Published online Jun 20, 2024. doi: 10.5662/wjm.v14.i2.92612
Revised: April 29, 2024
Accepted: May 23, 2024
Published online: June 20, 2024
Processing time: 134 Days and 11.3 Hours
The first wave of coronavirus disease 2019 (COVID-19) pandemic in Spain lasted from middle March to the end of June 2020. Spanish population was subjected to lockdown periods and scheduled surgeries were discontinued or reduced during variable periods. In our centre, we managed patients previously and newly diagnosed with cancer. We established a strategy based on limiting perioperative social contacts, preoperative screening (symptoms and reverse transcription-polymerase chain reaction) and creating separated in-hospital COVID-19-free pathways for non-infected patients. We also adopted some practice modifications (surgery in different facilities, changes in staff and guidelines, using continuously changing personal protective equipment…), that supposed new inconveniences.
To analyse cancer patients with a decision for surgery managed during the first wave, focalizing on outcomes and pandemic-related modifications.
We prospectively included adults with a confirmed diagnosis of colorectal, oesophago-gastric, liver-pancreatic or breast cancer with a decision for surgery, regardless of whether they ultimately underwent surgery. We analysed short-term outcomes [30-d postoperative morbimortality and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection] and outcomes after 3 years (adjuvant therapies, oncological events, death, SARS-CoV-2 infection and vaccination). We also investigated modifications to usual practice.
From 96 included patients, seven didn’t receive treatment that period and four never (3 due to COVID-19). Operated patients: 28 colon and 21 rectal cancers; laparoscopy 53.6%/90.0%, mortality 3.57%/0%, major complications 7.04%/25.00%, anastomotic leaks 0%/5.00%, 3-years disease-free survival (DFS) 82.14%/52.4% and overall survival (OS) 78.57%/76.2%. Six liver metastases and six pancreatic cancers: no mortality, one major complication, three grade A/B liver failures, one bile leak; 3-year DFS 0%/33.3% and OS 50.0%/33.3% (liver metasta
Some patients lost curative-intent surgery due to COVID-19 pandemic. Despite practice modifications and 43.8% delays higher than 4 weeks, surgery was resumed with minimal changes without impacting outcomes. Clean pathways are essential to continue surgery safely.
Core Tip: In our department, during coronavirus disease 2019 (COVID-19) first wave, all surgery was discontinued and resumed later for cancer patients. To minimise perioperative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, we established a physically separated clean pathway and adopted some practice modifications. We evaluated 96 malignancies; 88 underwent surgery (one SARS-CoV-2 positive): 49 colorectal, 20 breast, 12 liver-pancreatic and 7 oesophago-gastric. Three never received surgery because they contracted COVID-19. 78.2% were treated in alternative buildings, 43.8% waited more than 4 wk, two additional stomas were constructed, and laparoscopy decreased. None contracted perioperative SARS-CoV-2. Clean pathways are essential to continue cancer surgery during pandemics. Despite practice changes, we obtained comparable to standard outcomes.
- Citation: Trébol J, Carabias-Orgaz A, Esteban-Velasco MC, García-Plaza A, González-Muñoz JI, Sánchez-Casado AB, Parreño-Manchado FC, Eguía-Larrea M, Alcázar-Montero JA. Digestive and breast cancer patients managed during the first wave of COVID-19 pandemic: Short and middle term outcomes. World J Methodol 2024; 14(2): 92612
- URL: https://www.wjgnet.com/2222-0682/full/v14/i2/92612.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i2.92612
At the end of 2019, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was discovered in China. It causes coronavirus disease 2019 (COVID-19). The virus spread rapidly, and on 11 March 2020, the World Health Organization declared a global pandemic.
In Spain, the health crisis broke out during the first two weeks of March 2020 and officially lasted until July 5, 2023. There were six main waves until March 28, 2022 and, according to official data, it caused about 122000 deaths[1]. The first wave of the COVID-19 pandemic in Spain lasted from the beginning of the outbreak until June 21, 2020. The Spanish Government declared a state of alarm and total confinement of the population on March 14, 2020, with closure of the borders until 26 April. On 2 May, a de-escalation plan was approved according to epidemiological data, with four phases during the following 6–8 wk.
To better understand the first wave in Spain, we highlight two pieces of data: (1) The number of deaths ranged from 27127 (Health Department) to 45684 [Statistics National Institute (INE)][2], considering a total population in March 2020 of 47318050 people[3]. The discrepancy in these numbers could be due to the significant rise in observed deaths (compared with other years). It is suspected that many people died at home without any diagnostic test, and these data were considered by some institutions such as the INE; and (2) Until 10 May 2020, the last day these data were published separately, there were 40961 infections in healthcare professionals, of whom 4188 (10.5%) required hospital admission, 310 (1.1%) management in intensive care units (ICU) and 52 (0.1%) died[4].
It is also necessary to contextualise the situation concerning patients with digestive and breast cancers and their surgical treatment during the first wave. Especially during March and April 2020, the public health system experienced a massive influx of patients with COVID-19, which overwhelmed hospitals. Healthcare managers had to adopt some measures to preserve and expand wards and ICU beds such as modifying the hospital structure and organisation, opening field hospitals and assigning professionals from specific areas and specialties to the care of these patients. This led to variable cancellation of ordinary activities such as outpatient consultations and scheduled surgeries, to free up staff, resources (e.g. ventilators) and space (e.g. operating rooms were converted to ICUs). The initial calculations, based on mathematical models, of expected surgery cancellations or delays during the initial 12 wk of the COVID-19 pandemic were 72.3% globally, including 37.7% of cancer-related surgeries (2324070 of 6162311)[5]. So, access to cancer diagnosis and treatment was limited.
During the early period of the COVID-19 pandemic, healthcare workers were considered essential, and infection among them was frequent. Thus, surgeons were advised to take precautions to avoid exposure to COVID-19, including cancelling non-urgent and non-cancer surgeries. Moreover, in the beginning, there were concerns about possible virus aerosolisation with surgical smoke and pneumoperitoneum (based on previous reports with other viruses)[6]. This led some influential scientific societies such as the Association of Surgeons of Great Britain and Ireland to temporarily recommend against laparoscopy, a recommendation that was subsequently corrected. Later, a consensus was reached: It was considered safe increasing the usual preventive measures and implementing others[7] including specific personal protective equipment (PPE), smoke evacuators, aspirate the pneumoperitoneum and test patients before surgery. We modified our protocols to follow these guidelines. Some of these measures (such as PPE) were simplified later as our understanding of SARS-CoV-2 pathophysiology improved. It is also important to highlight that there was a widespread shortage of PPE, including in our setting[8]. These changing recommendations likely affected the care we could give to our patients.
Another issue that concerned surgeons was the possible perioperative morbimortality among patients infected by SARS-CoV-2 undergoing major cancer surgery. The first reports from China with surgical patients were quite worrisome. A major international collaborative scientific effort, the CovidSurg project, was undertaken to increase knowledge of how perioperative infection could affect surgical patients. The preliminary reports and the first publication at the end of May 2020 showed a 30-d mortality rate as high as 23.8%[9], so cancellation or postponement seemed to be a safe option for patients with cancer awaiting surgery. Another issue was to determine whether deferring cancer surgeries is safe and for how long. Clinical judgment argued against long delays, mainly for aggressive tumours such as hepatobiliopancreatic or oesophago-gastric cancer, time-critical diseases for which treatment delays are associated with disease progression and worse outcomes[10,11]. Many oncological associations opposed cancellations or delays[12] and proposed triage guidelines based on expert opinions rather than evidence-based data. Shinde et al[13] reviewed the studies addressing the impact of the waiting time to initiate therapy on cancer survival and prognosis and those triage recommendations. Several publications analysed these issues during the first wave of the COVID-19 pandemic, with continuous changes that added some confusion.
In this context, we had to manage patients awaiting their cancer surgery when the COVID-19 pandemic began. In addition, although there was a significant decrease (even cancellation on many days) of diagnostic tests, we had to face the new diagnoses of cancer with the abovementioned impediments.
Our hospital complex is a public university, teaching and fourth-level centre that provides health care to a province of 329245 people (on January 1, 2020). At that time, it comprised two acute care centres very close to each other with approximately 800 beds. Each one had its own critical care beds, postsurgical units, operating rooms and radiology equipment, while other resources (e.g. laboratory and blood bank) were shared. The General and Digestive Tract Surgery Department worked in the larger building. At specific moments of the first wave of the COVID-19 pandemic, some operating theatres and hospitalisation beds from a private centre were also employed. During the first wave, our hospital’s official data indicate that 1186 proven SARS-CoV-2-positive patients were admitted in conventional wards and 112 in ICUs, with 364 declared in-hospital deaths. The peak number of admitted infected patients was 382 in wards (as of April 3) and 56 in ICUs (as of April 5)[14].
This structure with two well-equipped acute care centres allowed our healthcare managers to allocate the larger building to patients with COVID-19 and the other to patients without COVID-19, with a clear physical barrier between the patient groups. The general and digestive surgery consultants (unlike all of our residents) were not assigned to COVID-19 wards, but attended emergencies not knowing whether the patients were infected and also surgical SARS-CoV-2 positive patients thus using the PPE dictated by the authorities and according to availability. During this period, 8 of the 29 consultants (27.6%) and 5 of the 9 resident doctors (55.6%) in our department contracted COVID-19 and were on sick leave[8]. However, both the anaesthesiologists and the nursing staff (ward and operating room) were assigned according to the needs to SARS-CoV-2-positive or SARS-CoV-2-negative patients.
In this context, we had to implement some modifications to continue treating our patients with cancer: patients not infected by SARS-CoV-2 were managed in a different building, with different operating theatres and wards. Although we tried to ensure that each patient was operated on by the usual subspecialised unit, due to sick leave this was sometimes not feasible for all the surgeries. In addition, we could not work with the usual anaesthesiologists and nurses. We faced another specific limitations: Sometimes the surgical devices and instruments were not what we usually used, and sometimes the recommended PPE had negative effects (heat, fog, weight, pressure, etc.) that made surgery difficult, as mentioned in some studies[15,16].
Our department cancelled all elective surgeries for 2 wk to analyse the evolution of COVID-19 hospitalisations. Surgery resumed at the beginning of April but only for patients with cancer who were previously screened for COVID-19 symptoms and with a polymerase chain reaction (PCR) test 48 h before surgery. If the PCR test result was positive, then the surgery was delayed for at least 4–8 wk, and the subsequent PCR test results had to be negative. During the first week of resumption, surgical activity was approximately 10% of the normal level. In the following 2 wk, it was at 10%–20% of the normal level and later stabilised at approximately 33%, when the COVID-19 admissions curve had flattened. By the beginning of June 2020, surgical activity exceeded 50% of the normal activity and some interventions returned to the larger building, in clearly demarcated COVID-19-free pathways. At the end of the first wave, activity returned to normal and in the usual building.
Initially, only surgeries for patients with cancer pathology was scheduled. Later, surgical programs were completed adding some benign cases with clinical priority. In mid-June some sessions with exclusively benign pathology were scheduled. Regarding cancer, we started with the pathology with the lowest complication risk and ICU bed need (colorectal and breast). When the COVID-19 admissions curve had stabilised, we resumed liver-pancreatic and oesophago-gastric surgeries, first treating those patients with the highest probability of long-term survival, with the lowest perioperative risks and with the most symptoms. Our goal was to offer our patients with cancer the same treatment they would have received without a health crisis.
In the present study, we analysed patients with digestive and breast cancer who had a decision for surgical resection managed at our department during the first wave of the COVID-19 pandemic. We focused on the outcomes and the possible impacts of the COVID-19 pandemic.
This prospective observational single-centre cohort study included consecutive elective and emergent patients with a confirmed diagnosis of digestive and breast cancer whose pre-COVID-19 pandemic therapeutic decision was surgical management, mostly with a curative intent, regardless of whether they finally had an operation. The study was approved by the local ethical review board (code 2020 04 479) and collected only anonymised data according to Spanish (organic law 3/2018 and law 41/2002) and European Union (EU regulation 2016/679) legislation on data protection and patient autonomy. Due to contagion risks and the policy of minimising personal contacts and physical documentation during the study period, an exception was made regarding obtaining written consent from some patients.
The short-term follow-up data are included in the National Institute for Health Research (NIHR) Global Research Unit on Global Surgery CovidSurg-Cancer, an international research collaborative[17]. The study was conducted according to the guidelines set by the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies[18].
The study included consecutive adult patients, aged ≥ 18 years, with a diagnosis of digestive (colorectal, oesophago-gastric and hepatobiliary) and breast cancer who were awaiting surgery when the COVID-19 pandemic was declared in Spain (March 14, 2020) and all the patients diagnosed during the first wave in Spain (until 21 June) at our centre. We considered all patients whose pre-pandemic therapeutic decision was surgery with a curative intent, regardless of whether they ultimately underwent surgery.
The short-term outcomes (for patients who underwent surgery) included the 30-day postoperative COVID-19 infection rate, postoperative mortality and complications. The long-term outcomes included adjuvant treatments received, cancer-related events (relapse and metastases), time to relapse, death, time to death, cause of death, and COVID-19 vaccination and infection and its consequences.
The baseline variables to account for perioperative and SARS-CoV-2 risk included patient demographics (age and sex), comorbidities, American Association of Anesthesiologists (ASA) grade, Eastern Cooperative Oncology Group performance and body mass index.
The cancer baseline details included the location, baseline tumor-node-metastasis (TNM) staging, date of diagnosis and treatment decision by a multidisciplinary team (MDT). Some cancers included more detailed data about histology (e.g. breast), options for resection (e.g. the National Comprehensive Cancer Network classification for pancreatic cancer) or preoperative treatments [e.g. biliary drainage in the pancreas; transarterial chemoembolisation (TACE) or radiofrequency ablation (RFA) in the liver].
The details for patients who underwent surgery included operative characteristics (procedure[s], urgency, approach, extent of resection, margin status, final TNM staging and final surgical intent), additional histological data in patients with breast cancer (oncotype), COVID-19 hospital situation on the day of surgery (according to the CRITCON levels), prior SARS-CoV-2 infection, changes potentially related to the COVID-19 pandemic (e.g. in patients with breast cancer whether reconstruction was performed immediately or deferred or in patients with colorectal cancer whether anastomosis was performed, etc.). The CRITCON levels were initially designed in London in response to the H1N1 (2009) influenza pandemic for critical care rationing. In 2020, the Intensive Care Society proposed CRITCON-Pandemic levels as a modification to face the needs and challenges of the COVID-19 pandemic[19,20].
Information collected of the short-term postoperative course: SARS-CoV-2 infection (defined by a laboratory test), mortality, reoperations, general and surgical complications [classified with the Clavien–Dindo (C-D) system[21]] and complications specific to liver and pancreas surgery [postoperative bleeding, bile leak, pancreatic fistula, post-hepatectomy liver failure (PHLF) and cholangitis]. Bile leak and PHLF were defined according to the International Study Group of Liver Surgery criteria[22,23]. Postoperative pancreatic fistula (POPF) was defined according to the International Study Group on Pancreatic Surgery criteria[24]. The postoperative length of stay was also analysed.
For patients who did not undergo an operation during the first wave we assessed whether the indication for surgery persisted and if it was performed, the reason(s) for changes in management, if new re-staging was done and changes attributable to the COVID-19 pandemic.
Information collected regarding the long-term follow-up: Adjuvant treatments; oncological events (local and regional relapses and metastases); time to relapse [disease-free survival (DFS) from surgery or diagnosis in non-operated]; whether the patient was alive or dead at the end of follow-up; reason for death; time to death [overall survival (OS)]; COVID-19 vaccination, doses and dates; and SARS-CoV-2 infection, type and sequelae.
Data were collected and managed by clinicians according to a prespecified protocol. The data were uploaded into a secure online Research Electronic Capture Database (REDCap)[25,26] hosted at University of Birmingham (UK) for the short-term follow-up and stored in a secure local Excel database for the long-term follow-up. No data identifying patients were uploaded.
SPSS v.28.0.0.1 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. The categorical data are presented as frequencies and percentages. Continuous data are summarised using the mean and standard deviation (SD) or median and interquartile range depending on their distribution. No analytic tests were performed due to the descriptive nature of the study, the small number of patients in some groups and because different periods were not compared.
This study included 96 patients with cancer managed in our department (54 colorectal, 20 breast, 9 oesophago-gastric, 7 liver and 6 pancreas). When the COVID-19 pandemic was declared, 40 patients were waiting for surgery (18 colorectal, 16 breast, 2 oesophago-gastric, 2 liver and 2 pancreas), so the other 56 were diagnosed during the first wave of the pandemic. Seven patients did not receive the planned treatment. Only one patient with perforated rectal cancer was operated on despite being SARS-CoV-2 positive.
Of the 54 patients, 33 had colon cancer and 21 rectal cancers. Considering the 53 SARS-CoV-2-negative patients, 49 (92.5%) – 29 with colon cancer and 20 with rectal cancer – underwent surgery during the first wave of the pandemic.
The patient demographic data and comorbidities are summarised in Tables 1 and 2. The most frequent comorbidities were hypertension (54.7%), diabetes (23.8%) and moderate to severe chronic kidney disease (13.2%). All patients had at least one comorbidity (mean 3.13, SD 1.34, range 1–6).
| Colorectal | HBP | OG | Breast | ||
| Total number | 53 (100) | 13 (100) | 9 (100) | 20 (100) | |
| Age (yr) | 40-49 | 3 (5.7) | 2 (15.4) | 5 (25.0) | |
| 50-59 | 3 (5.7) | 2 (15.4) | 8 (40.0) | ||
| 60-69 | 13 (24.5) | 5 (38.5) | 5 (55.6) | 3 (15.0) | |
| 70-79 | 22 (41.5) | 3 (23.1) | 3 (33.3) | 4 (20.0) | |
| 80-89 | 11 (20.8) | 1 (7.7) | 1 (11.1) | ||
| 90-99 | 1 (1.9) | ||||
| Sex | Female | 18 (34) | 7 (53.8) | 2 (22.2) | 19 (95.0) |
| Male | 35 (66) | 6 (46.2) | 7 (77.8) | 1 (5.0) | |
| ECOG perfor-mance score | 0 | 13 (24.5) | 5 (38.5) | 3 (33.3) | 19 (95.0) |
| 1 | 21 (39.6) | 6 (46.2) | 4 (44.4) | 1 (5.0) | |
| 2 | 17 (32.1) | 2 (15.4) | 2 (22.2) | ||
| 3 | 2 (3.8) | ||||
| ASA | I | 7 (13.2) | 1 (7.7) | 0 | 4 (20.0) |
| II | 25 (47.2) | 6 (46.2) | 6 (66.7) | 13 (65.0) | |
| III | 17 (32.1) | 5 (38.5) | 2 (22.2) | 3 (15.0) | |
| IV | 4 /7.5) | 1 (7.7) | 1 (11.1) | ||
| BMI | < 18.5 | 0 | 1 (7.7) | ||
| 18.5-24.9 | 10 (18.9) | 4 (30.8) | 3 (33.3) | 7 (35.0) | |
| 25-29.9 | 24 (45.3) | 5 (38.5) | 5 (55.6) | 8 (40.0) | |
| 30-34.9 | 15 (28.3) | 1 (7.7) | 1 (11.1) | 1 (5.0) | |
| 35-39.9 | 4 (28.3) | 1 (7.7) | 3 (15.0) | ||
| ≥ 40 | 0 | 1 (7.7) | 1 (5.0) | ||
| Number of comorbidities | Mean (SD), range | 3.13 (1.34) 1-6 | 2.85 (1.4), 1-5 | 3 (1.22) 1-5 | 1.85 (1.14) 0-4 |
| n (%) | CR, n = 53 | HBP, n = 13 | OG, n = 9 | Breast, n = 16 |
| Smoker | 5 (9.4) | 2 (15.4) | 2 (22.2) | 3 (18.8) |
| Chronic kidney disease (moderate/severe) | 7 (13.2) | 1 (7.7) | 2 (22.2) | 1 (6.3) |
| Chronic obstructive pulmonary disease | 1 (1.9) | 1 (7.7) | 0 | 0 |
| Asthma | 1 (1.9) | 1 (7.7) | 1 (11.1) | 0 |
| Congenital abnormality - cardiac | 1 (1.9) | 0 | 0 | 0 |
| Hypertension | 29 (54.7) | 5 (38.5) | 6 (66.7) | 7 (43.8) |
| Congestive heart failure | 6 (11.3) | 1 (7.7) | 0 | 0 |
| Ischemic heart disease | 4 (7.5) | 1 (7.7) | 0 | 0 |
| Peripheral vascular disease | 2 (3.8) | 0 | 0 | 0 |
| Stroke/TIA | 4 (7.5) | 1 (7.7) | 0 | 1 (6.3) |
| Dementia | 1 (1.9) | 0 | 0 | |
| Diabetes mellitus | 15 (28.3) | 3 (23.1) | 2 (22.2) | 1 (6.3) |
| HIV infection | 0 | 0 | 0 | 0 |
| Others | 39 (73.6)1 | 8 (61.5)2 | 6 (66.7)3 | 11 (68.8)4 |
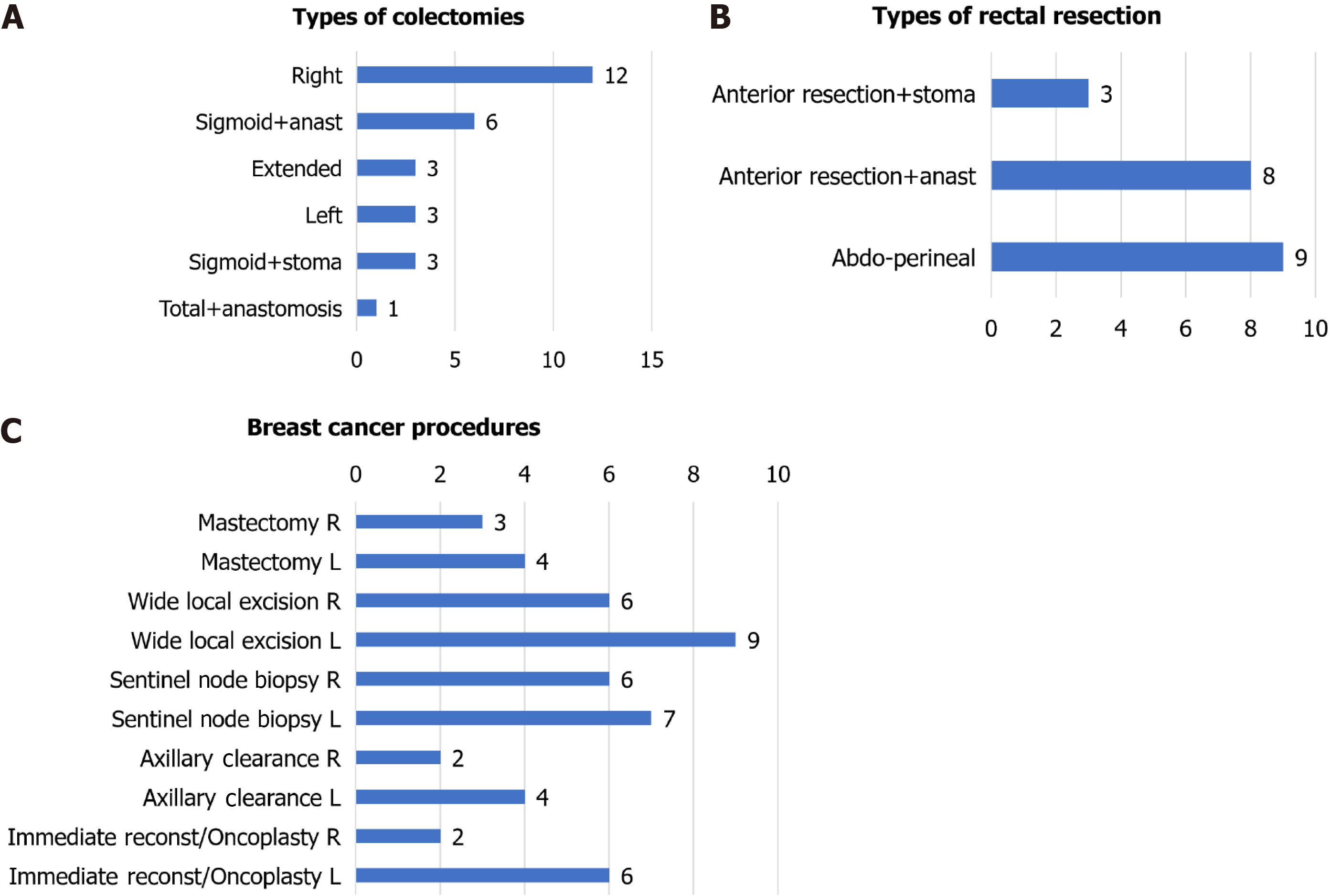
The cancer and surgery characteristics (including stoma and anastomosis features) are summarised separately for colon and rectal cancer in Table 3. The surgical procedures, operating surgeon and neoadjuvant therapies are described as follows: Colon cancer procedures (Figure 1A): Right colectomy (12/29, 41.4%), sigmoidectomy with anastomosis (6/29, 20.7%) or stoma (3/29, 10.3%), left colectomy (3/29, 10.3%), extended right colectomy (3/29, 10.3%) and total colectomy with ileorectostomy (1/29, 3.45%). Moreover, two partial cystectomies, one segmental ileal resection, one prosthetic eventroplasty and one cholecystectomy were associated in four patients. Fifteen of 25 anastomoses (60.0%) were handsewn and 10 (40%) were stapled. Non-colorectal consultants and colorectal trainees performed three (10.7%) and two (7.1%) of the procedures, respectively, with fewer trainees performing surgeries compared with the pre-COVID-19 pandemic period. The rectal cancer procedures (Figure 1B) included abdominoperineal resection (9/20, 45.0%) and anterior resection with anastomosis (8/20, 40.0%) or ostomy (3/20, 15.0%). In addition, one hepatic metastasis procedure was performed, and one patient required an ileal segment resection. All anastomoses were stapled. Colorectal consultants (no trainees, a change in usual practice) performed all surgeries. Twelve of the 20 patients (60.0%) received neoadjuvant therapies: long-course chemoradiation (8/12, 67.7%), short-course radiotherapy (2/12, 16.7%), long-course chemotherapy + short-course radiation (1, 8.33%) or long-course chemotherapy (1/12, 8.33%).
| Colon, n (%) | Rectal, n (%) | ||
| Total n (%) | 32/28 operated | 21 (1 positive)3 | |
| Final disease stage | 0 | 1 (3.1) | 1 (4.8) |
| I | 4 (12.5) + 2 (6.2)1 | 3 (14.3) | |
| IIA | 6 (18.8) + 1 (3.1)1 | 7 (33.3) | |
| IIB | 6 (18.8) | 0 | |
| IIIA | 1 (3.1) | 4 (19.0) | |
| IIIB | 7 (21.7) + 1 (3.1)2 | 4 (19.0) | |
| IIIC | 3 (9.4) | 0 | |
| IVA | 0 | 2 (9.5) | |
| Resection | R0 | 28 (100) | 18 (90.0) |
| R1 | 0 | 2 (10.0) | |
| R2 | 0 | 0 | |
| CRITCON level | 0 | 13 (46.4) | 9 (45.0) |
| 1 | 4 (14.3) | 4 (20.0) | |
| 2 | 8 (28.6) | 4 (20.0) | |
| 3 | 3 (10.7) | 3 (15.0) | |
| Urgency | Urgent/Expedited | 4 (14.3) | 0 |
| Elective | 24 (85.7) | 20 (100) | |
| Operative approach | Open | 10 (35.7) | 1 (5.0) |
| MIS | 15 (53.6) | 18 (90) | |
| MIS-open | 3 (10.7) | 1 (5.0) | |
| Anastomosis | Yes, with protective stoma | 0 | 5 (25.0) |
| Yes | 25 (89.3) | 3 (15.0) | |
| No, end stoma only | 3 (10.7) | 12 (60.0) | |
| Changes in anastomosis practice | No, typical practice | 27 (96.4) | 18 (90.0) |
| Change unrelated to COVID | 0 | 1 (5.0) | |
| Change due to pandemic | 1 (3.6) | 1 (5.0) |
Short-term results: The overall 30-d mortality was 2.08% (1 of 48 patients), 1 of 48 patients (2.08%) was discharged to a rehabilitation centre and 27 of 48 patients (56.3%) had some postoperative complication (all grades), with only one anastomotic leak (1/33 anastomoses, 5 protected, 3.03%). Major complications (Clavien–Dindo III–V) occurred in 7 of 48 patients (3.36%). No patient contracted postoperative SARS-CoV-2. Complications, mortality and the length of stay are presented in Table 4. The patient who died had moderate-to-severe dementia, underwent a Hartmann procedure and died of bronchoaspiration pneumonia.
| Colorectal cancer, n (%) | Right colon | Left colon | Rectal | |
| Total, n | 15 | 13 | 20 | |
| Mortality | 0 | 1 (7.7) | 0 | |
| Discharged rehabilitation center | 0 | 1 (7.7) | 0 | |
| Reoperation (CD III) | 0 | 1 (7.7) | 4 (20.0) | |
| Unplanned critical care admission (CD IV) | 0 | 0 | 1 (5.0) from theatre | |
| Any complication | 6 (40.0) | 9 (69.2) | 13 (65.0) | |
| Anastomotic leak | 0 | 0 | 1 (5.0) | |
| Bleeding requiring transfusion | 1 (6.7) | 2 (15.4) | 2 (10.0) | |
| Ileus | 3 (20.0) | 0 | 5 (25.0) | |
| Superficial/deep SSI | 1 (6.7) | 3 (23.1) | 3 (15.0) | |
| Organ/space SSI | 0 | 1 (7.7) | 2 (10.0) | |
| Wound dehiscence | 0 | 0 | 0 | |
| Acute kidney injury | 4 (26.7) | 3 (23.1) | 5 (25.0) | |
| Pneumonia | 0 | 1 (7.7) | 0 | |
| Other organ injury | 0 | 0 | 1 (5.0) | |
| Other | 0 | 4 (30.8) | 7 (35.0) | |
| Length of stay (d) | mean (SD) | 6.27 (2.76) | 6.36 (1.91) | 8.8 (7.36) |
Changes relatable to pandemic in management: In the 29 patients with colon cancer who underwent surgery, in our opinion, 19 (65.5%) experienced a delay to surgery. Objectively, 9 of 22 patients scheduled for surgery (40.9%) had to wait more than 4 wk in surgical waiting list, including four who had to wait for more than 2 months. Twenty-three of 29 patients (79.3%) underwent surgery in an alternative building (COVID-19 free). One patient underwent a stoma at the beginning of the first wave, which seems very unlikely in the pre-COVID-19 context, and one surgeon changed his usual handsewn ileocolonic anastomosis to a mechanical one. In both cases, these choices were made to avoid possible complications requiring critical care, wishing to reduce hospital stay and/or based on the initial guidelines. There were three patients who underwent open surgery by general surgery consultants; some of these surgeries probably could have been laparoscopic without pandemic. Four patients did not have surgery during the first wave of the COVID-19 pandemic: Two died while awaiting surgery, one in hospital due to severe COVID-19, and the other rejected surgery and died at home with COVID-19. Another two patients refused surgery: One never underwent surgery (and died due to COVID-19 pneumonia in February 2021) and the other finally accepted surgery in August 2020.
All patients with rectal cancer underwent surgery. In our opinion, 13 of 20 patients (65.0%) suffered a delay to surgery. Objectively, 9 of 20 (45.0%) scheduled patients had to wait more than 4 wk on the waiting list, including four for more than 2 months, excluding window periods for long-course (8 wk in our hospital) or short-course (4 wk) radiotherapy, which were accomplished. Sixteen of 20 patients (80.0%) were operated on in the COVID-free building and two (10%) experienced delays after their diagnosis due to cancellation of outpatient clinics or endoscopic therapies. Twelve end stomas were performed, nine because of abdominoperineal resection, and five of eight anastomoses were protected with an ileostomy. In two cases the stoma was an intraoperative decision: one due to an adverse event (distal rectum perforation) and the other one attributable to the COVID-19 pandemic (to avoid possible complications requiring critical care).
Long-term follow-up: Concerning the oncological outcomes, the adjuvant therapies are schematised by disease stage in Table 5; the pre-pandemic indications were maintained. In the 28 patients with colon cancer, four patients with high-risk stage II and all stage III–IV suitable patients received chemotherapy (in total, 11/28, 39.29%). In the patients with rectal cancer, the patient with a complete remission was added (16/21, 76.19%). At the end of the follow-up, five patients with colon cancer (17.9%) and nine patients with rectal cancer (42.9%) had experienced a relapse. There were four cancer-related deaths (8.16%, two patients with colon cancer and two patients with rectal cancer), and another patient died due to COVID-19. For the patients with colon cancer who relapse, the mean DFS was 8.01 (range 1.56–18.5) months and in those who die (all causes), the mean OS was 16.66 (range 0.33–28.0) months. The 3-year DFS and OS were 82.1% and 78.6%, respectively. For the patients with rectal cancer, the mean DFS was 13.6 (range 2.07–36.0) months and the mean OS was 18.3 (range 7.63–34.5) months. The 3-year DFS and OS were 52.4% and 76.2%, respectively.
| Colorectal | Colon | Rectal | ||||||
| Chemo | Relapse | Death | Chemo | RT | Relapse | Death | ||
| I | No | 4 | 3 | 2 | 2 | 2 | 3 | |
| Yes | 0 | 0 | 2 (PO/?) | 1 | 1 (S) | |||
| Unknown | 0 | 1 | ||||||
| IIA | No | 5 | 6 | 5 | 3 | 4 | 6 | |
| Yes | 1 | 0 | 1 (COVID) | 4 | 2 (L+R/L) | 1 (Infection) | ||
| Unknown | 1 | |||||||
| IIB | No | 3 | 5 | 5 | ||||
| Yes | 3 | 1 (R) | 1 (CA) | |||||
| IIIA | No | 0 | 1 | 1 | 0 | 4 | 3 | |
| Yes | 1 | 0 | 0 | 4 | 1 | 0 | 1 (RAD) | |
| IIIB | No | 3 | 5 | 6 | 0 | 0 | 2 | |
| Yes | 4 | 2 (R+S/S) | 1 (CA) | 4 | 4 (S) | 2 (CA/PO) | ||
| IIIC | No | 1 | 1 | 2 | ||||
| Yes | 2 | 2 (S) | 1 (?) | |||||
| IVA | No | 0 | 0 | 0 | 0 | 0 | 1 | |
| Yes | 0 | 0 | 0 | 2 | 2 (R+S/S) | 1 (CA) | ||
| O | No | 1 | 0 | 1 | 0 | 1 | 1 | |
| Yes | 0 | 0 | 0 | 1 | 0 | |||
Regarding COVID-19, 42 of the 49 patients who underwent surgery (85.7%) got vaccinated between March and May 2021. Five patients were not vaccinated, and data for the other two were lost. Of the five unvaccinated, four died (not due to COVID-19) before they could get vaccinated; the reason why the other was not vaccinated is unknown. Sixteen of 49 patients (32.7%) contracted COVID-19 during the follow-up: nine after their vaccination, five before vaccination, one who did not get vaccinated and one whose vaccination data was lost. Only two of these 16 patients (12.5%) required hospitalisation due to pneumonia (one before and other after vaccination); the last died due to bilateral pneumonia being cancer-free. 60% had asymptomatic or very oligosymptomatic clinical pictures.
The SARS-CoV-2-positive patient who underwent surgery did not have COVID-19 symptoms. He presented with T4aN1bM1 (liver) perforated superior rectal cancer with faecal diffuse peritonitis. He underwent the Hartmann procedure and had complicated recovery due to septic shock. He received adjuvant capecitabine and liver surgery (February 2021), but in May 2021, multiple liver metastases appeared. He died due to cancer progression 17 months after the index surgery. He was vaccinated in May 2021 and he did not became infected with SARS-CoV-2 again before his death.
There were seven patients with liver cancer and six with pancreatic cancer; 12 of them (92.3%) underwent surgery during the first wave of the pandemic. Their demographic data and comorbidities are described in Tables 1 and 2; all of them had at least one of the registered comorbidities (mean 2.85, SD 1.4, range 1–5). The cancer and surgical treatment characteristics are presented in Table 6.
| n (%) | Pancreas, n = 6 | Liver, n = 6 | |||
| Histology | Adenocarcinoma | 4 (66.7) [1DCC] | Tumour type | CR metastasis | 6 (100.0) |
| Neuroendocrine | 2 (33.3) | Other | 0 | ||
| Resectability (NCCN classification) | Resectable | 3 (50.0) | Pre-COVID Extent of resection | Minor | 2 (33.3) |
| Borderline resectable (vein) | 2 (33.3) | Major | 3 (50.0) | ||
| Borderline resectable (artery) | 0 | Extra major | 1 (16.7) | ||
| Locally advanced | 1 (16.7) | ||||
| Preoperative biliary drainage | 0 | 0 | |||
| Previous COVID | 0 | 0 | |||
| Neoadjuvant therapy | Long-course Chemo | 1 (16.7) | 4 (57.14) | ||
| Short-course Chemo | 1 (14.3) | ||||
| Radiochemotherapy | 1 (16.7) | ||||
| CRITCON level | 0 | 3 (50) | 4 (66.7) | ||
| 1 | 1 (16.7) | 2 (33.3) | |||
| 2 | 2 (33.3) | ||||
| Operative approach | Open | 6 (100) | 5 (83.3) | ||
| MIS | 1 (16.7) | ||||
| Resection margin status | R0 | 3 (50.0) | 4 (66.6) | ||
| R1 | 3 (50.0) [2 NE] | 1 (16.7) | |||
| Imaging complete | 0 | 1 (16.7) | |||
| Final staging | IB | 2 (33.3) | |||
| IIB | 2 (33.3) [1DCC] | ||||
| III | 2 (33.3) [2 NE] | ||||
In the six patients with pancreatic cancer, all the surgeries were elective, open and with a final curative intent. The following surgeries were performed: Left pancreatectomy (3/6, 50.0%; one with a total splenectomy), total pancreatectomy (2/6, 33.3%; one with a total splenectomy) and pancreaticoduodenectomy (1/5, 16.7%). None of these patients contracted COVID-19 during the first month after surgery and all were discharged home (length of stay: mean 8, SD 2.37, range 5–12 d).
All seven patients with liver cancer had colorectal metastases. One of them, scheduled for one-stage liver and colon surgery (preoperative T4N1 cancer), had his surgery cancelled and chemotherapy was resumed; in the re-staging he had developed bone metastases, so he never received surgery with a curative intent. All surgeries were elective and had a final curative intent. No patient received preoperative RFA/TACE. The following surgical procedures were performed: right hepatectomy (3/6, 50.0%; two with left-lobe metastasectomies and one with cholecystectomy), multiple metastasectomies (2/6, 33.3%) and laparoscopy-aided microwave ablation (1/6, 16.7%). No patient contracted COVID-19 and all were discharged home (length of stay: mean 6.33, SD 3.61, range 2–11 days).
Short-term results: The overall 30-d mortality was 0% and the complication rate (all grades) was 58.3%; it was higher among patients who underwent liver surgery (Table 7). Major complications (C-D grades III–V) occurred in 1 of 12 (8.33%); the highest complication grade was IIIA. Grade B/C PHLF affected 1 of 6 (16.7%) susceptible patients and there were no POPF.
| Pancreas and liver cancer, all results presented as n (%) unless specified | Pancreas, n = 6 | Liver, n = 6 | ||
| Overall complications | Mortality (CD V) | 0 (0) | 0 (0) | |
| Patients with complications | 3 (50.0) | 4 (66.7) | ||
| Unplanned ICU admission (CD IV) | 0 | 0 | ||
| Planned ICU admission | 4 (66.7) | 4 (66.7) | ||
| Reintervention | 1 (16.7) (III-A) | 0 | ||
| Any major complication (CD III-V) | 1 (16.7) III-A | 0 | ||
| Acute kidney injury | 2 (66.7) | 2 (50.0) | ||
| Ileus | 1 (33.3) | 1 (25.0) | ||
| Bleeding requiring transfusion | 0 | 1 (25.0) | ||
| Superficial/Deep SSI | 0 | 1 (25.0) | ||
| Other | 2 (66.7)1 | 0 | ||
| HBP complications | Postoperative bleeding | No | 6 (100) | 6 (100) |
| Bile leak | No | 6 (100) | 5 (83.3) | |
| Grade A | 0 | 1 (16.7) | ||
| Readmission with cholangitis | 0 | 0 | ||
| Pancreatic specific complications | Postoperative pancreatic fistula | No | 6 (100) | - |
| Liver specific complications | Post-hepatectomy liver failure | No | - | 3 (50.0) |
| Grade A | - | 2 (33.3) | ||
| Grade B | - | 1 (16.7) | ||
| Grade C | 0 | |||
| Length of stay (d) | Mean (SD) | 8 (2.37) | 6.33 (3.61) | |
Changes relatable to COVID-19 pandemic in management: For patients with pancreatic cancer, two (33.3%) had a delay, including one (16.7%) who was on the waiting list for more than 4 wk. Four of six patients (66.7%) were operated on in the alternative building, and one with a final R1 resection could not benefit from usual intraoperative margin status assessment. For patients with liver cancer, two (33.3%) had their planned surgery cancelled at the beginning of the study period. Chemotherapy was resumed in both; one progressed and never had surgery, and the other underwent surgery during the first wave. Four surgeries were delayed (66.7%, one for more than 4 wk), two patients (33.3%) underwent a longer course of neoadjuvant chemotherapy and two patients (33.3%) were operated on in an alternative hospital building.
Long-term follow-up: Concerning oncological outcomes, five of the 6 (83.3%) liver metastases patients who underwent surgery received adjuvant chemotherapy and all of them relapsed (five at a systemic location and one locally). The mean DFS was 8.32 (range 3.47–19.9) months. Four of these patients died (mean survival 22.12, range 14.4–36.07 months) due to cancer progression; the 3-year OS was 50%, and the 3.5-year OS was 33.3%. We will present the six patients with pancreatic cancer separated by histology. Concerning pancreatic carcinoma (Table 8), the 3-year DFS and OS were 33.3%. One patient was disease free at the end of the follow-up (this patient was also diagnosed of lung cancer in 2022), one died at 1.8 months due to pancreatitis and the other died at 14.3 months due to cancer. Both patients with neuroendocrine tumours were stage III and were disease free and alive at the end of the follow-up. The patient with distal cholangiocarcinoma was stage IIB, received adjuvant chemotherapy and radiotherapy, relapsed (DFS 14.6 months) and died due to cancer progression at 20.93 months.
| Pancreas carcinoma | Breast | |||||||
| Chemo | Relapse | Death | Chemo | RT | HNT | Relapse | ||
| IA | No | 5 | 1 | 2 | 9 | |||
| Yes | 5 | 9 | 8 | 1 (S) | ||||
| IB | No | 1 | ||||||
| Yes | 2 | 1 | 2 (CA/PANC) | |||||
| IIA | No | 0 | 1 | 0 | 3 | |||
| Yes | 3 | 2 | 3 | 0 | ||||
| IIB | No | 1 | 1 | |||||
| Yes | 1 | 0 | ||||||
| IIIA | No | 1 | ||||||
| Yes | 1 | 1 | 1 | 0 | ||||
| IIIB | No | 1 | 1 | 1 | 1 | |||
| Yes | 1 | 1 | 1 | 1 (L) | ||||
| IIIC | No | |||||||
| Yes | ||||||||
| IV | No | |||||||
| Yes | 1 | 1 | 1 | 1 (R+S) | ||||
| O | No | 2 | 0 | 2 | 3 | |||
| Yes | 1 | 3 | 1 | 0 | ||||
Regarding COVID-19, 11 of the 13 patients (84.6%) got vaccinated between April and June 2021; the other two died, not due to COVID-19, before vaccination started. Five of the 13 patients (38.5%) contracted the infection during the follow-up after immunisation. Of these five patients, three (60%) were asymptomatic or mildly symptomatic, without hospital admissions, sequelae or death.
There were seven patients with gastric cancer and two with oesophageal cancer. Of them, seven (77.8%) underwent surgery in May and June, when the pandemic was more stabilized at our setting. Table 1 provides the patient demographic data. All of the patients had at least one comorbidity (mean 3, SD 1.22, range 1–5; Table 2). The cancer and surgery characteristics are presented in Table 9.
| Gastric and oesophageal cancer [n (%)] | Gastric, n = 7 (5 operated) | Oesophageal, n = 2 | |
| Final TNM staging | IB | 1 (14.3)1 | |
| I-E | 1 (14.3)2 | ||
| IIA | 2 (28.6), 13 | ||
| IIIA | 1 (14.3)3 | ||
| IIIB | 2 (100) | ||
| IIIC | 2 (28.6) | ||
| Location | Middle third | 1 (50.0) | |
| Lower third – GEJ | 1 (50.0) | ||
| Proximal | 2 (40.0) | ||
| Distal | 3 (60.0) | ||
| Histology | Adenocarcinoma | 3 (60.0) | 1 (50.0) |
| Squamous cell | 1 (50.0) | ||
| Other | 1 GIST (20.0) 1 lymphoma (20.0) | ||
| Neoadjuvant therapy | Long-course RT+CT | 2 | |
| Long-course CT | 2 (40%) | ||
| Previous COVID | 1 (20.0%) | 0 | |
| CRITCON level | 0 | 3 (60.0) | 1 (50.0) |
| 1 | 1 (20.0) | 1 (50.0) | |
| 2 | 1 (20.0) | ||
| Urgency | Urgent/Expedited | ||
| Elective | |||
| Operative approach | Open | 1 (20.0) | 2 (100.0) |
| MIS | 3 (60.0) | 0 | |
| MIS-open | 1 (20.0) | 0 | |
| Resection | R0 | 5 (100) | 1 (50.0) |
| R1 | 0 | 1 (50.0) | |
| R2 | 0 | 0 | |
Of the seven patients with gastric cancer, five (71.4%) underwent an operation. One of these five patients (20.0%) had mild COVID-19 4 wk before surgery. All surgeries were elective except one (20.0%, due to obstruction) and had a definitive curative intent. The following surgical procedures were performed: total gastrectomy (2/5, 40.0%), atypical resections (2/5, 40.0%) and distal gastrectomy (1/5, 20.0%). Additional procedures included one feeding jejunostomy and one Dor fundoplication in a proximal atypical resection. No patient contracted COVID-19 during the first month after surgery and all were discharged home (length of stay: Mean 8.4, SD 3.13, range 5–13 days).
In the two patients with oesophageal cancer, the surgeries were elective and had a curative intent; neither patient had a previous SARS-CoV-2 infection. Both patients underwent oesophagogastrectomy, one with a total gastrectomy and one with a gastroplasty (three and two fields, respectively). One thorax was approached by thoracoscopy and the other by thoracotomy; both abdomens by open surgery. Neither patient contracted COVID-19 during the first month. One patient was discharged home but the other died on postoperative day 9 due to an anastomotic leak (length of stay: Mean 14, SD 7.77, range 9–19 days).
Short-term results: The overall 30-day mortality was 14.3% and complications (all grades) affected three of seven (42.9%), with the highest rate among patients with oesophageal cancer (Table 10). Major complications (C–D grades III–V) occurred in two of seven patients (28.6%).
| Gastric and oesophageal cancer, all results presented as n (%) unless specified | Gastric, n = 5 | Oesophagus, n = 2 | |
| Overall complications | Mortality (CD V) | 0 (0) | 1 (50.0) |
| Patients with complications | 1 (20.0) | 2 (100.0) | |
| Unplanned critical care admission (CD IV) | 0 | 0 | |
| Planned ICU admission | 2 (40.0) | 2 (100.0) | |
| Reintervention (CD III) | 0 | 1 (50.0) – IIIa (stent) | |
| Any major complication (CD III-V) | 0 | 2 (100.0) | |
| Acute kidney injury | 2 (100.0) | ||
| Pulmonary embolism | 1 (50.0) | ||
| Septic shock | 1 (50.0) | ||
| Anastomotic leak | 2 (100.0) | ||
| Ileus | 0 | ||
| Bleeding requiring transfusion | 0 | 0 | |
| Superficial/deep SSI | 1 (20.0) | 1 (50.0) | |
| Other | 1 (20.0)1 | 1 (50.0)2 | |
| Length of stay (d) | Mean (SD) | 8.4 (3.13) | 14 (7.7) |
Changes potentially relatable to COVID-19 pandemic in management: For patients with gastric cancer, two patients were not resected during the first wave. One contracted severe COVID-19 after the first neoadjuvant chemotherapy cycle and required hospitalisation. The MDT decision changed to surgery and when exploratory laparoscopy could be performed (17 June), peritoneal carcinomatosis was found. The other patient refused surgery and did not receive specific treatment but changed his mind in September 2020 and underwent surgery; an unresectable mass was found, and a palliative surgery was performed. For the five patients who underwent surgery, 3 (60.0%) took place in the COVID-free building, two (40%) had a delay [one patient with gastrointestinal stromal tumour (GIST) waited more than 4 wk on the waiting list] and one patient (20.0%, the first case operated) would likely had received a laparoscopic approach in the pre-COVID-19 setting. Concerning the two oesophageal cancer patients, both had a delayed surgery (more than 4 wk) and a very long period from the completion of neoadjuvant therapy to surgery. One patient (50.0%) was operated on in the alternative building. Perhaps without a health crisis, more minimally invasive approaches could have been intended.
Concerning oncological outcomes and therapies, the three patients with gastric carcinoma who underwent surgery received adjuvant chemotherapy. One case with stage IIIC disease relapsed at 27.6 months (systemic) and died at 33.8 months due to cancer. The other two were disease free and alive at the end of the follow-up; thus, the 3-year DFS and OS were 66.7%. The patient with lymphoma received chemotherapy, had a systemic relapse at 5.83 months and died due to hemophagocytic syndrome at 7.27 months. The patient with GIST was disease free and alive at the end of the follow-up. The case of squamous cell oesophageal cancer died due to postoperative complications. Finally, the patient with stage IIIB oesophageal adenocarcinoma received no chemotherapy, relapsed at 4.67 months (local and regional) and died at 5.36 months due to bowel ischaemia.
Regarding COVID-19, four of the seven patients (57.1%) who underwent surgery were vaccinated between March and May 2021; the other three patients died before vaccination started. Two of the seven patients (28.6%) contracted COVID-19 during the follow-up: one after getting immunised and the other on an unknown date prior to vaccination (diagnosed as IgG+). One patient was asymptomatic and the other had a mild infection.
Twenty patients with breast cancer underwent elective surgery. The demographic data are described in Table 1. In contrast to the previous groups, 4 of the 20 patients (20.0%) had no comorbidities (mean 1.85, SD 1.14, range 0–4; Table 2). One patient had COVID-19 5 wk before undergoing surgery.
The cancer and surgical treatment characteristics are presented in Table 11. The principal surgical procedures performed were: partial breast excisions (15/20, 75.0%), total mastectomies (3/20, 15.0%) and skin-sparing mastectomy (2/20, 10.0%); two patients (10.0%) had bilateral mastectomies. The complete procedures (including associated axillae and reconstructive manoeuvres) are presented in Figure 1C. Except for one patient who had hormone-sensitive metastatic cancer for a long time, in whom mastectomy was indicated to control local symptoms, the intention of all surgeries was curative.
| All results presented as n (%) unless specified | Breast, n = 20 | |
| Postoperative TNM staging | 0 | 3 (15.0) |
| IA | 10 (50.0) | |
| IIA | 3 (15.0) | |
| IIIA | 1 (5.0) | |
| IIIB | 2 (10.0) | |
| IV | 1 (5.0) | |
| Histology | Invasive carcinoma | 19 (95.0) |
| Ductal carcinoma in situ (high grade) | 1 (5.0) | |
| Estrogen receptor (+/-) | 15/5 | |
| Progesteron receptor | 11/9 | |
| Her-2 (+/-) | 3/16 | |
| Nottingham prognostic index (N=19). [Mean/SD/Range] | 3.65/1.11/ 2 - 6.60 | |
| Oncotype score (N=7). [Mean/SD/Range] | 48.86/23.53/10 - 80 | |
| Menopausal status1 | Post/Pre-menopausal | 15 (75.0)/4 (20.0) |
| Neoadjuvant therapies | Long-course chemotherapy | 3 (15.0) |
| Previous COVID | 1 (5.0) | |
| CRITCON level | 1 | 2 (10.0) |
| 2 | 10 (50.0) | |
| 3 | 8 (40.0) | |
| Resection | R0 | 17 (85.0) |
| R1 | 2 (10.0) | |
| R2 | 1 (5.0) | |
As expected, the short-term outcomes were favourable. No patient contracted COVID-19 during the 30-d postoperative period, and all of them were discharged home (length of stay: mean 1, SD 0.560, range 0–2 d), no patient required a re-intervention or ICU admission, and mortality was 0%. Five (25.0%) had a total of six mild complications (CD grade I): Three superficial surgical site infections and three seromas.
There were some changes potentially associated with the COVID-19 pandemic. Nineteen of the 20 patients (95%) were operated on in an alternative COVID-19-free building (including six in a private hospital) and twelve (60%) had to wait more than 4 wk on the waiting list. But the decision to perform oncoplastic surgery or a reconstruction was not modified in any case due to the pandemic.
Concerning the oncological outcomes, the adjuvant therapies are schematised by disease stage in Table 8. Of the 20 patients, 12 (60.0%) received chemotherapy, 17 (85.0%) radiation and 15 (75.0%) hormone treatment, maintaining pre-pandemic indications. At the end of the follow-up, only 3 of the 20 patients (15.0%) had experienced a relapse. There were no cancer-related deaths and all patients were alive, with a 3-year DFS and OS of 85.0% and 100%, respectively.
Regarding COVID-19, 100% of the patients were vaccinated between February and June 2021. Eleven patients (55.0%) contracted COVID-19 during the follow-up, all after getting immunised. All were symptomatic being the most frequent cold symptoms (63.6%) and fever (9.0%). No patient required hospitalisation, had sequelae or died.
A specific board including hospital managers and physicians decided to resume cancer surgical care after analysing the evolution of the COVID-19 pandemic at our institution during the first weeks. Based on the available literature and recommendations during that period, a strategy based on limiting perioperative social contacts for patients (including avoiding family caregivers at the hospital), preoperative swab testing (48–72 h before surgery), establishing clearly defined COVID-19-free surgical pathways (including operating theatres, critical care areas and inpatient wards) and limiting infection among health professionals with the continued use of PPE was implemented. We believe that the physical structure of our hospital at that time, with two fully equipped separate buildings, facilitated establishing clean areas. In terms of postoperative SARS-CoV-2 infection, the strategy proved to be safe, as none of the patients who underwent surgery contracted COVID during the first postoperative month. Later, some of these measures were shown to not be sufficiently effective[27]; however, screening based on symptoms, preoperative testing (to identify carriers)[28] and clean areas[29] (including for urgent surgery[30]) were proved to be effective even for cancer surgery.
Our 0% postoperative SARS-CoV-2 infection rate is quite notable. It is lower than in some of the CovidSurg-Cancer reports (3.8% for patients with colorectal cancer[31] and 6.2% for patients with liver and pancreatic cancer[32]). Very poor outcomes were reported initially in global studies with all type of surgical patients with perioperative SARS-CoV-2 infection, with mortality as high as 23.8%[9] or 19.8% in a meta-analysis of 2947 patients[33]. These results improved slightly in later publications with more selected populations. For example, mortality in patients with colorectal[31] or liver and pancreatic[32] cancer was 19.2% and 9.4%, respectively. In a comparative study from the United States (with 1:1 propensity-score matching to patients without COVID-19), mortality was 12% compared with 8.1% (P < 0.001) and postoperative complications, especially thromboembolic, were also higher[34].
The COVID-19 pandemic, especially the first waves, had a huge impact on cancer care: cancellation of consultations, screening and diagnostic tests and surgeries; medical material scarcity; fear to go to the hospital; and patients who contracted COVID-19, among others[35,36]. In our series, seven patients lost their opportunity to undergo surgery with a curative intent during the first wave. Two patients with colorectal cancer died due to COVID-19 before their surgery was scheduled. Two patients refused surgery: One never underwent surgery (died posteriorly due to COVID-19), and the other underwent surgery 4 months later without a clear impact on his oncological course. One patient with liver metastasis had his surgery cancelled and progressed (bone metastases) under chemotherapy; it is likely that his oncological course would not have been favourable even with liver surgery. In patients with gastric cancer, one patient contracted severe COVID-19 and could not undergo chemotherapy or surgery; when she recovered, peritoneal carcinomatosis was found. The other patient with gastric cancer refused surgery and when he changed his mind, an unresectable mass was found.
Other aspects could have impacted our patients. A high proportion (36/78, 46.2%) were on the surgical waiting list for more than 4 wk, considering that since their diagnosis some more weeks had passed. We used the date of waiting list inclusion to exclude the effect of the time for neoadjuvant therapies if indicated. Before the COVID-19 pandemic, although common sense dictated that surgical delay led to poorer outcomes, mostly for aggressive cancers, the available evidence was surprisingly scant, contradictory and difficult to decipher. This issue has been deeply analysed during this pandemic. Currently, we know that in patients with colorectal cancer, delays longer than 4 wk do not seem to affect complete resection rates or short-term outcomes (CovidSurg results)[37]. However, a meta-analysis confirmed than longer delays were significantly associated with worse OS (but not DFS) with a number needed to harm for a 1- and 3-month delay of 35 and 10, respectively[38]. A 2021 meta-analysis including 18 studies and 2533355 patients observed that delaying surgery for 12 wk decreased OS in breast cancer [hazard ratio (HR) 1.46, 95% confidence interval (CI) 1.28–1.65], lung cancer (HR: 1.04, 95%CI: 1.02–1.06) and colon cancer (HR: 1.24, 95%CI: 1.12–1.38)[39]. So, the COVID-19 pandemic likely negatively impacted the oncological outcomes of our cohort.
In addition, we had to make some modifications in our previous working methods: surgery in a different building and facility, continuous changes in all the staff, limitations in availability and changes in devices and instrumentation and new difficulties related to the use of PPE[15,16]. Moreover, at least at the beginning, there were continuous changes in the recommendations from scientific societies regarding surgical approaches, anastomoses, etc. Below, we discuss whether the combined effect of these aspects impacted the outcomes of our patients.
For colorectal cancer, apart from the treatment delays and changes in the location where the patients were treated, three patients with colon cancer were operated on by general surgery consultants (directly via open surgery), and we think that two definitive stomas were performed because of the pandemic situation. Our principal results are comparable with the outcomes published by the European Society of Coloproctology (ESCP) 2015 Right Hemicolectomy Audit[40], 2017 Left Colon, Sigmoid and Rectal Resections Audit[41,42] and elective colorectal cancer data from CovidSurg-Cancer[31], as presented in Table 12. The ESCP audits included contemporaneous data from more than 5000 patients from 54 countries. Although not significantly different from the rates published by the ESCP or CovidSurg, our rate of open surgery for colon cancer during the first wave of the COVID-19 pandemic was higher than usual in our institution (as an example in rectal cancer cohort laparoscopic rate is 90%). The anastomotic leak rate was 0% in patients with colon cancer but 12.5% (considering only performed anastomoses) in patients with rectal cancer. Moreover, mortality was < 2% for all types in both audits, but 7.7% for us in patients with left colon cancer (probably highly influenced by the low number of included patients). Concerning the long-term outcomes, we can compare our results with contemporary OS data obtained from the United States surveillance, epidemiology, and end results (SEER) program for cancer[43]. In 2019, the 3-year OS for colon cancer was 93.2%–93.5% (stages I–II), 78.9%–79.4% (stage III) and 23.2%–23.7% (stage IV). In our study, considering only deaths attributable to cancer, the 3-year OS was 93.7% for stages I–II and 90.9% for stage III. For rectal cancer, the 3-year OS in SEER was 91.9%–92.4% for stages I–II (100% in our study), 80.7%–81.4% for the regional stage (80% in our study) and 27.9%–28.8% for stage IV (50% in our study).
| Right colon | Left colon | Rectal | |||||||
| % | ESCP | CSURG | Ours | ESCP | CSURG | Ours | ESCP | CSURG | Ours |
| N | 2225 | 724 | 15 | 989 | 367 | 13 | 2579 | 935 | 20 |
| MIS | 54.4 | 54.7 | 53.3 | 53.6 | 62.9 | 53.8 | 54.2 | 57.8 | 90 |
| Open | 36.5 | 41 | 33.3 | 36.8 | 29.7 | 38.4 | 35.8 | 38.0 | 5 |
| Conv | 9.1 | 4.3 | 13.3 | 9.6 | 7.4 | 7.7 | 10 | 4.2 | 5 |
| ANAST | 98.6 | 93.5 | 100 | 93.3 | 86.1 | 76.9 | 42.8 | 37.4 | 27.3 |
| ANAST +DEF | 0.3 | 1.4 | 0 | 1.8 | 5.7 | 0 | 33.5 | 35.4 | 45.451 |
| End stoma | 1.1 | 5.1 | 0 | 4.9 | 8.2 | 23.1 | 23.7 | 27.2 | 27.31 |
| Leak | 6.5 | 3.6 | 0 | 7.5 | 4.1 | 0 | 9.2 | 6.5 | 5; 12.52 |
| Death | 1.7 | 1.2 | 0 | 0.7 | 1.6 | 7.7 | 0.8 | 2.0 | 0 |
For liver and pancreatic cancer, we are limited by a very small number of patients. Postoperative mortality was 0%, in line with recent national studies of unselected hospitals reporting in-hospital mortality rates after pancreatic surgery of 3.2%–8.6%[44,45] and 3.4%–5.8% for liver surgery[46,47], and with those reported from CovidSurg-Cancer (3.4% and 1.8%, respectively, for SARS-CoV-2-negative patients)[32]. Our incidence of major complications (8.33%) is also similar or even better than in CovidSurg-Cancer data (13.2%)[32]. Concerning the long-term oncological outcomes, our 3-year OS for patients with liver metastases (50.0%) is worse than in some population-based studies (approximately 70.0%)[48,49], but these results depend more on patient characteristics than in surgery itself. Our results in patients with pancreatic carcinoma (3-year OS 33.3%) are similar to those based on SEER database for stages Ib and IIb (20.0%–40.0%)[50].
Considering gastric and oesophageal cancer, the very low number of patients included limited our ability to compare the results. Both patients with oesophageal cancer had poor outcomes and major complications; one of them died. The poor outcomes are probably due to the high number of comorbidities (both were ASA III), advanced stage (both were IIB) and treatment delays. In the patients with gastric cancer, the results are more comparable with the standards (20% complications, 0% major complications and 0% deaths) from population-based studies (17.1% major complications and 3.3% mortality)[51] or with cases operated on during 2020 in a Chinese hospital (5% and 0%, respectively)[52]. For gastric carcinoma, in the SEER database the 3-year OS is 74.3%–75.4% for stages I–II and 40%–41.2% for stage III. With only one stage IIA and two stage IIIC patients, our 3-year OS was 66.7%, slightly longer than in the SEER database[43].
As expected, the patients with breast cancer had fewer comorbidities, and we registered no mortality or major complications. Only 25.0% of the patients had mild complications that needed no invasive manoeuvres. We tried to maintain European quality indicators in breast cancer care[53] and the requirements of a specialised centre[54]. There were no relevant changes, other than some delays, in the surgeries or oncological therapies. Eighty per cent of the patients had localised disease (stage 0–II), which explains our excellent 3-year OS (100%) and DFS (85.0%), comparable to or better than the SEER database (for localised disease 99.7%–99.8%, for regional disease 91.1%–91.4% and for metastatic cases – one case in our series – 43.5%–44.3%)[43].
This study has several limitations. First, due to its merely descriptive nature, we did not accurately compare the results or waiting times with pre- or post-lockdown periods. We could only use the approximation of being on the surgical waiting list for less than or more than 4 wk, and we could not compare, for example, the rates of minimally invasive surgery or the outcomes. We were only able to compare with the standards or multinational audits. Second, the low number of included patients, especially for liver, pancreatic, gastric and oesophageal cancer, significantly limited our ability to draw definitive conclusions. Third, to understand the real impact of the first wave of the COVID-19 pandemic, we would need to have information about all patients who had the types of cancer we included. In the present study, we only included patients who were diagnosed and proposed for surgery.
Some patients lost their opportunity to undergo surgery with a curative intent due to the first wave of the COVID-19 pandemic. Our results reaffirm the idea that clearly established clean areas and pathways, even with physical barriers, were essential to continue treating patients with digestive and breast cancers and to minimise perioperative SARS-Cov-2 infection and its possible consequences. Although the practice recommendations and availability of PPE changed continuously, and we had to face some relevant alterations in our previous clinical practice, our department could offer a surgical management of digestive and breast cancers with minimal changes compared with the pre-COVID-19 pandemic period, and with outcomes comparable to the standards.
Authors gratefully acknowledge all the Departments professionals, anesthesiologist, nurses and healthcare workers implicated in the management of the patients included in this study.
| 1. | Centro Nacional de Epidemiología. Instituto de Salud Carlos III. Ministerio de Sanidad. Gobierno de España. Informes covid-19. Periodical reports. Report nº 182: Covid-19 in spain at 5th july 2023. Cited December 13, 2023. Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx. |
| 2. | Cobarsí Morales J, Calvet Liñan L, Segundo Martín E. Muertes por COVID-19 en España durante la ‘primera ola’: Datos cuantitativos y su tratamiento periodístico. Rev Gen Inf Doc. 2022;32:61-91. [DOI] [Full Text] |
| 3. | Instituto Nacional de Estadística. Banco de series temporales. Total Nacional. Cited 29 December 2023. Available from: https://www.ine.es/consul/serie.do?d=true&s=ECP320. |
| 4. | Centro Nacional de Epidemiología. Instituto de Salud Carlos III. Ministerio de Sanidad. Gobierno de España. Informes covid-19. Periodical reports. Informes previos covid-19 profesionales sanitarios. Situación hasta 10 de mayo de 2020 (actualizada a 29 de mayo de 2020). Online; cited December 13, 2023. Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/Informes_Previos_COVID-19_Profesionales_Sanitarios_A%c3%b1o_2020.aspx. |
| 5. | COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 667] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 6. | Mowbray NG, Ansell J, Horwood J, Cornish J, Rizkallah P, Parker A, Wall P, Spinelli A, Torkington J. Safe management of surgical smoke in the age of COVID-19. Br J Surg. 2020;107:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 7. | Vigneswaran Y, Prachand VN, Posner MC, Matthews JB, Hussain M. What Is the Appropriate Use of Laparoscopy over Open Procedures in the Current COVID-19 Climate? J Gastrointest Surg. 2020;24:1686-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. |
|
| 9. | COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1185] [Cited by in RCA: 1220] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 10. | Müller PC, Hodson J, Kuemmerli C, Kalisvaart M, Pande R, Roberts KJ. Effect of time to surgery in resectable pancreatic cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2020;405:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Chen EY, Mayo SC, Sutton T, Kearney MR, Kardosh A, Vaccaro GM, Billingsley KG, Lopez CD. Effect of Time to Surgery of Colorectal Liver Metastases on Survival. J Gastrointest Cancer. 2021;52:169-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Finley C, Prashad A, Camuso N, Daly C, Aprikian A, Ball CG, Bentley J, Charest D, Fata P, Helyer L, O'Connell D, Moloo H, Seely A, Werier J, Zhong T, Earle CC. Guidance for management of cancer surgery during the COVID-19 pandemic. Can J Surg. 2020;63:S2-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Shinde RS, Naik MD, Shinde SR, Bhandare MS, Chaudhari VA, Shrikhande SV, Dcruz AK. To Do or Not to Do?-A Review of Cancer Surgery Triage Guidelines in COVID-19 Pandemic. Indian J Surg Oncol. 2020;11:175-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Junta de Castilla y León. Datos abiertos, situación epidemiológica del coronavirus (covid-19) en castilla y león. Situación de hospitalizados por coronavirus en Castilla y León. Cited Jan 10, 2024. Available from: https://analisis.datosabiertos.jcyl.es/explore/dataset/situacion-de-hospitalizados-por-coronavirus-en-castilla-y-leon/export. |
| 15. | Yánez Benítez C, Güemes A, Aranda J, Ribeiro M, Ottolino P, Di Saverio S, Alexandrino H, Ponchietti L, Blas JL; International Cooperation Group on PPE and Emergency Surgery, Ramos JP, Rangelova E, Muñoz M, Yánez C Sr. Impact of Personal Protective Equipment on Surgical Performance During the COVID-19 Pandemic. World J Surg. 2020;44:2842-2847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Alarfaj MA, Foula MS, Alshammary S, Nwesar FA, Eldamati AM, Alomar A, Abdulmomen AA, Alarfaj L, Almulhim A, Alarfaj O, Zakaria HM. Impact of wearing personal protective equipment on the performance and decision making of surgeons during the COVID-19 pandemic: An observational cross-sectional study. Medicine (Baltimore). 2021;100:e27240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | NIHR Global Health Research Unit on Global Surgery. Globalsurg-covidsurg-cancer study webpage. Cited 27 December 2023. Available from: https://globalsurg.org/cancercovidsurg/. |
| 18. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6863] [Article Influence: 623.9] [Reference Citation Analysis (0)] |
| 19. | Harvey D, Gardiner D, McGee A, DeBeer T, Shaw D. CRITCON-Pandemic levels: A stepwise ethical approach to clinician responsibility. J Intensive Care Soc. 2022;23:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Intensive Care Society. CRTICON levels. Cited Jan 3, 2024. Available from: https://ics.ac.uk/resource/critcon-levels.html. |
| 21. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24776] [Article Influence: 1179.8] [Reference Citation Analysis (0)] |
| 22. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1408] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 23. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1723] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 24. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 25. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36728] [Article Influence: 2295.5] [Reference Citation Analysis (0)] |
| 26. | Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 14627] [Article Influence: 2437.8] [Reference Citation Analysis (0)] |
| 27. | COVIDSurg Collaborative; GlobalSurg Collaborative. Effects of pre-operative isolation on postoperative pulmonary complications after elective surgery: an international prospective cohort study. Anaesthesia. 2021;76:1454-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | COVIDSurg Collaborative. Preoperative nasopharyngeal swab testing and postoperative pulmonary complications in patients undergoing elective surgery during the SARS-CoV-2 pandemic. Br J Surg. 2021;108:88-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Glasbey JC, Nepogodiev D, Simoes JFF, Omar O, Li E, Venn ML, Pgdme, Abou Chaar MK, Capizzi V, Chaudhry D, Desai A, Edwards JG, Evans JP, Fiore M, Videria JF, Ford SJ, Ganly I, Griffiths EA, Gujjuri RR, Kolias AG, Kaafarani HMA, Minaya-Bravo A, McKay SC, Mohan HM, Roberts KJ, San Miguel-Méndez C, Pockney P, Shaw R, Smart NJ, Stewart GD, Sundar Mrcog S, Vidya R, Bhangu AA; COVIDSurg Collaborative. Elective Cancer Surgery in COVID-19-Free Surgical Pathways During the SARS-CoV-2 Pandemic: An International, Multicenter, Comparative Cohort Study. J Clin Oncol. 2021;39:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 30. | Boffa DJ, Judson BL, Billingsley KG, Del Rossi E, Hindinger K, Walters S, Ermer T, Ratner E, Mitchell MR, Laurans MS, Johnson DC, Yoo PS, Morton JM, Zurich HB, Davis K, Ahuja N. Results of COVID-minimal Surgical Pathway During Surge-phase of COVID-19 Pandemic. Ann Surg. 2020;272:e316-e320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | COVIDSurg Collaborative. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis. 2020;23:732-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | McKay SC; COVIDSurg Collaborative. Outcomes of patients undergoing elective liver and pancreas cancer surgery during the SARS-CoV-2 pandemic: an international, multicentre, prospective cohort study. HPB (Oxford). 2022;24:1668-1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Abate SM, Mantefardo B, Basu B. Postoperative mortality among surgical patients with COVID-19: a systematic review and meta-analysis. Patient Saf Surg. 2020;14:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Argandykov D, Dorken-Gallastegi A, El Moheb M, Gebran A, Proaño-Zamudio JA, Bokenkamp M, Renne AM, Nepogodiev D, Bhangu A, Kaafarani HMA; COVIDSurg Collaborative. Is perioperative COVID-19 really associated with worse surgical outcomes? A nationwide COVIDSurg propensity-matched analysis. J Trauma Acute Care Surg. 2023;94:513-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Milgrom ZZ, Milgrom DP, Han Y, Hui SL, Haggstrom DA, Fisher CS, Mendonca EA. Breast Cancer Screening, Diagnosis, and Surgery during the Pre- and Peri-pandemic: Experience of Patients in a Statewide Health Information Exchange. Ann Surg Oncol. 2023;30:2883-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Doeve BH, Bakx JAC, Siersema PD, Rosman C, van Grieken NCT, van Berge Henegouwen MI, van Sandick JW, Verheij M, Bijlsma MF, Verhoeven RHA, van Laarhoven HWM. The impact of the COVID-19 pandemic on the diagnosis, stage, and treatment of esophagogastric cancer. J Gastroenterol. 2023;58:965-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 37. | COVIDSurg Collaborative. The impact of surgical delay on resectability of colorectal cancer: An international prospective cohort study. Colorectal Dis. 2022;24:708-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Whittaker TM, Abdelrazek MEG, Fitzpatrick AJ, Froud JLJ, Kelly JR, Williamson JS, Williams GL. Delay to elective colorectal cancer surgery and implications for survival: a systematic review and meta-analysis. Colorectal Dis. 2021;23:1699-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Johnson BA, Waddimba AC, Ogola GO, Fleshman JW Jr, Preskitt JT. A systematic review and meta-analysis of surgery delays and survival in breast, lung and colon cancers: Implication for surgical triage during the COVID-19 pandemic. Am J Surg. 2021;222:311-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 40. | 2015 European Society of Coloproctology Collaborating Group. Predictors for Anastomotic Leak, Postoperative Complications, and Mortality After Right Colectomy for Cancer: Results From an International Snapshot Audit. Dis Colon Rectum. 2020;63:606-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 41. | 2017 European Society of Coloproctology (ESCP) Collaborating Group. The 2017 European Society of Coloproctology (ESCP) international snapshot audit of left colon, sigmoid and rectal resections - Executive Summary. Colorectal Dis. 2018;20 Suppl 6:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | 2017 European Society of Coloproctology (ESCP) collaborating group. An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis. 2018;20 Suppl 6:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Surveillance Research Program National Cancer Institute. SEER*explorer: An interactive website for seer cancer statistics [internet]. Cited Jan 26, 2024. Available from: https://seer.cancer.gov/statistics-network/explorer/. |
| 44. | Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann Surg. 2016;264:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 45. | van Roessel S, Mackay TM, van Dieren S, van der Schelling GP, Nieuwenhuijs VB, Bosscha K, van der Harst E, van Dam RM, Liem MSL, Festen S, Stommel MWJ, Roos D, Wit F, Molenaar IQ, de Meijer VE, Kazemier G, de Hingh IHJT, van Santvoort HC, Bonsing BA, Busch OR, Groot Koerkamp B, Besselink MG; Dutch Pancreatic Cancer Group. Textbook Outcome: Nationwide Analysis of a Novel Quality Measure in Pancreatic Surgery. Ann Surg. 2020;271:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 46. | Farges O, Goutte N, Bendersky N, Falissard B; ACHBT-French Hepatectomy Study Group. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697-704; discussion 704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Filmann N, Walter D, Schadde E, Bruns C, Keck T, Lang H, Oldhafer K, Schlitt HJ, Schön MR, Herrmann E, Bechstein WO, Schnitzbauer AA. Mortality after liver surgery in Germany. Br J Surg. 2019;106:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 48. | Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, Cottier B, Poston G. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 49. | Angelsen JH, Horn A, Sorbye H, Eide GE, Løes IM, Viste A. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br J Surg. 2017;104:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol. 2017;24:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (2)] |
| 51. | Putila E, Helminen O, Helmiö M, Huhta H, Jalkanen A, Kallio R, Koivukangas V, Kokkola A, Laine S, Lietzen E, Louhimo J, Meriläinen S, Pohjanen VM, Rantanen T, Ristimäki A, Räsänen JV, Saarnio J, Sihvo E, Toikkanen V, Tyrväinen T, Valtola A, Kauppila JH. Population-based nationwide incidence of complications after gastrectomy for gastric adenocarcinoma in Finland. BJS Open. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Sun Y, Chen C, Hou L, Zhao E. Short-term and long-term outcomes of patients with gastric cancer during versus before the COVID-19 pandemic: cohort study using propensity score matching method. BMC Cancer. 2023;23:913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 53. | Del Turco MR, Ponti A, Bick U, Biganzoli L, Cserni G, Cutuli B, Decker T, Dietel M, Gentilini O, Kuehn T, Mano MP, Mantellini P, Marotti L, Poortmans P, Rank F, Roe H, Scaffidi E, van der Hage JA, Viale G, Wells C, Welnicka-Jaskiewicz M, Wengstöm Y, Cataliotti L. Quality indicators in breast cancer care. Eur J Cancer. 2010;46:2344-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Wilson AR, Marotti L, Bianchi S, Biganzoli L, Claassen S, Decker T, Frigerio A, Goldhirsch A, Gustafsson EG, Mansel RE, Orecchia R, Ponti A, Poortmans P, Regitnig P, Rosselli Del Turco M, Rutgers EJ, van Asperen C, Wells CA, Wengström Y, Cataliotti L; EUSOMA (European Society of Breast Cancer Specialists). The requirements of a specialist Breast Centre. Eur J Cancer. 2013;49:3579-3587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |