Published online Sep 20, 2024. doi: 10.5662/wjm.v14.i3.93854
Revised: April 22, 2024
Accepted: May 11, 2024
Published online: September 20, 2024
Processing time: 110 Days and 17.3 Hours
Low back pain (LBP) is a prevalent issue that orthopedic surgeons frequently address in the outpatient setting. LBP can arise from various causes, with stiffness in the paraspinal muscles being a notable contributor. The administration of Botulinum toxin type A (BoNT-A) has been found to alleviate back pain by relaxing these stiff muscles. While BoNT-A is approved for use in numerous conditions, a limited number of randomized clinical trials (RCTs) validate its efficacy specifically for treating LBP.
To study the safety and the efficacy of BoNT-A in minimizing pain and improving functional outcomes in patients of chronic LBP (CLBP).
In this RCT, adults aged 18-60 years with mechanical LBP persisting for at least six months were enrolled. Participants were allocated to either the Drug group, receiving 200 Ipsen Units (2 mL) of BoNT-A, or the Control group, which received a 2 mL placebo. Over a 2-month follow-up period, both groups were assessed using the Visual Analog Scale (VAS) for pain intensity and the Oswestry Disabi
The study followed 40 patients for two months, with 20 in each group. A clinically significant reduction in pain was observed in 36 participants. There was a statistically significant decrease in both VAS and ODI scores in the groups at the end of two months. Nonetheless, when comparing the mean score changes, only the reduction in ODI scores (15 in the placebo group vs 16.5 in the drug group, clinically insignificant) was statistically significant (P = 0.012), whereas the change in mean VAS scores was not significant (P = 0.45).
The study concludes that BoNT-A does not offer a short-term advantage over placebo in reducing pain or improving LBP scores in CLBP patients.
Core Tip: This randomized clinical trial investigated Botulinum toxin type A (BoNT-A) for treating chronic low back pain (CLBP) in adults aged 18-60 years old with symptoms persisting for over six months. Participants were divided into two groups: one receiving BoNT-A and the other a placebo, with outcomes measured using the Visual Analog Scale for pain and the Oswestry Disability Index (ODI) for disability. After two months, both groups showed pain reduction, but only the decrease in ODI scores was statistically significant (but clinically insignificant). Ultimately, BoNT-A did not demonstrate a short-term advantage over placebo in reducing pain or improving disability scores in CLBP patients.
- Citation: Jain M, Khan S, Varghese P, Tripathy SK, Mangaraj M. Botulinum toxin type A for treating chronic low back pain: A double blinded randomized control study. World J Methodol 2024; 14(3): 93854
- URL: https://www.wjgnet.com/2222-0682/full/v14/i3/93854.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i3.93854
Globally, low back pain (LBP) is a leading cause of pain and disability, significantly impacting individuals' workability worldwide[1]. The influx of LBP cases in the orthopedic outpatient departments of hospitals is on the rise, now representing nearly a third of all consultations. This trend incurs a huge healthcare expenditure. While acute instances of LBP tend to resolve promptly, a notable proportion, between 30%-40%, evolves into chronic LBP (CLBP), characterized by enduring and incapacitating symptoms. CLBP adversely affects individuals' psychosocial, behavioral, and vocational aspects, undermining their productivity and quality of life[2]. Effective management of CLBP is pivotal for diminishing its morbidity and the overall financial strain on healthcare systems[3]. The correlation between the severity of LBP and lumbar stiffness, primarily attributed to the contraction of the erector spinae muscles, highlights the demand for localized muscular interventions. These interventions include physical therapy, rehabilitation exercises, infrared therapy, and Botulinum toxin type A (BoNT-A)[4-7].
BoNT-A has demonstrated efficacy in managing various musculoskeletal conditions, including cervical dystonia, cerebral palsy, and spasticity. Although a few cohort studies have reported its potential advantages for CLBP, the supporting evidence remains limited in quality, signaling an urgent need for further randomized controlled trials (RCTs) to establish more definitive conclusions[8-10]. The current literature comprises only a modest number of RCTs with inconsistent findings[11-13]. Consequently, this study aims to explore the efficacy of BoNT-A in minimizing pain and improving functional outcomes in patients suffering from CLBP, endeavoring to enrich the existing evidence on its therapeutic value.
The study received ethical clearance from the institutional ethics committee (No. T/IM-F/21-22/03) and was officially registered in a clinical trial registry (CTRI/2022/08/044530, https://ctri.nic.in/Clinicaltrials/login.php). It was conducted in the Department of Orthopaedics at our institution, targeting patients with CLBP. Participation was contin
The sample size was estimated based on a previous study by Jazayeri et al[14] in 2011. Assuming a difference of 37.5% (50% for the drug group and 12.5% for the control group, with a power of 80% and significance level of 5%, the sample size was calculated to be 40 (i.e., 20 for each group). With a dropout of 20%, a sample of 44 were included in our study:
Total sample = 16 × [P1 × (100 - P1) + P2 × (100 - P2)]/(P1 – P2)2.
The participants were allocated to either arm with an allocation ratio of 1:1. The patients and the researcher (who assessed for scoring) were both blinded. Only the person doing the intervention (not involved in collecting scores) opened the sealed envelope and gave the drugs. The patients were divided into 2 groups.
Drug arm (Group B): The BoNT-A (Botox, Allergan India private limited) was re-constituted using frozen-dried toxin and mixed with 2 mL of preservative-free 0.9% normal saline to a strength of 100 Ipsen units/mL. The mixture was drawn into a 2 mL syringe fitted with a 22-gauge needle. Four equidistant points on paraspinal muscles, 1.5-2 inches away from the midline, bilaterally, were chosen, and 50 Ipsen units were inserted at each site. Tender or trigger points were preferred, if any. Care was taken to inject the toxin into the muscles' core while avoiding its spillover into the vascular compartment. All injections were performed without electromyographic (EMG) guidance.
Control arm (Group C): A similar dose of normal saline was injected at four points in the paraspinal muscles.
Both the groups received analgesics for not more than seven days, a weekly vitamin D supplement (60 K) for 8 wk, and taught home-based isometric back strengthening exercises to be done twice daily for a period of 15 min.
Outcome measures for the patients included an evaluation of pain intensity using the visual analog scale (VAS) on a Likert scale from 1 to 10, where "1" represented the least pain and "10" was the worst pain. Additionally, physical impairment and disability were quantified using the Oswestry Disability Index (ODI) questionnaire, with scores out of 100. Assessments were conducted at the initial consultation (baseline) and at the conclusion of the treatment period (eight weeks). At the eight-week follow-up, patients were asked about the duration of pain relief, specifically if pain had recurred. A significant improvement was defined as a greater than 50% change in scores before and after treatment.
Participants were advised to refrain from using opioid medications and from undergoing any other treatments not specified in the study protocol, such as facet joint block injections, throughout the study. The safety profile of the treatment was also monitored by documenting any side effects experienced by the participants.
The data was compiled in the Excel sheet. Results were analyzed using SPSS software version 25. The normality testing was done using the Shapiro-Wilk test. The VAS and ODI scores were compared at baseline and at eight weeks using the paired t-test. The comparison among different groups was performed using the student t-test. A P value of less than 0.05 was considered significant.
