Published online Sep 20, 2024. doi: 10.5662/wjm.v14.i3.92344
Revised: May 1, 2024
Accepted: May 22, 2024
Published online: September 20, 2024
Processing time: 154 Days and 4.9 Hours
Immunization is a key component of primary health care and an indisputable human right. Vaccines are critical to the prevention and control of infectious disease outbreaks. The coronavirus disease 2019 (COVID-19) pandemic and asso
To evaluate the immunization coverage among 12-23-month-old children in the rural areas of Community Health Centre (CHC) Dighal and to determine the factors influencing the existing immunization coverage.
A coverage evaluation survey was conducted according to the 30-cluster sampling technique, which is the standard methodology for such surveys devised by World Health Organization. A total of 300 children aged 12-23 months were included, whose immunization details were noted from their immunization cards.
Full immunization rate was noted in 86.7% of the children, with partial and non-immunized children accounting for 9% and 4.3% respectively. The full immunization dropout rate was 4.2%. The common reasons for partial or non-immunization were family problem including illness of mother, vaccine not being available and child being ill. Place of birth (P = 0.014) and availability of immunization card (P < 0.001) were significant predictors of the immunization status. Since the study was conducted in 2020/2021, health services were disrupted due to the COVID-19 lockdown.
Due to the coverage being higher than the national average, it was concluded that the immunization coverage was optimal and not affected by the COVID-19 pandemic.
Core Tip: Cluster sampling technique is a unique method of probability sampling. It has immense scope in being utilized for healthcare delivery service coverage. Each cluster is crucial in representing a geographically diverse population under study. This sustains uniform representation along with statistical correctness. This technique has been employed to evaluate the immunization coverage among children in a rural setting in India, during the pandemic.
- Citation: Sharma A, Jain R, Satija J, Sharma A, Sharma A, Shekhawat S. Cluster sampling methodology to evaluate immunization coverage. World J Methodol 2024; 14(3): 92344
- URL: https://www.wjgnet.com/2222-0682/full/v14/i3/92344.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i3.92344
Immunization is a key component of primary health care and an indisputable human right. Immunization currently prevents 3.5-5 million deaths annually from diseases like diphtheria, tetanus, pertussis, influenza and measles[1]. Despite tremendous progress, vaccination coverage has plateaued in recent years and even dropped for the first time in a decade in 2020, with 23 million children missing their vaccination due to the coronavirus disease 2019 (COVID-19) pandemic[2]. Global immunization efforts have saved at least 154 million lives or an equivalent of 6 lives every minute every year, predominantly infants. Measles vaccination has been the most impactful in reducing infant mortality, accounting for 60% of the lives saved due to immunization[3].
Under the Universal Immunization Programme, the Government of India provides vaccination to prevent seven vaccine-preventable diseases, viz., diphtheria, pertussis, tetanus, polio, measles, a severe form of childhood tuberculosis and hepatitis B, haemophilus influenza type b and diarrhoea[4]. The immunization coverage for 12-23-month-old children under the National Family Health Survey (NFHS 5) is 83.8 %, reflecting a 5.9% increase from NFHS 4 figure[5]. In rural Haryana, the coverage is 80.8%[6], with the Jhajjar district recording a massive increase in coverage of 24.5% to 84.1%[7].
Immunization against vaccine-preventable diseases is a cost-effective and efficient tool to reduce morbidity and mortality in children. Assessing the immunization coverage offers an idea about the extent of its utilization, the beneficial impact of the vaccination program and planning appropriate action to enhance its overall efficiency. This has enhanced healthy survival in children. During the COVID-19 pandemic, routine immunization services were disrupted due to the lockdown. Though many studies were conducted on immunization coverage, few were carried out in the pandemic setting, especially in rural India. This study throws light on the current situation of immunization coverage in the study area, despite the constraints posed by the pandemic. Hence, the current study was planned to evaluate the immunization coverage in the rural areas of Community Health Centre (CHC) Dighal, district Jhajjar, Haryana, which is the field practice area attached to the Department of Community Medicine, Post-graduate Institute of Medical Sciences Rohtak. The study aimed to evaluate the immunization coverage among 12-23-month-old children in the rural areas of CHC Dighal and to determine the factors influencing it.
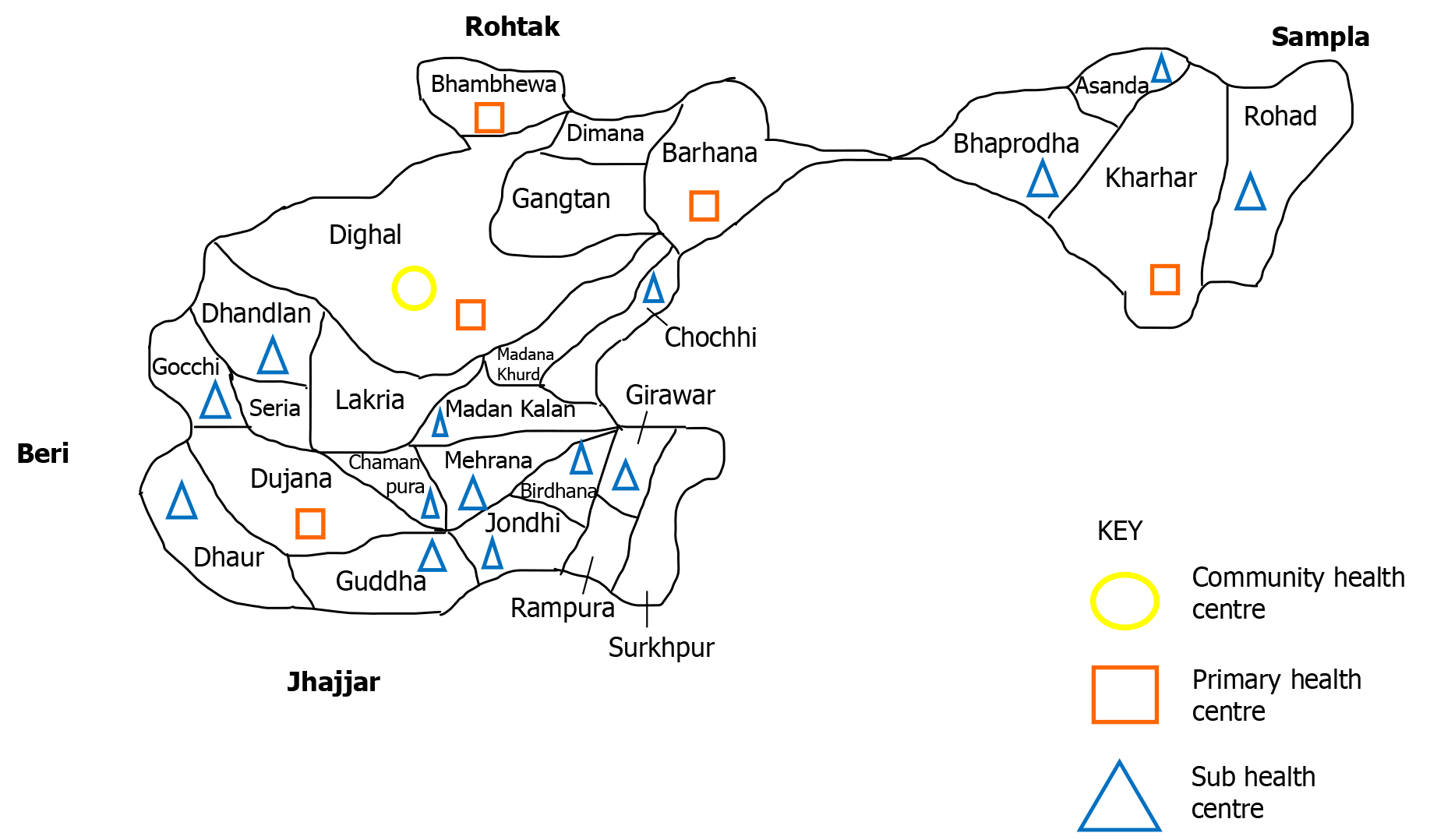
The study was conducted in the rural area of the CHC, Dighal (District Jhajjar), which is the field practice area attached to the Department of Community Medicine, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak. Twenty-six villages were provided health services through a network of five primary health centres – Dighal, Dujana, Kharhar, Barhana, and Bhambewa (Figure 1). The population of this block was 106654 as per the record of the CHC area till April 2020.
The present study was a cross-sectional, community-based study conducted from August 2021 to July 2022.
All children aged 12-23 months residing in the study area and children whose parents were willing to give written informed consent to participate in the study were included.
Severely sick children and parents of the children under the study who were unable to give relevant information for the study were excluded.
The coverage evaluation survey in the area was conducted according to the 30-cluster sampling technique, the standard methodology for such surveys devised by World Health Organization[8]. Ten children aged 12-23 months were selected from each of the selected clusters. If there were more than one eligible subject in any household, all the subjects were enrolled in the study. This sampling design estimated immunization coverage to within + 10 percentage points of true proportion, with 95% confidence. The 30 by 10 cluster survey was a two-stage cluster sampling. In the first stage, all the villages in the area were listed alphabetically. The population of each village was listed alphabetically and cumulative populations were calculated. The sampling interval was calculated by using the formula: Total cumulative population/30 (cluster) = sampling interval.
A four-digit random number was selected from the digits of any currency note, which was equal to or less than the sampling interval. Cluster no 1 was identified by locating the first village whose population was equal to or more
The cumulative population listed for that village was equal to or exceeded the calculated number. Clusters number
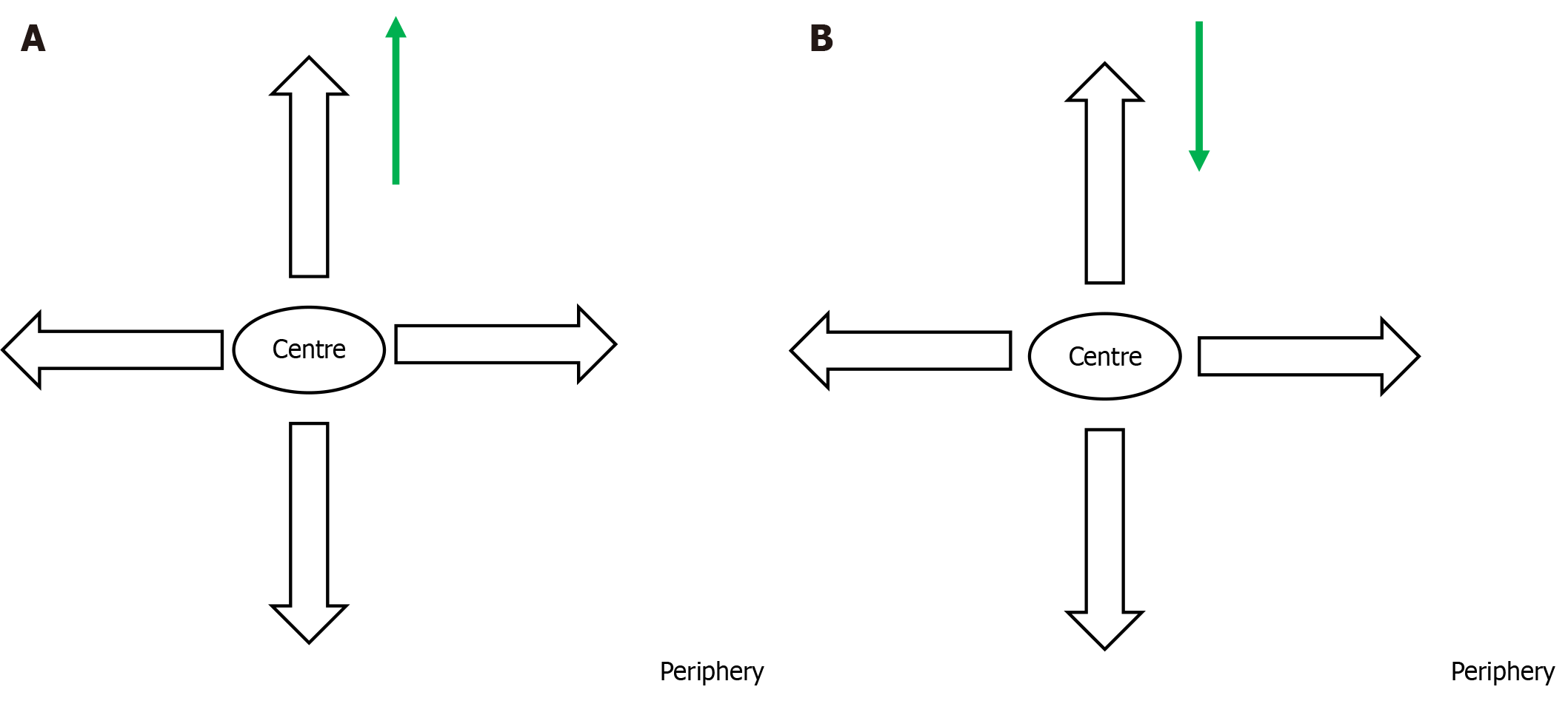
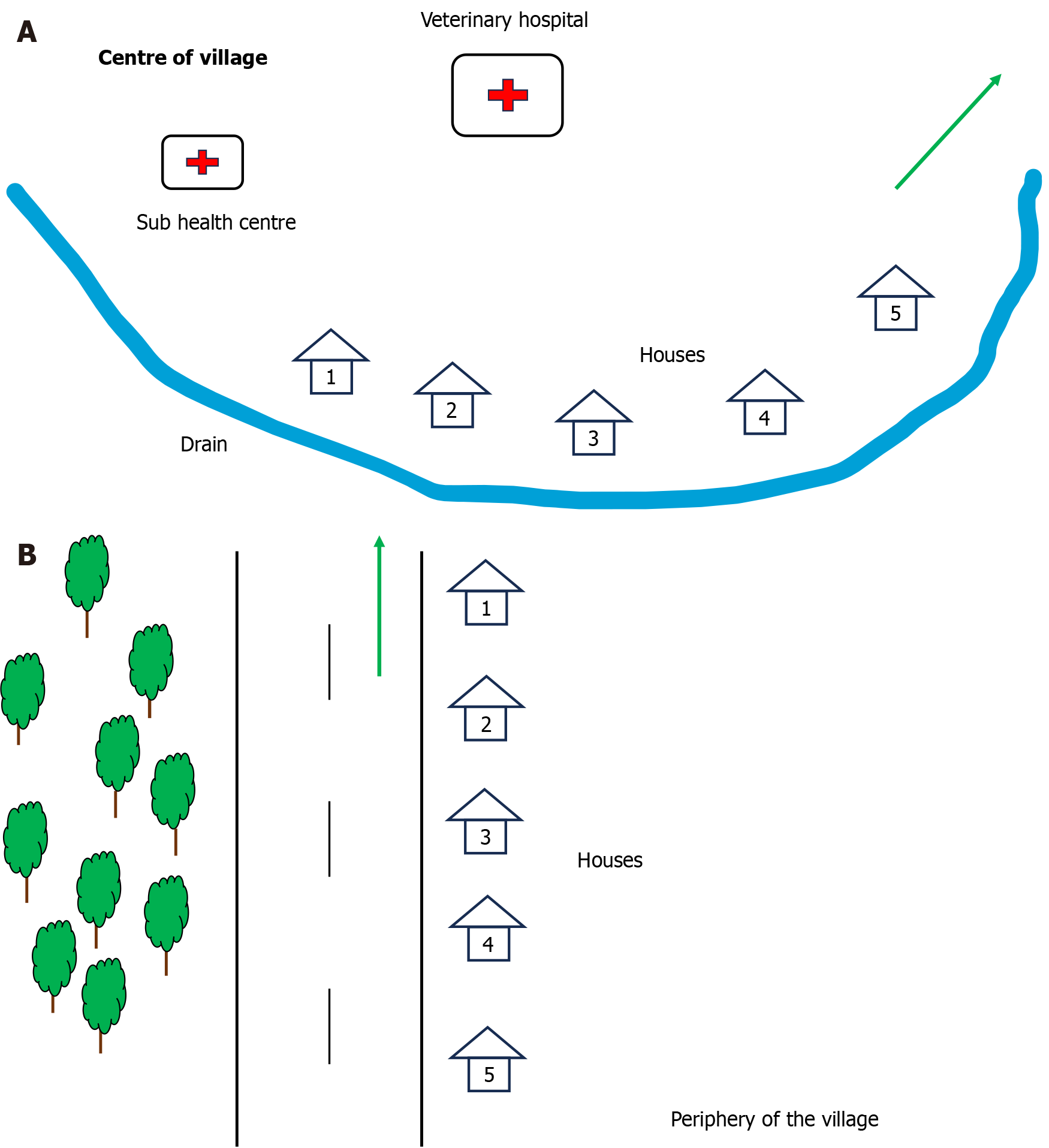
In the second stage of the cluster survey, the investigator chose a central point in the village and conducted the survey thereafter from house to house, till the desired sample size was reached, moving on to the adjacent street, if necessary (Figure 2A). In the next cluster, the investigator started from the periphery of the village, just to have a uniform sample of the total population (Figure 2B). This approach was adopted unlike the procedure for the previous cluster so that no particular section of the population was included in the study.
The investigator herself conducted the study through house-to-house visits and all the parents of the study subjects were fully informed about the purpose of the study. Written informed consent was obtained from the individuals before conducting the interview. Spot maps for two villages covered: Madana Kalan and Dhandlan (Figure 3).
This was an interview-based study and a semi-structured interview schedule based on the World Health Organization Universal Immunization Programme Coverage Household Survey form 2018, as relevant to the present study and incorporating questions related to the COVID-19 pandemic[8]. Vaccination cards were used to know the exact time of vaccination and in case cards were not available, history from either parent (mother/father)/reliable respondent was obtained and matched with the immunization record of the respective sub-centre. The reasons for refusal and dropouts were noted.
A Fully Immunized child is one who had received one dose of Bacillus Calmette Guerin (BCG), three doses of diphtheria pertussis tetanus (DPT) and oral polio virus (OPV) and one dose of Measles vaccine before one year of age. A partially immunized child is one who had been administered a vaccine but whose immunization is not complete. A non-immunized child is one who had not been given even a single dose of vaccine.
For full immunization dropouts: [(BCG - Measles) × 100]/BCG. For pentavalent (PENTA)/OPV/rotavirus (ROTA) virus vaccine dropouts: [(PENTA1 - PENTA3) × 100]/PENTA1.
The data so collected was compiled and entered as a master chart in an MS Excel spreadsheet. Analysis was carried out using the Statistical Package for the Social Science (SPSS) Version 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Data were analyzed using descriptive statistics, χ2 test and logistic regression statistical tests.
The total population covered (Table 1) comprised 53.7% male and 46.3% female children). The government hospital was where the majority of births took place. The median birth weight was 2.75 (1.6-4.0) kg. Most of the children were of the first or second birth order (86%). The mothers were mostly educated till senior secondary school (30.3%) or graduates (25.7%) and were mostly housewives (65.7%). Similarly, fathers were educated up to senior secondary school (32%) or graduation (34%) and commonly worked in the private sector (23.3%), followed by the labor market (22.7%). The joint family system was common in the area (59.7%). The majority of the children belonged to lower middle class and lower-class socioeconomic conditions, according to the Modified BG Prasad Socioeconomic Scale (Version 2020)[9] (Table 1). Immunization card was available with 94% of the parents. BCG scar was noted in 82% of the children. Most children took their vaccination at a government setup (97.6%). The full immunization rate was 86.7% with only 9% and 4.3% of the children being partially or non-immunized respectively.
| Sociodemographic variable | Number | Percentage (%) |
| Gender | ||
| Male | 161 | 53.7 |
| Female | 139 | 46.3 |
| Place of birth | ||
| Government hospital | 195 | 65.0 |
| Private hospital | 99 | 33.0 |
| Home | 6 | 2.0 |
| Age (months) | ||
| 12-17 | 108 | 36 |
| 18-23 | 192 | 64 |
| Birth weight | ||
| < 2.5 kg | 99 | 33 |
| ≥ 2.5 kg | 201 | 67 |
| Birth order | ||
| ≤ 2 | 252 | 86.0 |
| > 2 | 48 | 14.0 |
| Mother’s literacy level | ||
| Illiterate | 2 | 0.7 |
| Primary school (0-5 std) | 9 | 3.0 |
| Middle school (6-8 std) | 30 | 10.0 |
| High school (9-10 std) | 67 | 22.3 |
| Senior secondary school (11-12 std) | 91 | 30.3 |
| Graduate | 77 | 25.7 |
| Post-graduate | 24 | 8.0 |
| Mother’s occupation | ||
| Self-employed | 10 | 3.3 |
| Government job | 11 | 3.7 |
| Private job | 10 | 3.3 |
| Farmer | 37 | 12.3 |
| Laborer | 35 | 11.7 |
| Housewife | 197 | 65.7 |
| Father’s literacy level | ||
| Illiterate | 1 | 0.3 |
| Primary school (0-5 std) | 3 | 1.0 |
| Middle school (6-8 std) | 40 | 13.3 |
| High school (9-10 std) | 56 | 18.7 |
| Senior secondary school (11-12 std) | 96 | 32.0 |
| Graduate | 102 | 34.0 |
| Post-graduate | 2 | 0.7 |
| Father’s occupation | ||
| Unemployed | 11 | 3.7 |
| Self-employed | 57 | 19.0 |
| Government job | 60 | 20.0 |
| Private job | 70 | 23.3 |
| Farmer | 34 | 11.3 |
| Laborer | 68 | 22.7 |
| Type of family | ||
| Nuclear | 37 | 12.3 |
| Joint | 179 | 59.7 |
| Three-generation | 84 | 28.0 |
| Socio-economic class | ||
| Upper class | 15 | 5.0 |
| Upper middle class | 59 | 19.7 |
| Middle class | 54 | 18.0 |
| Lower middle class | 90 | 30.0 |
| Lower class | 82 | 27.3 |
The COVID-19 pandemic started in March 2020 and India is still witnessing a few hundred cases and deaths even today. The months of March to May 2020 witnessed a nationwide lockdown. A large number of migrant laborers traversed large stretches of the country to return to their native towns and villages[10]. From June 2020 onwards, there was relaxation in a few parts of the country, where transmission was minimal. The process of unlocking went on till December 2020. With the second wave hitting the country in March 2021, a nationwide lockdown was imposed again from April to June 2021, extending in some states, including Haryana till August 2021[11].
Vaccination services were hit, apart from other essential childhood care services like the Anganwadi centers. There was no vaccination at certain times due to the vaccination center being located in a hotspot, being declared a containment zone. Numerous people tested positive for the virus and were unable to get their children vaccinated during the periods of illness when they were quarantined in their homes. The mass movement of migrant laborers returning to their villages resulted in an increase in cases in rural areas. The contacts were advised to quarantine for 14 d, later reduced to 7 d. Those with a travel history were also quarantined. This resulted in their children missing essential childhood vaccination. Rumors were rife during the pandemic. This was a very important reason for children missing timely vaccination.
The immunization coverage from the present study suggests that the COVID-19 pandemic did not significantly affect the vaccination drive. The parents took their responsibility of getting their children immunized seriously. This further revealed the efforts undertaken by the peripheral health workers, who compensated for any hurdles faced due to the lockdown, managing timely immunization of the children.
A total of 210 children out of 300 missed one or the other vaccine mentioned in the National Immunization Schedule. The reasons for missing out any vaccine (n = 210), were lack of information (7.6%), lack of motivation (8.1%), obstacles like unavailability of the vaccine or the vaccinator (69.1%) and factors related to the COVID-19 pandemic (15.2%). The coverage for different vaccines is shown in Table 2. BCG, OPV, PENTA and ROTA had coverage > 90%. Measles rubella (MR) 1 had good coverage of 91.3%. MR 2 could be given between 16-24 months. Since the median age of the study participants was 19 months, some children still had to take the second dose of MR vaccine. Hence, its coverage could increase in the upcoming months. Hepatitis B and OPV birth doses had poor coverage, at 59% and 69.7% respectively. As the booster doses (DPT-B and OPV-B) could be given up to 2 years of age, the children still had time to be administered the same (Table 2). The dropout rates for different vaccines have been calculated as 1.05%, 0.35%, 0.71%, 1.78%, 3.18% for BCG-PENTA1, PENTA1-PENTA3, OPV1-OPV3, ROTA1-ROTA3, PENTA1-Measles dropouts. Full immunization dropout (BCG-Measles) was 4.2%.
| Vaccine | Number | Percentage (%) | |
| BCG | 286 | 95.3 | |
| OPV | OPV-1 | 282 | 94.0 |
| OPV-2 | 281 | 93.7 | |
| OPV-3 | 280 | 93.3 | |
| PENTA | PENTA-1 | 283 | 94.3 |
| PENTA-2 | 282 | 94.0 | |
| PENTA-3 | 282 | 94.0 | |
| ROTA | ROTA1 | 282 | 94.0 |
| ROTA2 | 280 | 93.3 | |
| ROTA3 | 277 | 92.3 | |
| f IPV | f IPV-1 | 280 | 93.3 |
| f IPV-2 | 274 | 82.3 | |
| PCV-1 | PCV-1 | 270 | 90.0 |
| PCV-2 | 255 | 85.0 | |
| MR | MR1 | 274 | 91.3 |
| MR2 | 152 | 50.7 | |
| HEP B-0 | 177 | 59 | |
| OPV-0 | 209 | 69.7 | |
| PCV-B | 239 | 79.7 | |
| DPT-B | 151 | 50.3 | |
| OPV-B | 151 | 50.3 | |
The association of variables with immunization coverage is shown in Table 3. Age (months), socioeconomic status, type of family and place of birth had a significant association (P < 0.05) with immunization coverage. The presence of BCG scar and the availability of immunization cards were highly significant indicators of immunization coverage (P < 0.001) (Table 3). However, in logistic regression analysis (Table 4), place of birth (P = 0.014) and availability of immunization card (P < 0.001) were found significant predictors of immunization status (Table 4).
| Characteristics | Partial & non immunized (n = 40) | Immunized (n = 260) | OR (95%CI) | P value |
| Gender | ||||
| Male | 24 | 137 | 1.35 (0.68-2.65) | 0.388 |
| Female | 16 | 123 | 1 (ref) | |
| Age (months) | ||||
| 12-17 | 20 | 88 | 1.96 (1.00-3.82) | 0.048a |
| 18-23 | 20 | 172 | 1 (ref) | |
| Birth weight (kg) | ||||
| < 2.5 | 14 | 85 | 1.11 (0.55-2.23) | 0.773 |
| ≥ 2.5 | 26 | 175 | 1 (ref) | |
| Mother’s education | ||||
| Illiterate/up to 8th std | 6 | 35 | 1.13 (0.44-2.90) | 0.792 |
| 9th std. & above | 34 | 225 | 1 (ref) | |
| Mother’s profession | ||||
| Employed (government/private job) | 3 | 18 | 1.09 (0.31-3.88) | 0.894 |
| Others1 | 37 | 242 | 1 (ref) | |
| Father’s education | ||||
| Illiterate/up to 8th std | 6 | 38 | 1.03 (0.41-2.70) | 0.553 |
| 9th std & above | 34 | 222 | 1 (ref) | |
| Father’s occupation | ||||
| Employed (government/private job) | 16 | 114 | 0.85 (0.43-1.68) | 0.648 |
| Others1 | 24 | 146 | 1 (ref) | |
| Socio-economic status | ||||
| Lower/lower middle class | 29 | 143 | 2.16 (1.03-4.50) | 0.026a |
| Middle/upper middle/high class | ||||
| Type of family | ||||
| Joint/three generations | 31 | 232 | 0.42 (0.18-096) | 0.039a |
| Nuclear | 9 | 28 | 1 (ref) | |
| Birth order | ||||
| > 2 | 9 | 39 | 1.64 (0.73-3.72) | 0.228 |
| ≤ 2 | 31 | 221 | 1 (ref) | |
| Availability of immunization card | ||||
| No | 13 | 5 | 3.40 (1.65-7.02) | < 0.001b |
| Yes | 27 | 255 | 1 (ref) | |
| Place of birth | ||||
| Home/private hospital | 8 | 97 | 0.42 (0.19-0.95) | 0.022a |
| Government hospital | 32 | 163 | 1 (ref) | |
| BCG scar | ||||
| No | 15 | 39 | 3.40 (1.65-7.02) | 0.001b |
| Yes | 25 | 221 | 1 (ref) | |
The present study reports the full immunization coverage as 86.7%, which is higher than the national average of 83.8%[5], revealing that priority was given to the Routine Immunization Programme, with regular sessions at a fixed date, time and venues. People of the area were aware of and accepted the Immunization Programme. The factors significantly associated with the coverage were the place of birth and the presence of a BCG scar.
The present study concluded that the majority of the participants were born in a government hospital (66.3%). There were no home deliveries. In a similar study conducted by Devasenapathy et al[12] in Delhi, only 73% of the children were born in a facility (government or private), as the study was conducted in urban slums. The association between place of birth and immunization status was found to be significant in the current research (P = 0.022) (P < 0.01).
In the present study, the median age of the study subjects was 19 months. In a study by Adedire et al[13] from Nigeria, 23.5%, 30.1%, and 46.4% of the children were in the age group of 12-15, 16-19 and 20-23 months respectively. Muluye et al[14] conducted a study in Ethiopia revealing 34.3%, 29.4%, 24.3%, and 12% of children in the 12-14, 15-17, 18-20- and 21-23-months age groups. A study from Indonesia by Herliana and Douiri[15] reported 28.8%, 24.6%, 23.3%, and 23.3% of children in the 12-23, 24-35, 36-47 and 48-59-months age groups. The association between the current age of the study subjects and immunization status proved to be significant in the current research (P = 0.048). Similarly, Herliana and Douiri[15] reported a significant association.
The joint family was the main type of family in the present study, at 59.7%. With high immunization coverage, this could suggest grandparents and relatives spending time on the child’s health care, in case the mother was unable to do so. In contrast, Devasenapathy et al[12] found the nuclear type to be more common (71.5%). This could be due to the study being conducted in urban slums. The current research found a significant association for the above (P = 0.039). Conversely, the above association was not significant in the study of Kumar et al[16].
Most of the study subjects belong to the lower middle- and middle class in this study. Still, with good immunization coverage (86.7%), it can be said that the health services were functional, accessible and acceptable to all strata of society. Similarly, class IV was the predominant socio-economic class in a study from Tripura by Datta et al[17]. The present study found the above association significant (P = 0.026). Kulkarni and Chavan[18] too found the association highly significant (P < 0.001).
About 94% of the study participants had the immunization card with them. This showed that parents understood the importance of maintaining vital health care documents. It was a valid piece of evidence marking the antigens already vaccinated against. This eliminated any duplications or omissions in the immunization of children. Similar findings were reported by Gupta et al[19]. About 84% of the parents had immunization cards with them. The present study identified a highly significant association between the availability of immunization cards and immunization status (P < 0.001). It was a valid piece of evidence marking the antigens already vaccinated against. Chhabra et al[20] too found the association significant (P < 0.01).
The BCG vaccine left a scar over the vaccination site (the deltoid muscle). This was an immunological response to the antigen. The BCG scar serves as a surrogate marker for vaccination against tuberculin antigen. It may be normally absent in up to 20% of the children[21]. Its absence does not necessarily signify immunization failure against TB antigen. This study finds 82% of children having the BCG scar. The present study found a highly significant association between the availability of immunization cards and immunization status (P < 0.001). Gupta et al[19] too found the association highly significant (P = 0.000).
Upon applying logistic regression analysis in the present study, the place of birth (P = 0.014) and availability of immunization cards (P < 0.001) were found to be significant factors for full immunization status. These factors revealed adequate access and acceptance of health services being provided in the study area. Saikia et al[22] highlighted that possession of child’s health card is the most significant factor for reducing the disparities in immunization coverage in India. In a study from Nigeria, Adedire et al[13] reported attendance of mothers at antenatal care centers [adjusted odds ratio (aOR) = 3.3, 95% confidence interval (CI): 1.1-8.3], maternal tetanus toxoid immunization (aOR = 3.2, 95%CI: 1.1-10.0), access to immunization information (aOR = 1.8, 95%CI: 1.1-2.5) and good knowledge of immunization in mothers (aOR = 2.4, 95%CI: 1.6-3.8) as significant determinants of full immunization.
Periodic intensification of routine immunization via Intensified Mission Indradhanush has improved vaccination coverage and timeliness[23]. Mobile messaging services such as the Kilkari application are an important source of awareness, timeliness and uptake of immunization services[24]. Newer tools such as automated incentivised mobile phone reminders, immunization due-list, computerized data tracking, community mobilization and campaigns improved vaccine coverage. Future work is needed to evaluate the effectiveness of identified technologies across diverse settings in India[25].
An area of particular interest for research can be previous adverse events causing fear in the community about vaccination. Parents whose children have been vaccinated and are doing well can be motivated to come forward and engage with parents having any apprehensions. The panchayat or village body has a great deal of support and is looked up to in the area. If its members pledge their support to routine immunization, many hurdles can be overcome.
The study was conducted in a rural block attached to a tertiary care hospital, and teaching and training center. Hence, the results cannot be generalized. Owing to the cross-sectional nature of the study, the direction of association can’t be ascertained.
The immunization coverage in the study area was significantly high and better than the national average, with minimal dropout rates. Priority was given to the Routine Immunization Program. The COVID-19 pandemic did not have any significant impact on immunization coverage. The parents were responsible for keeping abreast with the immunization schedule of their children. The health workers worked diligently to compensate for the missed vaccination during the lockdown period.
Sincere thanks to the Department of Community Medicine, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences Rohtak, Haryana, India.
| 1. | World Health Organization. Immunization coverage. [cited 15 December 2023]. Available from: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage. |
| 2. | World Health Organization. WHO and UNICEF warn of a decline in vaccinations during COVID-19. [cited 15 December 2023]. Available from: https://www.who.int/news/item/15-07-2020-who-and-unicef-warn-of-a-decline-in-vaccinations-during-covid-19. |
| 3. | World Health Organization. Global immunization efforts have saved at least 154 million lives over the past 50 years. [cited 29 April 2024]. Available from: https://www.who.int/news/item/24-04-2024-global-immunization-efforts-have-saved-at-least-154-million-lives-over-the-past-50-years. |
| 4. | Government of India Ministry of Health and Family Welfare. Mission Indradhanush. [cited 15 December 2023]. Available from: https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Mission_Indradhanush_Guidelines.pdf. |
| 5. | Government of India Ministry of Health and Family Welfare. National Family Health Survey (NFHS-5), 2019-21. India Report. [cited 20 December 2023]. Available from: http://rchiips.org/nfhs/NFHS-5Reports/NFHS-5_INDIA_REPORT.pdf. |
| 6. | Government of India Ministry of Health and Family Welfare. National Family Health Survey (NFHS-5), 2019-21. State Fact Sheet Haryana. [cited 20 December 2023]. Available from: http://rchiips.org/nfhs/NFHS-5_FCTS/Haryana.pdf. |
| 7. | Government of India Ministry of Health and Family Welfare. National Family Health Survey (NFHS-5), 2019-21. District Fact Sheet Jhajjar Haryana. [cited 20 December 2023]. Available from: http://rchiips.org/nfhs/NFHS-5_FCTS/HR/Jhajjar.pdf. |
| 8. | World Health Organization. World Health Organization vaccination coverage cluster surveys: reference manual. [cited 20 December 2022]. Available from: https://www.who.int/publications/i/item/WHO-IVB-18.09. |
| 9. | Mathiyalagen P, Davis P, Sarasveni M. Updated BG Prasad Socio-Economic Classification: The 2020 Update. Indian J Pediatr. 2021;88:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mohan M, Mishra S. India's Response to the COVID-19 Pandemic: A Frontal Assault on the "Historically Dispossessed". Int J Health Serv. 2021;51:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Singh KD, Goel V, Kumar H, Gettleman J. India, Day 1: World’s Largest Coronavirus Lockdown Begins. The New York Times. [cited 25 March 2024]. Available from: https://www.nytimes.com/2020/03/25/world/asia/india-lockdown-coronavirus.html. |
| 12. | Devasenapathy N, Ghosh Jerath S, Sharma S, Allen E, Shankar AH, Zodpey S. Determinants of childhood immunisation coverage in urban poor settlements of Delhi, India: a cross-sectional study. BMJ Open. 2016;6:e013015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Adedire EB, Ajayi I, Fawole OI, Ajumobi O, Kasasa S, Wasswa P, Nguku P. Immunisation coverage and its determinants among children aged 12-23 months in Atakumosa-west district, Osun State Nigeria: a cross-sectional study. BMC Public Health. 2016;16:905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Muluye M, Oljira L, Eyeberu A, Getachew T, Debella A, Deressa A, Dheresa M. Partial vaccination and associated factors among children aged 12-23 months in eastern Ethiopia. BMC Pediatr. 2022;22:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Herliana P, Douiri A. Determinants of immunisation coverage of children aged 12-59 months in Indonesia: a cross-sectional study. BMJ Open. 2017;7:e015790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Kumar D, Aggarwal A, Gomber S. Immunization status of children admitted to a tertiary-care hospital of north India: reasons for partial immunization or non-immunization. J Health Popul Nutr. 2010;28:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Datta A, Baidya S, Datta S, Mog C, Das S. A Study to Find Out the Full Immunization Coverage of 12 to 23-month old Children and Areas of Under-Performance using LQAS Technique in a Rural Area of Tripura. J Clin Diagn Res. 2017;11:LC01-LC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kulkarni SV, Chavan MK. A study to assess the immunization coverage in an urban slum of Mumbai by lot quality technique. Int J Med Public Health. 2013;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Gupta PK, Pore P, Patil U. Evaluation of immunization coverage in the rural area of pune, maharashtra, using the 30 cluster sampling technique. J Family Med Prim Care. 2013;2:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Chhabra P, Nair P, Gupta A, Sandhir M, Kannan AT. Immunization in urbanized villages of Delhi. Indian J Pediatr. 2007;74:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Dhanawade SS, Kumbhar SG, Gore AD, Patil VN. Scar formation and tuberculin conversion following BCG vaccination in infants: A prospective cohort study. J Family Med Prim Care. 2015;4:384-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Saikia N, Kumar K, Bora JK, Mondal S, Phad S, Agarwal S. What Determines the District-Level Disparities in Immunization Coverage in India: Findings from Five Rounds of the National Family Health Survey. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Summan A, Nandi A, Deo S, Laxminarayan R. Improving vaccination coverage and timeliness through periodic intensification of routine immunization: evidence from Mission Indradhanush. Ann N Y Acad Sci. 2021;1502:110-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Chakraborty A, Mohan D, Scott K, Sahore A, Shah N, Kumar N, Ummer O, Bashingwa JJH, Chamberlain S, Dutt P, Godfrey A, LeFevre AE; Kilkari Impact Evaluation Team. Does exposure to health information through mobile phones increase immunisation knowledge, completeness and timeliness in rural India? BMJ Glob Health. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Dudeja N, Khan T, Varughese DT, Abraham SG, Ninan MM, Prasad CL, Sarkar R, Kang G. Technologies for strengthening immunization coverage in India: a systematic review. Lancet Reg Health Southeast Asia. 2024;23:100251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |