Published online Dec 20, 2024. doi: 10.5662/wjm.v14.i4.95904
Revised: June 20, 2024
Accepted: July 3, 2024
Published online: December 20, 2024
Processing time: 95 Days and 11.8 Hours
End stage liver disease (ESLD) represents a growing health concern characterized by elevated morbidity and mortality, particularly among individual ineligible for liver transplantation. The demand for palliative care (PC) is pronounced in patients grappling with ESLD and acute on chronic liver failure (ACLF). Unfortunately, the historical underutilization of PC in ESLD patients, despite their substantial needs and those of their family caregivers, underscores the imperative of seamlessly integrating PC principles into routine healthcare practices across the entire disease spectrum.
To comprehensively investigate the evidence surrounding the benefits of inco
A systematic search in the Medline (PubMed) database was performed using a predetermined search command, encompassing studies published in English without any restrictions on the publication date. Subsequently, the retrieved studies were manually examined. Simple descriptive analyses were employed to summarize the results.
The search strategies yielded 721 references. Following the final analysis, 32 full-length references met the inclusion criteria and were consequently incorporated into the study. Meticulous data extraction from these 32 studies was undertaken, leading to the execution of a comprehensive narrative systematic review. The review found that PC provides significant benefits, reducing symptom burden, depressive symptoms, readmission rates, and hospital stays. Yet, barriers like the appeal of transplants and misconceptions about PC hinder optimal utilization. Integrating PC early, upon the diagnosis of ESLD and ACLF, regardless of transplant eligibility and availability, improves the quality of life for these patients.
Despite the substantial suffering and poor prognosis associated with ESLD and ACLF, where liver transplantation stands as the only curative treatment, albeit largely inaccessible, PC services have been overtly provided too late in the course of the illness. A comprehensive understanding of PC's pivotal role in treating ESLD and ACLF is crucial for overcoming these barriers, involving healthcare providers, patients, and caregivers.
Core Tip: This systematic review addresses the underexplored utilization of palliative care (PC) in patients with end stage liver disease (ESLD) and acute on chronic liver failure (ACLF), a demographic traditionally underserved. ESLD and ACLF are characterized by grim prognoses, substantial care costs, explicit patient suffering, and elevated mortality rates. Despite liver transplantation (LT) being a curative option, accessibility remains severely limited due to barriers such as donor scarcity, financial constraints, and inadequate social support. Even among those eligible for transplantation, a significant majority of ESLD patients are referred late for PC, typically within their final couple of weeks of life. PC offers notable benefits, including amelioration of symptom burden, reduced depressive symptoms, lower readmission rates, and shorter hospital stays. However, optimal utilization of PC faces barriers such as the allure of transplants and misconceptions about PC. A comprehensive understanding of the pivotal role of PC in ESLD and ACLF treatment is crucial for all stakeholders, including healthcare providers, patients, and caregivers, to overcome these barriers. Future prospective randomized studies, irrespective of LT eligibility, are needed to strengthen the evidence supporting early integration of PC in the management of ESLD/ACLF patients.
- Citation: Mafi VIP, Soldera J. Palliative care for end-stage liver disease and acute on chronic liver failure: A systematic review. World J Methodol 2024; 14(4): 95904
- URL: https://www.wjgnet.com/2222-0682/full/v14/i4/95904.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i4.95904
Patients suffering from decompensated liver cirrhosis or end-stage liver disease (ESLD) manifest a diverse prognosis, which has demonstrated improvement since the early 2000 s. This positive trend is ascribed to an estimated reduction of around 15% in mortality rates. The decline in mortality is a consequence of the elucidation and comprehension of acute on chronic liver failure (ACLF) and the increased accessibility of emergency liver transplantation (LT) for these patients[1].
ACLF, as expounded in a widely acknowledged prospective observational study encompassing 1343 cirrhosis cases known as ACLF in cirrhosis, delineates a syndrome characterized by the presence of acutely decompensated cirrhosis (DC) or ESLD, intertwined with the failure of multiple organs. This is accompanied by a noteworthy short-term mortality rate of ≥ 15% within 28 days[2].
The classification of ACLF is predicated on the extent of organ failure and the corresponding prognosis. This classification includes grade -1 (involving one organ failure, with a 28-day mortality rate of 22%), grade 2 (entailing two organ failures, with a 28-day mortality rate of 32%), and grade 3 (involving three or more organ failures, with a 28-day mortality rate of 73%). Moreover, patients with acute decompensation (AD) classified as non-ACLF exhibit an overall short-term mortality rate of 1.9%[2-5].
It is noteworthy that approximately half of the patients diagnosed with ACLF lack a previous history of DC. However, these patients manifest a more severe manifestation of the disease compared to those with a prior history of decompe
The contemporary understanding of the pathophysiological mechanisms underlying ACLF revolves around systemic inflammation as the cornerstone of its development. In contrast, ESLD or AD is considered a progression from its early asymptomatic state, termed compensated cirrhosis[1,2]. LT emerges as the exclusive curative option for individuals afflicted with either ESLD or ACLF[4]. However, the clinical implementation of this therapeutic intervention encounters various limitations attributable to factors such as elevated costs, which disproportionately impact developing nations, a scarcity of available donors, immunological rejection, disease or malignancy progression while on the transplant waiting list, active substance misuse, septic conditions, and involvement of extrahepatic organs[4].
Each patient diagnosed with ESLD and ACLF should undergo a comprehensive assessment for LT because of the significantly heightened 90-day mortality rates exceeding 20%. This imperative evaluation serves as a critical determinant in the overall management and decision-making process for patients grappling with these severe liver conditions. The recognition of these multifaceted factors underscores the complex landscape surrounding LT, necessitating a nuanced and comprehensive approach to address the diverse challenges associated with this life-saving procedure[1,2,4].
Patients diagnosed with ACLF grade 2 and grade 3 require immediate LT, given the substantial risk of short-term mortality, which escalates to 57% and 87%, respectively[2-5]. The attending physician might also consider a palliative care (PC) consultation in such a grim scenario. Historically, the utilization of PC services in managing patients with ESLD and ACLF has been found to be inadequate, primarily attributed to either sheer ignorance or a failure to comprehend the full spectrum of the underlying liver condition's progression[1-3].
PC constitutes an interdisciplinary medical approach that places emphasis on delivering the highest quality of life by optimizing symptom control and providing comprehensive psychosocial, spiritual, and practical support to both patients and their caregivers[3,4,6]. In the specific context of ESLD and ACLF, PC emerges as an alternative therapeutic avenue for patients deemed unsuitable for LT. The integration of PC into the overall management strategy acknowledges the multifaceted needs of these patients, addressing not only the physical symptoms but also the broader aspects of their well-being, ensuring a holistic and patient centered approach to care[2-5].
The intrinsic unpredictability that characterizes the trajectory of ESLD and ACLF underscores the rationale for the early integration of PC into the overall management framework. This proactive approach serves to alleviate the symptomatic toll associated with the condition, acknowledging the dynamic and evolving nature of ESLD and ACLF[3,4]. It is imperative that all patients grappling with ESLD and ACLF receive timely referral for PC consultation, encom
The challenges associated with providing PC services to ESLD and ACLF patients are multifaceted. These challenges include disparities in PC accessibility among individuals from low socioeconomic strata, insufficient referrals to PC services owing to the fluctuating course of ESLD, misconceptions regarding the role of PC, and an inability to pinpoint clinical triggers for PC consultation, such as the emergence of ascites and complications stemming from portal hypertension[3,4,6]. In ESLD patients awaiting LT, marked relief in symptom burden through the strategic integration of PC referrals within the routine outpatient assessment for those awaiting LT has been demonstrated[6].
This systematic narrative review delves into evidence-based literature, critically examining the advantages of integrating PC into the comprehensive management of patients with ESLD and/or ACLF. Through a meticulous analysis of the driving forces yielding heightened quality of life and the clinical justification for PC consultation referrals within this patient cohort, this review aims to contribute valuable insights to the evolving landscape of ESLD and ACLF management.
This narrative systemic review was meticulously conducted in strict adherence to the rigorous guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[7,8].
In formulating the search strategy, the command employed was as follows: ("end-stage liver disease" OR "ESLD" OR "cirrhosis" OR "acute on-chronic liver failure" OR "liver transplantation" OR "ACLF") AND ("palliative care" OR "end-of-life care" OR "hospice care" OR "palliative medicine" OR "comfort care"). The searches were conducted in September 2023, utilizing the electronic databases Medline (PubMed) and Google Scholar.
No constraints were placed on the date of publication, facilitating a thorough exploration of both historical and contemporary literature. However, language restrictions were implemented, confining the search to studies published in the English language. The inclusion of studies in the review adhered to the predefined Patients, Intervention, Comparison, Outcome criteria, ensuring a methodologically rigorous selection process.
For this systematic review, studies focusing on patients with ESLD exhibiting evidence of at least one of the following criteria were considered for inclusion: Liver cirrhosis accompanied by a clinical manifestation of decompensation; liver cirrhosis designated for LT; liver cirrhosis undergoing evaluation for LT; liver cirrhosis characterized by a Child-Turcotte-Pugh (CTP) classification predominantly falling within category B or C within the study's cohort (Child A ≤ 40%, or mean/median CTP score > 7); patients depicted with chronic and advanced liver ailments referred for LT evaluation.
On the other hand, studies were excluded if they were composed in languages other than English, involved parti
The initial screening of studies retrieved through the search command, focusing on titles and abstracts, aimed to discern papers aligned with the predefined inclusion criteria. It was imperative to exclude studies that did not adhere to the inclusion criteria, particularly those categorized as reviews and guidelines. This rigorous screening process ensured the selection of studies that distinctly met the criteria, fostering precision and relevance in the ensuing stages.
Following the preliminary screening, the abstracts of the short-listed studies underwent a comprehensive review to ascertain their potential relevance to the research objectives. This step involved a meticulous evaluation to further refine the selection, considering the depth and specificity of each study in relation to the research focus.
The final screening phase involved a detailed assessment of the full-length papers, emphasizing the meticulous elimination of studies that did not align with the predefined inclusion criteria. This thorough scrutiny aimed to uphold the scientific rigor of the study by ensuring that the selected papers contribute meaningfully to the research objectives.
Subsequently, a comprehensive analysis of each eligible study was conducted, facilitating the standardized extraction of essential data encompassing population characteristics, study design, structural elements, results, and a concise summary. This systematic data extraction process served as a foundation for the subsequent quantitative and qualitative analysis.
The extracted data was meticulously tabulated to facilitate a structured and organized representation, enabling the identification of emerging trends and patterns within the selected studies.
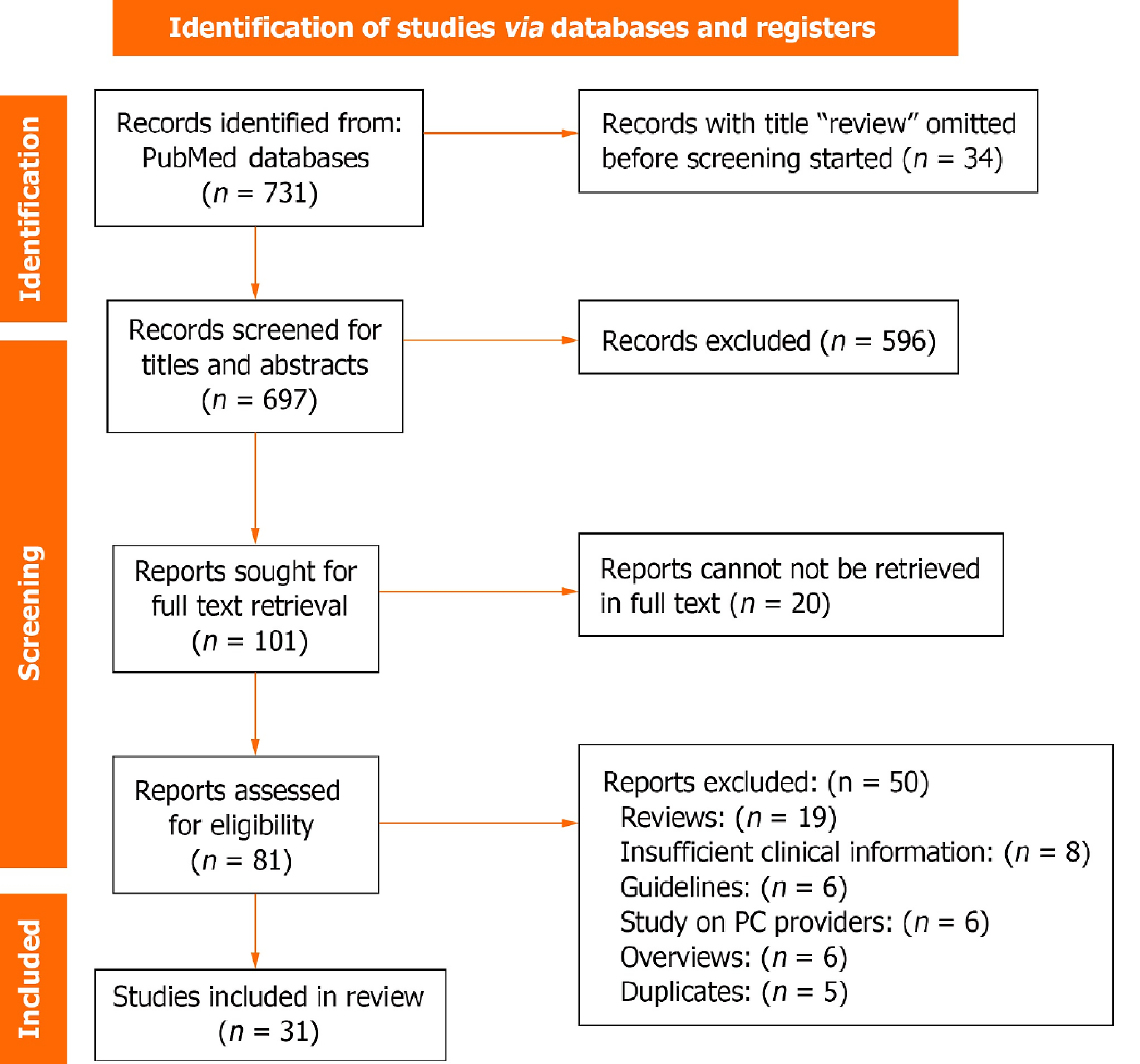
The search strategy yielded a total of 731 references. To refine the pool of potential studies, 34 records identified as reviews during the title review stage were promptly excluded, leaving 697 records for subsequent titles and abstracts screening. During this phase, adhering strictly to the predefined inclusion criteria, 101 records emerged as eligible, advancing to the next stage for full-text retrieval. Notably, 591 records were excluded during the title and abstract screening due to a lack of alignment with the inclusion criteria.
As part of the comprehensive evaluation, full-text retrieval was conducted for the 101 eligible records and 20 records were excluded at this stage due to unavailability in full text, leaving 81 reports for in-depth analysis. Within this stage, a rigorous application of the inclusion criteria led to the exclusion of 50 papers, ensuring the final selection of studies closely aligned with the research objectives.
The subsequent data extraction phase involved 31 studies, the detailed outcomes of which are presented in Table 1. To provide a visual representation of the search strategy, a PRISMA flow diagram, depicted in Figure 1, elucidates the sequential stages of study selection and exclusion. This graphical representation serves as a transparent and comprehensive overview of the search process.
| Ref. | Population | Study design | Structure | Results | Conclusion |
| Bauman et al[6], 2015 | 79 ESLD patients referred for LT evaluation or awaiting transplant between July 2013 and May 2014 | Longitudinal, multidisciplinary, EPCI for one week pre-transplant evaluation with outpatient consultations following national guidelines. GOC discussed with patients and families until October 2014 | Patients underwent EPCI with formal assessments of depression, liver-specific symptoms, psychosocial well-being, and spiritual health | Post-EPCI, there was a significant 50% reduction in symptom burden (P < 0.05) and a 43% decrease in depressive symptoms (P = 0.003). Patients with moderate to severe symptoms showed greater improvement. Assessment tools included ESAS, EPCI, and quality of life | Palliative care is underused in ESLD due to a lack of suitable distress assessment tools. EPCI effectively improves symptom burden and mood in ESLD patients awaiting transplant |
| Shinall et al[63], 2019 | Patients with ESLD admitted to an urban, academic referral center. Total patients: 398293 | RCT | Inclusion criteria involved ESLD diagnosis and expectation of potential mortality within 1 year. Patients were randomized into usual care or PC intervention groups with primary and secondary outcome assessments over 6 months | Among 293 eligible patients, 63 were enrolled in the RCT (31 in intervention, 32 in control). PC intervention showed reduced readmission risk (HR = 0.36, P = 0.017) and more days alive outside the hospital (OR = 3.97, P = 0.030) compared to control | PC demonstrated extended time before readmission and increased days alive outside hospital, highlighting its benefit for ESLD patients |
| Gupta et al[10], 2022 | Patients admitted with decompensated alcohol-associated cirrhosis who received PC consultation | Retrospective multicenter observational study | Analysis included 78 million discharged patients (2007-2014) from national databases with ESLD diagnosis criteria including alcoholic cirrhosis or other cirrhosis with alcohol disorder and decompensation events | Out of 1,421,849 hospitalized ESLD patients, 62782 received PC. Factors like advanced age, lower income, Medicaid coverage, urban location, prolonged hospital stay, and ventilation increased odds of receiving PC. Patients treated in facilities with PC services had lower 30-day readmission rates | Increasing inpatient PC consultation for decompensated ArLD correlates with reduced 30-day readmissions, shorter hospital stays, and lower costs |
| Poonja et al[11], 2014 | Adults with ESLD who were removed from or declined LT between January 2005 and December 2010 (n = 102) | Retrospective observational study | Primary outcome focused on DC patients referred and receiving PC Secondary outcomes included time from LT decline to death, rehospitalizations, ICU admissions, and place of death | Common reasons for LT removal or decline included noncompliance/substance abuse (26%) and severe illness/organ dysfunction (25%). Among delisted patients, 17% required renal replacement therapy, 48% had ICU admissions (median 14 days), but only 11% were referred for PC | Patients with DC declined or delisted from LT have low rates of PC referral (11%) and unclear GOC. Collaboration between LT and PC services can enhance quality of life for this patient group |
| Patel et al[25], 2017 | Hospitalized adults with terminal DC (ESLD). Total patients: 59887 | Cross-sectional observational study patient cohort | ESLD patients were identified using ICD-9 codes. Main outcomes included PC consultation rates during terminal hospitalization and total incurred costs, alongside demographic and comorbidity data | 29.1% of hospitalized ESLD adults received PC with an average cost of $49167. Urban residents had higher PC rates, while African Americans, Hispanics, and Asians had lower rates (racial disparity). PC was associated with reduced procedure burden and cost savings of $8892 | PC consultation during terminal hospitalization for ESLD is linked to cost reduction and lower procedure burden, despite racial disparities in PC access |
| Ufere et al[26], 2019 | Hepatologists and gastroenterologists (396) providing care for patients with ESLD | Cross-sectional observational study patient cohort | Survey of AASLD members assessing barriers to PC and ACP delivery in ESLD patients | Most respondents (95%) cited cultural factors as a barrier to PC delivery, followed by unrealistic expectations (93%) and time constraints (91%). ACP barriers included communication issues (84%) and lack of cultural competency training (81%) | Substantial barriers hinder PC and ACP services for ESLD patients according to hepatologists and gastroenterologists. Strategies are needed to improve timely and high-quality end-of-life care for these patients |
| Patel et al[27], 2021 | Study included 88 participants: 46 LT center physicians and 42 decompensated cirrhotic patients from 3 LT centers | Qualitative study using face-to-face semi-structured interviews | Interviews conducted with ESLD patients meeting specific criteria (consent, age 18 +, English proficiency, cirrhosis diagnosis, and specific symptoms) and clinicians (transplant hepatologists, surgeons, coordinators, social workers) exploring aspects of ACP | Study identified five themes related to ACP experiences: (1) Patients often considered values and preferences outside of clinical visits; (2) Optimism from transplant teams hindered discussions about dying; (3) Clinicians used discussions about death to encourage behavioral change; (4) Transplant teams avoided discussing nonaggressive treatment options; and (5) Surrogate decision-makers lacked preparation for end-of-life decisions | Decompensated cirrhotic patients lack adequate ACP throughout their illness trajectory, leading to overly aggressive end-of-life treatments. This highlights the need for improved ACP practices in this population |
| Lin et al[12], 2020 | Total of 903 patients: 214 from Wang Fang Hospital and 689 from Taipei Medical University | Retrospective cohort study | Inclusion criteria: Adults (> 18 years) with CLD and specific conditions, with EMR data available within 24 hours of admission. Exclusion criteria included pregnancy, cancer, and LT history | ESLD patients were categorized into acute death (within 30 days), PC (death within 1-9 months), and survived. Overall mortality rates were 283% in the training set and 22.6% in the validation set | Machine learning monitoring systems offer a comprehensive approach to assessing ESLD patient conditions. Supervised machine-learning models demonstrate superior predictive performance compared to traditional statistical methods |
| Vieira da Silva et al[22], 2023 | ESLD patients presenting to a university hospital and LT center from November 2019 to September 2020 | Prospective observational single-center study | Study excluded patients with previous LT, isolated acute liver failure, or another terminal disease (except hepatocellular carcinoma, HCC). PC criteria screening used the NECPAL CCOMS-ICO tool to identify patients needing PC and predicting mortality. Specific PC needs were assessed using the IPOS questionnaire for symptoms and mood | Among 54 patients, 9.3% were on the LT waiting list and 14.8% were under evaluation. NECPAL CCOMS-ICO identified 42.6% needing PC. Clinicians identified functional markers and comorbidities (47.8%) as indicating PC needs. IPOS identified multiple needs, with weakness (77.8%), reduced mobility (70.3%), and pain (48.1%) being prominent | All ESLD patients, including those awaiting transplantation, demonstrated significant PC needs. This highlights the importance of addressing PC needs across all stages of ESLD care |
| Beck et al[28], 2016 | Survey conducted among 200 LT patients | Qualitative survey study | Web-based survey evaluating attitudes of LT providers and barriers to PC for patients. Examined variables included provider attitudes toward symptom management and documentation practices | Response rate was 44% (88/200). Providers agreed that LT and PC are not mutually exclusive (86%). Most suggested PC referral when death is imminent (78%). Many providers recognized patient depression (66%), but fewer consulted PC for depression (28%). Attending physicians were identified as the main barrier to involving PC (84%) | PC in LT patients a ligns with LT goals even during listing. Barriers include confusion over referral criteria |
| Puentes et al[13], 2018 | Hospitalized patients with hepatic cirrhosis between January 2015 and December 2016, classified using the Child–Pugh score and MELD/Na score in January 2018 | Retrospective study | Child–Pugh score assessed cirrhosis prognosis using specific parameters (TB, Bil, Albumin, PT, ascites, encephalopathy). MELD/Na score predicted survival based on Na, Cr, Bil, and INR | Patients were classified into Child–Pugh Class A (17%), Class B (48.9%), and Class C (34%). MELD/Na scores ranged > 9 (2.15%), 10–19 (46.8%), 20–29 (27.7%), 30–40 (19.1%), and > 40 (4.3%). About 51.1% had MELD/Na > 20. The study showed 59.6% of patients needing PC died within 12 months | Child–Pugh and MELD/Na scores are valuable tools to identify PC needs in liver cirrhosis patients. Early identification and comprehensive care improve quality of life by addressing physical, spiritual, family, and social needs |
| Hudson et al[14], 2017 | Study included all emergency admissions with a cirrhosis diagnosis over two distinct 90-day periods (n = 83) | Retrospective study | Patients were assessed for five criteria independently scored by clinicians: (1) Child-Pugh score C; (2) ≥ 2 admissions within last 6 months; (3) Ongoing alcohol use with ArLD; (4) Unsuitability for LT; (5) WHO Performance status 3 or 4. One-year mortality was calculated using cumulative prognostic score out of 5 | Analysis of 73 admissions (79.5% male, 63% ArLD, median age 54) showed that presence of ≥ 3 poor-prognosis criteria predicted 1-year mortality with sensitivity, specificity, and positive predictive value of 72.2%, 83.8%, and 81.3%, respectively. This triggered implementation of supportive care interventions | Identifying high-risk cirrhosis patients using specific criteria allows for early implementation of supportive care alongside active management to improve outcomes |
| Lamba et al[21], 2011 | Study included 79 LT patients and 104 ESLD patients (n = 183) | Prospective, observational pre/post study | Interdisciplinary PC model integrated into LT service and surgical intensive care unit, focusing on family support, prognosis, and patient preferences (Part I) and interdisciplinary family meetings (Part II) within 72 hours | Baseline group (LT patients) had 21 deaths, and intervention group had 31 deaths. 85% received Part I and 58% received Part II of the intervention. GOC discussions on physician rounds increased significantly during the intervention. Do not resuscitate status increased from 52% to 81%, and withdrawal of life support increased from 35% to 68 | Early integration of PC alongside curative care in surgical intensive care unit can improve EOL practices without changing mortality rates in LT patients. Interdisciplinary communication interventions facilitate consensus on GOC for dying LT patients |
| Medici et al[15], 2008 | 157 ESLD patients admitted to hospice service | Observational retrospective analysis | Recorded patient details included age, gender, main diagnosis, comorbidities, length of hospice stay, and development of ESLD-specific complications during hospice stay (e.g., gastrointestinal bleeding, hepatic encephalopathy, tense ascites with dyspnea). MELD scores were computed at hospice entry and eventual LT | Most patients were male (67.5%) with a mean age of 57 years. Common ESLD causes were alcohol cirrhosis, hepatitis C virus infection, or both. HCC complicated 28% of cases. Common comorbidities included diabetes (18.2%), vasculopathy/hypertension (16.3%), kidney failure (11.3%), and chronic obstructive pulmonary disease (4.4%). A majority (78%) of patients died during the observation period | MELD scores can guide clinician recommendations for hospice care, aiming to increase duration beyond the current median of 2-3 weeks. Hospice referral for high MELD score patients is an effective strategy to enhance care for ESLD patients awaiting transplantation |
| Deng et al[16], 2021 | 233 adult cirrhosis patients evaluated for LT out patiently from July 1, 2019 to September 30, 2019 | Observational retrospective analysis | Patients excluded if severe hepatic encephalopathy or non-English/Spanish speakers. Frailty assessed using liver frailty index. Symptom scores ≥ 4 triggered comprehensive assessment. ESAS score ≥ 7 indicated high symptom burden and decreased functioning. Demographic and clinical data (etiology, HCC, ascites) collected from medical records | Median age 61 years, 43% female. Frailty distribution: 22% robust, 59% pre-frail, 19% frail 38% reported ≥ 1 severe symptom (based on ESAS). Higher frailty associated with increased prevalence of pain, dyspnea, fatigue, nausea, poor appetite, drowsiness, depression, and poor well-being (all P < 0.05) | Frailty strongly linked to physical/psychological symptoms (pain, depression) and poor quality of life in cirrhosis patients. Frail cirrhosis patients may benefit from PC co-management to address symptoms and improve quality of life |
| Jung et al[72], 2020 | 101 patients with liver disea | Retrospective descriptive survey analyzing decision-making practices and outcomes of life-sustaining treatment in ESLD patients with POLST | Case report form based on POLST and EMR, including patient characteristics, POLST details, life-sustaining treatment regimen, treatment outcomes, and withdrawals | 63 patients (62.4%) completed their own POLST; 3 patients withdrew (2 for LT, 1 for chemotherapy). Majority were male (81.2%) with an average age of 61.8 years | Emphasizes the importance of considering LT for ESLD patients before deciding on life-sustaining treatment. Supports patient self-determination and highlights the need for effective guidelines in this context |
| Kathpalia et al[17], 2016 | The study included 683 adult cirrhotic patients listed for LT at a large United States center from 2013 to 2014 | Descriptive retrospective study | The study included adult cirrhotic patients newly listed for LT who either died before transplant or were too ill for transplant. Patients with certain exclusion criteria were not included. Patient demographics, disease etiology, MELD score, education level, Child-Pugh Score, presence of HCC, and insurance type were recorded from EMR | Among the 683 Listed patients, 16% (107) either died (62 patients) or were removed due to clinical decompensation (45 patients) before receiving a LT. The median age was 58 years, and most patients were male (66%), Caucasian (53%), and had Child C cirrhosis (61%) or hepatocellular carcinoma (52%) | PC services are underutilized in older, non-white patients with cirrhosis awaiting LT. Early integration of PC into transplant decision-making is recommended |
| Adejumo et al[69], 2020 | The study included 67480 hospitalized patients with ESLD | Retrospective longitudinal analysis of ESLD patients hospitalized from January 2010 to January 2014 | The study used a database with a 90-day follow-up after discharge, matching patients who received PC to those who did not (1:1) using propensity scores | Among the ESLD hospitalizations, 5.3% (3485) received PC, showing an annual increase from 3.6% to 6.7%. Average 30- and 90-day readmission rates were 362% and 54.6%, respectively. PC was associated with a lower risk of 30- and 90-day readmission | PC was linked to reduced readmission rates, potentially lessening the burden on healthcare resources and improving cost savings during subsequent readmissions |
| Low et al[68], 2017 | The study involved 66 individuals with cirrhosis who died between April 2010 and September 2011, and 22 liver health professionals who participated in focus groups or interviews | This study used mixed methods, including retrospective case note review, qualitative focus groups, individual interviews, and qualitative focus group discussions | Researchers selected 30 out of the 66 deceased individuals with cirrhosis for data collection from their records in the 12 months before death. Additionally, semi-structured interviews and focus groups were conducted with health professionals involved in care | The study highlighted high rates of hospital admissions and symptom burden among participants with cirrhosis. Clinicians demonstrated reluctance to discuss prognosis or future care preferences due to lacking skills and confidence, resulting in delayed provision of PC | People with cirrhosis experience unpredictable disease trajectories marked by frequent hospitalizations and worsening symptoms nearing death. The study recommends implementing clinical tools to identify irreversible deterioration and fostering collaboration between liver services and PC to enhance care quality |
| Walling et al[58], 2015 | The study compared 49 hospitalized veterans to 61 pre-quality improvement project veterans | This was a comparative study that retrospectively evaluated PC consultation rates among care management recipients compared to a prospective cohort of LT identified using the same ICD-9 codes and screening criteria over a one-year period | Veterans with cirrhosis were identified using specific ICD-9 codes and screened for ESLD based on medical records at a VA hospital. A care coordinator followed veterans from hospitalization through April 2013, encouraging LT evaluation consults for those with a MELD ≥ 14 and PC consults for those with a MELD ≥ 20 or inoperable HCC | During the intervention period, hospitalized veterans were more likely to be considered for LT (77.6% vs 31.1%, P < 0.001) and to receive PC consultation, though the latter finding did not reach statistical significance (62.5% vs 47.1%, P = 0.38) | Active case finding increased consideration for LT without reducing PC consultation rates |
| Donlan et al[29], 2021 | The study involved ESLD patients and their informal caregivers | This was a qualitative study involving semi-structured interviews with 15 ESLD patients and 14 informal caregivers | Interviews were conducted by a team including a gastroenterologist, study coordinator, psychologist, and oncologist, aiming to explore participants' perceptions of PC and when it should be introduced to ESLD patients | Transplant-listed patients were concerned that PC referral might impact their chance of receiving a LT. Most participants felt that ESLD patients should learn about PC soon after diagnosis | Study participants often equated PC with hospice care initially. However, after receiving education on PC, nearly all participants, including transplant-eligible and ineligible patients, supported the early introduction of PC in ESLD care |
| Shinall et al[9], 2022 | The study included 24 participants from 11 institutions across the United States and Canada who participated in three focus groups | The study involved qualitative analysis of transcripts from provider focus groups followed by a community engagement studio with patients and caregivers | Three Zoom focus groups were conducted with hepatologists and PC specialists using open-ended questions. Qualitative data coding and analysis were performed following COREQ guidelines by the Vanderbilt University Qualitative Research Core, led by a PhD-level psychologist | The focus groups identified elements of specialist PC beneficial for LT patients. They also highlighted barriers to integrating PC, such as role boundaries, differences in clinical cultures, limited time and staff, and competing goals and priorities | Hepatologists, PC specialists, patients, and caregivers identified key barriers in LT patient care that specialist PC could address, including role boundaries, clinical culture differences, limited resources (time and staff), and conflicting priorities |
| Tombazzi et al[62], 2022 | The study included all patients discussed in the LTC at an academic medical center in the United States between August 2018 and May 2020, totaling 769 patients | This was a retrospective descriptive study followed by cohort analysis | Out of 135 patients declined for LT, 37 (27%) received a referral to PC. Data collected included baseline demographics, MELD score, decompensation events, and reasons for transplant ineligibility. Primary outcome was PC referral, and secondary outcomes included survival from LTC decision, time from LTC decision to PC referral, and code status at PC referral | Among 769 patients discussed at LTC, 135 were declined for transplantation, and 37 (27%) received a PC referral. Patients with higher MELD scores (21-30 and > 30) had significantly higher odds of PC referral compared to those with MELD score < 20 | Only a minority (27%) of patients declined or delisted for LT were referred to PC. MELD score and degree of decompensation were important factors associated with PC referral. Further exploration of this data is needed to inform future studies and establish criteria and timing for PC referral |
| Holden et al[73], 2020 | The study included 397 patients with DC admitted to Indiana University Hospital in 2012 | Retrospective cohort study | Patients were identified using EMR and confirmed for cirrhosis through manual chart review. Exclusions were patients under 18 years and those with prior LT. Primary outcome: Referral to PC. Secondary outcomes included hospitalization duration, medical interventions, code status limitations, LT, and mortality | Among 397 patients, 61 (15.4%) were referred to PC, 71 (17.9%) to hospice, and 99 (24.9%) to PC and/or hospice. Within one year, 50.4% of patients died. Referrals to PC and hospice were predominantly late, with 68.5% and 62.7% respectively | PC and hospice services are underutilized for patients with DC, with most referrals occurring late. Referral to PC was associated with increased comorbidity, while hospice referral was associated with greater comorbidities according to multivariable logistic regression |
| Peng et al[65], 2020 | The study included all adult patients with ESLD who died during hospitalization from 2010 to 2013 in Taiwan (n = 14,247) | Retrospective cohort study using the National Health Institute Research Database, adhering to STROBE guidelines | The study focused on ESLD patients aged 18 and older who died during hospitalization. Primary outcome was ICU admission during terminal hospitalizations. Secondary outcomes were cardiopulmonary resuscitation (CPR) and mechanical ventilation during terminal hospitalizations | Among ESLD patients, 60.8% had comorbid HCC. Patients without HCC were less likely to receive PC before terminal hospitalization compared to those with HCC. Those without HCC had higher rates of ICU admission, CPR, and mechanical ventilation during terminal hospitalization | Patients with ESLD not comorbid with HCC require more attention regarding PC needs and decision-making for intensive care utilization. Prior PC was associated with reduced probability of ICU admission |
| Patel et al[23], 2020 | The study included 167 veterans newly diagnosed with ESLD in 2012 at the Greater Los Angeles Healthcare System using the VA Corporate Data Warehouse | Prospective cohort study | Veterans were selected using ICD-9 codes for cirrhosis and liver decompensation. Different sampling techniques were applied to identify patients with ESLD using ICD-9 codes and chart abstraction | Among the identified patients, 62 met ESLD criteria after chart abstraction. The majority were male (98%), with a mean age at diagnosis of 61 years, and 74% were White. The quality indicator pass rate was 68%. Patients who received PC consultations were more likely to receive information care planning quality indicator | The study highlights inadequate quality of PC in veterans with ESLD. Patients who received specialty PC consultations and those affected by homelessness, drug, and alcohol abuse received better care. Combination of ICD-9 codes can effectively identify patients with ESLD |
| Orman et al[24], 2022 | The study included 679 adults (age ≥ 18) with cirrhosis admitted to Indiana University Hospital between June 2014 and July 2019 who received PC | Prospective cohort study | Patients were followed from admission through 90 days post-discharge to assess outcomes. PC consults were considered EOL care for patients with imminent in-hospital death Primary outcome was unplanned 30-day readmission. Secondary outcomes included hospital length of stay, intensive care utilization, inpatient costs, discharge medications, and 90-day post-discharge mortality and costs | Among 679 patients, 74 received PC, typically later in their hospitalization. Patients receiving PC had higher Charlson comorbidity index and greater impairments in activities of daily living, social activity, and quality of interactions | PC is underutilized and often initiated late in patients with severe liver disease and functional impairment. PC may reduce healthcare utilization and increase completion of advanced directives. RCTs are needed to further evaluate PC for this population |
| Thandassery et al[18], 2022 | The study compared patients with COVID-19 and cirrhosis (Group A, n = 1969) vs those with COVID-19 alone (Group B, n = 169257) | The study retrospectively analyzed a global multicenter database to assess mortality risk and PC referrals in patients with COVID-19 and cirrhosis | Data from 50 healthcare organizations worldwide were analyzed using a federated cloud-based network (TriNetX). Patients aged 18 to 90 years with COVID-19 were identified between January 20, 2020, and November 16, 2020 | Group A (COVID-19 and cirrhosis) had a higher mortality rate (8.9%) compared to Group B (COVID-19 alone, 5.6%). The hazard ratio (95%CI) for mortality with cirrhosis was 1.59 (1.26–1.99) (P = 0.01). PC referrals were more frequent in Group A (4.1%) compared to Group B (2.0%). The hazard ratio (95%CI) for PC referrals with cirrhosis was 2.02 (1.39–2.94) (P = 0.01) | Hospitalized patients with COVID-19 and cirrhosis are at high risk of mortality and should be considered for PC referrals |
| Brown et al[76], 2016 | The study included a cohort of ESLD patients (n = 22311) | This was a retrospective cohort study that identified hospitalized ESLD and HF patients between 2007 and 2011 | The study analyzed endpoints during index hospitalization, including mortality, discharge to hospice, and length of stay. Post-discharge endpoints included all-cause mortality, rehospitalization, hospice enroll | One year after discharge, ESLD patients had 209 days alive and out of hospital compared to 252 days alive and out of hospital for decompensated HF patients. Inpatient mortality for ESLD was 13.5%, with all-cause mortality at 64.9%, higher than HF rates. ESLD patients had a rehospitalization rate of 59.1%, slightly lower than HF patients | The study demonstrates substantial morbidity and mortality rates associated with end-of-life care in ESLD. There is a critical need for alternative approaches to manage the care of ESLD patients |
| Whitsett et al[30], 2022 | The study surveyed all United States transplant hepatology fellows enrolled in accredited fellowship programs during the 2020–2021 academic year | This was a qualitative study conducted using a national survey | The survey assessed the frequency of PC provision and fellows' comfort levels with physical and psychological symptom management, psychosocial care, communication skills, ACP, and end-of-life care | Out of 56 transplant hepatology fellows, 45 responded to the survey (79%), including 50% females (n = 22). Most fellows (67%, n = 29) trained at centers performing over 100 transplants per year. Additionally, 69% (n = 31) had a PC or hospice care rotation during residency, and 42% (n = 19) received education in PC during their transplant hepatology fellowship | The survey revealed gaps in PC experience and education during transplant hepatology fellowship, highlighting a lack of comfort in managing psychological distress and ACP. There is a desire among fellows to improve skills, particularly in symptom management |
| Ufere et al[20], 2020 | The study included patients with DC evaluated for LT between January 1, 2010, and December 31, 2017 (n = 230) | It was a retrospective analysis of all adults (age ≥ 18 years) evaluated for LT across a network of nine acute care hospitals | The study compared healthcare utilization in the last year of life and EOL care outcomes between transplant-listed (n = 133) and non-listed (n = 97) patients. Predictors of PC and hospice care utilization were examined using multivariate logistic regression | The majority of patients (80%) died in the hospital, with 70% of them in the ICU. About 70.0% received a life-sustaining procedure during their terminal hospitalization, which did not differ between transplant-listed and non-listed patients. Transplant-listed patients had lower odds of receiving specialty PC, while patients with HCC had higher odds of receiving hospice care | Patients with DC, regardless of transplant candidacy, exhibit high rates of healthcare utilization with low utilization of palliative and hospice care. They spend most of their last 90 days of life in the hospital, where they often eventually die |
Two experimental studies in patients with ESLD assessed the impact of specialist PC interventions. Shinall et al[9] conducted a randomized controlled trial (RCT) revealing that patients receiving PC had a lower hazard of readmission [hazard ratio (HR) = 0.36, 95%CI: 0.16-0.83, P = 0.017] and more days alive outside hospital (odds ratio = 3.97, 95%CI: 1.14-13.84, P = 0.030). However, this trial faced enrollment issues, ending prematurely with only 34% participation[9]. Bauman et al[6] highlighted underutilization of PC due to a lack of appropriate assessment tools for ESLD symptoms. They introduced an early PC intervention (EPCI), showing a 50% reduction in symptom burden (P < 0.05) and 43% reduction in depressive symptoms in ESLD patients awaiting LT. Limitations included a small sample size and limited follow-up, suggesting a need for future studies with larger samples and longer follow-up to validate EPCI benefits[6].
Several retrospective observational studies focused on assessing PC utilization and outcomes in patients with ESLD and related conditions were reviewed.
Gupta et al[10] found that inpatient PC consultation reduced 30-day readmission rates and hospital stays in alcohol-associated liver disease patients with ACLF scores ≥ 2, despite study limitations due to its retrospective nature. This study highlights the impact of PC on reducing healthcare utilization in specific ESLD subgroups.
Poonja et al[11] highlighted that many DC patients declined or delisted for LT were infrequently referred for PC, emphasizing the need for better symptom management tools and LT-PC partnership to enhance quality of life. The study identified prevalent symptoms among this patient population, underscoring the unmet need for comprehensive PC.
Lin et al[12] demonstrated machine learning's predictive capabilities in managing ESLD, aiding in mortality prediction and patient classification for acute or PC needs, with implications for wider use and validation across ethnicities. This study introduces innovative technology for improving risk stratification and personalized care in ESLD.
Puentes et al[13] revealed critical mortality rates among ESLD patients, emphasizing the practical use of Child-Pugh and MELD-Na scores for identifying PC candidates. These scoring systems serve as valuable tools for PC decision-making in ESLD.
Hudson et al[14] proposed prognostic screening tools for early intervention in cirrhotic patients upon hospital admission, offering a universally applicable strategy. This study proposes a practical approach to identifying high-risk ESLD patients for timely intervention.
Medici et al[15] emphasized the MELD score's potential utility for implementing PC, with acknowledgment of retrospective study limitations. The MELD score emerges as a practical clinical tool for guiding PC referrals in ESLD patients.
Deng et al[16] linked frailty to symptom burden in cirrhosis patients, highlighting the need for comprehensive assessment and PC co-management. This study underscores the importance of holistic care in managing frail ESLD patients.
Kathpalia et al[17] underscored the potential benefits of PC for cirrhotic patients facing LT-related mortality, advocating for early PC integration into decision-making. This study emphasizes the role of PC in improving outcomes for ESLD patients awaiting LT.
Thandassery et al[18] and Trebicka et al[19] linked ESLD and COVID-19 mortality, prompting PC considerations during pandemics, with retrospective study limitations. These studies highlight the unique challenges and considerations for PC in ESLD patients during public health emergencies.
Ufere et al[20] addressed gaps in PC utilization and training, necessitating better education and broader study designs to enhance care for advanced liver disease patients. This study emphasizes the need for improved PC education and integration into ESLD care.
Several prospective observational studies highlight the importance of integrating PC into the care of patients with ESLD.
Lamba et al[21] demonstrate that interdisciplinary communication interventions can facilitate early consensus on goals of care for terminally ill LT service patients, enhancing end-of-life care practices without altering mortality outcomes.
Vieira et al[22] emphasize the universal need for PC among ESLD patients, regardless of transplant eligibility. However, the study's limitations include a single-hospital sample and heterogeneous subgroup distribution, which could introduce bias.
Patel et al[23] identify inadequate PC quality in a veteran population diagnosed with ESLD, suggesting the use of International Classification of Diseases, Ninth Revision codes for patient cohort identification. The study's limitations include a small sample size and single-center data.
Orman et al[24] find that PC is underutilized and provided late to patients with severe liver disease, despite its association with reduced healthcare utilization and greater completion of advanced directives. Randomized trials are needed to evaluate PC's efficacy in this population.
A series of qualitative studies shed light on the challenges and barriers surrounding PC utilization and advance care planning (ACP) for patients with ESLD.
Patel et al[25] revealed low rates of PC consultation alongside high healthcare costs for ESLD patients, indicating racial and ethnic disparities in PC access. They propose targeted quality improvement initiatives to address these disparities.
Ufere et al[26] surveyed hepatologists and gastroenterologists, identifying cultural factors and unrealistic expectations as major barriers to PC and ACP utilization. Insufficient communication about end-of-life care and late engagement in ACP were also highlighted.
Patel et al[27] found that patients with DC received inadequate ACP, leading to overly aggressive life-sustaining treatments at the end of life. This study underscores the need for improved ACP throughout the illness trajectory.
Beck et al[28] suggested clear referral criteria, such as the MELD score, to expand PC access for LT patients. Educational interventions targeting attending physicians could enhance PC utilization.
Donlan et al[29] conducted educational interventions to dispel misconceptions about PC among ESLD patients and caregivers, emphasizing its role in enhancing illness understanding and coping mechanisms.
Shinall et al[9] identified barriers in LT patient care, including role boundaries and differences in clinical cultures, proposing comprehensive symptom assessment and support for caregivers to improve care quality.
Whitsett et al[30] highlighted gaps in PC education and confidence among transplant fellows, advocating for early consideration of palliative medicine and hospice in ESLD management due to its comparable morbidity and mortality rates to heart failure.
These qualitative studies collectively emphasize the need for targeted interventions, improved education, and enhanced interdisciplinary collaboration to optimize PC utilization and ACP for patients with ESLD.
ESLD is the apex of the progression of CLD to cirrhosis, decompensation, and chronic liver failure. Despite LT being proven curative for patients with ESLD, the availability of the procedure is restricted by donor availability, active substance abuse, financial constraints in developing countries, and progression of disease among wait-list patients including extrahepatic organ involvement and sepsis[3]. In this systemic review, we examined the evidence-based literature focusing on the advantages of integrating PC into the management of patients with ESLD and ACLF. PC encompasses achievable goals that include symptom control and interventions that favorably alter the natural course of the disease to offer curative intent[31]. More so in this review, we discuss the disease course of ESLD and ACLF, the role of PC in the management of these patients, the challenges, and barriers it encountered in evidence-based practice, an acknowledgment of the limitations of this study, and lastly the recommendations for future research on the integration of PC in the management of ESLD and or ACLF.
Unlike clinical treatment which aims at curing disease, PC is a medical specialty that focuses on helping people with serious illnesses get relief from the symptoms, pain, and side effects they experience. PC does not aim at curing disease so it can be provided along with curative treatment and may begin at the time of diagnosis. PC is appropriate for every stage of a serious illness, from diagnosis until the end of your life. In the context of ESLD and ACLF, PC emerges as an alternative therapeutic avenue for patients deemed unsuitable for LT[30,32-34].
Additionally, there is a clear unmet need for comprehensive PC, as evidenced by the infrequent referral of DC patients for PC, highlighting the necessity for better symptom management tools and collaboration between LT and PC services. Innovative approaches like machine learning show promising predictive capabilities in ESLD management, aiding in risk stratification and personalized care. Furthermore, the use of practical tools such as Child-Pugh and MELD-Na scores can effectively identify PC candidates, facilitating timely intervention and decision-making.
These studies underscore the impact of PC on reducing healthcare utilization, such as reducing readmission rates and hospital stays, particularly in specific ESLD subgroups like alcohol-associated liver disease with ACLF scores ≥ 2.
According to Bajaj et al[32], the guideline panel, comprising six experts in hepatology and two guideline methodologists, officially presented ACLF practice recommendations in the American Journal of Gastroenterology[32]. The authors provided insights into the unique similarities, differences, and limitations of the three most widely used ACLF definitions: European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF; Europe), Asian-Pacific Association for the Study of the Liver (Asia), and North American Consortium for the Study of ESLD (NACSELD; North America). They suggested defining ACLF as a "potentially reversible condition in patients with chronic liver disease with or without cirrhosis that is associated with the potential for multiple organ failure and mortality within 3 months in the absence of treatment of underlying liver disease, liver support, or LT"[32].
In the EASL-CLIF criteria, ACLF occurs in patients with DC who develop additional organ failures. The NACSELD criteria were established later, primarily for patients hospitalized with cirrhosis and bacterial infections. These two criteria are essentially modifications of the EASL-CLIF criteria, where ACLF is defined by the presence of at least two extrahepatic organ failures, including shock, HE grades 3 or 4, renal replacement, and/or mechanical ventilation[35].
The CLIF consortium (CLIF-C) organ failure is associated with high mortality, and the score determines individual thresholds for organ failures, which may include all organs, including the liver[36]. The concept of ACLF, although debated in the last decade, has gained momentum with the publication of clinical guidelines, signifying its global acceptance[36]. However, despite several clinical study attempts, disease-modifying therapies for ACLF, such as granulocyte-colony-stimulating factor[37], the unselective interleukin-1 (IL-1) inhibitor Anakinra[38], or selective IL-1 beta inhibitor canakinumab[39], have failed to establish due to the complexity of its underlying pathophysiology, which is not yet well understood[32].
Currently, there is no approved or broadly accepted standard treatment option to modify the disease course, and short-term mortality rates range between 30%-50%, making LT the only curative approach[33-36]. However, a shortage of donor organs and numerous contraindications limit access to transplantation to a small proportion of ACLF patients. The complexity of treatment strategies, involving multiple organ systems and various medical specialties, underscores the pressing need for guidelines for diagnostic and therapeutic procedures in ACLF[33-36].
The scores developed by the EASL-CLIF-C have been shown to be accurate predictors of patient mortality[40-43], even in specific events in the course of the disease, such as spontaneous bacterial peritonitis[44,45], acute variceal bleeding[46,47], sepsis[48] and hepatorenal syndrome[49,50]. It is no surprising at all that a very robust study found that a CLIF-C ACLF score cutoff ≥ 70 identified patients with a 100% mortality within 28 days, indicating that these patients may reach a threshold of futility for further ongoing intensive support, and the best treatment options may include PC[51]. A retrospective study was conducted on hospitalized patients with cirrhosis admitted between January 2015 and December 2016, utilizing the Child–Pugh score and the MELD/Na score in January 2018. Approximately 51.1% of the patients had a MELD/Na score > 20, while 48.9% had a score < 20. The study findings indicated that 59.6% of patients had died within 12 months, suggesting that a MELD score > 20 could serve as a referral threshold for LT patients to PC[13]. These results highlight the significant need for PC among this patient population.
The transition from compensated to DC and ESLD is universally recognized by the occurrence of clinical complications, including ascites, hepatic encephalopathy, gastrointestinal bleeding, and jaundice[52]. This transition process can follow two distinct pathways: AD and progressive, non-AD (NAD). AD is characterized by the first or recurrent grade 2 or 3 ascites within less than 2 weeks, the first or recurrent acute hepatic encephalopathy in patients with previously normal consciousness, acute gastrointestinal bleeding, and any type of acute bacterial infection[36]. AD and ACLF predominantly occur in patients with a history of previous decompensation, representing further decompensation. The most severe form of AD is ACLF, primarily triggered by alcoholic hepatitis or infections[36,52]. Regardless of the cirrhosis etiology, AD manifests as ACLF in approximately 16% of cases; pre-ACLF in 17%; unstable decompensation in 22%, characterized by persistent albeit unstable inflammatory status resulting in further AD events within 1 year; and stable decompensation in 48%, with a stable decrease of systemic inflammation and no further AD for at least 1 year[53].
In contrast, NAD is characterized by the progressive development of any single event (58%-72%) or any combination (28%-42%) of slow ascites formation, hepatic encephalopathy grade 1-2 or higher if manageable in an outpatient setting, or progressive jaundice in non-cholestatic cirrhosis[36]. NAD is mainly represented by the first decompensation, occurring in 58% to 72% of patients, and usually does not require hospitalization[52]. Even when presenting with two or more decompensating events, a significant proportion of these patients may be managed as outpatients or in the day-hospital setting. This also applies to many patients with further decompensation represented by refractory ascites and mild to moderate recurrent encephalopathy free of other complications[19]. Thus, it appears that AD and NAD impact the clinical course of cirrhosis differently, with AD mostly representing further decompensation and NAD mostly the first decompensating event.
These studies collectively emphasize the importance of holistic care, early PC integration into clinical decision-making, and the necessity for improved PC education and integration into ESLD care to enhance patient outcomes, especially during unique challenges like public health emergencies.
The American Association for the Study of Liver Diseases (AASLD) defines PC as “multidisciplinary, specialized medical care that addresses the physical, spiritual, and psychosocial needs of patients with serious illness and their caregivers”[31,54]. According to the World Health Organization, PC is “an approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering using early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial, and spiritual”[55]. AASLD emphasizes that PC can be provided at any stage of a serious illness and concurrently with disease-directed and curative treatments, including organ transplants[56]. Over the past decade, there has been an expansion in the body of evidence supporting early PC, extending to individuals with non-malignant conditions which carry a poor prognosis and intense suffering such as cirrhosis[56,57]. Some authors recognize the challenge clinicians face in determining the ideal timing for involving specialized PC teams due to the unpredictable disease trajectory and the potential for curative LT[58]. Overall, the majority of studies included in this review advocate for the early initiation of PC for patients with cirrhosis who develop ESLD or ACLF. This suggests that the diagnosis of cirrhosis represents the earliest point at which PC should be offered to these patients, making it the preferred stage to initiate PC.
From the outset, the aim of the study is to comprehensively investigate the evidence surrounding the benefits of incorporating PC care of patients with ESLD and/or ACLF. This systemic review addresses the underexplored utilization of PC in patients with ESLD and acute on chronic liver disease. The study focuses on the evidence based literatures that sought the advantages of integrating PC into the comprehensive management of patients with ESLD and ACLF. Thus aiming to contribute valuable insights to the evolving landscape of ESLD and ACLF management. In these studies, the diagnosis of ESLD and ACLF was made by a liver specialist or a qualified physician in the field, who then referred the patient to a PC at various stages of his or her life. The experience and education level of the healthcare personal who provide the PC are not even acknowledged in the studies included in this systemic review. PC management typically involves a team comprising physicians, nurses, chaplains, social workers, and other providers[31].
Globally, there is a growing recognition of the benefits of PC across disease states, including DC or ACLF. Evidently, a substantial body of evidence supports the integration of curative and PC approaches for patients with DC. Outpatient PC in patients with DC is associated with improved symptoms, enhanced care coordination, and better anticipatory planning[56,57]. The AASLD, through its Practice Guidelines Committee, has provided PC guidance specifically for patients with cirrhosis, addressing issues relevant to adult patients with DC. The decision to commission guidance rather than a guideline stems from the paucity of RCT on PC in DC[31]. While PC can be considered irrespective of the cirrhosis stage, the notable physical, psychosocial, and financial burden in this group has led to the development of guidance tailored to adult patients with DC[31].
PC, though underutilized, addresses the myriad challenges encountered by patients with DC or ESLD and their families. For example, a significant underutilization of PC services, particularly in older, non-white patients with cirrhosis on the LT waitlist was observed[11,59], which was corroborated by the National Inpatient Survey[60]. The lack of PC support has a negative impact on the quality of life of non-transplant candidates with ESLD[11,59]. Although an increase PC services utilization has been observed in the past decade[25,60], only a few ESLD patients declining or delisted for LT are referred for PC services[11,58,59], and they are commonly referred within a few days or weeks before their death[17,61]. Therefore, it is paramount to advocate for the systemic implementation of PC principles and resources to alleviate the physical and emotional burden on the ESLD patient population and their families[62].
Although, a very relevant limitation that might contribute to the underutilization of PC in ESLD is the lack of tools identifying distressing symptoms correlating with the optimal time for PC referral[6]. It is important to notice that low rates of PC consultation in patients with ESLD might incur into significant costs for these terminally ill patients[23,25,27,28,63,64] and an increase in undesired suffering, as ESLD patients receive ACP consultations too late in the course of their disease[23,26]. PC referral during hospital admissions that concludes in death is often belated and inefficacious, as it often fails to reduce anxiety symptoms[65]. Despite all the addressed above, the hepatology community recognizes PC as underutilized in ESLD and ACLF[65]. Nevertheless, pre-emptive PC intervention in the management of patients with ESLD can result in increase in time to the first readmission and more days alive outside the hospital for admitted ESLD patients[63].
LT stands as the sole cure for ESLD and ACLF, but annual LT rates remain stagnant due to various obstacles such as donor availability, financial constraints, active substance use, mental health disorders, and lack of social support[66]. Clinical scoring systems, including the CTP score, MELD, and ACLF grade, have been developed to predict mortality in cirrhosis patients[29]. ACLF grade at 3-7 days post-admission, MELD score and CLIF-C ACLF scores may identify patients for whom continued care is futile[6,34,51,67]. Although, it is rather challenging to identify the point of irreve
Disparities in PC referral extend to the delivery of PC in cirrhosis patients specifically. Financially and socially vulnerable groups are less likely to receive PC referrals upon hospital admission[36]. The misconception that PC is synonymous with end of life care persists in medical settings, that can be persistently propagated by attending physicians, potentially contributing to its underutilization in the transplant setting[28]. Also, the misconception that is a synonym to hospice care is also very common. It is important that fellow physicians, patients and their families receive education on PC, so to recognize its potential to support their understanding of illness and coping[26,29].
While the impact of PC on patients with ESLD and ACLF remains an understudied area, innovative care models have emerged to enhance the utilization of PC in these patients. For example, patients who denied or were delisted from LT or which experienced worsening MELD scores on the transplant list can enrolled in hospice care, and some of these patients might be successfully transplanted while in hospice care[39]. Also, PC consultation might reduce readmission rates and hospital stay in patients with cirrhosis[17,63], while improving physical and physiological suffering[6].
A study conducted by Adejumo et al[69] reported that PC consultation was associated with a lower risk for 30- and 90-day readmissions (HR = 0.42, 95%CI: 0.38–0.47 and 0.38, 95%CI: 0.34–0.42, respectively). This association resulted in reduced burden on healthcare resource utilization and improvement in cost savings during subsequent readmissions[69]. Lamba et al[21] implemented a single-centered, 2-tiered structured intervention, assessing automatic PC integration for cirrhosis and LT patients admitted to the Surgical Intensive Care Unit (SICU)[21]. The first part focused on reviewing prognosis, advanced directives, and symptom management within 24 hours of SICU admission. The second part involved a family meeting with both primary and PC teams to review the goals of care. The outcomes of this intervention included increased goals of care meetings (from 2% to 38%), increased do not resuscitate rates (from 52% to 81%), increased withdrawal from life support (from 35% to 68% of cases), and a decreased SICU mean length of stay by 3 days, all achieved without a change in mortality rates. This suggests that only patients undergoing futile care had life-sustaining care withdrawn. Additionally, interdisciplinary communication interventions with physicians and families in the SICU may lead to earlier consensus around goals of care for dying LT service patients. Early integration of PC alongside aggressive, disease-focused, curative care in the SICU for LT patients can be accomplished without a change in mortality and can improve end of life care practices in such patients[21].
These studies collectively indicate a benefit with PC across the continuum of care in heterogeneous patient populations, particularly those struggling with uncertainties such as the population with DC, which experiences significant stress, anxiety, and decreased quality of life[67]. Early PC referral could provide a benefit to caregivers of patients with cirrhosis who also suffer while caring for loved ones with this illness. Early PC integration has been shown to reduce depression and anxiety among caregivers, such as spouses, children, and parents[70].
Cirrhosis is linked to a diminished health-related quality of life, posing challenges due to various stressors encompassing physical, cognitive, psychological, and social aspects. Studies have consistently revealed that patients with DC face variable mortality rates ranging from 20% to 80% over five years. The disease trajectory is progressive, marked by declining health, increasing symptom burden, and frequent hospitalizations. While LT has proven to be a curative option, only a minority of patients survive to receive it[70]. There is an unmet PC need for cirrhosis patients and their caregivers, with only a few of them receiving PC or hospice care referrals, often occurring late in the disease course[61,71,72].
Several identified barriers hinder the implementation of PC for patients with ESLD and/or ACLF, such as a shortage of specialty PC providers, the absence of evidence-based referral criteria, a lack of role clarity between specialists, the stigma associating PC with "giving up" on curative treatments, insufficient provider training, competing demands on providers' time, and prognostic uncertainty[26]. Additionally, many symptoms experienced by individuals with ESLD are highly liver-specific and are longitudinally managed by liver teams. The process of transplant waitlisting and evaluation itself acts as a barrier to PC due to potential conflicts between transplantation and PC, although these patients undergoing transplant evaluation tend to receive lower-quality end of life care[58,64,73]. Notably, PC competencies for hepatologists have not been developed, as hepatology training does not routinely include PC training.
AASLD guideline statements emphasize that patients with ESLD and their caregivers frequently face substantial unmet PC needs across psychological, physical, social, financial, and spiritual dimensions. The recommendations advocate for the assessment of unmet PC needs and the consideration of specialty PC consultation for all patients with DC and their caregivers. Importantly, the guidance clarifies that disease-directed care, such as transplantation evaluation and listing, should not preclude the delivery of PC or specialty PC consultation for patients with DC.
Clearly, obstacles impeding the delivery of quality PC for patients with ESLD or ACLF include a shortage of PC specialists and inadequate training for healthcare providers, including hepatologists, as PC training is typically not integrated into hepatology training programs. Therefore, it is imperative to mandate PC training for healthcare professionals in subspecialty hepatology departments who are directly responsible for the care of patients with ESLD/ACLF[74-77].
Acknowledging the shortage of specialty PC providers, the AASLD recommends that hepatology clinicians take a pivotal role in providing primary PC services to patients with cirrhosis. This role includes conducting symptom assessment and management, facilitating basic ACP activities such as identifying surrogate decision-makers, offering counselling, and making referrals for additional support when necessary and feasible[31].
Artificial intelligence (AI) holds significant promise in the field of healthcare, particularly in prognosticating patients with complex conditions such as LT, ESLD or ACLF[78-80]. By leveraging advanced algorithms and machine learning techniques, these systems can analyze vast amounts of patient data, including clinical variables, imaging studies, and laboratory results, to identify patterns and predict disease progression[81-84]. In the context of PC for patients with ESLD or ACLF, AI-driven prognostic models could assist healthcare providers in selecting those individuals who are most likely to benefit from PC interventions. This tailored approach may optimize resource allocation and improve outcomes by ensuring that PC services are directed towards those who stand to benefit the most.
Our study exhibits several limitations. Primarily, due to the retrospective nature of our discharge database, our analysis is susceptible to coding errors and missing data. While transplant patients represent a minority group, the non-transplant patient group is inadequately assessed in only a few studies. The prospective and retrospective studies utilized to elucidate the impact of PC in ESLD and/or ACLF predominantly consist of non-randomized trials, observational studies, or relatively small-scale investigations. Notably, the transplant and ineligible transplant populations differ, each harboring unique sources of suffering and care disparities. Consequently, findings from studies in one population may lack generalizability to the other, necessitating separate investigations.
Future directions for the effective implementation of PC into clinical hepatology involve commitments to bridge the research gap, advocate for essential health policy changes, instigate relevant cultural shifts, and foster essential clinical innovation. Prospective and randomized trials on integrated PC interventions for patients with ESLD and ACLF are crucial, with a specific focus on evaluating the PC needs of both transplant and non-transplant patient groups. Emphasis should be placed on assessing symptom burden and quality of life rather than solely focusing on the intervention's impact on mortality. Collaborative efforts between LT and PC services could enhance the quality of life in this patient population.
ESLD and ACLF impose a substantial burden of clinical complications, leading to patient suffering and poor prognosis. Despite LT being the sole recognized cure for ESLD and ACLF, it remains accessible only to a minority of patients. PC represents a specialized area of clinical intervention aimed at enhancing the quality of life for individuals with advanced cirrhosis. This involves advanced care planning, alleviation of physical symptoms through timely interventions, and provision of emotional support to both the patient and their family. Regrettably, the utilization of PC is infrequent, and referrals typically occur late in the course of patients with ESLD and ACLF. Ideally, PC should be initiated upon the diagnosis of ESLD or ACLF and be sustained throughout the entire trajectory of the illness. Overcoming existing barriers and integrating PC early in the treatment continuum, including alongside active interventions such as LT, would maximize benefits for patients before the end of life and reduce costs for the broader healthcare system. To facilitate improvement in this regard, there is a need for more researches particularly prospective randomized control trials assessing the impact of integrated PC services in the early management of patients with ESLD and ACLF, irrespective of transplant eligibility.
We extend our appreciation to the Faculty of Life Sciences and Education at the University of South Wales for the Acute Medicine MSc program and their invaluable support in our work. We sincerely acknowledge the efforts of the University of South Wales and commend them for their commitment to providing life-long learning opportunities and advanced life skills to healthcare professionals.
| 1. | Sacleux SC, Saliba F. How to Optimize the Results of Liver Transplantation for Acute-on-Chronic Liver Failure. Life (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Schulz MS, Gu W, Schnitzbauer AA, Trebicka J. Liver Transplantation as a Cornerstone Treatment for Acute-On-Chronic Liver Failure. Transpl Int. 2022;35:10108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 3. | Philips CA, Kedarisetty CK. Palliative Care for Patients with End-Stage Liver Disease. J Clin Exp Hepatol. 2023;13:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Mazzarelli C, Prentice WM, Heneghan MA, Belli LS, Agarwal K, Cannon MD. Palliative care in end-stage liver disease: Time to do better? Liver Transpl. 2018;24:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C, Solís-Munoz P, Saliba F, Zeuzem S, Albillos A, Benten D, Montero-Alvarez JL, Chivas MT, Concepción M, Córdoba J, McCormick A, Stauber R, Vogel W, de Gottardi A, Welzel TM, Domenicali M, Risso A, Wendon J, Deulofeu C, Angeli P, Durand F, Pavesi M, Gerbes A, Jalan R, Moreau R, Ginés P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 481] [Article Influence: 48.1] [Reference Citation Analysis (1)] |
| 6. | Baumann AJ, Wheeler DS, James M, Turner R, Siegel A, Navarro VJ. Benefit of Early Palliative Care Intervention in End-Stage Liver Disease Patients Awaiting Liver Transplantation. J Pain Symptom Manage. 2015;50:882-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123-e130. [PubMed] |
| 8. | Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J Public Health (Oxf). 2022;44:e588-e592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 226] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 9. | Shinall MC Jr, Bonnet K, Schlundt D, Verma M. Integrating Specialist Palliative Care in the Liver Transplantation Evaluation Process: A Qualitative Analysis of Hepatologist and Palliative Care Provider Views. Liver Transpl. 2022;28:678-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Gupta K, Hans B, Khan A, Sohail SH, Kapuria D, Chang C. A retrospective study on use of palliative care for patients with alcohol related end stage liver disease in United States. World J Hepatol. 2022;14:1817-1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Poonja Z, Brisebois A, van Zanten SV, Tandon P, Meeberg G, Karvellas CJ. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol. 2014;12:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Lin YJ, Chen RJ, Tang JH, Yu CS, Wu JL, Chen LC, Chang SS. Machine-Learning Monitoring System for Predicting Mortality Among Patients With Noncancer End-Stage Liver Disease: Retrospective Study. JMIR Med Inform. 2020;8:e24305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Puentes JCP, Rocha H, Nicolau S, Ferrão G. Effectiveness of the MELD/Na Score and the Child-Pugh Score for the Identification of Palliative Care Needs in Patients with Cirrhosis of the Liver. Indian J Palliat Care. 2018;24:526-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hudson BE, Ameneshoa K, Gopfert A, Goddard R, Forbes K, Verne J, Collins P, Gordon F, Portal AJ, Reid C, McCune CA. Integration of palliative and supportive care in the management of advanced liver disease: development and evaluation of a prognostic screening tool and supportive care intervention. Frontline Gastroenterol. 2017;8:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Medici V, Rossaro L, Wegelin JA, Kamboj A, Nakai J, Fisher K, Meyers FJ. The utility of the model for end-stage liver disease score: a reliable guide for liver transplant candidacy and, for select patients, simultaneous hospice referral. Liver Transpl. 2008;14:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Deng LX, Bischoff KE, Kent DS, O'Riordan DL, Pantilat SZ, Lai JC. Frailty is strongly associated with self-reported symptom burden among patients with cirrhosis. Eur J Gastroenterol Hepatol. 2021;33:e395-e400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Kathpalia P, Smith A, Lai JC. Underutilization of palliative care services in the liver transplant population. World J Transplant. 2016;6:594-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 18. | Thandassery RB, Sharma S, Syed M, Perisetti A. A global multicenter propensity-matched analysis of mortality risk and palliative care referral due to cirrhosis in hospitalized patients with COVID-19. J Clin Transl Res. 2022;8:414-420. [PubMed] |
| 19. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 20. | Ufere NN, Halford JL, Caldwell J, Jang MY, Bhatt S, Donlan J, Ho J, Jackson V, Chung RT, El-Jawahri A. Health Care Utilization and End-of-Life Care Outcomes for Patients With Decompensated Cirrhosis Based on Transplant Candidacy. J Pain Symptom Manage. 2020;59:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Lamba S, Murphy P, McVicker S, Harris Smith J, Mosenthal AC. Changing end-of-life care practice for liver transplant service patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012;44:508-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Vieira da Silva SN, Baptista P, Fonseca Silva I, Freire E, Pessegueiro Miranda H. Palliative Care in Advanced Liver Disease: Similar or Different Palliative Care Needs in Patients with a Prospect of Transplantation? Prospective Study from a Portuguese University Hospital and Transplantation Center. GE Port J Gastroenterol. 2023;30:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Patel A, Asch S, Antonio AL, Kanwal F, Lorenz K, Riopelle D, Dickey A, Larkin J, Lee M, Walling A. Measuring the Quality of Palliative Care for Patients with End-Stage Liver Disease. Dig Dis Sci. 2020;65:2562-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Orman ES, Yousef A, Xu C, Shamseddeen H, Johnson AW, Nephew L, Ghabril M, Desai AP, Patidar KR, Chalasani N. Palliative Care, Patient-Reported Measures, and Outcomes in Hospitalized Patients With Cirrhosis. J Pain Symptom Manage. 2022;63:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Patel AA, Walling AM, Ricks-Oddie J, May FP, Saab S, Wenger N. Palliative Care and Health Care Utilization for Patients With End-Stage Liver Disease at the End of Life. Clin Gastroenterol Hepatol. 2017;15:1612-1619.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Ufere NN, Donlan J, Waldman L, Dienstag JL, Friedman LS, Corey KE, Hashemi N, Carolan P, Mullen AC, Thiim M, Bhan I, Nipp R, Greer JA, Temel JS, Chung RT, El-Jawahri A. Barriers to Use of Palliative Care and Advance Care Planning Discussions for Patients With End-Stage Liver Disease. Clin Gastroenterol Hepatol. 2019;17:2592-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Patel AA, Ryan GW, Tisnado D, Chuang E, Walling AM, Saab S, Khemichian S, Sundaram V, Brook RH, Wenger NS. Deficits in Advance Care Planning for Patients With Decompensated Cirrhosis at Liver Transplant Centers. JAMA Intern Med. 2021;181:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Beck KR, Pantilat SZ, O'Riordan DL, Peters MG. Use of Palliative Care Consultation for Patients with End-Stage Liver Disease: Survey of Liver Transplant Service Providers. J Palliat Med. 2016;19:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Donlan J, Ufere NN, Indriolo T, Jackson V, Chung RT, El-Jawahri A, Traeger L. Patient and Caregiver Perspectives on Palliative Care in End-Stage Liver Disease. J Palliat Med. 2021;24:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Whitsett MP, Ufere NN, Patel A, Shea JA, Jones CA, Fix OK, Serper M. Palliative care experience and perceived gaps in training among transplant hepatology fellows: A national survey. Hepatol Commun. 2022;6:1680-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Rogal SS, Hansen L, Patel A, Ufere NN, Verma M, Woodrell CD, Kanwal F. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022;76:819-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 32. | Bajaj JS, O'Leary JG, Lai JC, Wong F, Long MD, Wong RJ, Kamath PS. Acute-on-Chronic Liver Failure Clinical Guidelines. Am J Gastroenterol. 2022;117:225-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 33. | Engelmann C, Berg T. Clinical practice guidelines for acute-on-chronic liver failure: are we ready for reaching global consensus? Hepatobiliary Surg Nutr. 2023;12:239-243. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 590] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 35. | O'Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, Subramanian RM, Kamath PS, Thuluvath P, Vargas HE, Maliakkal B, Tandon P, Lai J, Thacker LR, Bajaj JS. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 36. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2171] [Article Influence: 180.9] [Reference Citation Analysis (5)] |
| 37. | Engelmann C, Herber A, Franke A, Bruns T, Reuken P, Schiefke I, Zipprich A, Zeuzem S, Goeser T, Canbay A, Berg C, Trebicka J, Uschner FE, Chang J, Mueller T, Aehling N, Schmelzle M, Splith K, Lammert F, Lange CM, Sarrazin C, Trautwein C, Manns M, Häussinger D, Pfeiffenberger J, Galle PR, Schmiedeknecht A, Berg T. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study). J Hepatol. 2021;75:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Szabo G, Mitchell M, McClain CJ, Dasarathy S, Barton B, McCullough AJ, Nagy LE, Kroll-Desrosiers A, Tornai D, Min HA, Radaeva S, Holbein MEB, Casey L, Cuthbert J. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology. 2022;76:1058-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 39. | Vergis N, Patel V, Bogdanowicz K, Czyzewska-Khan J, Fiorentino F, Day E, Cross M, Foster N, Lord E, Goldin R, Forrest E, Thursz M. IL-1 Signal Inhibition In Alcoholic Hepatitis (ISAIAH): a study protocol for a multicentre, randomised, placebo-controlled trial to explore the potential benefits of canakinumab in the treatment of alcoholic hepatitis. Trials. 2021;22:792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Rashed E, Soldera J. CLIF-SOFA and CLIF-C scores for the prognostication of acute-on-chronic liver failure and acute decompensation of cirrhosis: A systematic review. World J Hepatol. 2022;14:2025-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 41. | Grochot RM, Luz LB, Garcia R, Balbinot RÂ, Balbinot SS, Soldera J. Acute–on–chronic liver failure data from a teaching hospital in Brazil. A Historical Cohort. Inter J Sci Res. 2020;9:1-6. |
| 42. | Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Grochot RM, Luz LB, Garcia R, Balbinot RA, Balbinot SS, Soldera J. CLIF-SOFA is superior to other liver-specific scores for predicting mortality in acute-on-chronic liver failure and decompensated cirrhosis. Austin J Gastroenterol. 2019;6:1105. |
| 44. | Oliveira Coberllini Jacques R, Silva Massignan L, Schumacher Winkler M, Sartori Balbinot R, Balbinot RA, Sartori Balbinot S, Soldera J. Liver‐specific scores as predictors of mortality in spontaneous bacterial peritonitis. GastroHep. 2020;2:224-231. [DOI] [Full Text] |
| 45. | Jacques ROC, Massignan LDS, Winkler MS, Balbinot RS, Balbinot SS, Soldera J. Acute-chronic liver failure is strongly associated with lower survival in patients with spontaneous bacterial peritonitis. Arq Gastroenterol. 2021;58:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 46. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Rost GL Jr, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Acute-on-chronic liver failure is independently associated with higher mortality for cirrhotic patients with acute esophageal variceal hemorrhage: Retrospective cohort study. World J Clin Cases. 2023;11:4003-4018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 47. | Terres AZ, Balbinot RS, Muscope ALF, Eberhardt LZ, Balensiefer JIL, Cini BT, Rost GL, Longen ML, Schena B, Balbinot RA, Balbinot SS, Soldera J. Predicting mortality for cirrhotic patients with acute oesophageal variceal haemorrhage using liver‐specific scores. GastroHep. 2021;3: 236-246. [DOI] [Full Text] |
| 48. | Ndomba N, Soldera J. Management of sepsis in a cirrhotic patient admitted to the intensive care unit: A systematic literature review. World J Hepatol. 2023;15:850-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 49. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Luis Rost G Jr, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Evidence-based protocol for diagnosis and treatment of hepatorenal syndrome is independently associated with lower mortality. Gastroenterol Hepatol. 2022;45:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Zulian Terres A, Sartori Balbinot R, Laura Facco Muscope A, Luisa Longen M, Schena B, Teston Cini B, Luis Rost G, Isabel Leichtweis Balensiefer J, Zanotto Eberhardt L, Angelo Balbinot R, Sartori Balbinot S, Soldera J. Predicting mortality for hepatorenal syndrome with liver‐specific scores. GastroHep. 2020;2:336-343. [DOI] [Full Text] |
| 51. | Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, Mookerjee RP. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 52. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 53. | Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 54. | Radbruch L, De Lima L, Knaul F, Wenk R, Ali Z, Bhatnaghar S, Blanchard C, Bruera E, Buitrago R, Burla C, Callaway M, Munyoro EC, Centeno C, Cleary J, Connor S, Davaasuren O, Downing J, Foley K, Goh C, Gomez-Garcia W, Harding R, Khan QT, Larkin P, Leng M, Luyirika E, Marston J, Moine S, Osman H, Pettus K, Puchalski C, Rajagopal MR, Spence D, Spruijt O, Venkateswaran C, Wee B, Woodruff R, Yong J, Pastrana T. Redefining Palliative Care-A New Consensus-Based Definition. J Pain Symptom Manage. 2020;60:754-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 535] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 55. | Verma M, Bakitas MA. Creating Effective Models for Delivering Palliative Care in Advanced Liver Disease. Curr Hepatol Rep. 2021;20:43-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Verma M, Tapper EB, Singal AG, Navarro V. Nonhospice Palliative Care Within the Treatment of End-Stage Liver Disease. Hepatology. 2020;71:2149-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Tandon P, Walling A, Patton H, Taddei T. AGA Clinical Practice Update on Palliative Care Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2021;19:646-656.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Walling AM, Schreibeis-Baum H, Pimstone N, Asch SM, Robinson L, Korlekar S, Lorenz K, Nwajuaku T, Rosenfeld K. Proactive case finding to improve concurrently curative and palliative care in patients with end-stage liver disease. J Palliat Med. 2015;18:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Oliveira HM, Miranda HP, Rego F, Nunes R. Palliative care and end stage liver disease: A cohort analysis of palliative care use and factors associated with referral. Ann Hepatol. 2024;29:101518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 61. | Kelly SG, Campbell TC, Hillman L, Said A, Lucey MR, Agarwal PD. The Utilization of Palliative Care Services in Patients with Cirrhosis who have been Denied Liver Transplantation: A Single Center Retrospective Review. Ann Hepatol. 2017;16:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 62. | Tombazzi CR, Howe CF, Slaughter JC, Obstein KL. Rate of and Factors Associated with Palliative Care Referral among Patients Declined for Liver Transplantation. J Palliat Med. 2022;25:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Shinall MC Jr, Karlekar M, Martin S, Gatto CL, Misra S, Chung CY, Porayko MK, Scanga AE, Schneider NJ, Ely EW, Pulley JM, Jerome RN, Dear ML, Conway D, Buie R, Liu D, Lindsell CJ, Bernard GR. COMPASS: A Pilot Trial of an Early Palliative Care Intervention for Patients With End-Stage Liver Disease. J Pain Symptom Manage. 2019;58:614-622.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 64. | Haire E, Mann M, Yeoman A, Atkinson C, Wright M, Noble S. Supportive and palliative care needs in advanced non-malignant liver disease: systematic review. BMJ Support Palliat Care. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Peng JK, Chang HH, Higginson IJ, Gao W. Intensive care utilization in patients with end-stage liver disease: A population-based comparative study of cohorts with and without comorbid hepatocellular carcinoma in taiwan. EClinicalMedicine. 2020;22:100357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 66. | Caulfield T, Murdoch B, Sapir-Pichhadze R, Keown P. Policy Challenges for Organ Allocation in an Era of "Precision Medicine". Can J Kidney Health Dis. 2020;7:2054358120912655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Rodrigue JR, Dimitri N, Reed A, Antonellis T, Hanto DW, Curry M. Quality of life and psychosocial functioning of spouse/partner caregivers before and after liver transplantation. Clin Transplant. 2011;25:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Low J, Davis S, Vickerstaff V, Greenslade L, Hopkins K, Langford A, Marshall A, Thorburn D, Jones L. Advanced chronic liver disease in the last year of life: a mixed methods study to understand how care in a specialist liver unit could be improved. BMJ Open. 2017;7:e016887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Adejumo AC, Kim D, Iqbal U, Yoo ER, Boursiquot BC, Cholankeril G, Wong RJ, Kwo PY, Ahmed A. Suboptimal Use of Inpatient Palliative Care Consultation May Lead to Higher Readmissions and Costs in End-Stage Liver Disease. J Palliat Med. 2020;23:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Goldberg DS, French B, Sahota G, Wallace AE, Lewis JD, Halpern SD. Use of Population-based Data to Demonstrate How Waitlist-based Metrics Overestimate Geographic Disparities in Access to Liver Transplant Care. Am J Transplant. 2016;16:2903-2911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 71. | Langberg KM, Kapo JM, Taddei TH. Palliative care in decompensated cirrhosis: A review. Liver Int. 2018;38:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Jung HJ, Park JY. Life-Sustaining Treatment in End-Stage Liver Disease Patients: Patients' Decisions and Results. Hanguk Hosupisu Wanhwa Uiryo Hakhoe Chi. 2020;23:85-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Holden JH, Shamseddeen H, Johnson AW, Byriel B, Subramoney K, Cheng YW, Saito A, Ghabril M, Chalasani N, Sachs GA, Orman ES. Palliative Care and Hospice Referrals in Patients with Decompensated Cirrhosis: What Factors Are Important? J Palliat Med. 2020;23:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Campbell M, Grande G, Wilson C, Caress AL, Roberts D. Exploring differences in referrals to a hospice at home service in two socio-economically distinct areas of Manchester, UK. Palliat Med. 2010;24:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Verma M, Taddei T, Volk M, Navarro V. Advance Care Planning: A Convincing Argument to Make It Part of Liver Transplant Evaluation. Liver Transpl. 2021;27:461-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Brown CL, Hammill BG, Qualls LG, Curtis LH, Muir AJ. Significant Morbidity and Mortality Among Hospitalized End-Stage Liver Disease Patients in Medicare. J Pain Symptom Manage. 2016;52:412-419.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, Chai E, Nelson JE. Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. JAMA. 2016;316:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 78. | Soldera J. Artificial intelligence as a prognostic tool for gastrointestinal tract pathologies. Med Vozandes. 2023;34:9-14. |
| 79. | Ballotin VR, Bigarella LG, Soldera J. Deep learning applied to the imaging diagnosis of hepatocellular carcinoma. Artif Intell Gastrointest Endosc. 2021;2:127-135. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 80. | Soldera J, Tomé F, Corso LL, Machado Rech M, Ferrazza AD, Terres AZ, Cini BT, Eberhardt LZ, Balensiefer JIL, Balbinot RS, Muscope ALF, Longen ML, Schena B, Rost GL Jr. , Furlan RG, Balbinot RA, Balbinot SS. Use of a Machine Learning Algorithm to Predict Rebleeding and Mortality for Oesophageal Variceal Bleeding in Cirrhotic Patients. EMJ Gastroenterol. 2020;9:46-48. |
| 81. | Rech MM, Corso LL, Dal Bó EF, Tomé F, Terres AZ, Balbinot RS, Muscope AL, Eberhardt LZ, Cini BT, Balensiefer JI, Schena B, Longen ML, Rost GL Jr, Balbinot SS, Balbinot RA, Soldera J. Prospective Validation of a Neural Network Model for the Prediction of 1-Year Mortality in Cirrhotic Patients with Acute Esophageal Variceal Bleeding. Gastroenterology. 2023;. |
| 82. | Soldera J, Tomé F, Corso LL, Ballotin VR, Bigarella LG, Balbinot RS, Rodriguez S, Brandão AB, Hochhegger B. Predicting 30 and 365-Day Mortality After Liver Transplantation Using a Machine Learning Algorithm. Gastroenterology. 2021;160:S-789. [DOI] [Full Text] |
| 83. | Soldera J, Corso LL, Rech MM, Ballotin VR, Bigarella LG, Tomé F, Moraes N, Balbinot RS, Rodriguez S, Brandão ABM, Hochhegger B. Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: A cohort study. World J Hepatol. 2024;16:193-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (1)] |
| 84. | Chongo G, Soldera J. Use of machine learning models for the prognostication of liver transplantation: A systematic review. World J Transplant. 2024;14:88891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Reference Citation Analysis (1)] |