INTRODUCTION
Pancreaticoduodenectomy (PD) is the procedure of choice for the surgical treatment of both benign and malignant lesions of the pancreatic head and the periampullary region. In recent years, significant progress has been made in regard to the outcomes of this highly demanding operation. Mortality rates of less than 5% have been reported among specialized centers worldwide with high hospital volume considered, at least in part, responsible for this impressive outcome[1,2]. However, despite this favorable development, morbidity remains a major issue after any kind of pancreatic surgery. The establishment of a postoperative pancreatic fistula (POPF) is considered the most common and, concomitantly, the most serious complication associated with PD, with incidence varying in the literature between 5 and 40%, depending on the definition used[3].
In 2005, the International Study Group of Pancreatic Fistula, aiming to overcome problems associated with the absence of a universally adopted definition, developed a definition and grading scheme of POPF[4]. According to this definition, a pancreatic fistula is defined as a drain output of any measurable volume of fluid starting from the third postoperative day with amylase content greater than 3 times the serum amylase activity. Subsequently, 3 different grades of POPF (grades A, B, and C) were defined based on the clinical impact of POPF on the patients’ clinical course. In 2016, the International Study Group of Pancreatic Fistula reconvened as the International Study Group of Pancreatic Surgery (ISGPS) to review the recent literature and update the 2005 definition and grading system of POPF. In the updated definition, the clinically relevant POPF is now redefined as a drain output of any measurable volume of fluid with an amylase level of more than 3 times the upper limit of the institutional normal serum amylase activity, associated with a clinically relevant condition related directly to the POPF. Therefore, the former grade A POPF is now called a “biochemical leak.” A grade B POPF requires the modification of the postoperative management while the drains are either left in place for more than 3 wk or are repositioned with the use of endoscopic or minimally invasive percutaneous procedures. Finally, patients with Grade C POPF require reoperation or have signs of organ failure[5].
In general, a pancreaticojejunostomy—that is, an anastomosis between the pancreatic stump and a jejunal loop—has been established as the standard and most commonly applied method of reconstruction following PD[6]. A POPF represents the clinical manifestation of a failing and inefficient anastomosis. The quest for either technical modifications or pharmaceutical interventions that could possibly aid in decreasing the incidence of this often-devastating complication appears justified. Performing a pancreaticogastrostomy over a pancreaticojejunostomy has been tested in this direction, but literature data in regard to the efficiency of the approach are contradictory[7]. Furthermore, the former seems to be associated with an increased incidence of post-pancreatectomy haemorrhage[7]. Similarly, the pancreatic duct occlusion or the use of fibrin glue to reinforce the anastomosis did not seem to have the desired results[8-10]. Apart from the various proposed technical modifications of the operative technique, pharmaceutical agents have been tested as well. In theory, somatostatin analogues could limit the incidence of POPF by decreasing exocrine pancreatic secretion. However, a recent meta-analysis demonstrated that the administration of somatostatin analogues such as octreotide did not affect the incidence of POPF and clinically relevant POPF after PD[11]. The stenting of the pancreatic duct, with the use of either internal or external stents, has also been evaluated because, at least in theory, it is an approach that could eliminate many pathophysiological factors responsible for the occurrence of a POPF. The purpose of the present review was to assess the role of pancreatic duct stenting on the incidence of POPF, after PD, by reviewing the relevant literature.
RISK FACTORS FOR POPF
The determination of risk factors for the development of a POPF has been a field of constant research. Ideally, a process of objectifying and easily reproducing the risk assessment could more accurately target possible interventions or deviations from the standard practice selectively to the high-risk patient groups. Therefore, the possible benefits from every intervention that could act protectively, against the development of a POPF, could be augmented. In 2013, Callery et al[12] proposed and validated a clinical risk score, that is, the fistula risk score that could objectively quantify the risk for POPF. The authors assessed and calibrated 4 distinct and widely acknowledged risk factors for POPF after PD, namely the small diameter of the pancreatic duct, the “soft” texture of the pancreatic parenchyma, the presence of high-risk pathology, and the excessive intraoperative blood loss. The combination of these factors, which correlated strongly with the occurrence of a POPF, afforded a 10-point fistula risk score of high predictive value. In general, patients with scores of 0 points, within the validation cohort, never developed a POPF, whereas fistulas occurred in all patients with a score of more than 9[12].
An alternative fistula risk score was proposed by Mungroop et al[13] in 2019 in an attempt to eliminate blood loss as a predictor for POPF. The blood loss factor had been only weakly correlated with the end point of POPF, and the authors aimed to test the hypothesis of developing a risk score taking into account only 3 predictors of POPF development, namely the pancreatic texture, the pancreatic duct diameter, and the body mass index. The alternative fistula risk score was externally validated in 2 independent databases (University Hospital of Verona and University Hospital of Pennsylvania), using both 2005 and 2016 ISGPS definitions, and its predictive value was adequately documented[13]. However, as the penetration of minimally invasive surgery in the field of pancreatic surgery was constantly increasing, the need to validate and optimize the alternative fistula risk score for patients undergoing minimally invasive PD also became mandatory. The updated alternative fistula risk score, which included male sex as a risk factor for POPF development, was the result of a validation study performed in a pan-European cohort of 952 consecutive patients undergoing minimally invasive PD in 26 centers from 7 countries[14].
PROS AND CONS OF PANCREATIC DUCT STENTING
The development of a POPF represents a major source of morbidity and even mortality after a PD[1-3]. The direct and indirect consequences of a POPF can significantly complicate the patient’s postoperative course. An intra-abdominal hemorrhage, an abscess formation, delayed gastric emptying, or the significant delay of bowel function in the postoperative period represent only some of the possible indirect dismal effects of a POPF. From the pathophysiological viewpoint, 3 important factors could be postulated in the aetiology of a pancreatic fistula: First, the poor surgical technique resulting in a not-watertight anastomosis that is, in turn, highly susceptible to leaks; second, the increased intraluminal pressure within the jejunal loop that is purposed to contain and propel the pancreatic juice; third, the destructive effect of the activated pancreatic enzymes on an immature anastomosis that can magnify clinically insignificant leaks.
In general, 2 stent types sized between 5 and 8 Fr, depending on the pancreatic duct size, have been tested in regard to their efficiency in reducing the incidence of POPF after PD, that is, internal and external stents. An external stent is a plastic catheter inserted into the main pancreatic duct and is purposed to drain the pancreatic juice originating from the main pancreatic duct directly outside the abdominal cavity. In contrast, an internal stent is similarly a plastic catheter, though significantly smaller in length than an external stent, purposed to direct the pancreatic juice into the intestinal lumen[15]. From the technical viewpoint, the use of stents, either internal or external, during the maturing process of a pancreaticojejunostomy can efficiently prevent the inadvertent iatrogenic occlusion of the main pancreatic duct, irrespective of the adopted technique[15].
In 1999, Roder and Stein set the scene for the introduction and the establishment of pancreatic stents in pancreatic surgery by reporting an impressive decrease in POPF rate, from 29.3% to 6.8%, with the use of external stents[15]. In general, the rationale for using an external stent is the increased short-term safety and, up to a point, guaranteed clinical stability in the immediate postoperative period. In support of this, one of the most decisive interventions in the therapeutic setting—that is, after a clinically significant POPF has already been established—is the external drainage, via a catheter, of the pancreatic juice[16]. Thus, proactively thinking, the use of an external stent during the index operation could effectively prevent the accumulation of pancreatic juice within the jejunal loop, which is anastomosed with the pancreatic stump, and subsequently disrupt the pathophysiologic cascade of events that eventually could result in the occurrence of a POPF[17]. The issue of the increased intraluminal pressure, as one of the causes of POPF, which is further magnified in the immediate postoperative period due to the decreased gastrointestinal motility, seems to be adequately addressed by the external stenting approach[18]. Furthermore, the complete diversion of pancreatic juice prevents the activation of the pancreatic enzymes by the enzyme, enterokinase, within the jejunal lumen[19]. In theory, protecting a healing anastomosis from the corrosive effect of highly active pancreatic enzymes could increase the likelihood of an uneventful maturing of the anastomosis.
However, there are also drawbacks associated with the approach of externally stenting the pancreaticojejunostomy. Digestive enzymes of significant physiological value are diverted and, ultimately, deprived from the gastrointestinal tract. Impairments on gastrointestinal tract motility and on the absorption of valuable, during the immediate postoperative course, nutrients should be anticipated with mainly unknown clinical implementations. In addition, the stent-related complications are not negligible. Drainage tube discomfort, displacement, and shedding or clogging resulting in peritonitis can all occur and significantly raise morbidity and mortality rates[20-22]. Finally, mechanical injury of the pancreatic duct, at the level of the anastomosis, may likely occur during stent removal, resulting in pancreatitis or obstruction of the pancreatic duct[23,24]. Ohwada et al[25] reported 2 cases (5.4%) of local peritonitis associated with the removal of external stents after PD.
That said, the use of an internal stent should be considered a less radical approach detached by the majority of the limitations associated with the use of external stents. Internal stents could in theory be associated with better long-term outcomes because they are associated with decreased risk of pancreatic duct dilation and endocrine dysfunction compared to external stents[26]. Guiding the pancreatic juice toward the appropriate direction rather than externally diverting it and aiding in performing a technically optimal anastomosis in cases of pancreatic ducts of small diameter are the rationale behind the use of an internal stent. Irrespective of their effectiveness in reducing the incidence of POPF, an internal stent does not have to be removed, and it is associated with fewer fluid losses, water–electrolyte imbalance, impaired gastrointestinal function, internal environment disturbance, malnutrition, and other risks[26]. Preoperative nutrition status plays an important role in predicting the risk of POPF, and several scores have been proposed so far[27].
TO STENT OR NOT TO STENT A PD
The goal behind the use of stents, inserted within the main pancreatic duct during the reconstruction process following PD, is to reduce the incidence of POPF. Several clinical controlled trials and 7 randomized controlled trials (RCTs) have been conducted to assess the impact of stents on this matter, although with conflicting results[21,26-32]. Recently, 2 meta–analyses were published with the aim of summarizing the currently available evidence.
In the meta-analysis by Jiang et al[33], 4 RCTs and 6 non-randomized trials with a total of 2101 patients were included. According to the results, the use of an external stent yielded superior results over the use of an internal stent, in terms of POPF grade C occurrence. However, the use of stent, irrespective of the type, did not reduce the rate of POPF grade B in all studies. The authors concluded that compared with internal stents, the use of external stent might be associated with a lower rate of pancreatic fistula grade C but underlined the need for more high-quality evidence to further explore the safety and efficacy of pancreatic duct external stents[33]. In 2022, Guo et al[34] published another meta-analysis including all the available RCTs and a total of 847 patients with more or less respective results. The authors reported no statistically significant difference between the stent group and non-stent group in the incidence of POPF, in-hospital mortality, reoperation, delayed gastric emptying rate, and wound infection. However, the subgroup analyses revealed that the use of an external stent significantly reduced the incidence of POPF.
DISCUSSION
The development of a clinically relevant POPF—that is, grade B or C, according to the most recent ISGPS definition—remains the most challenging complication after PD[5]. Practically, a pancreatic fistula represents the clinical manifestation of a failing pancreatico–enteric anastomosis. Multiple techniques and, in general, various strategies such as pancreatic duct stenting or the administration of somatostatin analogues have been tested in the direction of reducing the incidence of this troubling complication. However, until today, no single method has proved absolutely efficient. In 2017, the ISGPS published a position statement in regard to the optimal method of reestablishing the continuity of the pancreatic stump with the rest of the gastrointestinal tract after PD. According to this statement, there is no specific technique—that is, a pancreaticogastrostomy or a pancreaticojejunostomy—that can guarantee the complete elimination of the incidence of a clinically relevant POPF. Specifically, in regard to the suggested role of pancreatic stents during PD, the authors underlined the scarcity of high-quality data and the need for further research in the field[35].
In practice, there is low risk for the development of POPF patients, and things are relatively straightforward. The incidence of POPF is limited, and there is no innate need for utilizing adjuncts to improve the outcome. However, problems arise when a high risk is present for a POPF patient. The several published fistula risk scores are particularly aimed at accurately defining this high-risk patient group. Factors such as the soft texture of the pancreatic parenchyma, the small diameter of the main pancreatic duct, male gender, as well as certain anthropometric variables can predict an increased likelihood of a technically difficult pancreaticojejunostomy with high associated failure rate and, subsequently, POPF development[12-14]. In this setting, POPF rates of even more than 30% could be anticipated[14]. Studies regarding the use of fibrin sealants during pancreatic surgery to reduce POPF have been published with controversial results. In a Cochrane review in 2020, the researchers concluded that based on the then-current available evidence, fibrin sealants may have little or no effect on postoperative pancreatic fistula in people undergoing distal pancreatectomy[36].
An approach of utilizing adjuncts such as stents during PD, in these high-risk patients, appears justified[37]. Irrespective of the stent type that is highlighted in the literature as superior, there are reports that do underline the benefits of the approach. Jiang et al[38] analyzed a cohort of 172 patients at high risk for POPF and reported that the use of an external stent could, indeed, reduce the incidence of clinically relevant POPF in patients with a fistula risk score ≥ 4. Conversely, Kawai et al[39] highlighted the superiority of internal stents based on the results of a multicenter large cohort study using propensity score-matched analysis comparing internal and external stents for pancreatojejunostomy during PD. According to the results, clinically relevant POPF occurred in more patients in the external stents group than in patients in the internal stents group (28.7% vs 12.9%, P < 0.001). Particularly for the high-risk group (soft pancreas and no dilatation of the pancreatic duct), the rate of clinically relevant POPF in the internal and external stents groups was 18.8% and 35.4% respectively. The authors concluded that internal stents are safer than external stents for PD.
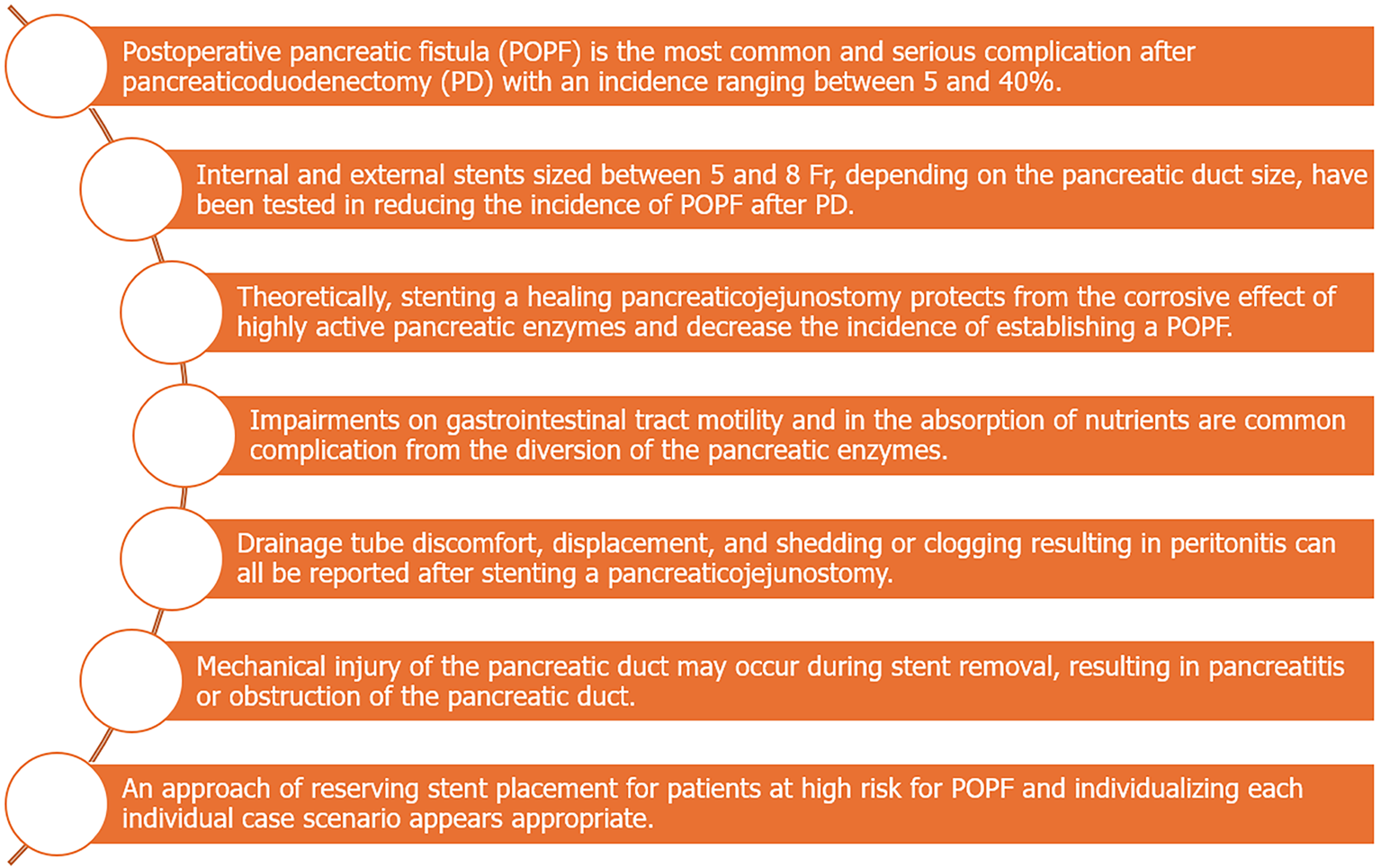
The task of summing up and analyzing all these controversial and confusing data is rather difficult (Figure 1). Drawing definite conclusions based on the existing evidence appears inappropriate. However, some factors can certainly be underlined. Performing a pancreaticojejunostomy over a stent, especially when conditions that do not guarantee a favorable outcome are present, can create the conditions for a safer and technically sound anastomosis. The inadvertent occlusion of a small (in diameter) pancreatic duct that can be prevented by the use of a stent could compromise the operative outcomes. In practice, when a small duct is the case, performing the pancreaticojejunostomy over a pancreatic stent has become commonplace in the majority of specialized centers worldwide[40,41].
Figure 1 Key-points of stenting a pancreaticojejunostomy after pancreatoduodenectomy.
CONCLUSION
In conclusion, literature reports seem to highlight, in their majority, the superiority of external stents over their internal counterparts in regard to the incidence of POPF, albeit at the cost of increased morbidity associated mainly with the stent removal. Certainly, the use of an internal stent is a less invasive approach with acceptable results and definitely lacking the drawbacks arising through the complete diversion of pancreatic juice from the gastrointestinal tract. Bearing in mind the scarcity of high-quality data on the subject, an approach of reserving stent placement for patients at high risk for POPF and individualizing the selection between the use of an internal or an external stent according to the distinct characteristics of each individual case scenario appears appropriate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Greece
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade D
Novelty: Grade C, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade C, Grade B
P-Reviewer: Shiryajev YN, Russia; Wani I, India S-Editor: Liu JH L-Editor: A P-Editor: Yu HG