Published online Sep 20, 2024. doi: 10.5662/wjm.v14.i3.92807
Revised: April 19, 2024
Accepted: May 11, 2024
Published online: September 20, 2024
Processing time: 140 Days and 1.4 Hours
The lymphocyte to monocyte ratio (LMR) is considered a marker of systemic infla
To investigate the predictive role of LMR in diabetic coronary artery disease pa
This cross-sectional study was conducted at tertiary care super-specialty hospital at New Delhi, India. A total of 200 angiography-proven coronary artery disease (CAD) patients were enrolled and grouped into two categories: Group I [CAD patients with type 2 diabetes mellitus (T2DM) and glycated hemoglobin (HbA1c) levels ≥ 6.5%], and Group II (CAD patients without T2DM and HbA1c levels < 6.5%). Serum lipoproteins, HbA1c, and complete blood count of enrolled patients were analyzed using fully automatic analyzers.
The logistic regression analysis showed an odds ratio of 1.48 (95%CI: 1.28-1.72, P < 0.05) for diabetic coronary artery disease patients (Group I) in unadjusted model. After adjusting for age, gender, diet, smoking, and hypertension history, the odds ratio increased to 1.49 (95%CI: 1.29-1.74, P < 0.01) in close association with LMR. Further adjustment for high cholesterol and triglycerides yielded the same odds ratio of 1.49 (95%CI: 1.27-1.75, P < 0.01). Receiver operating characteristic curve analysis revealed 74% sensitivity, 64% specificity, and 0.74 area under the curve (95%CI: 0.67-0.80, P < 0.001), suggesting moderate predictive accuracy for diabetic CAD patients.
LMR showed positive association with diabetic coronary artery disease, with moderate predictive accuracy. These findings have implications for improving CAD management in diabetics, necessitating further research and targeted interventions.
Core Tip: The lymphocyte to monocyte ratio (LMR) proves to be a potential marker of systemic inflammation in cardio
- Citation: Dabla PK, Shrivastav D, Mehra P, Mehta V. Role of lymphocyte-to-monocyte ratio as a predictive marker for diabetic coronary artery disease: A cross-sectional study. World J Methodol 2024; 14(3): 92807
- URL: https://www.wjgnet.com/2222-0682/full/v14/i3/92807.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i3.92807
In the general population, the coronary artery disease (CAD) is a major cause of morbidity and mortality worldwide. In India, the prevalence of cardiovascular disease among adults ≥ 45 years was 5.2% in 2019[1]. The hyperglycemia contri
The lymphocyte-to-monocyte ratio (LMR) is a newly recognized systemic inflammatory marker and demonstrated its usefulness as an indicator of the systemic inflammatory response and its potential as a prognostic factor in different types of cancer and cardiac diseases[7]. The emerging evidence from the experimental and clinical studies supports that LMR could be an independent risk factor for CAD[8]. The LMR has also been shown to correlate with the in-hospital death rate among patients experiencing acute type A aortic dissection[9]. Similarly, some studies have indicated a relationship between LMR and cardiovascular disease as well as adverse cardiovascular events[10-12]. However, there is paucity of data regarding the relationship of LMR in diabetic and non-diabetic CAD. Thus, the aim of this study is to investigate the association of LMR with diabetic CAD patients compared with non-diabetic CAD patients.
This cross-sectional study was conducted at the Department of Biochemistry at G.B. Pant Institute of Postgraduate Medical Education and Research in New Delhi, India. A total of 200 patients with angiographically proven CAD were enrolled from Cardiology outpatient and in-patient department. The study was conducted in accordance with internationally accepted recommendations for clinical investigation (Declaration of Helsinki of the World Medical Association, revised October 2013). The study was approved by the institutional ethical committee of Maulana Azad Medical College and associated hospitals, Delhi, India.
The inclusion criteria for the study were adult patients aged over 18 years, regardless of gender, who had been diagnosed with coronary heart disease based on resting electrocardiography and invasive coronary angiography with more than 50% stenosis in at least one coronary artery[13]. On the other hand, patients below the age of 18 years, patients with renal and hepatic impairment, patients who had undergone previous procedures such as coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, and stenting were excluded from this study.
The study subjects were required to complete a questionnaire to gather demographic information related to their age, gender, dietary habits, addictive habits, and history of diabetes, hypertension. Additionally, for patients with T2DM, the questionnaire also collected information regarding, duration of diabetes and symptoms experienced at the time of diagnosis.
Venous blood samples were collected from each participant under proper aseptic conditions. Three milliliters of blood were transferred to an EDTA vial for glycated hemoglobin (HbA1c) analysis, and the remaining sample was transferred to a citrate vial for blood sugar analysis. The enrolled patients were categorized into two groups based on their previous diabetes diagnosis and HbA1c levels: Group I consisted of patients with HbA1c levels ≥ 6.5% and a history of diabetes, while Group II consisted of individuals without a history of diabetes and HbA1c levels below 6.5%[6]. Lipid profile and complete blood count were measured by fully automatic analyzers.
The atherogenic indexes were calculated using the following formulas: NHC [non-high density lipoprotein (HDL) cholesterol] = Serum total cholesterol-serum high-density lipoprotein cholesterol (HDL-C). AC (Atherogenic coefficient) = Non-high-density lipoprotein cholesterol (NHC)/serum HDL-C. AIP (Atherogenic index of plasma) = Log (serum triglyceride/serum HDL-C). CRI-I (Castelli's Risk Index I) = Serum total cholesterol/serum HDL-C.CRI-II (Castelli's Risk Index II) = Serum low density lipoprotein cholesterol/serum HDL-C.
The data was analyzed using the statistical program for social science (SPSS) version 21, IBM Corp., Chicago, United States. The normality of the data was checked using the Shapiro-Wilk test. Student
The mean age of Group I (81 males and 19 females) and Group II (89 males and 11 females) were 54.2 ± 10.2 and 53.2 ± 10.3 years, respectively. Regarding dietary habits, most of the patients were non-vegetarian, 81 (81%) patients in Group I and 60 (60%) in Group II, P = 0.004. For addiction, the frequency of smokers were significantly higher in Group I (60%) compared with Group II (25%). Regarding history of hypertension, Group I had significantly higher (P = 0.003) number of hypertensive patients (39%) than Group II (20%). In biochemical parameters, the diabetes specific parameters i.e., HbA1c and random blood sugar levels were significantly higher (P < 0.001) in Group I. Further we observed significant differences (P < 0.05) in serum levels of total cholesterol (TC), triglycerides (TG), and very low-density lipoprotein cholesterol (VLDL) and non-HDL cholesterol (non-HDL-C) between Group I and Group II. In terms of white blood cell counts, Group I had significantly higher lymphocyte counts (3.49 ± 1.68 × 109/L) and LMR (6.19 ± 4.14) than Group II (2.46 ± 1.27 × 109/L) and LMR (4.19 ± 2.78), (P = 0.001, for both).
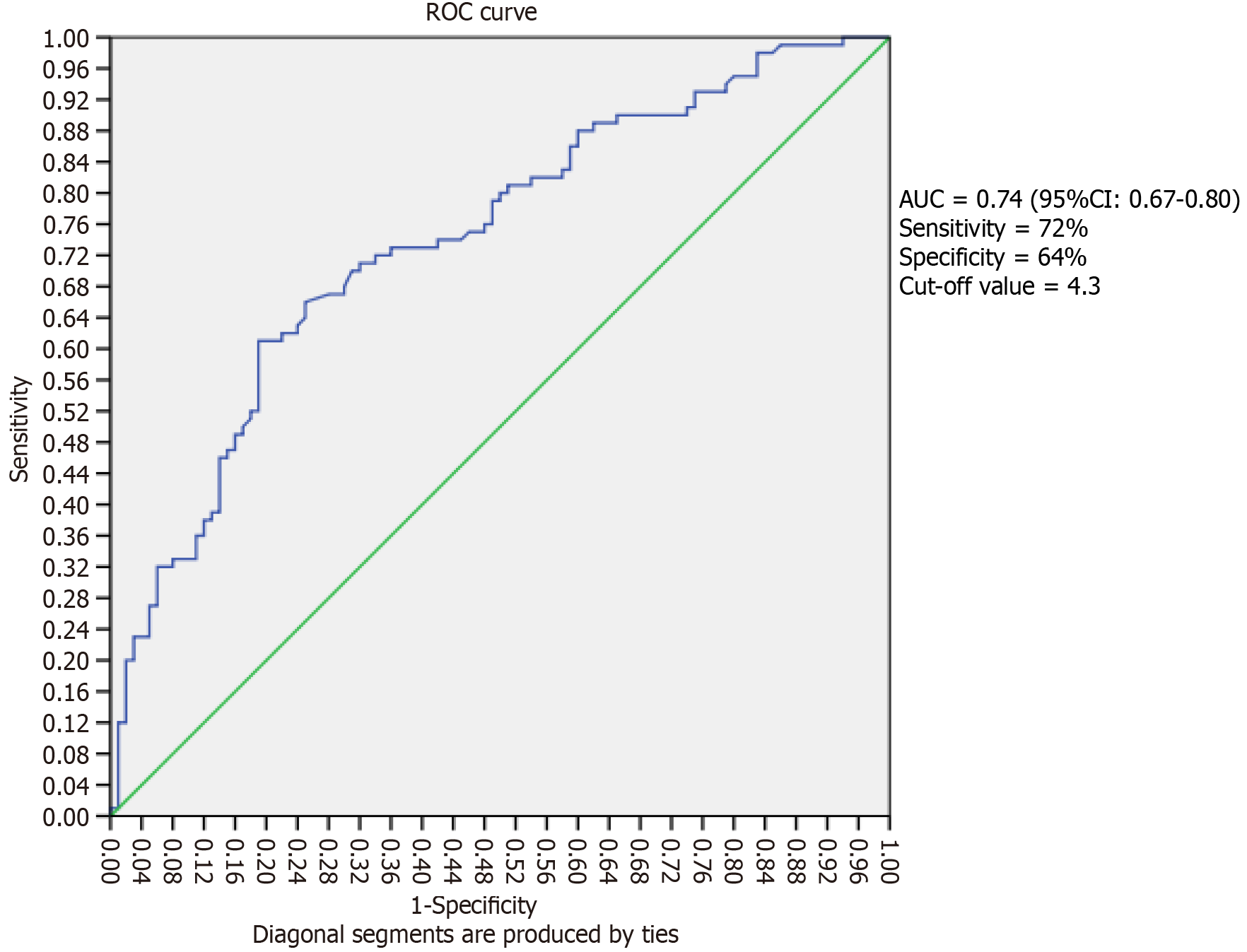
The comparison of demographic characteristics and biochemical parameters is given in Table 1. On comparison of lipid indices, the AIP, AC and CRI-I were significantly higher (P < 0.001) in Group I compared with Group II (Table 2). Logistic regression was used to estimate the odds ratio for CAD in T2DM; in unadjusted conditions the LMR increased the risk of CAD in T2DM, odd ratio 1.48 (95%CI: 1.28-1.72, P = 0.01). Further on adjusted cofounding variables model-1 (age, gender, diet, smoking, history of hypertension), the LMR increases the risk of CAD in T2DM with the odds ratio 1.49 (95%CI: 1.29-1.74, P = 0.001). Also, on adjusting the variable model 1 along with high TC and high TG, the odds ratio was 1.49 (95%CI: 1.27-1.75, P = 0.001) (Table 3). Furthermore, we observed the ROC curve analysis for estimating the threshold cut-off value of LMR for CAD with T2DM was 4.3. The area of the curve is 0.74 (95%CI: 0.67-0.80, P < 0.001) with sensitivity of 72% and specificity of 64%. The cut off value was 4.3 for LMR in diabetic coronary artery disease (Figure 1).
| Demographic parameters | Group I | Group II | P value |
| Age (yr) (mean ± SD) | 54.2 ± 10.2 | 53.2 ± 10.3 | 0.473 |
| Gender (male/female) | 81/19 | 89/11 | 0.82 |
| Diet (non-veg/veg) | 81/19 | 60/40 | 0.004 |
| Smoker (yes/no) | 65/35 | 50/50 | 0.022 |
| Alcoholic (yes/no) | 26/74 | 25/75 | 0.500 |
| Tobacco chewer (yes/no) | 49/51 | 39/61 | 0.100 |
| Hypertension history (hypertensive/normotensive) | 39/61 | 20/80 | 0.003 |
| RBS (mg/dL) (mean ± SD) | 220.63 ± 100.26 | 120.61 ± 42.52 | 0.001 |
| HbA1c (mean ± SD) | 8.83 ± 2.03 | 5.65 ± 0.38 | 0.001 |
| TC (mg/dL) (mean ± SD) | 154.86 ± 51.04 | 139.54 ± 55.23 | 0.006 |
| TG (mg/dL) (mean ± SD) | 176.03 ± 99.57 | 124.47 ± 67.05 | 0.001 |
| HDL (mg/dL) (mean ± SD) | 33.82 ± 9.02 | 35.15 ± 11.28 | 0.564 |
| LDL (mg/dL) (mean ± SD) | 84.74 ± 38.78 | 80.92 ± 43.55 | 0.278 |
| VLDL (mg/dL) (mean ± SD) | 34.97 ± 21.77 | 25.50 ± 14.56 | 0.001 |
| Hb in gm (mean ± SD) | 13.49 ± 2.10 | 13.87 ± 1.90 | 0.17 |
| WBC in 109/L (mean ± SD) | 9.92 ± 3.5 | 10.75 ± 7.5 | 0.75 |
| Neutrophils, 109/L (mean ± SD) | 6.25 ± 2.99 | 6.92 ± 4.85 | 0.64 |
| Lymphocytes, 109/L (mean ± SD) | 3.49 ± 1.68 | 2.46 ± 1.27 | 0.001 |
| Monocytes, 109/L (mean ± SD) | 0.58 ± 0.26 | 0.75 ± 0.66 | 0.13 |
| Eosinophils, 109/L (mean ± SD) | 0.27 ± 0.19 | 0.39 ± 0.59 | 0.572 |
| LMR (mean ± SD) | 6.19 ± 4.14 | 4.19 ± 2.78 | 0.001 |
| Atherogenic index | Group I | Group II | P value |
| Atherogenic index of plasma (mean ± SD) | 0.67 ± 0.24 | 0.51 ± 0.26 | 0.001 |
| Castelli's risk index I (mean ± SD) | 4.88 ± 1.98 | 4.37 ± 3.42 | 0.001 |
| Castelli's risk index II (mean ± SD) | 2.70 ± 1.50 | 2.60 ± 2.77 | 0.115 |
| Atherogenic coefficient (mean ± SD) | 3.88 ± 1.98 | 3.37 ± 3.42 | 0.001 |
| Non-HDL-C (mean ± SD) | 121.27 ± 50.39 | 104.38 ± 54.59 | 0.002 |
| Model | Exp(B) | 95%CI | P value | |
| Lower | Upper | |||
| LMR (model 1) | 1.48 | 1.28 | 1.72 | 0.001 |
| LMR + (model 1 + age, gender, diet, smoking, history of hypertension) | 1.49 | 1.29 | 1.74 | 0.001 |
| LMR + (model 2 + high TC, high TG) | 1.49 | 1.27 | 1.75 | 0.001 |
| Model 1: Unadjusted | ||||
| Model 2: Age + gender + diet + smoking + history of hypertension + model 1 | ||||
| Model 3: Model 2 + high TC, high TG | ||||
This study shows that the LMR was higher in diabetic CAD patients compared with non-diabetic CAD patients. The LMR ratio signifies inflammation and is likely a marker of atherosclerotic burden especially in the presence of multiple atherosclerotic risk factors. Notably, even after adjusting for confounding variables, the association of LMR with CAD remained statistically significant. This observation suggests that LMR may be a marker of higher inflammation along with higher atherosclerotic burden, ultimately signifying poor prognosis.
CAD is a major cause of mortality and morbidity worldwide and is increasingly becoming a major public health concern in the developing countries[14]. The atherosclerotic plaque formation and progression to CAD is primarily attributed to atherosclerosis, chronic inflammation, and endothelial dysfunction[15]. In the atherosclerotic process the inflammation plays an active role. However, it is unclear that which cell is primarily responsible for the initiation of these cascade processes.
In our study we found that diabetic CAD group had higher frequency of non-vegetarians, smokers and hypertensive individuals. Similar to our data, non-vegetarian diet, smoking, and hypertension have been shown to increase the risk of CAD in diabetes[16-18]. Studies show that elevated lipid levels are prevalent in diabetic CAD patients with high HbA1c levels, and they have more coronary arteries involved in atherosclerosis and often require coronary artery bypass graft surgery as a treatment option[19-20]. In our study, we observed similar results which show that the diabetic CAD patients have significantly elevated levels of diabetes specific parameters (blood sugar and HbA1c) and lipid profile (TC, TG, and VLDL).
We observed a significant increase in the risk of diabetic CAD associated with LMR. Importantly, this association remained statistically significant even after adjusting for confounding variables. The results were consistent with previous research including the study by Gong et al[12] which showed independent positive association between LMR and severity of coronary artery disease and suggested LMR could be a predictive biomarker for CAD. Hua et al[20] revealed that increased levels of subtypes of WBCs were positively associated with high risk of death in CAD. Further, we observed the utility of LMR as a predictor of diabetic coronary artery disease and found LMR cut-off of 4.3 can predict the presence of diabetic coronary artery disease in diabetics with a sensitivity of 74% and specificity of 64%. Several studies have highlighted the significance of LMR (lymphocyte-to-monocyte ratio) as a predictive factor for coronary artery disease. Gong et al[12] also reported that LMR value greater than 5.06 could predict atherosclerotic CAD even before angiography. Additionally, Si et al[8] identified a LMR value of 4.8 or lower as a novel and independent risk factor for CAD. Furthermore, Kose et al[21] suggested that LMR, an easily measurable and cost-effective laboratory parameter, exhibited a significant association with the presence of CAD and high SYNTAX scores in patients with stable angina pectoris. Furthermore, LMR is linked to the process of left ventricle remodeling, the recovery of the myocardium, the buildup of myofibroblasts, and the formation of new blood vessels[22].
Thus, we investigated the pivotal role of the LMR as a prognostic factor in diabetic patients with CAD. We revealed the compelling insights, particularly helping in understanding the prognosis and implications of this ratio in diabetic and non-diabetic CAD patients. It is noteworthy to emphasize that there is paucity of available data for the prognostic significance of the LMR ratio in both diabetic and non-diabetic CAD patients. Nevertheless, our findings underscore a substantial disparity in the LMR ratio between these two groups. This significant difference of LMR ratio between both diabetic and non-diabetic CAD patients highlights the burden of systemic inflammation in CAD patients. The results from the AUC analysis underscore the efficacy of the LMR ratio as a predictive marker in diabetic CAD subgroup. Further, ready availability and easy access for WBC count also adds to its value. The differential patterns observed in the LMR ratio between diabetic and non-diabetic CAD cohorts highlight the interplay between immune responses and CAD pro
This study investigated the role of the LMR among diabetic and non-diabetic coronary artery disease patients. The results emphasize the importance of inflammation, lipid profile, and LMR in the development and progression of CAD in individuals with diabetes. Risk factors such as non-vegetarian diet, smoking, hypertension, elevated triglyceride levels, low HDL-C levels, and high LMR were associated with a higher risk of CAD in diabetic patients. The study also demonstrated that LMR is higher in diabetic CAD group compared with non-diabetic CAD, suggesting its usefulness for risk assessment. These findings have implications for improving CAD management in diabetic patients and call for further research regarding the significance and mechanism of increased LMR in diabetic CAD subgroup.
| 1. | Kodali NK, Bhat LD, Phillip NE, Koya SF. Prevalence and associated factors of cardiovascular diseases among men and women aged 45 years and above: Analysis of the longitudinal ageing study in India, 2017-2019. Indian Heart J. 2023;75:31-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Shrivastav D, Dabla PK, Singh DD, Mehta V. Type 2 diabetes mellitus and coronary artery stenosis: a risk pattern association study. Explor Med. 2023;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481-3488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1182] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 4. | Khalid U, Hansen PR, Gislason GH, Lindhardsen J, Kristensen SL, Winther SA, Skov L, Torp-Pedersen C, Ahlehoff O. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Gurm HS, Bhatt DL, Gupta R, Ellis SG, Topol EJ, Lauer MS. Preprocedural white blood cell count and death after percutaneous coronary intervention. Am Heart J. 2003;146:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Núñez J, Miñana G, Bodí V, Núñez E, Sanchis J, Husser O, Llàcer A. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18:3226-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Gary T, Pichler M, Belaj K, Eller P, Hafner F, Gerger A, Brodmann M. Lymphocyte-to-monocyte ratio: a novel marker for critical limb ischemia in PAOD patients. Int J Clin Pract. 2014;68:1483-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Si Y, Liu J, Shan W, Zhang Y, Han C, Wang R, Sun L. Association of lymphocyte-to-monocyte ratio with total coronary plaque burden in patients with coronary artery disease. Coron Artery Dis. 2020;31:650-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Lin Y, Peng Y, Chen Y, Li S, Huang X, Zhang H, Jiang F, Chen Q. Association of lymphocyte to monocyte ratio and risk of in-hospital mortality in patients with acute type A aortic dissection. Biomark Med. 2019;13:1263-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Zhao Y, Hao C, Bo X, Lu Z, Qian H, Chen L. The prognostic value of admission lymphocyte-to-monocyte ratio in critically ill patients with acute myocardial infarction. BMC Cardiovasc Disord. 2022;22:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Kiris T, Çelik A, Variş E, Akan E, Akyildiz ZI, Karaca M, Nazli C, Dogan A. Association of Lymphocyte-to-Monocyte Ratio With the Mortality in Patients With ST-Elevation Myocardial Infarction Who Underwent Primary Percutaneous Coronary Intervention. Angiology. 2017;68:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Gong S, Gao X, Xu F, Shang Z, Li S, Chen W, Yang J, Li J. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine (Baltimore). 2018;97:e12813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Juan-Salvadores P, Jiménez Díaz VA, Iglesia Carreño C, Guitián González A, Veiga C, Martínez Reglero C, Baz Alonso JA, Caamaño Isorna F, Iñiguez Romo A. Coronary Artery Disease in Very Young Patients: Analysis of Risk Factors and Long-Term Follow-Up. J Cardiovasc Dev Dis. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35:72-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 679] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 15. | Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 293] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 16. | Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence. Curr Atheroscler Rep. 2012;14:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Yang Y, Peng N, Chen G, Wan Q, Yan L, Wang G, Qin Y, Luo Z, Tang X, Huo Y, Hu R, Ye Z, Qin G, Gao Z, Su Q, Mu Y, Zhao J, Chen L, Zeng T, Yu X, Li Q, Shen F, Zhang Y, Wang Y, Deng H, Liu C, Wu S, Yang T, Li M, Xu Y, Xu M, Zhao Z, Wang T, Lu J, Bi Y, Wang W, Ning G, Zhang Q, Shi L. Interaction between smoking and diabetes in relation to subsequent risk of cardiovascular events. Cardiovasc Diabetol. 2022;21:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Yen FS, Wei JC, Chiu LT, Hsu CC, Hwu CM. Diabetes, hypertension, and cardiovascular disease development. J Transl Med. 2022;20:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Hegde SS, Mallesh P, Yeli SM, Gadad VM, M GP. Comparitive angiographic profile in diabetic and non-diabetic patients with acute coronary syndrome. J Clin Diagn Res. 2014;8:MC07-MC10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hua Y, Sun JY, Lou YX, Sun W, Kong XQ. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int J Cardiol. 2023;379:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 21. | Kose N, Akin F, Yildirim T, Ergun G, Altun I. The association between the lymphocyte-to-monocyte ratio and coronary artery disease severity in patients with stable coronary artery disease. Eur Rev Med Pharmacol Sci. 2019;23:2570-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 22. | Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |