, Shimamoto T, Minatsuki C, Nakagawa H, Mizutani S, Tsuji Y, Sakaguchi Y, Tamura N, Yakabi S, Ohki D, Mizutani H, Niimi K, Wada R, Fujishiro M. Analysis of Barrett's Esophagus and Its Risk Factors: A Cross-Sectional Study of 10,122 Subjects at a Japanese Health Examination Center. Digestion. 2022;103:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Qumseya BJ, Bukannan A, Gendy S, Ahemd Y, Sultan S, Bain P, Gross SA, Iyer P, Wani S. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc. 2019;90:707-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Sharma N, Ho KY. Risk Factors for Barrett's Oesophagus. Gastrointest Tumors. 2016;3:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Eusebi LH, Telese A, Cirota GG, Haidry R, Zagari RM, Bazzoli F, Ford AC. Systematic review with meta-analysis: risk factors for Barrett's oesophagus in individuals with gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther. 2021;53:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Pelzner K, Fuchs C, Petersen M, Maus M, Bruns CJ, Leers JM. Sex- and gender-specific differences in symptoms and health-related quality of life among patients with gastroesophageal reflux disease. Dis Esophagus. 2024;37:doad064. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Xie M, Deng L, Fass R, Song G. Obesity is associated with higher prevalence of gastroesophageal reflux disease and reflux related complications: A global healthcare database study. Neurogastroenterol Motil. 2024;36:e14750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Lee SW, Lien HC, Chang CS, Chang CH, Ko CW, Yeh HZ. Differences of risk factors and clinical presentations in male and female Taiwanese individuals with Barrett's esophagus. J Chin Med Assoc. 2018;81:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Vaishnav B, Bamanikar A, Maske P, Reddy A, Dasgupta S. Gastroesophageal Reflux Disease and its Association with Body Mass Index: Clinical and Endoscopic Study. J Clin Diagn Res. 2017;11:OC01-OC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Zafar S, Haque IU, Tayyab GU, Rehman AU, Rehman AU, Chaudhry NU. Correlation of gastroesophageal reflux disease symptoms with body mass index. Saudi J Gastroenterol. 2008;14:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Steevens J, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, Goldbohm RA, van den Brandt PA. A prospective cohort study on overweight, smoking, alcohol consumption, and risk of Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2011;20:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 431] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 38. | Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett's oesophagus in women. Gut. 2009;58:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, Miller KK, Bredella MA. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 40. | Kendall BJ, Macdonald GA, Hayward NK, Prins JB, Brown I, Walker N, Pandeya N, Green AC, Webb PM, Whiteman DC; Study of Digestive Health. Leptin and the risk of Barrett's oesophagus. Gut. 2008;57:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Monardo A, McCullough J. Incidence of Dysplasia in Obese vs Nonobese Patients With Nondysplastic Barrett Esophagus. Ochsner J. 2019;19:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Friedenberg FK, Hanlon A, Vanar V, Nehemia D, Mekapati J, Nelson DB, Richter JE. Trends in gastroesophageal reflux disease as measured by the National Ambulatory Medical Care Survey. Dig Dis Sci. 2010;55:1911-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Cook MB, Shaheen NJ, Anderson LA, Giffen C, Chow WH, Vaughan TL, Whiteman DC, Corley DA. Cigarette smoking increases risk of Barrett's esophagus: an analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Smith KJ, O'Brien SM, Smithers BM, Gotley DC, Webb PM, Green AC, Whiteman DC. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 45. | Drahos J, Ricker W, Parsons R, Pfeiffer RM, Warren JL, Cook MB. Metabolic syndrome increases risk of Barrett esophagus in the absence of gastroesophageal reflux: an analysis of SEER-Medicare Data. J Clin Gastroenterol. 2015;49:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1056] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 47. | Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett's esophagus. Am J Epidemiol. 2005;162:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1149] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 49. | El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Arshad HMS, Farooq U, Cheema A, Arshad A, Masood M, Vega KJ. Disparities in esophageal cancer incidence and esophageal adenocarcinoma mortality in the United States over the last 25-40 years. World J Gastrointest Endosc. 2023;15:715-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (4)] |
| 51. | Banki F, DeMeester SR, Mason RJ, Campos G, Hagen JA, Peters JH, Bremner CG, DeMeester TR. Barrett's esophagus in females: a comparative analysis of risk factors in females and males. Am J Gastroenterol. 2005;100:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, Farahmand BY, Winchester CC, Roda E, Bazzoli F. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 53. | Roumans CAM, Zellenrath PA, Steyerberg EW, Lansdorp-Vogelaar I, Doukas M, Biermann K, Alderliesten J, van Ingen G, Nagengast WB, Karrenbeld A, Ter Borg F, Hage M, Ter Borg PCJ, den Bakker MA, Alkhalaf A, Moll FCP, Brouwer-Hol L, van Baarlen J, Quispel R, van Tilburg A, Burger JPW, van Tilburg AJP, Ooms AHAG, Tang TJ, Romberg-Camps MJL, Goudkade D, Bruno MJ, Rizopoulos D, Spaander MCW. Sex Differences in Neoplastic Progression in Barrett's Esophagus: A Multicenter Prospective Cohort Study. Cancers (Basel). 2022;14:3240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 54. | Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, Wani S. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117:559-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 242] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 55. | Weusten BLAM, Bisschops R, Dinis-Ribeiro M, di Pietro M, Pech O, Spaander MCW, Baldaque-Silva F, Barret M, Coron E, Fernández-Esparrach G, Fitzgerald RC, Jansen M, Jovani M, Marques-de-Sa I, Rattan A, Tan WK, Verheij EPD, Zellenrath PA, Triantafyllou K, Pouw RE. Diagnosis and management of Barrett esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2023;55:1124-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 56. | Haddad JD, Sawas T. Surveillance Intervals for Barrett’s Esophagus Need to be Changed-Surveillance Intervals are Too Long and Should be Shortened. Foregut: The Journal of the American Foregut Society. 2023;3:75-79. [DOI] [Full Text] |
| 57. | Eloubeidi MA, Provenzale D. Clinical and demographic predictors of Barrett's esophagus among patients with gastroesophageal reflux disease: a multivariable analysis in veterans. J Clin Gastroenterol. 2001;33:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Rubenstein JH, McConnell D, Waljee AK, Metko V, Nofz K, Khodadost M, Jiang L, Raghunathan T. Validation and Comparison of Tools for Selecting Individuals to Screen for Barrett's Esophagus and Early Neoplasia. Gastroenterology. 2020;158:2082-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Chien S, Glen P, Penman I, Cruickshank N, Bryce G, Crumley A, Phull P, Miller M, Fletcher J, Gunjaca I, Apollos J, Robertson K, Fullarton G. Oesophageal cell collection device and biomarker testing to identify high-risk Barrett's patients requiring endoscopic investigation. Br J Surg. 2024;111:znae117. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Sijben J, Rainey L, Peters Y, Fitzgerald RC, Wani S, Kolb JM, Broeders MJM, Siersema PD. Dutch, UK and US professionals' perceptions of screening for Barrett's esophagus and esophageal adenocarcinoma: a concept mapping study. BMC Cancer. 2023;23:1111. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Vantanasiri K, Kamboj AK, Kisiel JB, Iyer PG. Advances in Screening for Barrett Esophagus and Esophageal Adenocarcinoma. Mayo Clin Proc. 2024;99:459-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 62. | Kirtane T, Parasa S. The Role of Artificial Intelligence in Surveillance in Barrett’s Esophagus and Gastric Intestinal Metaplasia. Foregut: The Journal of the American Foregut Society. 2023;3:121-127. [DOI] [Full Text] |
| 63. | Tsai MC, Yen HH, Tsai HY, Huang YK, Luo YS, Kornelius E, Sung WW, Lin CC, Tseng MH, Wang CC. Artificial intelligence system for the detection of Barrett's esophagus. World J Gastroenterol. 2023;29:6198-6207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Bouzid K, Sharma H, Killcoyne S, Castro DC, Schwaighofer A, Ilse M, Salvatelli V, Oktay O, Murthy S, Bordeaux L, Moore L, O'Donovan M, Thieme A, Nori A, Gehrung M, Alvarez-Valle J. Enabling large-scale screening of Barrett's esophagus using weakly supervised deep learning in histopathology. Nat Commun. 2024;15:2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 65. | Zhang H, Kahn A. Developing the Ideal Barrett’s Esophagus Screening Program: Is it Time for Universal Screening? Foregut: The Journal of the American Foregut Society. 2023;3:107-113. [DOI] [Full Text] |
| 66. | Zilberstein N, Godbee M, Mehta NA, Waxman I. Advanced endoscopic imaging for detection of Barrett's esophagus. Clin Endosc. 2024;57:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 67. | Patil DT, Odze RD. Barrett's Esophagus and Associated Dysplasia. Gastroenterol Clin North Am. 2024;53:1-23. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Corley DA, Kubo A, DeBoer J, Rumore GJ. Diagnosing Barrett's esophagus: reliability of clinical and pathologic diagnoses. Gastrointest Endosc. 2009;69:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Jacobson BC, Gerson LB. The inaccuracy of ICD-9-CM Code 530.2 for identifying patients with Barrett's esophagus. Dis Esophagus. 2008;21:452-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Jiang Y, Damiris K, Ahmed AM, Chowdhury S, Xu B, Ahlawat S. S3143 Impact of Barrett’s Esophagus on Inpatient Outcomes in Patients Hospitalized With Atrial Fibrillation. Am J Gastroenterol. 2020;115:S6-S7. [DOI] [Full Text] |
| 71. | Chung H, Farraj K, Malik A, Amine M, Suliman I, Nadir A. S484 Barrett's Esophagus in the Asian Population: A National Cohort Study. Am J Gastroenterol. 2023;118:S352-S353. [DOI] [Full Text] |
| 72. | Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality. [cited May 15, 2024]. Available from: https://hcup-us.ahrq.gov. |