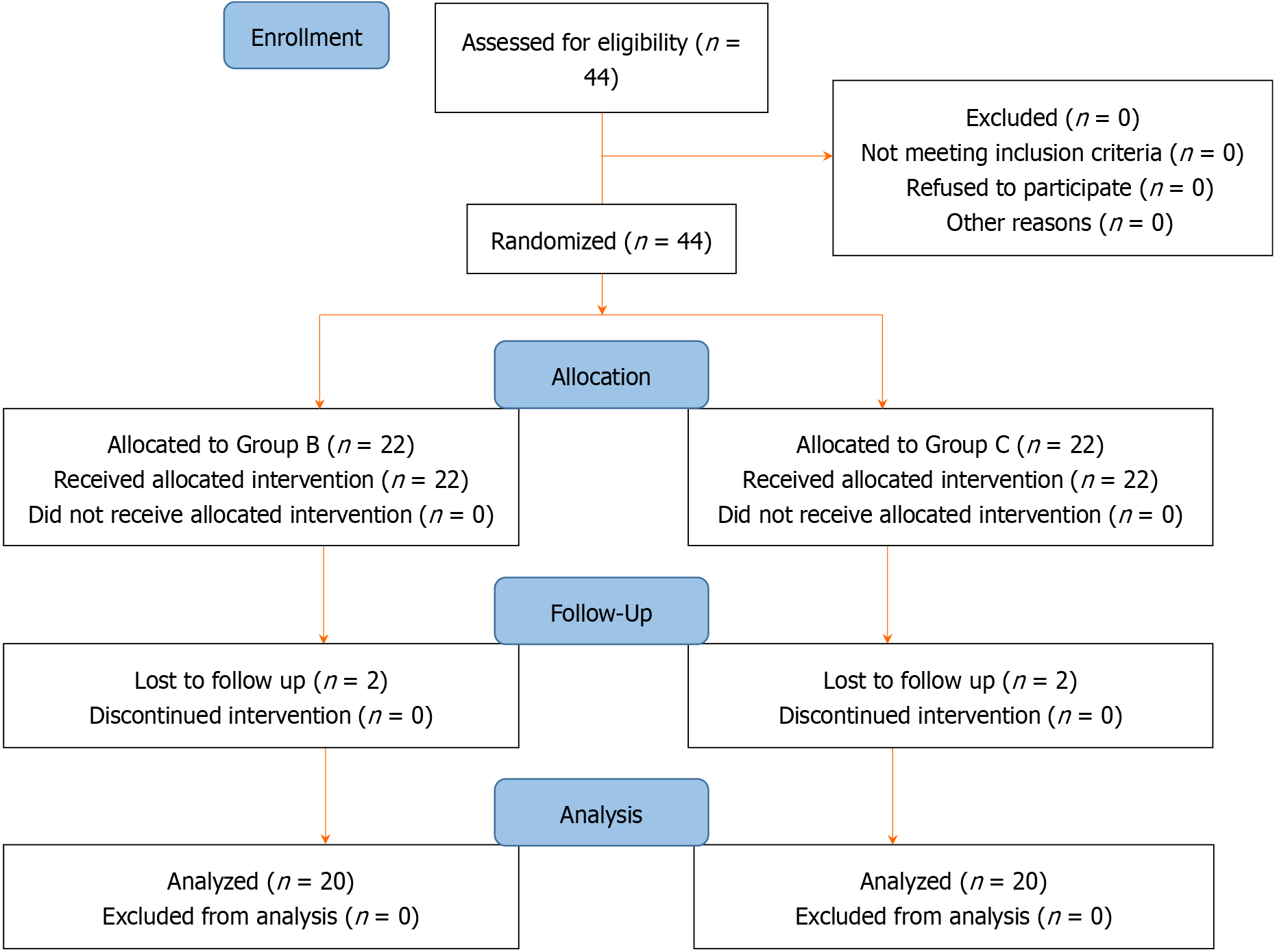
A total of 44 subjects with CLBP were included in the study. Two patients in each group did not follow up and were excluded. Forty patients were followed up for eight weeks. The details are given in Figure 1. The demographic profile of the patients was found to be similar, as depicted in Table 1. The biochemical parameters are also similar, but Vitamin D deficiency was seen in all patients of both groups (Table 2). The outcome is depicted in Table 3. All the patients had clinical reductions in pain and ODI scores at the last follow-up of eight weeks. A total of 36 patients had significant pain relief. Of these, 17 patients received BoNT-A injections, while the remaining 19 received a placebo. None of the subjects reported any adverse effects due to medication during the course of the study.
| Variables | Placebo | Botulinum toxin | P value |
| Age (mean ± SD) | 39.5 ± 7.55 | 40.62 ± 9.13 | 0.67 |
| Male:female | 9:11 | 13:7 | |
| Mean duration of pain (months) | 15.20 | 15.05 | 0.97 |
| Body mass index (mean ± SD) | 23.93 ± 4.23 | 23.94 ± 3.65 | 0.99 |
| Variables | Placebo (mean ± SD) | Botulinum toxin (mean ± SD) | P value |
| Serum calcium (mg/dL) | 9.29 ± 0.36 | 9.29 ± 0.65 | 1.00 |
| Serum alkaline phosphate (IU) | 90.1 ± 37.5 | 81.19 ± 25.20 | 0.38 |
| Serum phosphate (mg/dL) | 3.36 ± 0.61 | 3.43 ± 0.39 | 0.66 |
| Serum vitamin D (mg/dL) | 8.95 ± 4.15 | 10.05 ± 3.69 | 0.38 |
| Variables | Placebo (mean ± SD) | Botulinum toxin (mean ± SD) | ||||
| Pre | Post | P value | Pre | Post | P value | |
| VAS | 6.7 ± 0.92 | 2.85 ± 1.08 | < 0.001 | 6.25 ± 0.55 | 2.45 ± 0.99 | < 0.001 |
| ODI | 34 ± 4.58 | 19 ± 4.29 | 0.001 | 33.5 ± 3.76 | 17 ± 3.72 | 0.007 |
Both groups had significant reductions in VAS and ODI scores at the end of 8 wk (Table 3). On comparing the change in means in both the groups, it was found that there was a statistically significant reduction in ODI scores (though clinically insignificant). In contrast, the VAS score improvement was insignificant (Table 4).
| Variables | Placebo (mean ± SD) | Botulinum toxin (mean ± SD) | P value |
| Mean change in VAS | 3.85 ± 0.81 | 3.8 ± 0.89 | 0.427 |
| Mean change in ODI | 15 ± 1.50 | 16.5 ± 2.41 | 0.012 |
The cause of chronic LBP is multifactorial, but lumbar stiffness due to erector spinal muscle spasm is linked to the level of severity of LBP[5]. This reflex spasm model of back pain has been demonstrated by the EMG readings showing increased activity in the muscles exhibiting pain[15]. BoNT-A has a role as a muscle relaxant, an analgesic, and an anti-inflammatory. The mechanism of action is also diverse, from inhibiting the pain transmitters from the nerve endings and ganglions of peripheral nerves to blocking the release of acetylcholine from the neuromuscular junction[16].
BoNT-A is widely used in the management of many medical and cosmetic issues. The use of BoNT-A in cervical dystonia has yielded great results. Researchers have tried to use in several musculoskeletal ailments (refractory joint pain, tennis elbow, plantar fasciitis) with varying success[17]. Liu[18] tried its use in third transverse process syndrome and acupuncture and found it superior[18]. Fishman et al[19] successfully used it in treating piriformis syndrome in a 12-wk study[19]. However, De Andrés et al[20] compared BoNT-A with saline/bupivacaine in myofascial pain syndrome (iliopsoas and quadratus lumborum muscles) and found no significant difference[20].
Similarly, its use in chronic LBP has been variable. While the open-label trial has clearly shown some reversible beneficial effects in the short term, few RCTs have unpredictable responses, and the final verdict is far from any conclusion. In this further literature review, we chronologically study the outcome pattern in these two different categories of evidence. Jabbari et al[8] in 2006, studied 75 patients injected with BoNT-A 200-500 Ipsen units and followed up for 14 months[8]. The authors found 40 (53%) had substantial pain relief at two months, and 90% persisted at the final follow-up. In the same year, Ney et al[21] did another study in a cohort of 60 patients of CLBP wherein the authors injected 500 units of BoNT-A[21]. They found that BoNT-A significantly reduced LBP scores in about 58% of patients at the end of 8 wk, which gradually faded away with persistent results at only 16% at four and 8% in six months. Nagarajan et al[9] in 2007, conducted a study in Kuwait with eight CLBP subjects injected with 100 units of BoNT-A[9]. The authors found remarkable improvement in pain and functional scores in 63% (5/8) when followed up for 60 d. More recently, Sahoo et al[10] enrolled 19 patients with CLBP and injected them with 100 units of BoNT-A[10]. The authors found beneficial effects in them at two months, which persisted even at six months.
Foster et al[12] conducted the earliest double-blinded RCT study in the year 2001 with 31 patients of CLBP using 200 Ipsen units of BoNT-A[12]. At eight weeks, the BoNT-A group had more pain relief (9/15 vs 2/16, P = 0.009) and enhancement in the LPB functional scores (10/15 vs 3/16, P = 0.011). No patients experienced side effects. A year later, Subin et al[11] conducted a similar study wherein they compared nine patients of BoNT-A (100 Ipsen) to 10 patients of placebo and found pain reduction to be significant in the BoNT-A group (7/9 vs 0/10)[11]. About a decade later, Jazayeri et al[14] did a single-blinded RCT in 50 patients who again received 200 Ipsen units of BoNT-A[14]. The authors found that at 8 wk, patients had better pain relief (64% vs 12%, P = 0.001), and functional scores (68% vs 12%, P = 0.005) as compared to the saline group. Later, Machado et al[16] conducted a double-blinded RCT wherein 18 patients received a high dose (500-1000 units) of BoNT-A and 19 patients received normal saline[16]. The researchers only showed marginal improvement in the pain scores. Cogné et al[13] did a cross-over RCT wherein they proposed to have 60 patients; half of which were planned to receive 200 Ipsen units of BoNT-A, and another half were placebo with drugs change at 120 d[13]. The authors had to curtail their recruitment following no beneficial effect with a final size of 19. The authors concluded that there was no advantage of BoNT-A compared to placebo (30, 90, 120 d) regarding clinical outcome scores, quality of life, and spinal strength. Our study also showed no significant difference compared to the placebo regarding pain and functional scores similar to the above study. Clearly, the researchers have different dosages, with a range from 100-1000 units. The action is reversible, and therefore, the benefit has also been studied mostly in the short term (2 months), and this property has been used by Cogné et al[13] for drug crossover[13]. Only one study demonstrates benefits for up to 6 months[10].
No patient in our study had any adverse reaction. Jabbari et al[8] had 3 (4%) of patients, while Ney et al[21] had two patients with flu-like symptoms that resolved in 2-5 d[8,21]. Other researchers like Jazayeri et al[14] also did not report any complications[14].
Our study has a few limitations. CLBP is multifactorial, and this causal heterogeneity may affect the response to BoNT-A treatment. Better localization of the injected muscles with newer techniques, such as ultrasound/electromyogram, could help improve the results of BoNT-A. We have used a dose of 200 units, which can be considered inadequate in comparison to few studies that have utilized higher doses and found beneficial effects[8,16]. Nevertheless, future trials could be multicentric and conducted with larger doses to document any constructive effect. The strength of this study is it is an RCT. A negative finding of our study can help clinicians and researchers to consider against using a costly drug like BoNT-A for treating CLBP.
BoNT-A is found to have no advantage over the placebo in the short term for relieving pain and LBP scores in CLBP.
| 1. | Rossignol M, Abenhaim L, Séguin P, Neveu A, Collet JP, Ducruet T, Shapiro S. Coordination of primary health care for back pain. A randomized controlled trial. Spine (Phila Pa 1976). 2000;25:251-8; discussion 258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Foster NE, Thomas E, Bishop A, Dunn KM, Main CJ. Distinctiveness of psychological obstacles to recovery in low back pain patients in primary care. Pain. 2010;148:398-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1395] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 4. | Mellin G. Decreased joint and spinal mobility associated with low back pain in young adults. J Spinal Disord. 1990;3:238-243. [PubMed] |
| 5. | Chou R, Côté P, Randhawa K, Torres P, Yu H, Nordin M, Hurwitz EL, Haldeman S, Cedraschi C. The Global Spine Care Initiative: applying evidence-based guidelines on the non-invasive management of back and neck pain to low- and middle-income communities. Eur Spine J. 2018;27:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Ansari NN, Naghdi S, Naseri N, Entezary E, Irani S, Jalaie S, Hasson S. Effect of therapeutic infra-red in patients with non-specific low back pain: a pilot study. J Bodyw Mov Ther. 2014;18:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Difazio M, Jabbari B. A focused review of the use of botulinum toxins for low back pain. Clin J Pain. 2002;18:S155-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Jabbari B, Ney J, Sichani A, Monacci W, Foster L, Difazio M. Treatment of refractory, chronic low back pain with botulinum neurotoxin A: an open-label, pilot study. Pain Med. 2006;7:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Nagarajan V, Al-Shubaili A, Ayad YM, Alexander J, Al-Ramezi K. Low back ache treatment with botulinum neurotoxin type A. Local experience in Kuwait. Med Princ Pract. 2007;16:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Sahoo J, Jena D, Viswanath A, Barman A. Injection Botulinum Toxin A in Treatment of Resistant Chronic Low Back Pain: A Prospective Open-Label Study. Cureus. 2021;13:e17811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Subin B, Saleemi S, Morgan G, Zavisca F, Cork R. Treatment of Chronic Low Back Pain by Local Injection of BoNT-A. Internet J Pain, Symptom Control Palliat Care. 2002;4:1-5. |
| 12. | Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology. 2001;56:1290-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Cogné M, Petit H, Creuzé A, Liguoro D, de Seze M. Are paraspinous intramuscular injections of botulinum toxin a (BoNT-A) efficient in the treatment of chronic low-back pain? A randomised, double-blinded crossover trial. BMC Musculoskelet Disord. 2017;18:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Jazayeri SM, Ashraf A, Fini HM, Karimian H, Nasab MV. Efficacy of botulinum toxin type a for treating chronic low back pain. Anesth Pain Med. 2011;1:77-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Zedka M, Prochazka A, Knight B, Gillard D, Gauthier M. Voluntary and reflex control of human back muscles during induced pain. J Physiol. 1999;520 Pt 2:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Machado D, Kumar A, Jabbari B. Abobotulinum Toxin A in the Treatment of Chronic Low Back Pain. Toxins (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Singh JA. Use of botulinum toxin in musculoskeletal pain. F1000Res. 2013;2:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Liu Z. [Botulinum toxin A (BTX-A) point injection for treatment of the third lumbar transverse process syndrome]. Zhongguo Zhen Jiu. 2008;28:337-339. [PubMed] |
| 19. | Fishman LM, Anderson C, Rosner B. BOTOX and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002;81:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | De Andrés J, Adsuara VM, Palmisani S, Villanueva V, López-Alarcón MD. A double-blind, controlled, randomized trial to evaluate the efficacy of botulinum toxin for the treatment of lumbar myofascial pain in humans. Reg Anesth Pain Med. 2010;35:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Ney JP, Difazio M, Sichani A, Monacci W, Foster L, Jabbari B. Treatment of chronic low back pain with successive injections of botulinum toxin a over 6 months: a prospective trial of 60 patients. Clin J Pain. 2006;22:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |