Published online Sep 20, 2024. doi: 10.5662/wjm.v14.i3.91058
Revised: January 28, 2024
Accepted: March 21, 2024
Published online: September 20, 2024
Processing time: 187 Days and 9 Hours
Hepatitis C virus (HCV) infection progresses through various phases, starting with inflammation and ending with hepatocellular carcinoma. There are several invasive and non-invasive methods to diagnose chronic HCV infection. The invasive methods have their benefits but are linked to morbidity and complications. Thus, it is important to analyze the potential of non-invasive methods as an alternative. Shear wave elastography (SWE) is a non-invasive imaging tool widely validated in clinical and research studies as a surrogate marker of liver fibrosis. Liver fibrosis determination by invasive liver biopsy and non-invasive SWE agree closely in clinical studies and therefore both are gold standards.
To analyzed the diagnostic efficacy of non-invasive indices [serum fibronectin, aspartate aminotransferase to platelet ratio index (APRI), alanine aminotransferase ratio (AAR), and fibrosis-4 (FIB-4)] in relation to SWE. We have used an Artificial Intelligence method to predict the severity of liver fibrosis and uncover the complex relationship between non-invasive indices and fibrosis severity.
We have conducted a hospital-based study considering 100 untreated patients detected as HCV positive using a quantitative Real-Time Polymerase Chain Reaction assay. We performed statistical and probabilistic analyses to determine the relationship between non-invasive indices and the severity of fibrosis. We also used standard diagnostic methods to measure the diagnostic accuracy for all the subjects.
The results of our study showed that fibronectin is a highly accurate diagnostic tool for predicting fibrosis stages (mild, moderate, and severe). This was based on its sensitivity (100%, 92.2%, 96.2%), specificity (96%, 100%, 98.6%), Youden’s index (0.960, 0.922, 0.948), area under receiver operating characteristic curve (0.999, 0.993, 0.922), and Likelihood test (LR+ > 10 and LR- < 0.1). Additionally, our Bayesian Network analysis revealed that fibronectin (> 200), AAR (> 1), APRI (> 3), and FIB-4 (> 4) were all strongly associated with patients who had severe fibrosis, with a 100% probability.
We have found a strong correlation between fibronectin and liver fibrosis progression in HCV patients. Additionally, we observed that the severity of liver fibrosis increases with an increase in the non-invasive indices that we investigated.
Core Tip: The role of non-invasive indices (including serum fibronectin) was investigated to assess and differentiate liver fibrosis in untreated hepatitis C virus (HCV)-infected patients. The overall assessment and prediction process involved the correlation of fibronectin, alanine aminotransferase ratio, aspartate aminotransferase to platelet ratio index, and fibrosis-4 with severity staging performed through shear wave elastography. The role of non-invasive indices to assess and differentiate liver fibrosis is further validated through the calculation of diagnostic accuracy measured using various standard methods such as, sensitivity and specificity, Youden's index, area under receiver operating characteristic curve, and likelihood test. We have explored machine learning-based analysis using a Bayesian Network to predict and validate the diagnostic ability of non-invasive indices for predicting liver fibrosis in HCV patients.
- Citation: Kaur N, Goyal G, Garg R, Tapasvi C, Demirbaga U. Ensemble for evaluating diagnostic efficacy of non-invasive indices in predicting liver fibrosis in untreated hepatitis C virus population. World J Methodol 2024; 14(3): 91058
- URL: https://www.wjgnet.com/2222-0682/full/v14/i3/91058.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i3.91058
Hepatitis C virus (HCV) is linked to both acute and chronic hepatitis, which can be of varying severity ranging from a mild infection to a severe and long-lasting infection with the possibility of cirrhosis and cancer. According to the fact sheet published by World Health Organization (WHO), approximately 58 million people are living with chronic HCV infection in 2019; this includes approximately 1.5 million new infections every year[1,2]. In this fact sheet, it was approximated by WHO that HCV infection resulted in around 290000 deaths in 2019, mostly linked to cirrhosis and hepatocellular carcinoma. HCV is a blood-borne, enveloped, and single-strand ribonucleic acid (RNA) virus with at least six genotypes and numerous subtypes; it belongs to the hepacivirus genus in the Flaviviridae family[3,4]. The prevalence of HCV genotypes and subtypes varies geographically according to transmission and ethnicity. A significant portion of the Indian population is infected with HCV, with a prevalence ranging between 0.5% and 1.5%. In India, some areas (Punjab and the north-eastern region) may represent HCV hotspots compared to other parts of India. Here, the most common means of HCV transmission are related to blood transfusion and the insecure use of injections for therapeutic reasons[5].
HCV does not cause any significant symptoms, and people often remain unaware of the advancement of the infection. The liver disease arising from the HCV infection gradually progresses through various phases, including inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma. In the first phase, the liver becomes tender and expanded, depicting the immune system’s response to the offending toxins. The second phase (fibrosis) is triggered through chronic (long-term) inflammation, usually as a result of the healing process of the liver to regenerate the damaged areas of the liver. At a certain point, the liver reaches the stage of scarring and goes beyond its self-healing ability. This phase is known as cirrhosis. Interventions at the early stage of cirrhosis can stimulate healing and recovery from the infection. However, at a later stage and even when it reaches the final phase (carcinoma), the cells can not heal, which may lead to complete liver failure and eventually death. Looking into the progression phase, it is evident that by tracing the infection at the fibrosis phase, the possibility of improving the self-healing ability of the liver and stimulating the recovery process can be largely possible[6].
Thus, to diagnose chronic HCV infection at an early stage, invasive methods and non-invasive indices have been proposed and employed in clinical studies. Liver biopsy is the primary invasive method (often called a gold standard) to detect liver fibrosis. Although it has benefits, it is also linked to morbidity and complications (minor or significant); approximately a quarter of patients undergoing liver biopsy witness right upper quadrant pain[7]. In contrast, the non-invasive (blood-based) biomarkers proffer higher degree of patient acceptance over liver biopsy, alongside being safe and cost-effective. They are classified into two categories, serum biomarkers and imaging techniques.
Serum biomarkers are classified into two further forms, i.e. direct (class I) and indirect (class II) markers/indices. The class I indices are the direct fragments of components in the liver matrix. These fragments are produced by hepatic stellate cells during the remodeling process of the extra cellular matrix (ECM), hence reflecting the discharge of ECM. The class I indices (direct markers) are fibronectin, YKL-40, hyaluronic acid, laminin, procollagen type I carboxy-terminal peptide, and alpha-2-macroglobulin[8]. Among these, fibronectin is a glycoprotein of the ECM with a high molecular weight. Hepatocytes are the main cells responsible for a variety of cellular functions and protein synthesis. Serum fibronectin exists in two forms in the blood, i.e. cellular fibronectin and plasma fibronectin[9]. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum bilirubin are the mainly considered class II (indirect) indices in HCV, but they cannot distinguish between intermediate fibrosis stages[10].
Imaging techniques such as Fibroscan or transient elastography and shear wave elastography (SWE) are non-invasive methods to assess liver fibrosis. Although Fibroscan is prevalent in the United States, it has several limitations including cost of the equipment and lack of standardized cutoffs for the diagnosis of fibrosis stages. SWE is a non-invasive imaging tool that measures liver stiffness that, in turn, has been validated in clinical and research studies[11,12] as a surrogate marker of liver fibrosis. This technique can help to gather real-time images through a B-mode ultrasound probe[13]. Liver fibrosis determination by invasive biopsy and non-invasive SWE agree closely; thus, both methods are considered to be gold standards[14-16]. Existing studies[17-19] strongly suggest that SWE is accurate and has diagnostic effectiveness in predicting and staging biopsy-proven liver fibrosis patients across varied populations worldwide.
The interest in non-invasive markers or indices for predicting fibrosis in chronic HCV subjects has recently increased. However, these indices’ validity is limited, restricting their adoption in clinical applications. Thus, the present study investigated the role of non-invasive biomarkers (including serum fibronectin) to assess the presence of a severity category of fibrosis vs the absence of fibrosis in untreated HCV-infected patients. The overall assessment process involved the correlation of fibronectin, alanine aminotransferase ratio (AAR), aspartate aminotransferase to platelet ratio index (APRI), and fibrosis-4 (FIB-4) with the severity staging performed through SWE. In this study, SWE is considered an alternative gold standard to liver biopsy proven through different existing works[12-19]. This is further validated by calculating diagnostic accuracy measured using standard methods such as sensitivity and specificity, Youden’s index, area under receiver operating characteristic curve (AUROC), and likelihood test[20].
Most of the existing studies rely on statistical analysis for evaluation. However, these days artificial intelligence (AI)-based techniques are very popular for analyzing the diagnostic ability of clinical indices in patient-based studies[21]. This is because once the AI model is trained to behave in a certain way using existing diagnostic data, then analyzing the clinical data is far more accurate and faster as compared to standard statistical methods[22]. Researchers from the University of Florida used the data related to HCV recorded in the national HCV registry to train the AI models that were further used to predict various risk factors pertaining to HCV treatment[2]. Thus, we have explored AI-based analysis using a Bayesian Network to validate the diagnostic ability of non-invasive indices for predicting liver fibrosis in HCV patients. Moreover, Bayesian networks reveal the conditional dependence between the non-invasive indices by creating parent-child relationships between them[23]. Furthermore, this method provides a diagnostic range for the values of non-invasive indices that clinicians and medical practitioners can use to know the disease progression and detect the severity of liver fibrosis in HCV patients.
A hospital-based observational study was performed on one hundred indoor (hospitalized) and outdoor (outpatients) adult untreated HCV patients who attended the Department of Medicine, Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India. Based on the availability and feasibility of the participants, a non-random convenient sampling technique was adopted. The samples were collected from the Malwa population (prone to insecure use of injections for therapeutic reasons), one of the reasons prevalent in HCV hotspots[5]. The patients who tested positive for HCV-RNA using real-time quantitative Polymerase chain reaction - RTPCR (TracQ-C) assay (with detection limit ≥ 15 IU/mL), were included in this study. The viral load of each patient was recorded. The exclusion criteria were followed and patients with comorbidities were excluded from the study. The HCV diagnosed patients were further recruited according to their LSM category (Table 1) derived from the SWE measurement until the limit of the specific fibrosis category was reached (approximately 25 ± 1 individuals each), after which no more in the category were recruited. All the recruited subjects gave informed consent, regardless of sex or age. The work was undertaken following permission from the Institutional Ethics Committee established within the University.
| Severity of fibrosis | METAVIR stages | LSM in kPa |
| Normal | F0-F1 | 2 - 7 |
| Mild fibrosis | F2 | 7.1-11 |
| Moderate fibrosis | F3 | 11.1-21 |
| Severe fibrosis | F4 | > 21 |
Exclusion criteria: The co-infected patients (like hepatitis B or human immunodeficiency virus (HIV), or hepatocellular carcinoma) who were detected as positive using a Triple H card were not considered in the present study. None of the considered subjects was linked to ALT flare (values five-fold the top ceiling normal (45 U/mL) measured using AU480 Beckman Coulter fully automated machine), failed or unreliable liver stiffness calculation (using SWE), having more than one HCV genotype infection, and metabolic dysfunction-associated steatotic liver disease (MASLD). We diagnosed MASLD based on cardiometabolic criteria and ruled out patients with Type 2 diabetes mellitus (+ve), body mass index (≥ 25 kg/m2), and metabolic abnormalities (such as waist circumference (≥ 94/80 in men/women), blood pressure (≥ 130/85 mmHg), plasma triglycerides (≥ 150 mg/dL), HDL cholesterol [> 40/50 mg/dL for men/women), HOMA-insulin resistance score (≥ 2.5)]. The above test was conducted on an AU480 Beckman Coulter fully automated machine. We used an alcohol use questionnaire to rule out serious alcohol use (> 50/60 g/d for men/women) in the study. Additionally, pregnant women were also excluded from the study. We formalized all the above observations when testing positive for HCV PCR.
The proposed study considered several non-invasive indices for routine and special investigations discussed below.
Routine investigations: All the samples were subjected to routine investigation, including AST, ALT, alkaline phosphata
Special investigations: Fibrosis-4 (FIB-4): Developed in the APRICOT study by Sterling et al[10] to predict fibrosis and cirrhosis for patients co-infected by HCV/HIV. Eventually, this marker was further validated through several studies concerning HCV patients[24,25]. FIB-4 = [AST (IU/mL) × age (years)]/[platelet count (*109/L) × ALT 1/2 (IU/mL)].
AST to Platelet Ratio Index (APRI): A simple method developed by Wai et al[26], calculated using the routine parame
AST/ALT ratio (AAR): The proportion of AST and ALT concentrations in the blood measured using a blood test.
Plasma fibronectin: Estimated using the Qayee Bio kit on the ELISA Reader. It is based on the double antibody sandwich enzyme-linked immunosorbent method[27].
SWE: This technique was used for the liver stiffness measurement (LSM) using the ultrasound machine Philips Affiniti 70 (USG). SWE is one of the clinical researchers’ most popular non-invasive methods for measuring liver stiffness[11].
A 10 mL venous blood sample was collected after cleaning the venipuncture site with a spirit swab. The sample was then put into a different vacutainer depending on the type of test and allowed to clot, and then the serum was separated by centrifugation and analyzed. Ethylenediaminetetraacetic acid tubes-BD Vacutainer was used for hematology tests (Hb, TLC and platelet count), sodium citrate vacutainer for PT test, plain red vacutainer for TSP, albumin, bilirubin, AST, ALT, and ALP assays. The viral markers were performed first, and then HCV RNA by reverse transcriptase - polymerase chain reaction was performed and estimated from a Nationally Accredited Laboratory recognized and approved by the Punjab Government. The LSM has been performed using SWE. The USG machine (Philips Affiniti 70) was used to visualize the right lobe of the liver (from the intercostal space) with patients lying with their right arm in maximum abduction while in the supine position. The patients were asked to hold their breath for approximately 5 s to perform imaging. With the visual depth of the system set at 8 cm visual depth, the region of interest was fixed at 1-2 cm below the right liver capsule, with intra-hepatic vessels and gallbladder at a distance apart. The system was calibrated to adjust the sample volume depth at 4 cm or under. The stiffness of the liver was computed automatically by the calibrated system, and the results were generated as the velocity of the shear wave (represented as vs-m/s). Further, the mean elastic modulus (in kPa) was calculated automatically inside the region of interest. The specific segment of the liver was shot 10-12 times, with average result reliability considered with ten successful shots, and the measurement success rate of > 80% was acquired.
In this study, the proposed cutoffs for LSM are described according to the degree of fibrosis in reference to METAVIR stages shown in Table 1. For a diagnostic test, the cutoff value is not universal and is different based on the region, machine and disease condition[28]. So, the cutoff used in the present study was determined according to the USG machine and the range provided for that machine by the relevant vendor. We even verified it with some existing studies[12,24] that suggested standardization of these values and fibrosis range calculation.
The statistical calculations were performed on the estimated data using SPSS tool version 21 for Windows (IBM Corp., Armonk, NY, United States) to understand the correlation and significance. The results were presented as median ± inter-quartile range (IQR) or mean ± standard deviation. The correlation was calculated for LSM as an ordinal variable. We used the Kolmogorov-Smirnov test to check the normality of data. The comparison of quantitative variables between the study groups was performed using ANOVA-Kruskal-Wallis test.
Then, we calculated the diagnostic accuracy based on sensitivity and specificity, Youden’s index, ROC and AUROC, and the Likelihood test. A multiple linear regression analysis of the clinical results followed this. The multi-linear regression was implemented using Python using ordinary least square regression technique. The model calculates the best fit using least squared method. A probability value (P value) less than 0.05 was considered statistically significant.
We used a Bayesian network to analyze the data for probabilistic analysis to uncover the complex relationship of non-invasive indices with disease progression. We implemented a Tree-Augmented Na¨ıve Bayesian model in the Genie platform (https://www.bayesfusion.com/). The details of this technique are explained in the following sections.
Tree-augmented Na¨ıve Bayesian network: Bayesian networks, also known as belief networks, are direct acyclical graphs that show the independence of the connection probability distribution over the set of variables[29]. Bayesian networks have several benefits over other machine learning techniques[30], including dealing with uncertainty and incomplete data, incorporating prior knowledge and domain expertise, providing a graphical representation of the relationships between variables, and quickly computing conditional probabilities. Additionally, Bayesian networks are effective for time-series data and dynamic systems, can handle continuous and discrete variables, and produce predictions that are easy to understand. Considering such benefits, we implemented the Tree Augmented Naive Bayes model (TAN), a semi-Naive Bayesian Learning method, to reveal the complex and hidden relation- ship between non-invasive indices for probabilistically detecting liver fibrosis severity in HCV patients. In the TAN model, the class variable (severity) is the parent of all other variables[31]. Among other variables, a parent-child relationship is created by learning from the data depending on the class variable, i.e. the severity. The relationships between the variables are classified as below:
Independence: No direct connection between them.
Dependence: Direct relationship with each other.
Conditional dependence: Dependent on each other conditionally.
Here, we have used four methods to validate the diagnostic accuracy of fibronectin, APRI, and FIB-4. The four methods are discussed below.
Sensitivity and specificity: Sensitivity and specificity are calculated as follows. Sensitivity = TP/(TP + FN). Specificity = TN/(TN + FP). Where, TP = true positive, FN = false negative, TN = true negative, and FP = false positive.
Youden’s index: A test is said to have poor diagnostic accuracy if the value of Youden’s index (J) equals 0, and a perfect diagnostic test if Youden’s index equals 1[20]. It is defined as: J = (Sensitivity + Specificity) – 1.
ROC and AUROC: The AUROC can have a value between 0 and 1, and it acts as a good indicator to depict the goodness of the test[18]. If the curve is closer to the left-upper quadrant and covers a larger area, it tends closer to 1. This shows that the test better discriminates between fibrosis and no fibrosis cases.
Likelihood tests: Two types of likelihood tests were performed: (1) Positive likelihood test (LR+); and (2) Negative likelihood test (LR-). The larger the LR+, the more informative the test. Similarly, the smaller the LR-, the more informative the test. In simple words, LR+ > 10 and LR- < 0.1 are considered good diagnostic test ratios[32,20]. They are defined as: LR+ = sensitivity/(1 − specificity). LR− = (1 − sensitivity)/specificity.
The results obtained after the experimental evaluation and statistical analysis are in the subsequent sections.
The present study included 100 HCV Patients, 66 males and 34 females, with a mean age of 42.7± 13 years. According to SWE, based on LSM values, 25 HCV patients were non-fibrotic, while the other 75 patients had liver fibrosis (24 mild, 25 moderate, and 26 severe). We have considered an equal distribution (25 ± 1) across absent, mild, moderate and severe fibrosis categories by considering the patients testing HCV RNA positive jointly with SWE results.
Table 2 shows the Median ± IQR of biological variables in all four categories (i.e. non-fibrotic, mild, moderate, and severe fibrosis). The P value (< 0.05) was considered significant to differentiate the degree of fibrosis among the four categories. In routine investigations, the viral load, AST, ALT, ALP, TLC and platelet count were found to be significant (P < 0.05) as median ± IQR was found to be raised with the severity of fibrosis (Table 2). In special investigations, serum fibronectin, APRI, and FIB-4 were considered significant (P < 0.05) in differentiating the degree of fibrosis across the four study groups (no fibrotic, mild, moderate, and severe fibrosis).
| Indices | No fibrosis0 | Mild fibrosis1 | Moderate fibrosis2 | Severe fibrosis3 | P value | ||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Routine investigations | |||||||||
| Viral load | 112792 | 56721.5-131470.5 | 308255 | 231893.25-424659.75 | 1050000 | 696516.5-1334612.5 | 4956005 | 3170155.25-9357337 | 0.000a |
| Hb | 13.2 | 11.35-15 | 13 | 11.07-14.7 | 13 | 10.65-14 | 12.95 | 10.95-13.4 | 0.674 |
| INR | 1 | 1-1.1 | 1.1 | 1.0175-1.3 | 1.1 | 1-1.185 | 1.09 | 1-1.1775 | 0.478 |
| TSP | 6.9 | 6.55-7.155 | 6.9 | 6.6-7.2 | 7.1 | 6.65-7.72 | 7 | 6.55-7.2 | 0.683 |
| Albumin | 3.9 | 3.55-4.5 | 3.8 | 3.5-4.175 | 4.1 | 3.55-4.3 | 3.85 | 3.375-4.3 | 0.849 |
| Bilirubin | 0.68 | 0.4-0.7 | 0.7 | 0.5-0.895 | 0.7 | 0.4-0.95 | 0.8 | 0.475-1 | 0.488 |
| AST | 43 | 31.5-63 | 53.5 | 30.5-92.75 | 97 | 70.5-115.5 | 108.5 | 62.5-172.5 | 0.000a |
| ALT | 50 | 36-77 | 60 | 40.25-93.3 | 92 | 56-175.5 | 106.5 | 85.25-151 | 0.030a |
| ALP | 85 | 77.5-88 | 82 | 78.25-88 | 102 | 97.5-131 | 150 | 129.5-168 | 0.000a |
| TLC | 8800 | 7700-9450 | 8050 | 6750-9075 | 7200 | 6600-8500 | 7200 | 6500-7725 | 0.000a |
| Plt count | 216 | 158-279.5 | 195 | 138.75-235 | 160 | 111.5-210 | 130 | 102-160.25 | 0.000a |
| Special investigations | |||||||||
| Fibronectin | 98 | 89.82-101.25 | 118 | 113.4-126.57 | 147 | 138.75-154 | 204.5 | 188.975-222.25 | 0.000a |
| AAR | 0.83 | 0.64-1.08 | 0.86 | 0.71-1.21 | 0.89 | 0.724-1.26 | 1.82 | 1.159-4.37 | 0.704 |
| APRI | 0.52 | 0.33-0.69 | 0.6 | 0.495-1.84 | 1.67 | 0.95-2.291 | 1.82 | 1.159-4.37 | 0.000a |
| FIB-4 | 1 | 0.63-1.29 | 1.44 | 0.98-2.29 | 2.52 | 2.04-3.918 | 3.77 | 2.68-6.6 | 0.000a |
We have calculated Pearson’s correlation coefficient (R)[33] and the significance of investigations concerning LSM.
Routine investigations: The correlation coefficients and significance with regard to the routine investigations concerning LSM are shown in Table 3. It can be seen that viral load, AST, ALT, ALP, TLC, and platelet count are highly significant (P < 0.05). At the same time, all other indices are insignificant (P > 0.05) according to the obtained results (Table 3).
| Indices | R | P value |
| Routine investigations | ||
| Viral load | 0.663 | 0.000a |
| Hb | -0.169 | 0.092 |
| INR | 0.041 | 0.688 |
| TSP | 0.081 | 0.425 |
| Albumin | -0.06 | 0.552 |
| Bilirubin | 0.149 | 0.14 |
| AST | 0.428 | 0.000a |
| ALT | 0.3 | 0.000a |
| ALP | 0.747 | 0.000a |
| TLC | -0.391 | 0.000a |
| Plt count | -0.473 | 0.000a |
| Special investigations | ||
| Serum fibronectin | 0.929 | 0.000a |
| AAR | 0.127 | 0.207 |
| APRI | 0.574 | 0.000a |
| FIB-4 | 0.586 | 0.000a |
Special investigations: Table 3 shows the correlation and significance concerning special investigations for all cases with respect to LSM.
Serum fibronectin levels: The validity assessment of fibronectin for predicting fibrosis was also done through correlation. Table 3 depicts a high correlation (r = 0.929) for serum fibronectin levels with respect to LSM. A statistically high significance was found, i.e. P < 0.05.
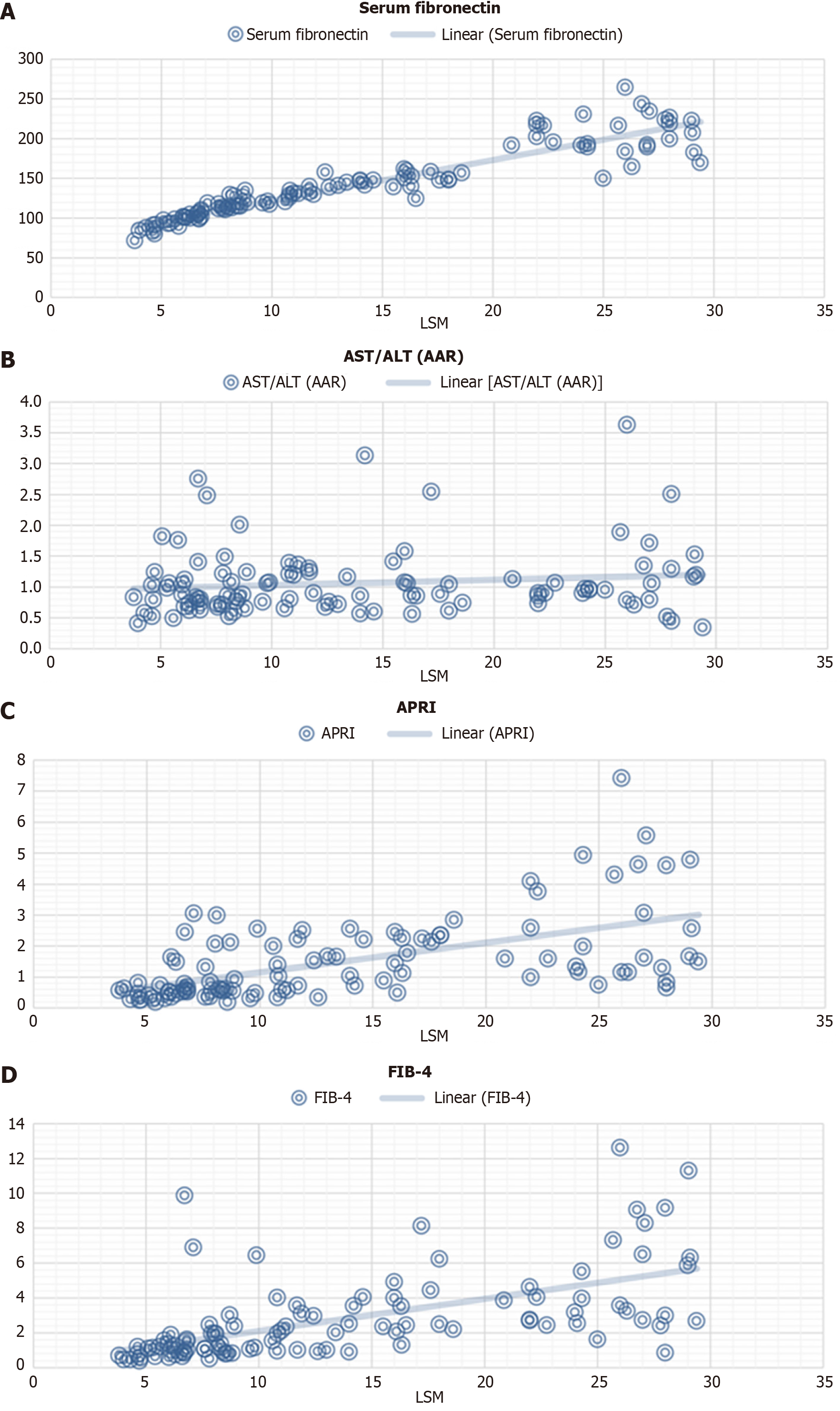
Fibrotic scores: These indices were validated with respect to LSM based on correlation. The results show that APRI and FIB-4 depict a positive correlation (r = 0.574 for APRI and r = 0.586 for FIB-4) trend, but AAR depicts a low positive correlation (r = 0.127). The results are depicted in Table 3. When compared with LSM values, APRI and FIB-4 were highly significant (P < 0.05). However, AAR proved to be non-significant (P > 0.05) (Table 3). Figure 1 depicts the correlation plot depicting r and P value considering special investigations (including fibronectin) vs LSM.
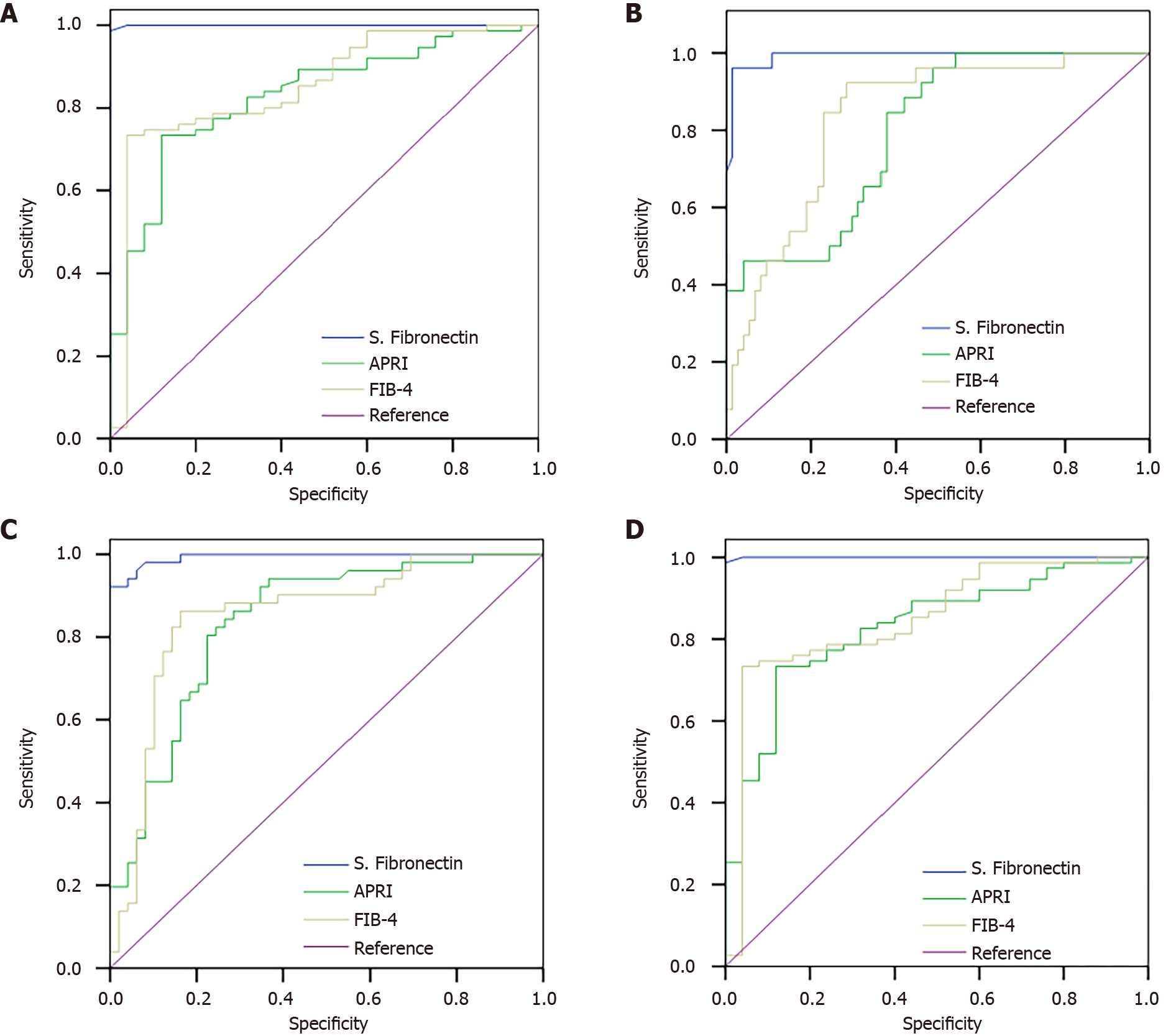
Table 4 compares laboratory investigations for the diagnostic accuracy methods discussed earlier. We want to highlight that sensitivity and specificity are expressed as a decimal and not a percentage in the calculation of Youden’s Index. Moreover, Figure 2A-D show the ROC curve of fibronectin, APRI, and FIB-4 in all cases and also in differentiating fibrosis. The cutoffs for fibronectin, APRI and FIB-4, based on which diagnostic analysis was performed, include mild (> 110, > 0.824, > 1.90), moderate (> 140, > 1.19, > 2.45) and severe fibrosis (> 180, > 1.38, > 2.7), respectively. These cutoffs are based on Youden’s index.
| Indices | S. Fibronectin, % | APRI, % | FIB-4, % |
| Fibrosis | |||
| Sensitivity | 98.70 | 73.30 | 73.30 |
| Specificity | 100 | 88 | 96 |
| Youdens index | 0.987 | 0.613 | 0.693 |
| AUROC | 1 | 0.829 | 0.85 |
| LR+ | > 10 | < 10 | > 10 |
| LR- | 0.013 | 0.303 | 0.277 |
| Mild fibrosis | |||
| Sensitivity | 100 | 45.80 | 45.80 |
| Specificity | 96 | 88 | 96 |
| Youdens index | 0.96 | 0.338 | 0.418 |
| AUROC | 0.999 | 0.672 | 0.725 |
| LR+ | > 10 | < 10 | > 10 |
| LR- | 0 | 0.615 | 0.564 |
| Moderate fibrosis | |||
| Sensitivity | 92.20 | 80.40 | 82.40 |
| Specificity | 100 | 77.60 | 85.70 |
| Youdens index | 0.922 | 0.579 | 0.681 |
| AUROC | 0.993 | 0.836 | 0.853 |
| LR+ | > 10 | < 10 | < 10 |
| LR- | 0.0784 | 0.252 | 0.205 |
| Severe fibrosis | |||
| Sensitivity | 96.20 | 84.60 | 92.30 |
| Specificity | 98.60 | 62.20 | 71.60 |
| Youdens index | 0.948 | 0.468 | 0.639 |
| AUROC | 0.922 | 0.796 | 0.835 |
| LR+ | > 10 | < 10 | < 10 |
| LR- | 0.038 | 0.247 | 0.107 |
As we know that correlation just provides the degree to which variables are associated to one another. But, in most of the occasions, just providing simple association is not enough evidence for the overall analysis. So, we used multi-linear regression to explain the variation in one variable, i.e. dependent variable with respect to other variables. The multiple linear regression is defined on the basis of the equation. yi = β0 + β1xi1 + β1xi2 + ... + βpxip + ∈. Where yi represents the dependent variable, xi depicts the explanatory variables, β0 represents y-intercept, βp represents slope coefficients for each explanatory variable, and ∈ is the residual.
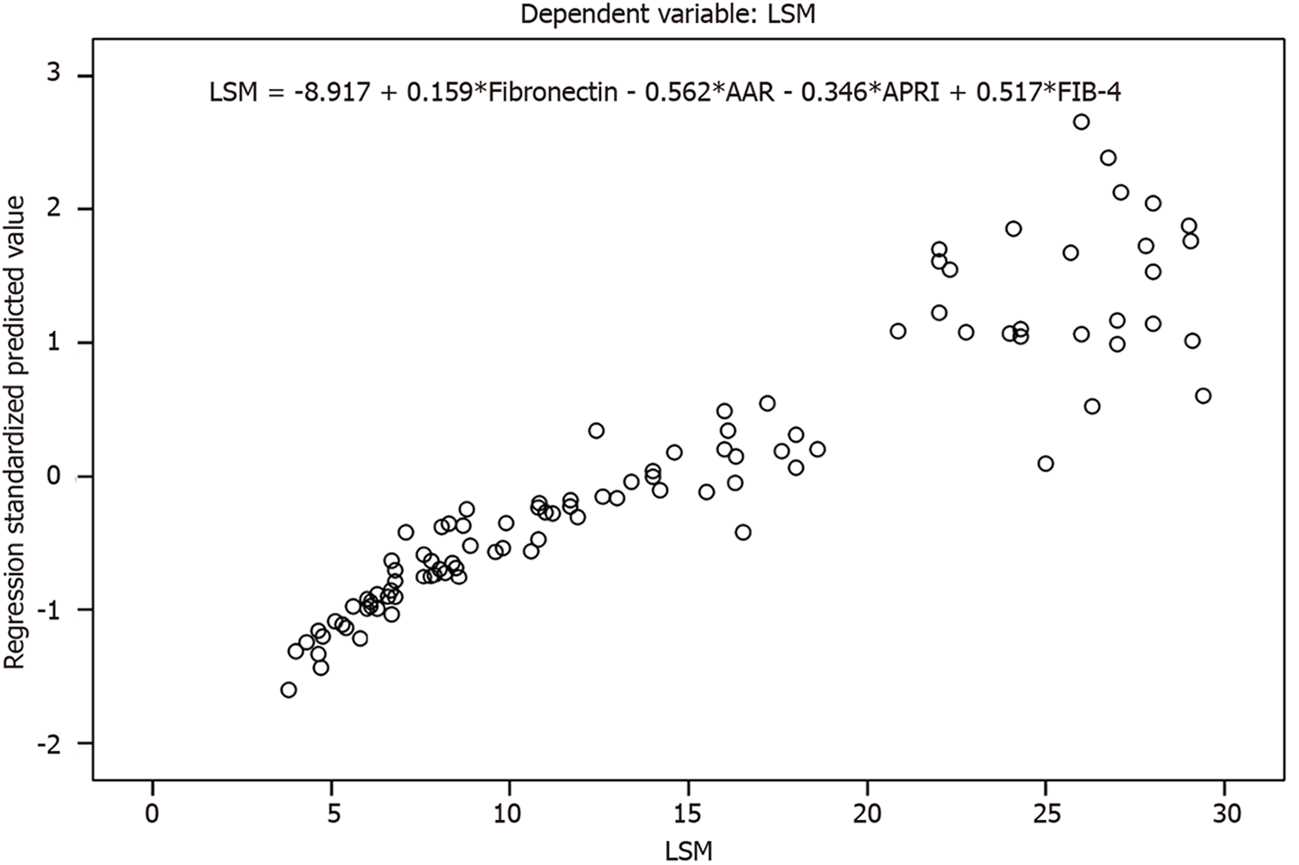
The variables that we are trying to predict or analyze are called dependent variable as they are dependent on other variables. Here, LSM is considered as the dependent variable. The variables that are considered to analyze or predict the dependent variables are known as explanatory variables. These variables are independent of other variables. Here, the independent variables considered to quantify the relationship include the special investigations, i.e. fibronectin, AAR, APRI, and FIB-4. The results obtained from linear regression show that fibronectin (P < 0.05) was statistically significant with respect to LSM (dependent variable) as compared to AAR, APRI, and FIB-4. The results obtained using linear regression depicted the p-value of various indices, fibronectin (0.000), AAR (0.430), APRI (0.442), and FIB-4 (0.073). The goodness of the fit (accuracy of the model) comes out to be 0.870 based on R-squared value. Figure 3 depicts the plots showing the regression plot (including the regression equation).
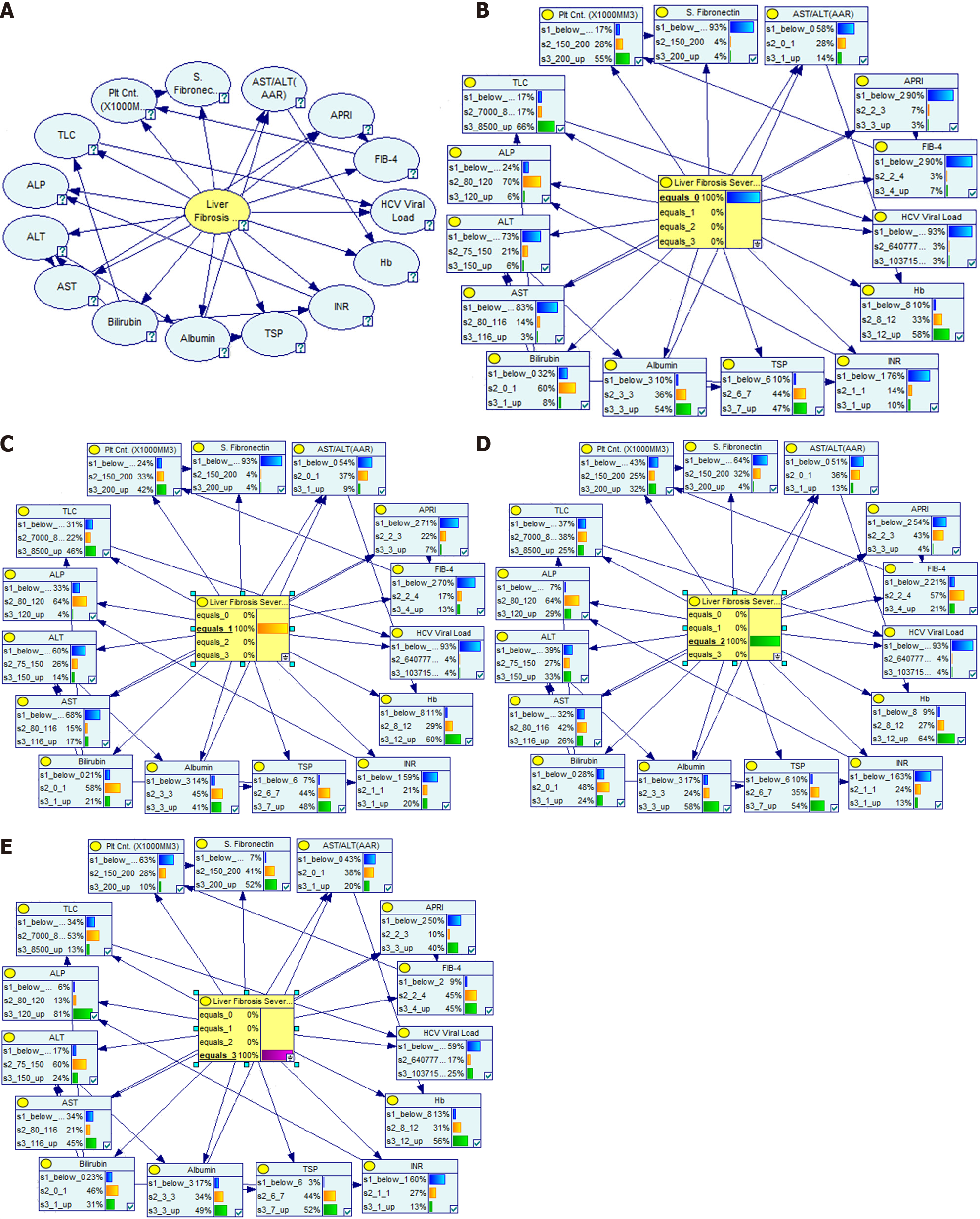
The Bayesian Network uses the labeled data for training based on the SWE cutoffs. Once the model is trained, it can automatically detect/predict the relationship of any test data input to the model. In our implementation, we specifically opted for the information-based binning method to determine the cutoffs (known as discretization or binning in Bayesian Networks). This approach ensures that the discretization process is driven by statistical measures, enabling us to identify the most informative cutoff points. By prioritizing information preservation, the information-based binning method offers distinct advantages in accuracy, interpretability, and the ability to capture underlying patterns in the data. Therefore, we believe that using information-based binning for the Bayesian Network cutoffs enhances our approach’s reliability and effectiveness. We have constructed a Bayesian graph consisting of nodes and the directed connections between them, where nodes represent the non-invasive indices, and the edges between the two nodes represent potential dependencies between them. The direction of the arrow goes from the influencing variable (parent) to the affected variable (child). Figure 4A shows the dependency network for the Tree-Augmented Na¨ıve Bayesian model implemented in the Genie platform. This dependency network was exploited with all the probabilities for Liver Fibrosis Severity levels ranging from 0-3 (where 0 is no fibrosis), as shown in Figure 4B-E.
As seen in Figure 4B, when Liver Fibrosis Severity is 0, the probability of serum fibronectin being lower than 150 is 93%. Likewise, the probability of AAR (below 1), APRI (below 2), and FIB-4 (below 2) is likely to be 58%, 90%, and 90%, respectively. Similarly, the probabilistic dependence for other non-invasive indices is also depicted in Figure 4B. Looking at Figure 4E, the liver fibrosis severity is maximum when the probability of serum fibronectin being at maximum values is 52% (with 93% for value above 150). This kind of variation is also visible for FIB-4.
Tables 5 and 6 show the probabilities of Liver Fibrosis severity based on the values of serum fibronectin and a combination of special investigations, namely serum fibronectin, AAR, APRI, and FIB-4. It is seen that there is a direct relation between these non-invasive indices and the severity of Liver Fibrosis. While these indices are at their lowest, the Liver Fibrosis severity tends to decrease. Likewise, when these values increase, the Liver Fibrosis severity also increases.
| Fibronectin | Fibronectin < 150, % | Fibronectin 150-200, % | Fibronectin > 200, % |
| LF severity (0) | 37 | 1 | 1 |
| LF severity (1) | 36 | 1 | 1 |
| LF severity (2) | 25 | 42 | 1 |
| LF severity (3) | 2 | 57 | 98 |
| Fibronectin | Fibronectin < 150, % | Fibronectin 150-200, % | Fibronectin > 200, % |
| AAR | AAR (< 1) | AAR (1) | AAR (> 1) |
| APRI | APRI (< 2) | APRI (2-3) | APRI (> 3) |
| FIB-4 | FIB-4 (< 2) | FIB-4 (2-4) | FIB-4 (> 4) |
| LF severity (0) | 59 | 0 | 0 |
| LF severity (1) | 31 | 0 | 0 |
| LF severity (2) | 8 | 77 | 0 |
| LF severity (3) | 2 | 23 | 100 |
Infection with HCV is a common problem worldwide. Most cases progress to chronic infection with its complications. Due to progressive HCV, liver fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma may occur[34]. In the present study, 100 newly diagnosed HCV patients were considered with a mean age of 42.7 ± 13 years, of which 66% were males and 34% were females. This is found to be similar to other studies that have considered the elderly population in their HCV studies and also have more males as compared to females[12,35,36]. Because treatment of HCV will lead to a reversal of non-invasive markers, only untreated HCV patients were included in our study. Taneja et al[37] stated that consuming alcohol > 30 g/d may lead to high fibrosis. So, we have excluded alcoholics (> 80 g/d) from our study. SWE cutoffs were determined using the USG machine ‘Philips Affinity 70’ (Table 1 depicts the cutoff ranges). Similar cutoffs were defined by Jeong et al[12] (F0-F1: 6.77 ± 1.72, F2: 9.98 ± 3.99, F3: 15.8 ± 7.73 and F4: 22.09 ± 10.09).
We have found a strong association between liver fibrosis and liver function tests and routine investigations such as AST, ALT, ALP, platelet count and TLC (Table 2). Fibronectin, APRI, and FIB-4 are found to be highly significant (P < 0.05) according to the degree of fibrosis. Similar findings were found in various existing proposals. For example, Tada et al[25] found high significance for platelet count and ALT regarding liver fibrosis (P = 0.003) in HCV cases. Also, Tamaki et al[35] found a strong correlation between age and platelet count with liver fibrosis grading (P < 0.05), taking SWE as the main standard method. In the present study, serum fibronectin was highly significant according to the degree of fibrosis (P < 0.05) compared with SWE (Table 2).
Furthermore, compared with SWE, serum fibronectin showed a high positive correlation (r = 0.929; P < 0.05). However, APRI and FIB-4 display a positive correlation but are not as strong as fibronectin (Table 2). Attallah et al[38] evaluated the diagnostic value of fibronectin as a predictor of liver fibrosis in patients with chronic HCV infection. Also, they have incorporated a fibronectin discriminant score along with Albumin and APRI, which can decrease the demand for liver biopsy. It was revealed by Yamauchi et al[39] that the fibronectin receptor was increased in fibrotic areas and on the plasma membrane of hepatocytes of the fibrotic liver. Also, a positive correlation was obtained between fibronectin and the severity of the liver disease assessed by ALT, AST and serum bilirubin, thus making it suitable to differentiate fibrosis staging in HCV Patients[40]. In our study, AUROC of serum fibronectin, APRI and FIB-4 were calculated as 0.99, 0.67 and 0.725 with cut off of > 110, > 0.82, and > 1.90, respectively, to predict patients with mild liver fibrosis. Our results are similar to Jeong et al[12]. They also found low diagnostic accuracy for APRI (0.691) compared to SWE for predicting mild fibrosis. Moreover, Kujur et al[24], and Tada et al[25] showed mild diagnostic accuracy, i.e. (0.842, 0.874) and (0.809, 0.803), respectively, for APRI and FIB-4 in mild fibrosis prediction. In the present study, to detect moderate fibrosis, the AUROC of fibronectin, APRI, and FIB- 4 were calculated as 0.99, 0.83 and 0.853 with a cutoff value of > 140, > 1.19, and > 2.45, respectively. Serum fibronectin showed excellent diagnostic accuracy, while APRI and FIB-4 showed moderate diagnostic accuracy. Our findings are similar to the findings of Kujur et al[24], de Oliveira et al[41], and Lin et al[42]. The AUROC of fibronectin, APRI and FIB-4 were calculated as 0.992, 0.796 and 0.835 with cutoffs of > 180, > 1.38, and > 2.7, respectively, in predicting severe liver fibrosis. Existing studies[41,42] had reported similar results indicating moderate accuracy for APRI and FIB-4 in predicting severe fibrosis. Similar findings for fibronectin were evaluated in[43,44] showing that the level of fibronectin increases significantly with the progression of fibrosis staging. Moreover, Ghafar et al[43] showed that fibronectin had a 65% sensitivity with a cutoff of 85.6 and stated that fibronectin is associated with significant fibrosis. A retrospective study conducted by Cassinotto et al[45] stated that with SWE as a reference method, FIB-4 had a sensitivity, specificity, and AUROC as 71.4%, 91.4%, and 0.837, respectively. A recent study conducted by Thanapirom et al[46] to assess liver fibrosis using non-invasive markers. In this study, APRI and FIB-4 correlated well with SWE. Using magnetic resonance elastography as a reference method, the diagnostic performance of SWE, APRI and FIB-4 was 0.87, 0.83, and 0.79 in differentiating mild fibrosis, and 0.96, 0.89, and 0.91 for cirrhosis, respectively.
In the present study, we have analyzed the diagnostic accuracy to validate the effectiveness of the performed tests for discriminating the patients according to fibrosis staging. Looking at sensitivity and specificity, it is evident that serum fibronectin shows high diagnostic accuracy in discriminating between non-fibrosis, mild, moderate, and severe fibrosis cases. However, APRI and FIB-4 show different variations. They depict lower values for mild fibrosis, which increase as the disease severity increases (Table 3). Thus, APRI and FIB-4 do not strongly discriminate between different stages of fibrosis severity. Youden’s Index also depicts a similar trend and advocates fibronectin as a strong diagnostic predictor, with results ranging between 92.2% and 98.7% for mild to severe fibrosis cases (Table 3). Finally, the likelihood tests also support fibronectin as a strong diagnostic marker for predicting liver fibrosis for HCV patients (Table 3). Additionally, increased levels of fibronectin are reported in patients with fibronectin glomerulopathy (autosomal disease), Duchenne muscular dystrophy, rheumatoid vasculitis, preeclampsia, and collagen vascular diseases[47]. In the present study, we affirmed that none of the patients suffered from any other disease that are associated with fibronectin levels. However, we suggest future studies to ensure the validity of this fact with more accuracy. Kim et al[47] stated that fibronectin increases in hepatocellular carcinoma and there is a strong correlation between the liver scarring and fibronectin. The authors mentioned that it may be useful to study fibronectin in the early stages of liver disease. The present study positively correlates with the findings in[47], as the fibronectin has proved to be a valid marker in assessing liver fibrosis.
The present study used a Bayesian Network to understand the hidden relationship between the non-invasive indices. The results depict a strong probabilistic relationship between them in discriminating liver fibrosis stages. The Bayesian Network also provides a trend of the variation in the values of non-invasive indices on liver fibrosis staging. An increase in the values of fibronectin is probabilistically related to an increase in the severity of the disease. Thus, we could predict different diagnostic ranges (cutoffs) for the investigations in relation to the different stages of disease progression (Tables 5 and 6). These cutoffs can be used by clinicians to interpret the results obtained in their equivalent local setting. This may vary if local settings are not standardized and the AI model is trained using totally varied data. It would be suitable to use these cutoffs in conjunction with positive HCV PCR to handle this issue. As visible from Table 5, if we rely just on fibronectin as a diagnostic test, there is a 98% probability that serum fibronectin (> 200) is related to patients with severe fibrosis. This is in line with the statistical findings that suggest fibronectin value > 180 is associated with severe fibrosis. If the fibronectin value is below 150, there is a 37% chance of no fibrosis, a 36% chance of mild fibrosis, and 25% chance of moderate fibrosis, and just 2% chance of severe fibrosis. This means a fibronectin value below 150 is not related to severe fibrosis, as depicted by the AUROC. Similarly, a fibronectin value between 150-200 shows a 42% probability of moderate fibrosis and a 57% probability of severe fibrosis. Table 6 depicts the results where Bayesian Network considers data corresponding to all four special investigations. It shows that if fibronectin (>200), AAR (>1), APRI (>3), and FIB-4 (>4), then there is 100% chance of severe fibrosis. These findings validate the findings based on AUROC and statistical analysis. But, we suggest this aspect needs further validation as this is the first study that suggests diagnostic cutoffs using AI. The accuracy for the Bayesian network was just above 90%, so a larger data size (with high data quality) can be used in the future to provide a strong validation to support the findings of this work.
We concluded that serum fibronectin is a strong non-invasive marker in predicting and differentiating the degree of fibrosis among HCV patients. This conclusion is supported by several diagnostic validators, all of them supporting the role of fibronectin as a potential alternative for diagnosing liver fibrosis in the HCV population. Moreover, this study also includes a timely and novel AI technique known as Bayesian Network to understand and uncover the hidden relationship between various routine and special investigations conducted for detecting liver fibrosis in HCV patients. It depicts a strong relationship between fibronectin and liver fibrosis severity stages. This study also analyses the trend of variation of values of routine and special investigations to uncover the tentative range that can be used by diagnostics and clinicians for further studies or for detecting fibrosis stages in HCV patients. However, it is suggested that further studies considering larger sample size and demographic diversity should be conducted.
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
| 1. | World Health Organization. Hepatitis C: Key facts. World Health Organization; 18 July 2023. Date accessed: December 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
| 2. | McNemar E. Florida Health System Uses AI to Predict Hepatitis C Treatment Outcomes. Health IT Analytics; 15 February 2022. Date accessed: December 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
| 3. | Lindenbach BD, Rice CM. Flaviviridae: The viruses and Their replication. In: KDM Fields BN, Howley PM, Griffin DE, Lamb RA, Mar- tin MA, Roizman B, Strauss SE, editors. Fields Virology. Philadelphia: Lippincott- Raven. 2001; 991-1042. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (34)] |
| 4. | Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 5. | Puri P. Tackling the Hepatitis B Disease Burden in India. J Clin Exp Hepatol. 2014;4:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Kaur N, Goyal G, Garg R, Tapasvi C, Chawla S, Kaur R. Potential role of noninvasive biomarkers during liver fibrosis. World J Hepatol. 2021;13:1919-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1569] [Article Influence: 98.1] [Reference Citation Analysis (1)] |
| 8. | Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Lucena S, Arocha Piñango CL, Guerrero B. [Fibronectin. Structure and functions associated to hemostasis. Review]. Invest Clin. 2007;48:249-262. [PubMed] |
| 10. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3523] [Article Influence: 185.4] [Reference Citation Analysis (0)] |
| 11. | Suda T, Okawa O, Masaoka R, Gyotoku Y, Tokutomi N, Katayama Y, Tamano M. Shear wave elastography in hepatitis C patients before and after antiviral therapy. World J Hepatol. 2017;9:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Jeong JY, Kim TY, Sohn JH, Kim Y, Jeong WK, Oh YH, Yoo KS. Real time shear wave elastography in chronic liver diseases: accuracy for predicting liver fibrosis, in comparison with serum markers. World J Gastroenterol. 2014;20:13920-13929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 13. | Gharibvand MM, Asare M, Motamedfar A, Alavinejad P, Momeni M. Ultrasound shear wave elastography and liver biopsy to determine liver fibrosis in adult patients. J Family Med Prim Care. 2020;9:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Zayadeen AR, Hijazeen S, Smadi M, Fayyad L, Halasa M, AlQusous S, AlRabadi O, Hijazeen R, Ajlouni Y, Tulenko K, Malik P. Compar- ing shear wave elastography with liver biopsy in the assessment of liver fibrosis at King Hussein Medical Center. EGLJ. 2022;12:1-8. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Osman AM, El Shimy A, Abd El Aziz MM. 2D shear wave elastography (SWE) performance versus vibration-controlled transient elastography (VCTE/fibroscan) in the assessment of liver stiffness in chronic hepatitis. Insights Imaging. 2020;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 17. | Jamialahmadi T, Nematy M, Jangjoo A, Goshayeshi L, Rezvani R, Ghaffarzadegan K, Nooghabi MJ, Shalchian P, Zangui M, Javid Z, Doaei S, Rajabzadeh F. Measurement of Liver Stiffness with 2D-Shear Wave Elastography (2D-SWE) in Bariatric Surgery Candidates Reveals Acceptable Diagnostic Yield Compared to Liver Biopsy. Obes Surg. 2019;29:2585-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Galal SM, Soror SM, Hussien O, Moustafa EF, Hassany SM. Noninvasive assessment of liver fibrosis in children with chronic hepatitis C: Shear wave elastography and APRI versus liver biopsy. Arab J Gastroenterol. 2020;21:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 20. | Šimundić AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC. 2009;19:203-211. [PubMed] |
| 21. | Edeh MO, Dalal S, Dhaou IB, Agubosim CC, Umoke CC, Richard-Nnabu NE, Dahiya N. Artificial Intelligence-Based Ensemble Learning Model for Prediction of Hepatitis C Disease. Front Public Health. 2022;10:892371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Doyle OM, Leavitt N, Rigg JA. Finding undiagnosed patients with hepatitis C infection: an application of artificial intelligence to patient claims data. Sci Rep. 2020;10:10521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Stephenson TA. An introduction to Bayesian network theory and usage. IDIAP, 2000. [DOI] [Full Text] |
| 24. | Kujur KK, Garg R, Kaur S, Aggarwal S, Tapasvi C, Chawla SP. Comparison of APRI, FIB-4 with Shear Wave Elastography in Assessment of Liver Fibrosis in Untreated Chronic Hepatitis C Patients. JIACM. 2020;21:3-4. |
| 25. | Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Okuda S, Tsuji N, Imayoshi Y, Yasuda E. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: Comparison of liver fibrosis indices. Hepatol Res. 2015;45:E122-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3229] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 27. | Moradi Z, Moradi P, Hassan Meshkibaf M, Aleosfoor M, Sharafi M, Jafarzadeh S. The comparison of plasma fibronectin in term and preterm delivery: A cross-sectional, descriptive-analytical study. Int J Reprod Biomed. 2019;18:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb). 2016;26:297-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 465] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 29. | Zhang X, Mahadevan S. Bayesian network modeling of accident investigation reports for aviation safety assessment. Reliab Eng Syst Safe. 2021;209:107371. [DOI] [Full Text] |
| 30. | Mitra K, Saguna S, Åhlund C, Ranjan R. Alpine: a Bayesian system for cloud performance diagnosis and prediction. In 2017 IEEE International Conference on Services Computing (SCC) 2017 Jun 25 (pp. 281-288). IEEE. [DOI] [Full Text] |
| 31. | Jiang L, Cai Z, Wang D, Zhang H. Improving Tree augmented Naive Bayes for class probability estimation. Knowledge-Based Syst. 2012;26:239-245. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1093] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 33. | Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69-71. [PubMed] |
| 34. | Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, Lok AS, Hussain KB, Gish R, Van Thiel DH, Younossi Z, Tong M, Hassanein T, Balart L, Fleckenstein J, Flamm S, Blei A, Befeler AS; Liver Cancer Network. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Tamaki N, Kurosaki M, Matsuda S, Nakata T, Muraoka M, Suzuki Y, Yasui Y, Suzuki S, Hosokawa T, Nishimura T, Ueda K, Tsuchiya K, Nakanishi H, Itakura J, Takahashi Y, Matsunaga K, Taki K, Asahina Y, Izumi N. Prospective comparison of real-time tissue elastography and serum fibrosis markers for the estimation of liver fibrosis in chronic hepatitis C patients. Hepatol Res. 2014;44:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Li J, Gordon SC, Rupp LB, Zhang T, Boscarino JA, Vijayadeva V, Schmidt MA, Lu M; Chronic Hepatitis Cohort Study (CHeCS) Investigators. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Taneja S, Tohra S, Duseja A, Dhiman RK, Chawla YK. Noninvasive Assessment of Liver Fibrosis By Transient Elastography and FIB4/APRI for Prediction of Treatment Response in Chronic Hepatitis C-An Experience from a Tertiary Care Hospital. J Clin Exp Hepatol. 2016;6:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Attallah AM, Abdallah SO, Attallah AA, Omran MM, Farid K, Nasif WA, Shiha GE, Abdel-Aziz AA, Rasafy N, Shaker YM. Diagnostic value of fibronectin discriminant score for predicting liver fibrosis stages in chronic hepatitis C virus patients. Ann Hepatol. 2013;12:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Yamauchi M, Nakajima H, Ohata M, Hirakawa J, Mizuhara Y, Nakahara M, Kimura K, Fujisawa K, Kameda H. Detection of fibronectin receptor in sera: its clinical significance as a parameter of hepatic fibrosis. Hepatology. 1991;14:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Zahran FM, EL-Dosoky I, Shiha GE, Keshta AT, Omran MM, Attallah AM. Detection of fibronectin in SERA of HCV infected patients with liver fibrosis. Biochemistry Letters. 2008;4:38-54. [DOI] [Full Text] |
| 41. | de Oliveira AC, El-Bacha I, Vianna MV, Parise ER. Utility and limitations of APRI and FIB4 to predict staging in a cohort of nonselected outpatients with hepatitis C. Ann Hepatol. 2016;15:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 785] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 43. | Ghafar AA, Ghoneem E, Agwa RH, Akef A, Hasan AS, Abdallah N. Value of serum fibronectin for assessment of liver fibrosis in chronic hepatitis C virus patients. EJIM. 2019;31:465-472. [DOI] [Full Text] |
| 44. | Junge J, Horn T, Christoffersen P. The occurrence and significance of fibronectin in livers from chronic alcoholics. An immunohistochemical study of early alcoholic liver injury. APMIS. 1988;96:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Cassinotto C, Boursier J, Paisant A, Guiu B, Irles-Depe M, Canivet C, Aube C, de Ledinghen V. Transient Versus Two-Dimensional Shear-Wave Elastography in a Multistep Strategy to Detect Advanced Fibrosis in NAFLD. Hepatology. 2021;73:2196-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Thanapirom K, Suksawatamnuay S, Tanpowpong N, Chaopathomkul B, Sriphoosanaphan S, Thaimai P, Srisoonthorn N, Treeprasertsuk S, Komolmit P. Non-invasive tests for liver fibrosis assessment in patients with chronic liver diseases: a prospective study. Sci Rep. 2022;12:4913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Kim H, Park J, Kim Y, Sohn A, Yeo I, Jong Yu S, Yoon JH, Park T, Kim Y. Serum fibronectin distinguishes the early stages of hepatocellular carcinoma. Sci Rep. 2017;7:9449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |