Published online Sep 20, 2024. doi: 10.5662/wjm.v14.i3.91832
Revised: January 29, 2024
Accepted: March 1, 2024
Published online: September 20, 2024
Processing time: 170 Days and 19.1 Hours
Diabesity (diabetes as a consequence of obesity) has emerged as a huge healthcare challenge across the globe due to the obesity pandemic. Judicious use of anti
To study the real-world benefits and side effects of using semaglutide to manage patients with diabesity.
We evaluated the efficacy and safety of semaglutide use in managing patients with diabesity in a large academic hospital in the United States. Several para
106 patients (56 males) with type 2 diabetes mellitus (T2DM), mean age 60.8 ± 11.2 years, mean durations of T2DM 12.4 ± 7.2 years and mean semaglutide treatment for 2.6 ± 1.1 years were included. Semaglutide treatment was associated with significant improvement in diabesity outcomes such as mean weight reductions from baseline 110.4 ± 24.6 kg to 99.9 ± 24.9 kg at 12 months and 96.8 ± 22.9 kg at latest follow up and HbA1c improvement from baseline of 82 ± 21 mmol/mol to 67 ± 20 at 12 months and 71 ± 23 mmol/mol at the latest follow up. An insulin dose reduction from mean baseline of 95 ± 74 units to 76.5 ± 56.2 units was also observed at the latest follow up. Side effects were mild and mainly gastrointestinal like bloating and nausea improving with prolonged use of semaglutide.
Semaglutide treatment is associated with significant improvement in diabesity outcomes such as reduction in body weight, HbA1c and insulin doses without major adverse effects. Reviews of largescale real-world data are expected to inform better clinical practice decision making to improve the care of patients with diabesity.
Core Tip: Rational medical management of diabesity, i.e., diabetes resulting from obesity, involves judicious use of antidiabetic drugs which should ideally help body weight loss while controlling hyperglycemia. Although semaglutide use has been associated with significant improvements in body weight and glycated hemoglobin (HbA1c) in multiple randomized controlled trials (RCTs) and prospective observational studies, more real-world data from day-to-day medical practice would inform better clinical decision making. We report our retrospective study data that reveals better diabesity outcomes compared to RCTs with a mean weight loss of 12.3%, HbA1c reduction of 13.7% and insulin dose reduction of 19.5% with semaglutide treatment.
- Citation: Alkhalifah M, Afsar H, Shams A, Blaibel D, Chandrabalan V, Pappachan JM. Semaglutide for the management of diabesity: The real-world experience. World J Methodol 2024; 14(3): 91832
- URL: https://www.wjgnet.com/2222-0682/full/v14/i3/91832.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i3.91832
The global obesity pandemic in the past few decades has resulted in a substantial increase in the prevalence of patients with type 2 diabetes mellitus (T2DM) across the world. According to the International Diabetes Federation (IDF) estimates, there were 537 million adults worldwide living with diabetes in the year 2021, the majority of whom were suffering from T2DM[1]. In a significant proportion of patients with T2DM, diabetes occurs as a direct consequence of obesity or abdominal adiposity. Diabesity is an important concept to denote the strong pathobiological interlink between obesity and T2DM, which has important therapeutic implications as control of both diseases becomes imperative in the optimal care of these patients[2]. However, glycemic management is often given priority even by diabetologists while managing diabesity, which can potentially worsen obesity as several of the antidiabetic medications including insulins may cause weight gain. Moreover, obesity is usually a progressive disease in many individuals, and therefore, diabesity is very likely to worsen over time unless the appropriate management strategies are adopted early in the course of illness by managing obesity with lifestyle and pharmacological interventions.
The novel antidiabetic medications belonging to the glucagon like peptide-1 receptor agonist (GLP-1RA) class were available for managing diabesity over more than one and a half decades, and newer agents are currently being introduced into the global market, some of which are also used for weight management even in patients without T2DM. This class of drugs acts through pancreatic and extra-pancreatic mechanisms causing meal-related pancreatic insulin secretion and suppression of endogenous glucagon, appetite suppression, and early satiety by central and peripheral mechanisms (by delaying gastrointestinal nutrient transit), and with a strong tendency for body weight reduction[2,3]. Semaglutide is one of the latest additions to the list of GLP-1RA molecules and has been in use for over the past 6 years in many countries including the United Kingdom (UK). The Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) Trials, and the Peptide Innovation for Early Diabetes Treatment (PIONEER) Trials tested the efficacy of semaglutide (1 mg) subcutaneously weekly for the management of T2DM, while Semaglutide Treatment Effect in People with Obesity (STEP) Trial tested the efficacy of the molecule (at 2.4 mg weekly dose) for body weight management[4]. These large multinational randomized controlled clinical trials (RCTs), viz., SUSTAIN 1-11, PIONEER 1-12 and STEP 1-6 trials, demonstrated the efficacy and safety of semaglutide as an antidiabetic medication with good weight loss potential beyond doubt.
As the settings of RCTs and prospective observational studies are well-supervised and often rigorously scrutinized by research teams, the study results captured by these methods may not always reflect the actual real-world clinical picture in our day-to-day medical practice. Therefore, it is imperative to have real-world clinical data for truly appraising the actual immediate and long-term efficacy and safety of semaglutide to aid therapeutic decision making for patients with diabesity and T2DM. The present study is such an attempt to gather the real-world data from patients managed on long-term basis with semaglutide for diabesity in a large academic teaching hospital in the UK.
This is a retrospective clinical study by review of the clinical and therapeutic data of all patients managed with any one of the injectable (brand: Ozempic), or oral (brand: Rybelsus) semaglutide agents, between January 1, 2019 and May 31, 2023 at Lancashire Teaching Hospitals NHS Trust (LTHTR). The endocrine and metabolic service of this hospital provides comprehensive diabetic care for patients in the Central Lancashire and South Cumbria regions of the UK, with a population of about 0.4 million people. The study was approved by the institutional audit/research committee (No: DIAB/CA/2022-23-08).
All adult patients (aged ≥ 18 years) with T2DM treated by one of the above GLP-1RA molecules for management of their diabetes were considered for inclusion in the study.
Electronic medical records were searched for all patients with a diagnosis of diabetes mellitus treated with semaglutide during the study period. The total number of cases in this category were further reviewed for inclusion in the study.
(1) Patients with a diagnosis of T2DM managed with injectable or oral semaglutide; and (2) Participants with predefined primary outcome measures (HbA1c alteration from baseline values to different follow-up periods) and/or secondary outcomes such as alterations in body weight, as well as reduction in insulin dose – all these outcomes with meaningful data in at least one of the follow-up periods (6 months, 12 months and/or at the last follow up just prior to completion of the study). Additional factors examined were blood pressure, renal functions, and urine albumin creatinine ratio.
(1) Patients with a diagnosis of type 1 diabetes mellitus; (2) Incomplete study data to obtain meaningful outcome measures as specified above; and (3) Follow-up duration less than 6 months.
Data was collected by reviewing each case fulfilling the inclusion criteria identified from the total number of cases in the electronic patient record system (Flex) of the LTHTR. Microsoft Excel spreadsheet was used for data compilation and rechecked for logical inconsistency and entry error. Data was cleaned accordingly before analysis.
Data management and statistical analysis were done using statistical package for social sciences (SPSS software Version 26). The analysis included frequency distribution, cross-tabulation as well as descriptive statistical analysis. Paired t-test was performed to ascertain statistical significance of change in glycemic status, weight, and dose of insulin before and after semaglutide therapy. The results were presented in tables and graphs.
A total of 106 patients with T2DM on semaglutide were included in this study. 56 were males and the remainder females. Table 1 shows their baseline clinical and biochemical characteristics. The data analysis has been done separately for male and female patients for each of the variables. The mean (SD) duration of diabetes was 12.4 (7.2) years, and the mean follow up duration after initiation of semaglutide was 2.6 (1.1) years, and the Table 2 shows other different antidiabetic medications received by patients prior to the initiation of semaglutide. Among different anti-diabetic drugs, 69.8% patients were on metformin, 69.8% were on insulin and 51.9% were on sodium glucose cotransporter 2 (SGLT2) inhibitors.
| Attributes | Male | Female | All | Reference value (if applicable) | |||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | ||
| Patient age (years) | 60.88 | (10.04) | 60.64 | (12.43) | 60.76 | (11.18) | |
| Systolic blood pressure (mm Hg) | 140.43 | (18.42) | 136.79 | (15.18) | 138.86 | (17.02) | |
| Diastolic blood pressure (mm Hg) | 80.32 | (10.82) | 71.32 | (10.62) | 76.45 | (11.56) | |
| Patient baseline weight (kg) | 111.45 | (21.07) | 109.05 | (28.63) | 110.36 | (24.56) | |
| Patient baseline HbA1c (mmol/mol) | 84.18 | (22.20) | 79.46 | (19.40) | 81.96 | (20.96) | 20-41 mmol/mol |
| Serum creatinine (μmol/L) | 101.16 | (53.98) | 75.33 | (37.18) | 89.34 | (48.52) | Male: 59-104 μmol/L; Female: 45-84 μmol/L |
| Urine ACR (mg/mmol) | 21.96 | (38.26) | 28.74 | (58.59) | 24.97 | (47.81) | Male: < 2.5 mg/mmol; Female: < 3.5 mg/mmol |
| Total cholesterol (mmol/L) | 4.10 | (1.47) | 4.53 | (1.16) | 4.29 | (1.35) | < 5.01 mmol/L |
| HDL (mmol/L) | 1.07 | (0.34) | 1.16 | (0.36) | 1.11 | (0.35) | > 1 mmol/L |
| Triglyceride (mmol/L) | 4.82 | (5.79) | 5.02 | (2.62) | 4.89 | (4.60) | < 2.3 mmol/L |
| ALT (U/L) | 25.50 | (15.04) | 23.73 | (11.17) | 24.69 | (13.33) | 0-33 U/L |
| Medication | Total patients | Percentage (%) |
| Metformin | 74 | 69.8 |
| SGLT2 inhibitors | 55 | 51.9 |
| Sulfonylureas | 27 | 25.5 |
| Pioglitazone | 7 | 6.6 |
| Meglitinides | 1 | 0.9 |
| DPP4 inhibitors | 6 | 5.7 |
| Insulin | 74 | 69.8 |
| Anti-obesity medications | 1 | 0.9 |
98 (92.5%) patients were on subcutaneous semaglutide while the remaining 8 were on the oral drug. Eight (7.5%) patients experienced gastrointestinal side effects (mainly nausea and bloating) after initiation of semaglutide but could tolerate the drug later (4 patients had to stop the drug within 3 months of initiation of semaglutide and have not been included in the analysis).
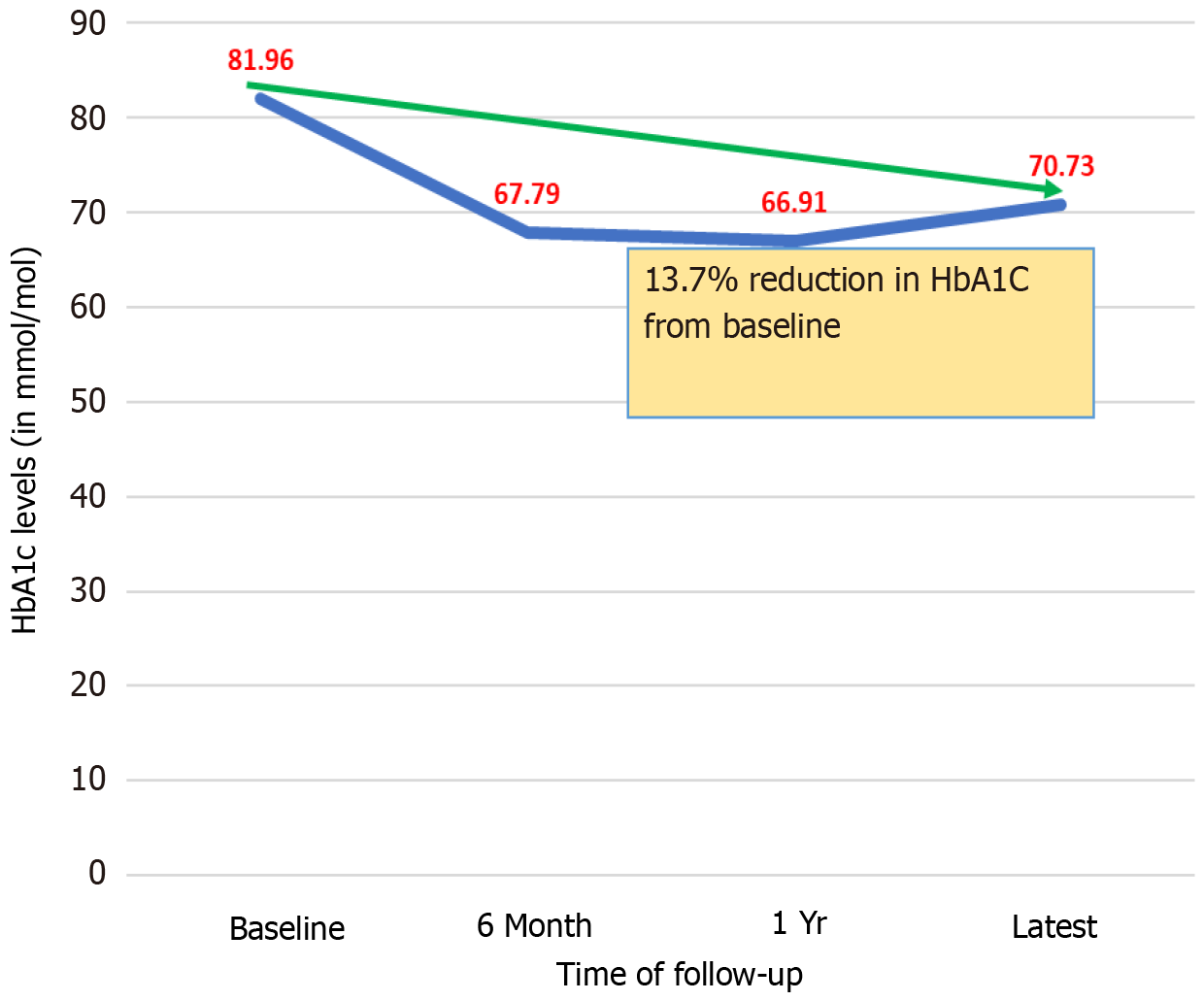
Table 3 focuses on the baseline HbA1c and its changes over various time intervals after initiating semaglutide. Baseline mean HbA1c was 81.96 (SD 20.96) mmol/mol which reduced to 66.91 (SD 19.88) mmol/mol after one year. Figure 1 displays the HbA1c reduction trend.
| Attributes | Male | Female | All | Reference value (if applicable) | |||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | ||
| Patient baseline HbA1c (mmol/mol) | 84.18 | (22.20) | 79.46 | (19.40) | 81.96 | (20.96) | 20-41 mmol/mol |
| HbA1c after 6 months (mmol/mol) | 68.52 | (21.06) | 67.02 | (15.66) | 67.79 | (18.53) | 20-41 mmol/mol |
| HbA1c after 1 yr (mmol/mol) | 71.42 | (21.94) | 63.27 | (17.48) | 66.91 | (19.88) | 20-41 mmol/mol |
| Latest HbA1c (mmol/mol) | 72.98 | (25.60) | 68.2 | (19.80) | 70.73 | (23.04) | 20-41 mmol/mol |
Table 4 shows the paired t-test for HbA1c, and compares HbA1c at baseline to 6 months, 1 year and at the latest follow-up separately. P value was < 0.05 in each of the scenarios.
| HbA1c | Mean | SD | df | Sig. (2-tailed) P value |
| HbA1c baseline to 6 month | 12.31 | 17.926 | 47 | 0.000 |
| HbA1c baseline to 1 yr | 14.456 | 19.428 | 47 | 0.000 |
| HbA1c baseline to latest | 9.644 | 20.174 | 47 | 0.002 |
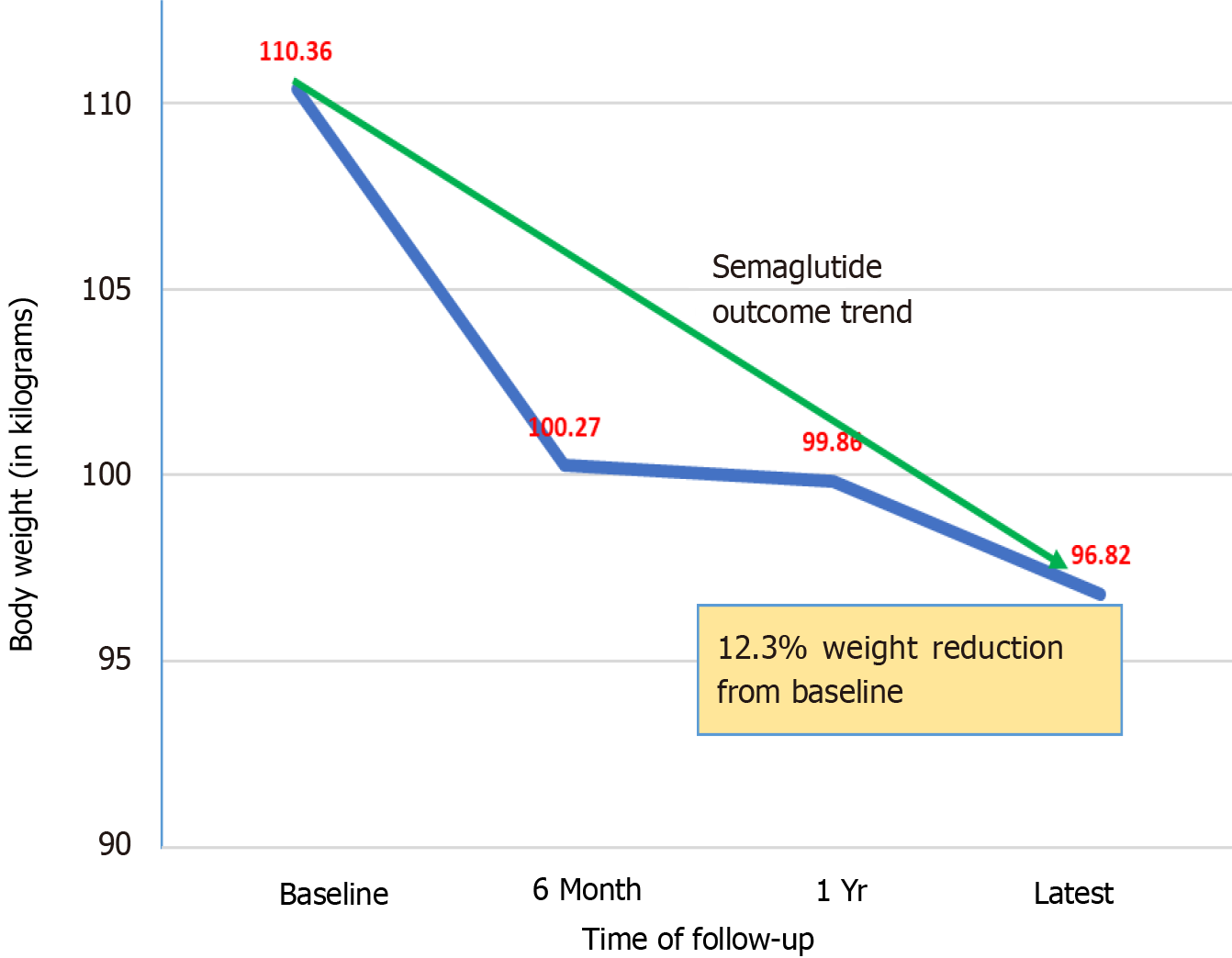
Table 5 displays the baseline body weight and its changes over time after initiating semaglutide.
| Attributes | Male | Female | All | |||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Patient baseline weight (kg) | 111.45 | (21.07) | 109.05 | (28.63) | 110.36 | (24.56) |
| Weight after 6 months (kg) | 100.19 | (20.98) | 100.39 | (38.57) | 100.27 | (29.13) |
| Weight after 1 year (kg) | 102.10 | (25.96) | 98.07 | (25.35) | 99.86 | (24.94) |
| Latest weight (kg) | 101.74 | (18.21) | 90.21 | (22.93) | 96.82 | (22.88) |
Although the mean weight reduction from baseline to follow-up at 6 months and 12 months did not show statistical significance, weight loss at the latest follow-up period showed a tendency to reach significance (P = 0.057). The mean weight reduction was 12.3% from baseline. The weight loss tendency plateaued between 6-12 months. The mean decline in weight during the first 6 months was more profound than the decline which happened after 1 year to the latest follow-up. The weight reduction trend is shown in Figure 2.
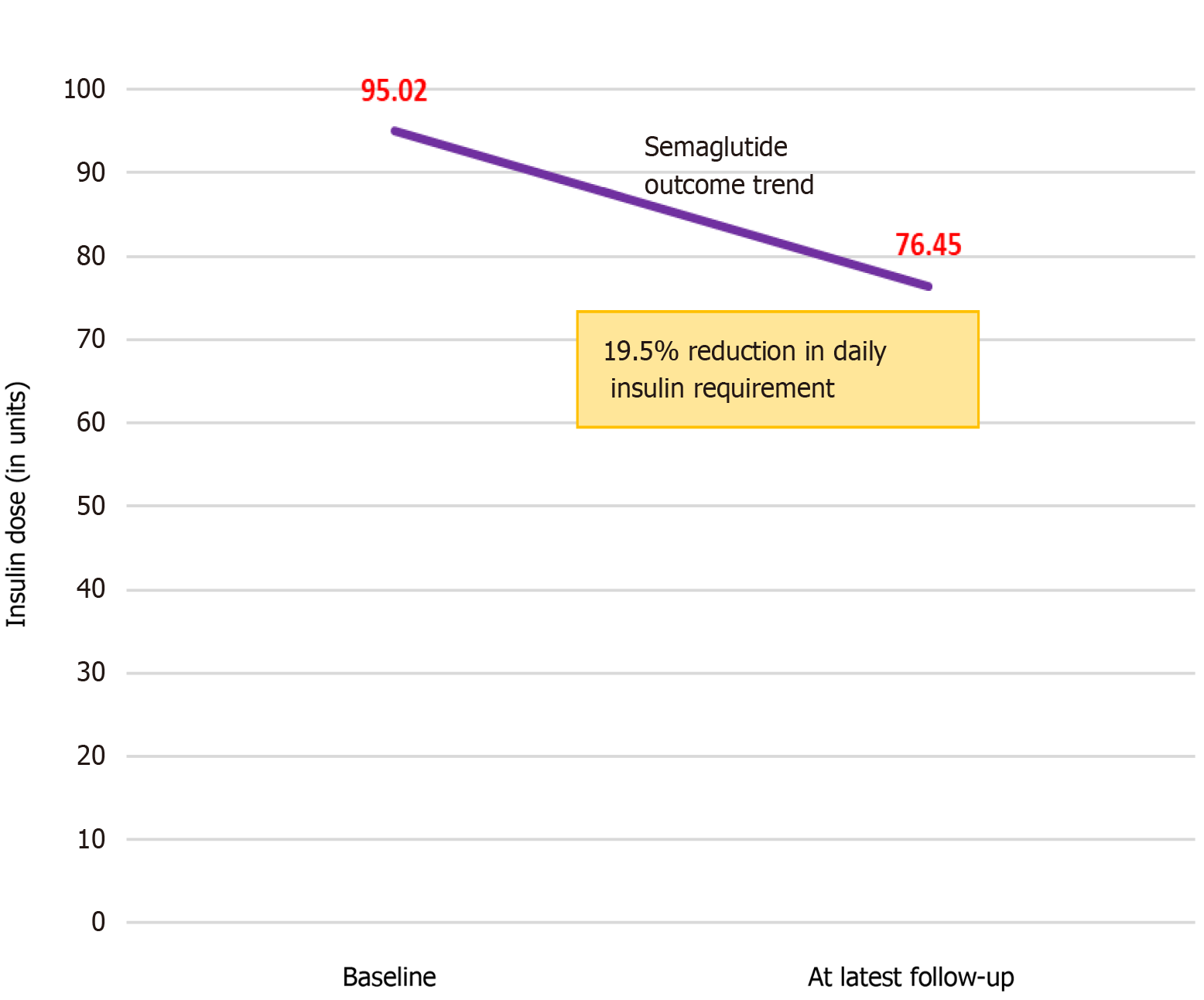
Table 6 displays daily insulin requirements in the patients before and after starting semaglutide. We noted that the baseline insulin dose was lower in the females compared to males. Additionally, the dose reductions in units were greater among females compared to males. Four patients could completely stop insulin after treatment with semaglutide. The total insulin dose reduction from baseline to last follow-up was statistically significant (P = 0.024) (Figure 3).
| Attributes | Male | Female | All | |||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Daily insulin dose baseline (units) | 106.00 | (83.59) | 81.89 | (58.48) | 95.02 | (74.02) |
| Daily insulin dose latest (units) | 92.2 | (62.86) | 62.14 | (46.21) | 76.45 | (56.17) |
Several previous studies including multiple RCTs, and observational studies illustrate consistent beneficial effects of semaglutide therapy particularly in patients with diabesity. Our data revealed significant improvements in diabesity outcomes such as a mean weight reduction of 12.3% and a mean HbA1c reduction of 13.7% from baseline at the latest follow up period at a mean follow up duration of 2.6 ± 1.1 years. A mean 19.5% reduction of total insulin dose was possible in those patients who were already established on insulin management for longstanding inadequately controlled T2DM, and 4 patients could even completely stop insulin. This real-world data reinforces our understanding on the therapeutic benefits of this new GLP-1RA molecule, semaglutide, for optimal management of diabesity in day-to-day medical practice. Unfortunately, we couldn’t procure enough data in this audit sufficiently powered to analyze the cardiovascular and metabolic implications of semaglutide treatment as observed in major RCTs. However, we observed remarkably great improvements in diabesity outcomes such as HbA1c reduction and weight loss compared to those observed in large RCTs.
A recent systematic review of major clinical trials showed a mean HbA1c reduction of 0.97% (95%CI: -1.33 to -0.62) and 1.36% (95%CI: -1.59 to -1.13) with 0.5 mg and 1.0 mg of semaglutide respectively as subcutaneous (S/c) weekly injections compared to placebo for treatment of patients with T2DM[5]. This study also very clearly showed better efficacy of this GLP-RA molecule compared to other antidiabetic medications for glycemic control with HbA1c reductions of 0.56% and 0.63% respectively when treated with 0.5 mg and 1.0 mg weekly of S/c semaglutide respectively. Our patients achieved greater mean HbA1c reduction (15.05 mmol/mol – equivalent to approximately 1.4%) at one year of follow up compared to the above meta-analysis-based study results for management of T2DM as all our patients were already on other antidiabetic medications. We acknowledge that our patients had higher baseline HbA1c (81.96 ± 20.96 mmol/mol) which could be the reason for better HbA1c reduction compared to the data revealed by clinical trials in which the baseline HbA1c was found to be comparatively lower (median HbA1c: 8.1–8.5%; 64.8–69.6 mmol/mol)[6]. Higher baseline HbA1c is known to be associated with better HbA1c reduction when treated with any antidiabetic agent including GLP-1RA as demonstrated by previous studies[7,8], which likely further explain our results with considerable HbA1c reduction. Moreover, other antidiabetic agents used by our patients (such as metformin and SGLT-2 inhibitors) would have resulted in better glycemic control. This is comparable to the observations from the SUSTAIN 9 trial, which showed that adding injectable semaglutide to patients treated for at least 90 days with SGLT-2i resulted in a significant reduction in HbA1c of an average of 1.42% (−15·55 mmol/mol)[9].
We observed a gradual worsening of metabolic control with an increase in HbA1c levels from 66.91 mmol/mol at one year to 70.73 mmol/mol in the last follow up at 2.6 ± 1.1 years. GLP-1RA medications were found to have lower efficacy in controlling chronic hyperglycemia in patients with T2DM after years of use because of various reasons such as antibody formation against some of the molecules, gradual decline in beta cell function is most patients with T2DM as part of the pathobiological behavior of the disease, and possibly a decrease in effector response to GLP-1RA drugs at the molecular level[10-13]. We believe that the slight worsening of metabolic control with a gradual increase in HbA1c level observed in our cohort at the latest follow-up could be explained by similar mechanisms.
Insulin dose reduction in a good proportion of patients was one of the interesting observations of our study which has great metabolic connotations in managing patients with diabesity. Marked improvement of glycemic control with semaglutide treatment helped insulin dose reduction in many patients and even total discontinuation of insulin in 4 patients in this cohort. Insulin dose reduction was reported by some of the RCTs and some cohort studies with the addition of semaglutide in the treatment regime[14-18]. However, we observed a mean insulin dose reduction from 95.02 to 76.45 units which is higher than the mean insulin dose reduction of 4-6 units observed by the SUSTAIN-5 Trial[15] and the study reported by Ares-Blanco et al[18] (16 units of insulin dose reduction) from Spain. Again high baseline HbA1c (82 mmol/mol) levels with a marked improvement in the metabolic control following initiation of semaglutide would explain this discrepancy, as other studies reported mean baseline HbA1c of 64 to 72 mmol/mol at the time of commenc
In our cohort we observed a weight reduction of 10.5 kg at 12 months’ follow up with a further improvement of body weight by another -3.04 kg at the latest follow up (a total mean weight loss of 13.54 kg) at a mean 2.6 ± 1.1 years. The reported mean weight loss observed in major RCTs with semaglutide in patients with diabesity ranged from 2.32 to 3.99 kg in an updated meta-analysis[5]. Real-world data shows variable weight loss response ranging from 4.2 kg to 9.0 kg[19,20]. Weight loss response in patients with T2DM following semaglutide therapy could be related to various factors such as baseline body weight, ethnic-specific incretin response and the glycemia-related fluctuations in incretin physiology[13]. Higher degree of weight loss observed in our cohort could also be due to the co-administered antidiabetic medi
The side effects experienced by patients in our study were mainly gastrointestinal and mild, which improved with ongoing treatment. The low rate of side effects we observed in this study compared to various other studies[5,21,22] could be related to the poor reporting of adverse events in the clinical records during follow up of the patients, a limitation unavoidable in retrospective studies.
We acknowledge a few limitations to our work which are inherent to retrospective cohort studies. Because of inadequate data, we had to exclude several cases from the analysis. Although several patients had at least annual follow-up prior to the last follow up we documented in our study, we were unable to get adequate data for analysis of these periodic follow-ups in obtaining meaningful statistical outcomes in this study. We were also unable to procure adequate data on cardio-metabolic parameters such as improvement of blood pressure, lipid profile and renal outcomes because of inadequate documentation of these data in the follow-up period. Extrapolating the marked weight reduction of 12.3% into cardiometabolic outcomes from previous studies, we would have expected significant improvements in the above parameters which were not documented well in our patients’ clinical records, and this remains as a major pitfall of the study. Inadequate reporting of adverse events in the case records could have been the reason for the low incidence of side effects observed in this study.
However, the remarkable improvements in body weight, HbA1c and insulin dose reduction observed in our study bring forth the importance of appropriate use of GLP-1RA molecules including semaglutide in our day-to-day clinical practice. In fact, our data shows more profound improvements in diabesity outcomes such as marked weight loss and HbA1c reduction compared to RCT-based study data. This has important clinical implications for future research as significant weight loss in appropriately selected patients with early onset T2DM could result in diabetes remission or even reversal at least in a proportion of patients. Moreover, such patients may also have better cardiovascular benefits compared to patients with longer duration of diabetes. Therefore, compiling more real-world data with this kind of observational studies should enhance our current knowledge-base to inform better medical practice decision making in the future.
Our data shows that semaglutide use is associated with better clinical and biochemical outcomes in the real-world management of diabesity compared to the RCTs and other observational studies, with higher mean weight reduction of 12.3%, improvement of mean HbA1c by 14.5 mmol/mol (at one year), and mean insulin dose reduction of 18.6 units. Although we could not get adequate data on cardiometabolic outcomes, extrapolating the benefits of > 10% weight loss in clinical settings would have improved several of these outcomes. More data from real-world observational studies is expected to improve our understanding in using semaglutide and other newer GLP-1RA molecules for judicious management of diabesity to address the alarming rise in this clinical problem across the globe secondary to the obesity pandemic. Future research should evaluate the feasibility of early initiation of GLP-1RA such as semaglutide in patients with diabesity to see if that improves clinical and therapeutic outcomes.
Diabesity, diabetes as a consequence of obesity, is a huge healthcare challenge across the globe and judicious use of antidiabetic medications like semaglutide is important for the optimal management as shown in randomized controlled trials (RCTs).
Real-world data on management of diabesity with semaglutide are also crucial for appropriate clinical practice decision making.
We aimed to study the real-world benefits and side effect profile of using semaglutide to manage patients with diabesity.
In a retrospective study, we evaluated the efficacy and safety of semaglutide for managing patients with diabesity bet
Among 106 patients (56 males) with T2DM with a mean age and diabetes duration 60.8 ± 11.2 years, 12.4 ± 7.2 years respectively treated with semaglutide for a mean 2.6 ± 1.1 years, significant improvements in diabesity outcomes such as a mean weight reduction of 12.3% and HbA1c reduction of 13.7% from baseline at the latest follow-up period were observed. A mean insulin dose reduction of 19.5% from baseline was also observed at the latest follow-up as an additional benefit of semaglutide treatment. Mild gastrointestinal side effects like bloating and nausea, improving with prolonged use of semaglutide were also observed in this study.
As RCTs are performed in strictly controlled research environments, the results may not always reflect patient outcomes of real-world clinical practice settings. Reviews of large-scale cohort data from real-world settings as in our study would inform better clinical practice decision making to improve the care of patients with diabesity.
Significant improvements in diabesity outcomes such as reductions in body weight, HbA1c, and insulin doses were observed with semaglutide treatment, without major adverse effects in a real-world clinical practice setting.
We thank Dr. Marina George Kudiyirickal for providing us the audio for the core tip.
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | IDF Diabetes Atlas 2021. IDF Atlas 10th Edition, International Diabetes Federation. Accessed on 9th December 2023. Available from: https//:diabetesatlas.org/atlas/tenth-edition. |
| 2. | Michaelidou M, Pappachan JM, Jeeyavudeen MS. Management of diabesity: Current concepts. World J Diabetes. 2023;14:396-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (4)] |
| 3. | Scheen AJ, Lefèbvre PJ. Glucagon, from past to present: a century of intensive research and controversies. Lancet Diabetes Endocrinol. 2023;11:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Singh G, Krauthamer M, Bjalme-Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med. 2022;70:5-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 5. | Hu S, Su X, Fan G. Efficacy and tolerability of the Subcutaneous Semaglutide for type 2 Diabetes patients: an updated systematic review and meta-analysis. Diabetol Metab Syndr. 2023;15:218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Caruso I, Di Gioia L, Di Molfetta S, Cignarelli A, Palmer SC, Natale P, Strippoli GFM, Perrini S, Natalicchio A, Laviola L, Giorgino F. Glucometabolic outcomes of GLP-1 receptor agonist-based therapies in patients with type 2 diabetes: a systematic review and network meta-analysis. EClinicalMedicine. 2023;64:102181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 7. | DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med. 2010;27:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Gentilella R, Romera I, Nicolay C, Buzzetti R, Vázquez LA, Sesti G. Change in HbA(1c) Across the Baseline HbA(1c) Range in Type 2 Diabetes Patients Receiving Once-Weekly Dulaglutide Versus Other Incretin Agents. Diabetes Ther. 2019;10:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, Thrasher J, Woo V, Philis-Tsimikas A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 10. | Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L; DURATION-1 Study Group. Exenatide once weekly vs twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 802] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 11. | Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Jones B. The therapeutic potential of GLP-1 receptor biased agonism. Br J Pharmacol. 2022;179:492-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Nauck MA, Müller TD. Incretin hormones and type 2 diabetes. Diabetologia. 2023;66:1780-1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 14. | Giugliano D, Longo M, Caruso P, Di Fraia R, Scappaticcio L, Gicchino M, Petrizzo M, Bellastella G, Maiorino MI, Esposito K. Feasibility of Simplification From a Basal-Bolus Insulin Regimen to a Fixed-Ratio Formulation of Basal Insulin Plus a GLP-1RA or to Basal Insulin Plus an SGLT2 Inhibitor: BEYOND, a Randomized, Pragmatic Trial. Diabetes Care. 2021;44:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, Araki E, Chu PL, Wijayasinghe N, Norwood P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J Clin Endocrinol Metab. 2018;103:2291-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 16. | Yale JF, Catarig AM, Grau K, Harris S, Klimek-Abercrombie A, Rabasa-Lhoret R, Reardon L, Woo V, Liutkus J. Use of once-weekly semaglutide in patients with type 2 diabetes in routine clinical practice: Results from the SURE Canada multicentre, prospective, observational study. Diabetes Obes Metab. 2021;23:2269-2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Rana KB, Britnell SR, Gilbertson ME, Ibrahim SL. Comparison of the Effectiveness of Liraglutide vs Semaglutide in a Veteran Population. J Pharm Pract. 2023;36:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 18. | Ares-Blanco J, Pujante-Alarcón P, Lambert C, Morales-Sánchez P, Delgado-Álvarez E, Menéndez-Torre EL. Real-life effects of adding weekly subcutaneous semaglutide to insulin for the treatment of type 2 diabetes mellitus. Rev Clin Esp (Barc). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Napoli R, Berra C, Catarig AM, Di Loreto C, Donatiello E, Berentzen TL, Pitocco D, Giorgino F. Once-weekly semaglutide use in patients with type 2 diabetes: Real-world data from the SURE Italy observational study. Diabetes Obes Metab. 2023;25:1658-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Milenkovikj T, Chekorova Mitreva B, Jovanovska Mishevska S, Bitoska-Mileva I, Ahmeti I; MIRAGE study group. Once-weekly semaglutide use in glucagon-like peptide-1 receptor agonist naïve patients with type 2 diabetes in North Macedonia: Real-world data from the MIRAGE study. Diabetes Res Clin Pract. 2023;206:111018. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Patel T, Nageeta F, Sohail R, Butt TS, Ganesan S, Madhurita F, Ahmed M, Zafar M, Zafar W, Zaman MU, Varrassi G, Khatri M, Kumar S. Comparative efficacy and safety profile of once-weekly Semaglutide vs once-daily Sitagliptin as an add-on to metformin in patients with type 2 diabetes: a systematic review and meta-analysis. Ann Med. 2023;55:2239830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Zhang Z, Zhang Q, Tan Y, Chen Y, Zhou X, Liu S, Yu J. GLP-1RAs caused gastrointestinal adverse reactions of drug withdrawal: a system review and network meta-analysis. Front Endocrinol (Lausanne). 2023;14:1149328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |