Published online Mar 20, 2024. doi: 10.5662/wjm.v14.i1.88518
Peer-review started: September 29, 2023
First decision: December 6, 2023
Revised: December 27, 2023
Accepted: February 3, 2024
Article in press: February 3, 2024
Published online: March 20, 2024
Processing time: 160 Days and 1.2 Hours
It was reported that rikkunshito (TJ-43) improved the cisplatin-induced decreases in the active form of ghrelin in plasma; however, other effects on gastrointestinal hormones have not been investigated.
To investigate the effects of TJ-43 on peripheral levels of incretin hormones, including gastric inhibitory polypeptide (GIP) and glucagon-like polypeptide-1 (GLP-1), in humans and rats.
Patients were divided into two groups, namely patients who received TJ-43 immediately following surgery [TJ-43(+) group] and those who received TJ-43 on postoperative day 21 [TJ-43(-) group], and the plasma levels of active GIP and active GLP-1 were assessed. In animal experiments, rats were treated with TJ-43 [rat (r)TJ-43(+) group] or without [rTJ-43(−) group] by gavage for 4 wk, and the plasma active GIP and active GLP-1 levels were measured. The expression of incretin hormones in the gastrointestinal tract and insulin in the pancreas were investigated by immunohistochemistry. Furthermore, the cyclic adenosine monophosphate activities were assessed in pancreatic tissues from rats treated with or without TJ-43 in vivo, and the blood glucose levels and plasma insulin levels were measured in rats treated with or without TJ-43 in oral glucose tolerance tests.
In humans, the active incretin hormone levels increased, and values were significantly greater in the TJ-43(+) group compared those in the TJ-43(-) group. In rats, the plasma active incretin levels significantly increased in the rTJ-43(+) group compared with those in the rTJ-43(-) group. GIP and GLP-1 expressions were enhanced by TJ-43 treatment. Moreover, plasma insulin levels increased and blood glucose levels were blunted in the rTJ-43(+) group.
The results show that TJ-43 may be beneficial for patients who undergo pancreatic surgery.
Core Tip: This study aimed to investigate the effects of rikkunshito (TJ-43) on gastrointestinal hormones, including gastric inhibitory polypeptide and glucagon-like peptide-1, in humans and rats. As a result, TJ-43 increased incretin hormones, and insulin expression, and suppressed increases in blood glucose levels in human and animal models. Thus, TJ-43 may provide benefits after pancreatic surgery.
- Citation: Kono H, Furuya S, Akaike H, Shoda K, Kawaguchi Y, Amemiya H, Kawaida H, Ichikawa D. Rikkunshito increases peripheral incretin-hormone levels in humans and rats. World J Methodol 2024; 14(1): 88518
- URL: https://www.wjgnet.com/2222-0682/full/v14/i1/88518.htm
- DOI: https://dx.doi.org/10.5662/wjm.v14.i1.88518
The Japanese traditional herbal medicine named “rikkunshito” (TJ-43) is generally obtained in the form of a powdered extract consisting of eight crude medicines[1] and is used to treat various gastrointestinal (GI) disturbances[2-4]. TJ-43 was also shown to promote gastric emptying in rats[5]. Furthermore, using TJ-43 in combination with an anti-emetic drug prevented anorexia and vomiting in advanced breast cancer patients undergoing chemotherapy[6]. The preventive effects of TJ-43 on chemotherapy-induced nausea, vomiting, and anorexia were also reported in patients treated with cisplatin-based chemotherapy[7]. Moreover, TJ-43 is administered to patients undergoing GI surgery, including pancreatico-duodenectomy[1], and significant effects of TJ-43 have been reported. TJ-43 increased peripheral acylated-ghrelin levels, improved delayed gastric emptying (DGE), and ameliorated postoperative GI symptoms in patients undergoing gastrectomy[8]. In patients undergoing pylorus-preserving pancreatico-duodenectomy (PpPD), postoperative oral food intake does not increase easily because of DGE. Considering that TJ-43 increases active ghrelin levels, which increases food intake, TJ-43 is often administered in patients undergoing PpPD. In addition, it is necessary to control blood glucose levels in patients undergoing PpPD.
The GI hormones constitute a group of hormones that control functions of the digestive organs and are secreted by enteroendocrine cells in the GI tract and pancreas[9]. They exert autocrine and paracrine actions that help integrate the GI functions. Most gut peptides, including secretin, cholecystokinin, or substance P, play the roles of neurotransmitters and neuromodulators in the central and peripheral nervous systems[10]. Enteroendocrine cells do not form glands but are spread throughout the GI tract. It is well known that TJ-43 increases the secretion of the GI hormone “ghrelin,” an endogenous ligand of the growth hormone secretagogue receptor. It consists of 28 amino acids and is secreted mainly from the stomach[11].
Incretin hormones are also GI hormones and have received significant attention because of their important roles in glucose homeostasis, type 2 diabetes, and potentially other metabolic disorders[12]. Glucose intake leads to a stimulation of insulin secretion than an intravenous administration of glucose[13]. This is known as the incretin effect, which is attributed to the fact that oral glucose intake leads to the release of incretin hormones, including gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), from entero-endocrine cells in the gastrointestinal tract. In contrast, intravenous glucose administration does not induce the release of incretin hormones[13]. The GI hormones released in response to nutrient absorption act as endocrine signals to the pancreatic islet cells, augmenting insulin secretion. In this study, TJ-43 increased peripheral incretin-hormone levels in patients undergoing PpPD; however, the effects and significance of TJ-43 on GI hormones, including incretin hormones, which are related to insulin secretion, have not been elucidated in previous reports. Therefore, the effects of TJ-43 on incretin hormones were also investigated in rats herein.
This was a retrospective observational study. Blood samples were obtained from 41 patients who underwent PpPD at the University Hospital (Table 1). The study was conducted following the ethical standards outlined in the Declaration of Helsinki and approved by the University Ethics Committee (Chief, Prof. Zentaro Yamagata; No. 820). Upon admission, informed consent was obtained from all patients. The tumor stage was evaluated according to the Union for International Cancer Control classification[14]. Moreover, the histological and pathological diagnoses of the specimens were confirmed using the World Health Organization classification criteria.
| Variable | TJ-43− (n = 20) | TJ-43+ (n = 21) | P value | |
| Age | Year | 67 7.0 | 66 7.7 | 0.962 |
| Sex | Male | 10 (50) | 14 (67) | 0.199 |
| Female | 10 (50) | 7 (33) | ||
| Disease | Pancreas Ca. (Ph) | 7 (35) | 8 (38) | |
| IPMC | 2 (10) | 2 (10) | ||
| IPMA | 2 (10) | 1 (5) | ||
| CBD Ca. | 5 (25) | 5 (24) | ||
| Vater Ca. | 3 (15) | 4 (19) | ||
| Panaceas-NET | 1 (5) | 0 (0) | ||
| GB Ca. | 0 (0) | 1 (5) | ||
| UICC tumor stage; pancreas Ca. (0/I/IIA/IIB); other Ca. (0/I/II/III) | Pancreas Ca. (Ph) | (0/0/1/6) | (0/0/3/5) | |
| IPMC | (1/0/1/0) | (0/0/1/1) | ||
| IPMA | N/A | N/A | ||
| CBD Ca. | (0/1/4/0) | (0/3/2/0) | ||
| Vater Ca. | (0/1/2/0) | (0/2/2/0) | ||
| P-NET | (0/1/0/0) | N/A | ||
| GB Ca. | N/A | (0/0/1/0) | ||
| Tumor differentiation (well/mod./poor) | Pancreas Ca. (Ph) | (3/2/2) | (1/6/1) | |
| IPMC | (0/2/0) | (1/0/1) | ||
| IPMA | N/A | N/A | ||
| CBD Ca. | (0/5/0) | (2/2/1) | ||
| Vater Ca. | (2/1/0) | (1/3/0) | ||
| P-NET | N/A | N/A | ||
| GB Ca. | N/A | (0/1/0) | ||
| Time of operation | Min | 500 ± 56 | 509 ± 0.72 | 0.299 |
| Blood loss | mL | 692 ± 0.54 | 959 ± 0.66 | 0.182 |
| HbA1c | % | 5.6 ± 2.2 | 5.7 ± 2.3 | 0.892 |
| Tumor markers | CEA (ng/mL) | 3.1 ± 1.3 | 3.7 ± 1.1 | 0.872 |
| CA19-9 (U/mL) | 455 ± 23 | 451 ± 29 | ||
| DGE | % | 25 | 19 | 0.773 |
| POPF | 0.886 | |||
| Grade A | 15 (75) | 16 (76) | ||
| Grades B and C | 5 (25) | 5 (24) | ||
| Postoperative pneumonia | 1 (5) | 1 (4.8) | 0.889 |
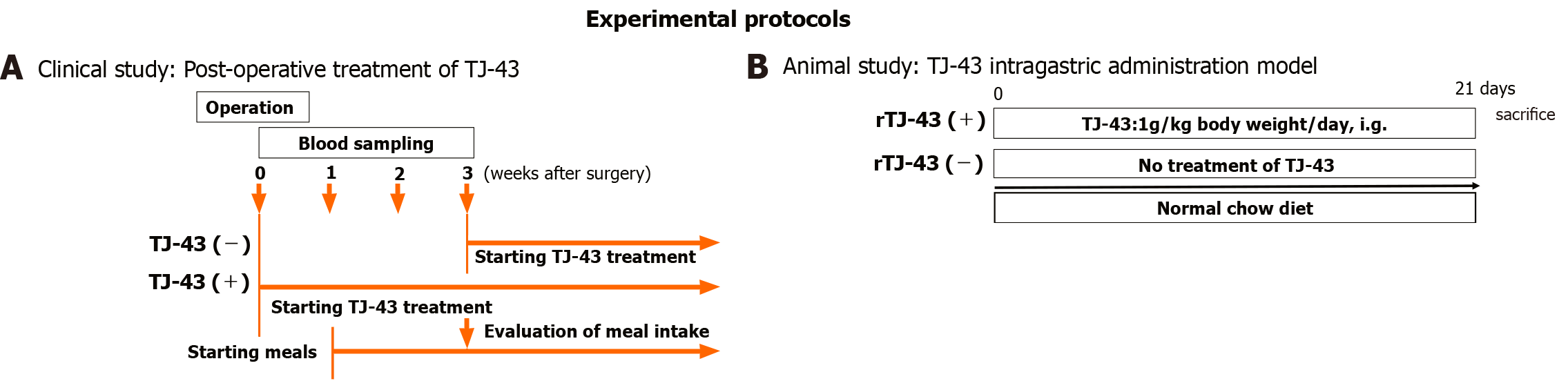
The patients were preoperatively enrolled into the two groups based on treatments of TJ-43; the TJ-43(-) group (n = 20) was treated from the postoperative day (POD) 21 with TJ-43 (7.5 g/d) using an enteral feeding catheter or by oral administration, representing the conventional treatment, and the TJ-43(+) group (n = 21) was treated daily with TJ-43 (7.5 g/d) from POD 1, representing the modified treatment (Figure 1). Enteral feeding of 900 kcal/day was started from POD 1 and continued throughout the study period. The postoperative meals were started at POD 7 in all cases. Three weeks after surgery, the total oral calorie intake was evaluated in both groups, which represented the primary endpoint of this study.
For the definition of complications, DGE was defined based on international criteria[15]. Postoperative pancreatic fistula (POPF) was classified into grades based on the guidelines established by the International Study Group on Pancreatic Fistula[16,17]. Grade A POPF, known as a biochemical fistula, has no clinical impact on the normal postoperative pathway. Clinically significant POPFs are classified as grades B and C. Grade B POPF requires one of the following conditions: an endoscopic or radiological intervention, a drain in situ for longer than 3 wk, clinical symptoms without organ failure, or a clinically relevant change in POPF management. Whenever a major change in clinical management or deviation from the normal clinical pathway is required, or organ failure occurs, the classification shifts to grade C POPF[17].
Blood samples were collected before and after the operation designated time points. Plasma samples were stored at −80°C until further analysis.
The plasma levels of active GIP (RayBiotech Life Inc., Peachtree Corners, GA, United States), and GLP-1 (Invitrogen, Waltham, MA, United States) levels were evaluated[18].
Immunoreactive insulin (IRI) was assessed by clinical laboratory analysis. The amount of glucose in the serum was measured using the Glucose B Test (Wako Pure Chemicals Co., Ltd., Tokyo, Japan).
The experiments were performed according to protocols approved by the university review board (#3-38). The protocols followed our institutional criteria and the National Research Council criteria for the care and use of laboratory animals in research. Male Sprague–Dawley rats (180–200 g body weight, Japan SLC Inc., Shizuoka, Japan) were used. Animals were housed in sterilized cages in a facility with a 12-h night/d cycle. The staff and veterinarians in the animal laboratory maintained the animal facilities and were always available to ensure animal health. All animals were provided humane care in compliance with governmental regulations and institutional guidelines.
Rats were treated with TJ-43 once a day (1 g/kg body weight) [rat (r)TJ-43(+) group] by gavage for 4 wk (Figure 1B). On the sacrifice date, the rats fasted for 8 h before TJ-43 treatment. The rats were fed regular chow diets 1 h after TJ-43 treatment. Blood samples were collected from the aorta 3 h after TJ-43 treatment. Immediately after blood collection, the blood samples were centrifuged. A DPP-4 inhibitor (Sigma-Aldrich, Milwaukee, WI) was added to the plasma at a concentration of 50 nM to 1 mL for measurement of incretin hormones. These samples were stored at −80°C.
Some tissue sections were stained with hematoxylin and eosin to assess inflammation and necrosis. Pathology was evaluated in a blinded fashion by an expert in rodent pathology.
Tissue specimens were cut into 4-mm serial sections. Each section was treated in antigen retrieval solution for 15 min at 120°C using Dako REAL Target Retrieval Solution (Dako, Carpinteria, CA, United States). Then, sections were blocked using 5% normal blocking serum for 20 min and incubated with rabbit polyclonal antibody. The following antibodies were used: anti-GIP diluted 1:300 (bs-0098R, Bioss, Woburn, MA, United States), anti-insulin diluted 1:2000 (ab181547, Abcam, Cambridge, United Kingdom), and anti-GLP diluted 1:200 (ab218532, Abcam). Sections in which the primary antibody had been substituted by non-immune serum served as negative controls. Following incubation, immune-peroxidase staining was completed using a Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA, United States) and 3,3’-diaminobenzidine-tetrachloride as a chromogen[19].
The density of stained cells in three different areas per section was quantified and standardized (per 250000 mm2) using the BZ-H3C measurement module (Hybrid Cell Count Ver.1.1, Keyence)[20,21].
Cyclic adenosine monophosphate (cAMP) determination was performed using a cAMP ELISA kit (#581001, Cayman Chemical Company, Ann Arber, MI, United States) following the manufacturer’s instructions. In another set of experiments, pancreatic tissues were harvested from rats treated with TJ-43. The frozen tissues were weighed and dropped into volumes (5–10 mL of solution/gram of tissue) of 5% trichloroacetic acid (TCA) in water. The samples were homogenized on ice using a Polytron-type homogenizer. The precipitate was removed by centrifugation at 1500 g for 10 min, and the supernatant was carefully transferred to a clean test tube. The TCA was extracted from the samples using water-saturated ether. Five volumes of ether were added to one volume of supernatant and mixed for 10 s, and then the organic and aqueous phases were allowed to separate. The top of the ether layer was carefully removed and discarded. The extraction was repeated two more times. The residual ether was removed from the aqueous layer by heating the sample to 70°C for 5 min. All ether must be removed because even trace amounts can interfere with the assay. All the samples had a protein concentration greater than 1 mg/mL. Finally, 100 mL of each sample was assayed for cAMP levels according to the measurement method described in the handling instructions.
In the rTJ-43(+) group, animals received intragastric TJ-43 treatment for 3 wk. All rats fasted for 12 h before experiments, in both groups. Then, glucose dissolved in distilled water (1 g/mL) was administered (2 g/kg body weight) by gavage 3 h after TJ-43 administration in the rTJ-43(+) and rTJ-43(-) groups.
Blood samples were taken from the tail vein before and 30, 60, and 120 min after the administration of glucose. The amount of glucose in the serum was measured using the Glucose B Test (Wako Pure Chemicals Co., Ltd., Tokyo, Japan).
Statistical analyses were performed using EZR software (Saitama Medical Center, Saitama, Japan)[22]. Data were presented as the mean ± standard error of the mean. P < 0.05 was considered a significant difference.
Adverse events were not observed by TJ-43 treatments (Table 1).
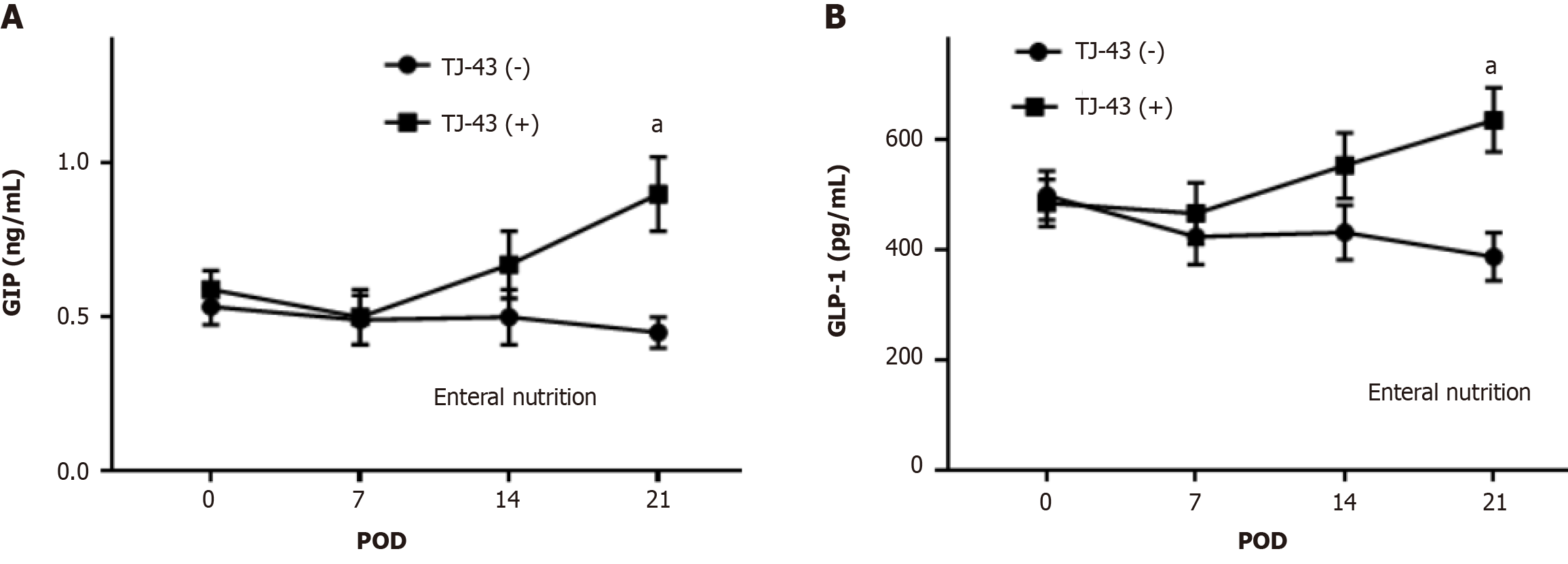
Plasma incretin hormone levels after TJ-43 treatment were significantly greater compared with those before TJ-43 treatment (Figure 2).
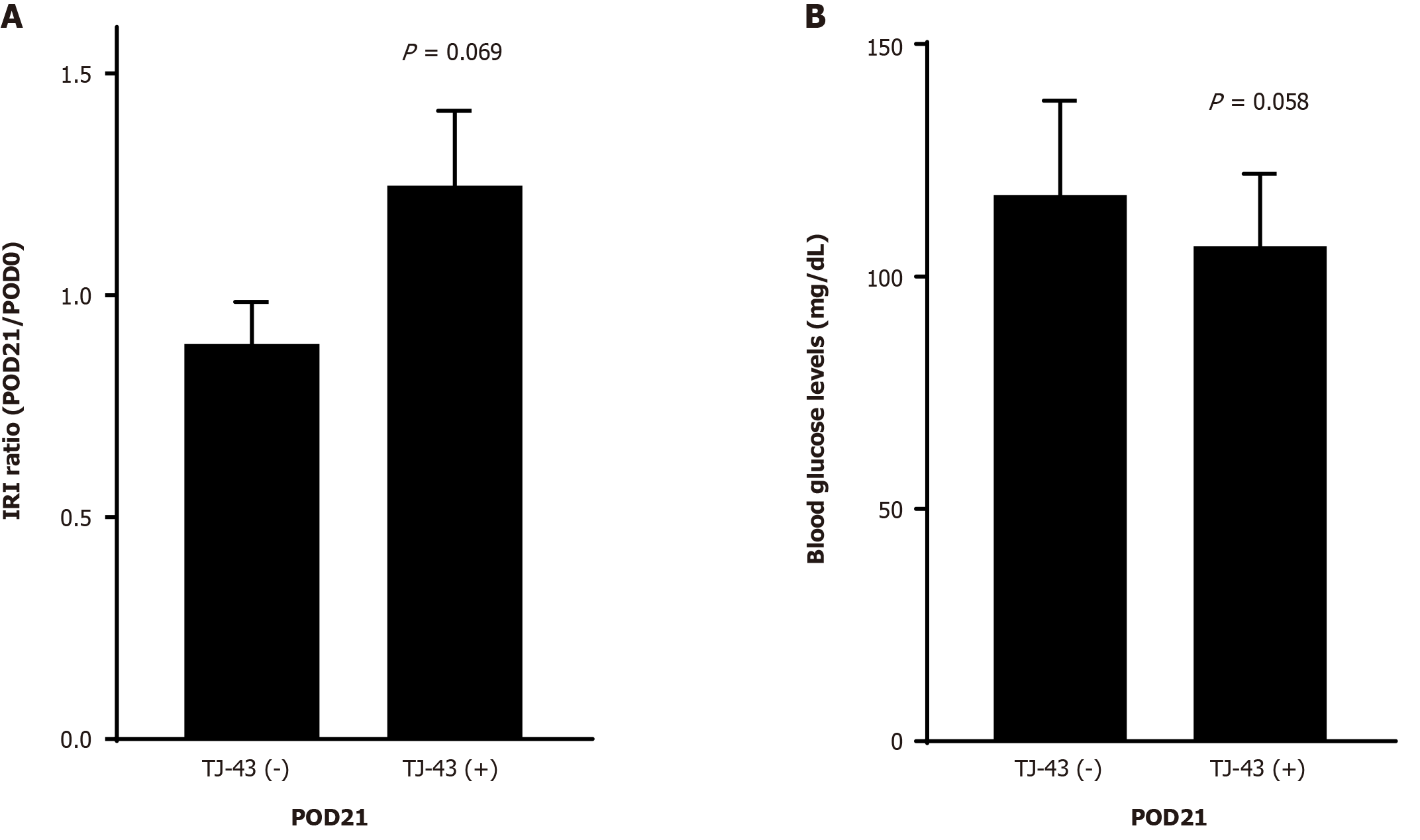
The IRI levels in the TJ-43(+) group were greater, but not significant, compared with those in the TJ-43(−) group (Figure 3A). There were no significant differences in blood glucose levels between the two group (Figure 3B).
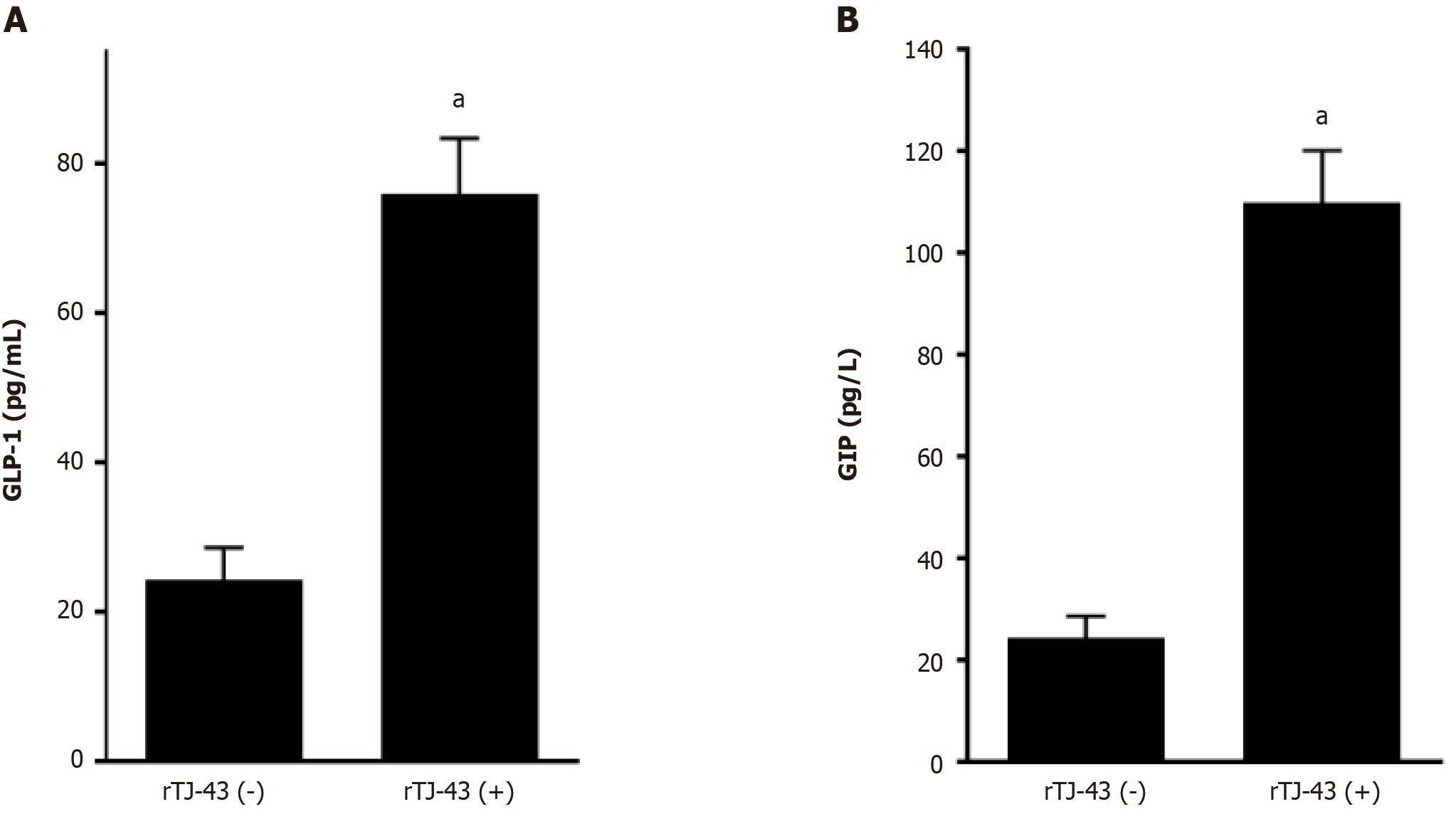
Plasma incretin-hormone levels increased significantly after TJ-43 treatment (Figure 4).
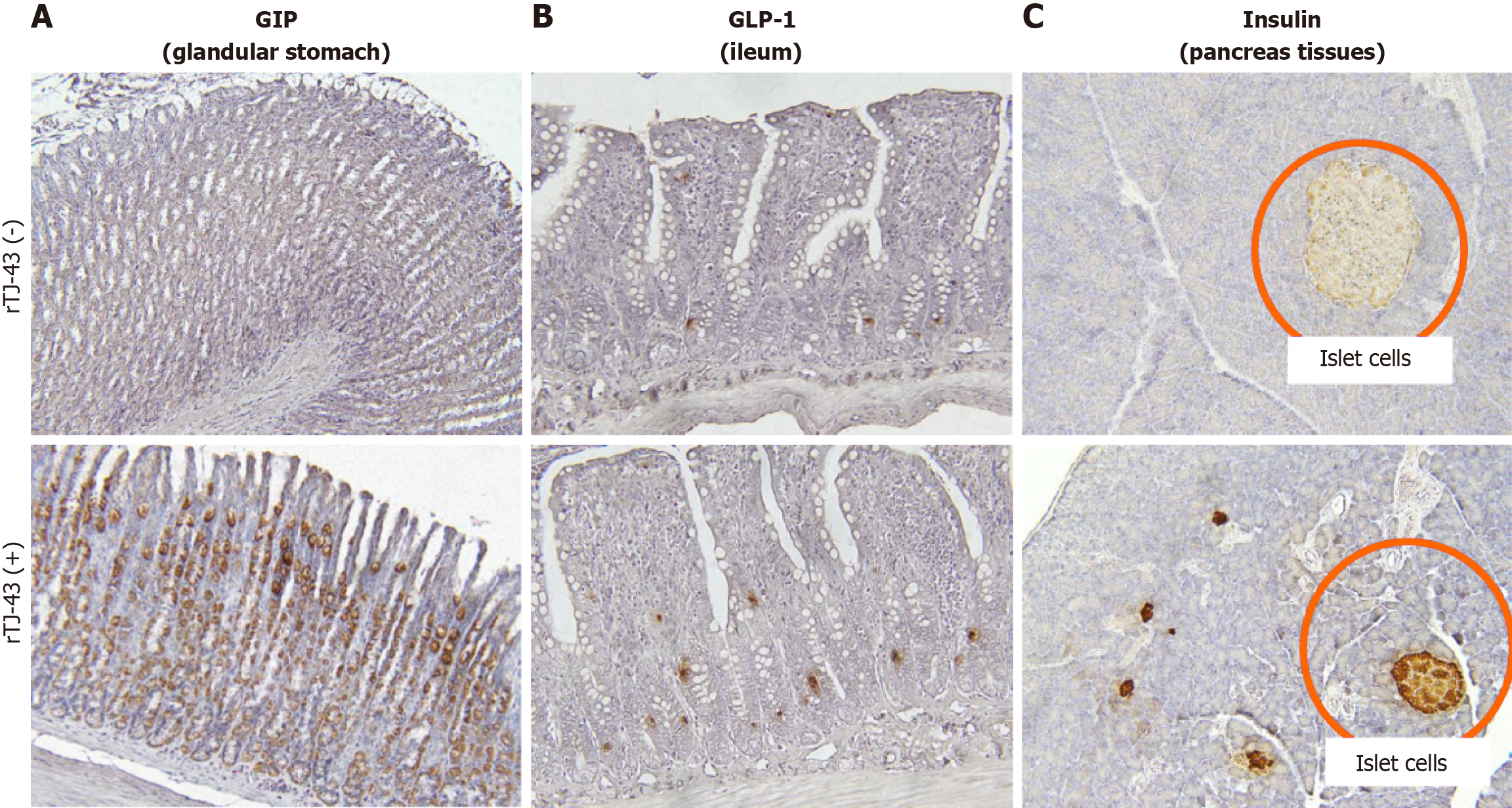
Immunohistochemical analysis revealed that GIP expression was mainly detected in the glandular stomach in rats without TJ-43 treatment (Figure 5). After 4 wk of TJ-43 treatment, the expression markedly increased. Moreover, GLP-1 expression was mainly detected in the ileum and enhanced after TJ-43 treatment.
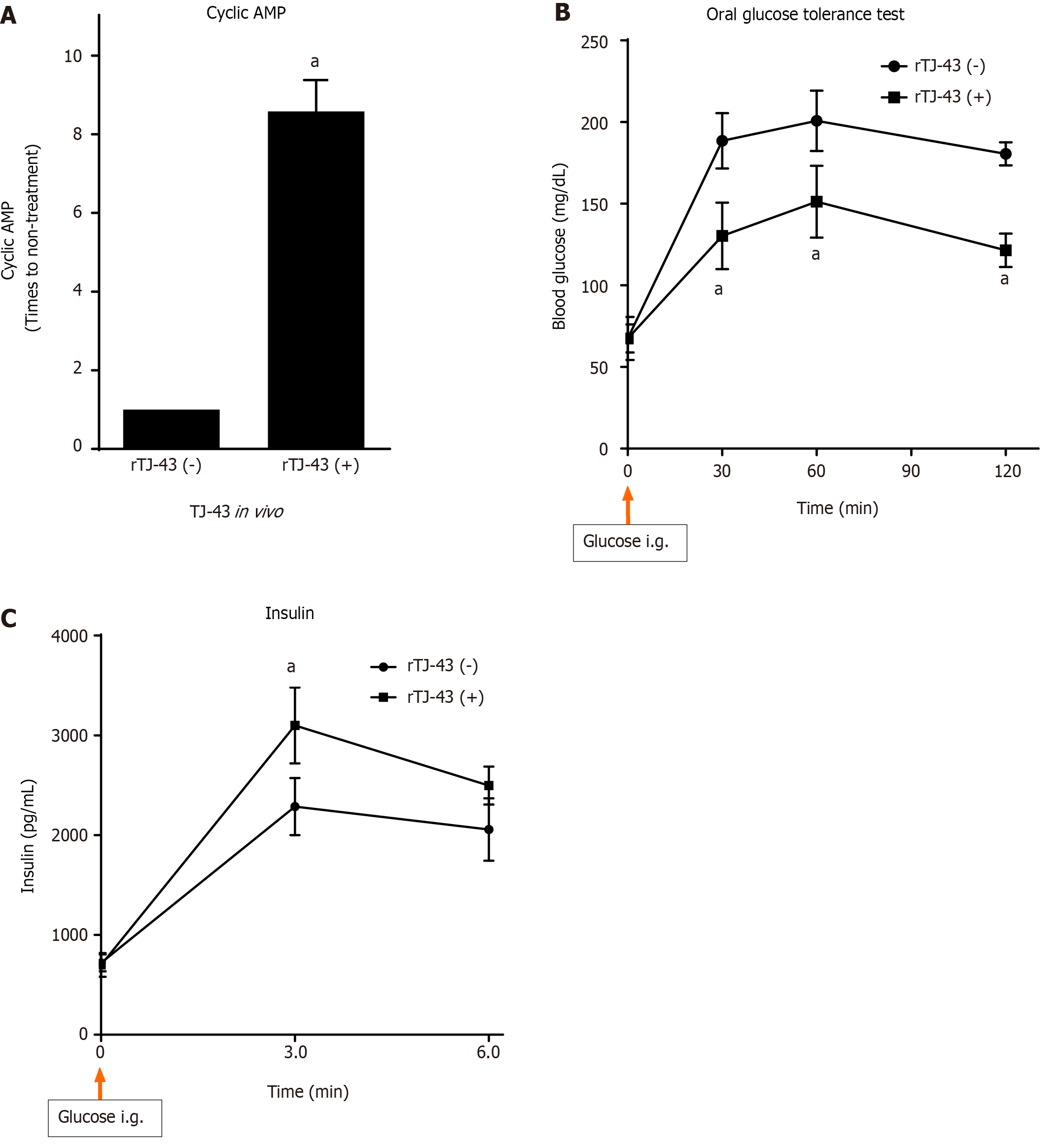
The cAMP activity was measured in the pancreatic tissues of normal rats (Figure 6). However, the activity in the tissues of rats treated with TJ-43 significantly increased compared with those in the tissues of rats without TJ-43 treatment in vivo.
As a result of the cAMP activity in the pancreatic tissues, the expression of insulin in the pancreatic islet cells was markedly enhanced in the cells isolated from animals treated with TJ-43 compared with those isolated from animals without TJ-43 treatment (Figure 5).
The results of the simultaneous administration of glucose and TJ-43 to rats are shown in Figure 6. In the rTJ-43(−) group, the blood glucose levels rose immediately after administration, reaching a maximum value (232.5 ± 22.9 mg/dL) at 30 min and 211.2 ± 3.20 mg/dL at 60 min, followed by a gradual decrease, almost reaching the pre-administration level at 120 min.
In contrast, the blood glucose levels in the rTJ-43(+) group at 30 and 60 min after administration were 194.5 ± 26.1 and 194.0 ± 16.1 mg/dL, respectively. These values were significantly blunted compared with those in the rTJ-43(-) group (P < 0.05).
The results of blood insulin levels in rats treated with the simultaneous administration of glucose are shown in Figure 6. In the rTJ-43(-) group, blood insulin levels rose immediately after glucose administration, reaching a maximum value (3110 ± 456 mg/dL) at 30 min and 2286 ± 298 mg/dL at 60 min. Then the levels gradually decreased to the pre-administration level at 120 min (data not shown).
Moreover, the blood insulin levels at 30 and 60 min after glucose loading in the rTJ-43(+) group were significantly greater than those in the rTJ-43(-) group (P < 0.05).
Incretins are GI polypeptide hormones secreted after nutrient intake[13,23]. One of the incretins, GIP, is secreted from K cells in the upper intestine. Another incretin, GLP-1, is secreted from L cells in the lower intestine. Insulin is secreted from the pancreatic islet b-cells in a blood glucose-dependent manner, suggesting that hypoglycemia rarely occurs without meal consumption. In addition, incretins inhibit gastric acid secretion but do not affect gastric emptying[24]. Thus, incretin hormones are attracting attention for their clinical application in diabetes treatment.
In this study, TJ-43 increased plasma incretin-hormone levels in the patients undergoing PpPD (Figure 2). Incretin is quickly destroyed by the DPP-4 enzyme. Therefore, incretin is less likely to be destroyed, and postprandial glucose levels after are reduced. The clinically available drug that suppresses this enzyme is referred to as a DPP-4 inhibitor. Although it is less effective than the direct injection of GLP-1, it can easily be started as an oral medication. The development of GLP-1 agonists gained more attention after it was demonstrated that exogenous GLP-1 can lower both fasting-induced and postprandial glycemia[25]. During fasting, the glucose-lowering effect of GLP-1 is predominantly mediated by effects on islet cell function, increasing insulin[26]. Accordingly, incretin hormones and their agonists are unlikely to cause hypoglycemia. Collectively, these new drugs are called “incretin-related drugs.” In this study, TJ-43 treatment increased the plasma incretin levels (Figures 2 and 4), and thus, TJ-43 treatment may be useful in combination with other incretin-related drugs.
Herbal medicines stimulate incretin secretion in mice[27,28]. In that study, TJ-43 did not affect postprandial glucose, active GLP-1, triglyceride, or remnant-like particle cholesterol responses[29]. In contrast, the plasma insulin levels 60 min after TJ-43 administration were significantly higher than those measured immediately after TJ-43 administration. Additionally, the free fatty acid levels were reduced 60 to 180 min after the intake of TJ-43. It was concluded that active GLP-1 does not contribute to enhanced insulin secretion 60 min after ingestion of solid test meals. A prokinetic effect of TJ-43 may alter insulin secretion after ingestion of solid test meals. In this study, TJ-43 increased the incretin levels after the pancreatic operation (Figure 2), but the increase in GIP was greater than that in active GLP-1 (Figure 2), suggesting that the effect of TJ-43 on the increase in GIP is stronger compared that in GLP-1.
Both GIP and GLP-1 hormones improve glucose tolerance. GIP appears to be the most effective, particularly regarding insulin secretion, whereas the action of GLP-1 is mainly associated with the inhibition of glucagon secretion[30]. In the present study, plasma active GIP and active GLP-1 Levels were increased by TJ-43 treatment in patients undergoing PpPD (Figure 2). Furthermore, insulin secretion increased, and blood glucose levels decreased in patients with TJ-43 treatment after PpPD (Figure 3), although there were no significant differences among the clinical cases. Notably, the effects of TJ-43 treatment still need to be investigated in normal physiological conditions. Moreover, an animal study is required to clarify whether the increases in incretin-hormone and IRI levels are caused by TJ-43 treatment. Therefore, a study using animal models is currently underway.
The plasma levels and intestinal expression of incretin hormones significantly increased in rats. Furthermore, the degree of increase in GIP was significantly higher than that in GLP-1. Moreover, the activation of cAMP, insulin expression in the pancreas tissues, and blood insulin levels were markedly enhanced by continuous TJ-43 administration by gavage (Figures 4-6). Conversely, the blood glucose levels were significantly lower in the rTJ-43(+) group compared with those in the rTJ-43(-) group after performing oral glucose tolerance tests (Figure 6). Thus, TJ-43 can increase plasma incretin hormone levels, the expression of insulin, and inhibit elevation of blood glucose levels. Hence, TJ-43 may be beneficial for patients who undergo pancreatic resection.
Although TJ-43 may have useful effects on insulin secretion after pancreatic surgery, only a small number of clinical cases were considered in this retrospective study. Therefore, it is difficult to establish a definite conclusion, and a randomized control study with a large number of clinical cases is needed, in addition to the animal study, to verify the effects of TJ-43 and clarify the mechanisms. Overall, oral dietary intake of TJ-43 may be advantageous for increasing blood levels of incretin hormones and acylated ghrelin after pancreatic surgery, which reduces the total volume of pancreas tissue.
The results suggest that TJ-43 is beneficial for patients who undergo pancreatic surgery.
It was reported that rikkunshito (TJ-43) improved the cisplatin-induced decreases in the active form of ghrelin in plasma; however, other effects on gastrointestinal hormones have not been investigated. In patients undergoing pylorus-preserving pancreatico-duodenectomy (PpPD), postoperative oral food intake can be hindered by delayed gastric emptying (DGE). In addition to ghrelin, the effects of TJ-43 on gastrointestinal hormone levels are investigated herein.
It is necessary to resolve the issue of DGE after PpPD.
This basic study aimed to investigate the effects of TJ-43 on peripheral levels of incretin hormones, including gastric inhibitory polypeptide (GIP) and glucagon-like polypeptide-1 (GLP-1), in humans and rats.
Patients were divided into two groups, namely patients who received TJ-43 immediately following surgery [TJ-43(+) group] and those who received TJ-43 on postoperative day (POD) 21 [TJ-43(-) group], and the plasma levels of active GIP and active GLP-1 were assessed. In animal experiments, rats were treated with TJ-43 [rat (r)TJ-43(+) group] or without [rTJ-43(−) group] by gavage for 4 wk, and the plasma active GIP and active GLP-1 Levels were measured. The expression of incretin hormones in the gastrointestinal tract and insulin in the pancreas were investigated by immunohistochemistry. Furthermore, the cyclic adenosine monophosphate activities were assessed in pancreatic tissues from rats treated with or without TJ-43 in vivo, and the blood glucose levels and plasma insulin levels were measured in rats treated with or without TJ-43 in oral glucose tolerance tests.
The active GIP and active GLP-1 Levels increased, and the values at POD 21 were significantly greater in the TJ-43(+) group than those in the TJ-43(-) group. In the rat model, the plasma active incretin levels significantly increased in the rTJ-43(+) group compared with those in the rTJ-43(-) group, although GIP and GLP-1 expressions were enhanced by TJ-43 treatment in both groups. Moreover, plasma insulin levels increased and blood glucose levels were blunted in the rTJ-43(+) group.
The results suggest that TJ-43 is beneficial for patients who undergo pancreatic surgery.
To verify the effects and clarify the mechanisms of TJ-43 after pancreatic surgery, a prospective study is required.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lin SR, Taiwan S-Editor: Liu JH L-Editor: A P-Editor: Guo X
| 1. | Takiguchi S, Hiura Y, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Mori M, Hosoda H, Kangawa K, Doki Y. Effect of rikkunshito, a Japanese herbal medicine, on gastrointestinal symptoms and ghrelin levels in gastric cancer patients after gastrectomy. Gastric Cancer. 2013;16:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Inokuchi K, Masaoka T, Kanai T. Rikkunshito as a Therapeautic Agent for Functional Dyspepsia and its Prokinetic and Non-Prokinetic Effects. Front Pharmacol. 2021;12:640576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Yada T, Kohno D, Maejima Y, Sedbazar U, Arai T, Toriya M, Maekawa F, Kurita H, Niijima A, Yakabi K. Neurohormones, rikkunshito and hypothalamic neurons interactively control appetite and anorexia. Curr Pharm Des. 2012;18:4854-4864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Hoshino N, Nishizaki D, Hida K, Obama K, Sakai Y. Rikkunshito for upper gastrointestinal symptoms: A systematic review and meta-analysis. Complement Ther Med. 2019;42:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Kido T, Nakai Y, Kase Y, Sakakibara I, Nomura M, Takeda S, Aburada M. Effects of rikkunshi-to, a traditional Japanese medicine, on the delay of gastric emptying induced by N(G)-nitro-L-arginine. J Pharmacol Sci. 2005;98:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Jo T, Shigemi D, Konishi T, Yamana H, Michihata N, Kumazawa R, Yokoyama A, Urushiyama H, Matsui H, Fushimi K, Nagase T, Yasunaga H. Antiemetic Effect of Rikkunshito, a Japanese Kampo Herbal Medicine, on Cisplatin-induced Nausea And Vomiting: A Nationwide Database Study In Japan. Intern Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Hirose C, Iihara H, Funaguchi N, Endo J, Ito F, Yanase K, Kaito D, Sasaki Y, Gomyo T, Sakai C, Ohno Y, Suzuki A. Prophylactic effect of rikkunshito, an herbal medicine, for chemotherapy-induced nausea in thoracic cancer patients receiving carboplatin-based chemotherapy. Pharmazie. 2019;74:620-624. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Wagner M, Probst P, Haselbeck-Köbler M, Brandenburg JM, Kalkum E, Störzinger D, Kessler J, Simon JJ, Friederich HC, Angelescu M, Billeter AT, Hackert T, Müller-Stich BP, Büchler MW. The Problem of Appetite Loss After Major Abdominal Surgery: A Systematic Review. Ann Surg. 2022;276:256-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Tack J, Verbeure W, Mori H, Schol J, Van den Houte K, Huang IH, Balsiger L, Broeders B, Colomier E, Scarpellini E, Carbone F. The gastrointestinal tract in hunger and satiety signalling. United European Gastroenterol J. 2021;9:727-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Makris AP, Karianaki M, Tsamis KI, Paschou SA. The role of the gut-brain axis in depression: endocrine, neural, and immune pathways. Hormones (Athens). 2021;20:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Schulz C, Vezzani C, Kroemer NB. How gut hormones shape reward: A systematic review of the role of ghrelin and GLP-1 in human fMRI. Physiol Behav. 2023;263:114111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Goldenberg RM, Teoh H, Verma S. Glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide receptor co-agonists for cardioprotection, type 2 diabetes and obesity: a review of mechanisms and clinical data. Curr Opin Cardiol. 2023;38:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Camilleri M. The role of gastric function in control of food intake (and body weight) in relation to obesity, as well as pharmacological and surgical interventions. Neurogastroenterol Motil. 2024;36:e14660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Lüttges J. [What's new? The 2010 WHO classification for tumours of the pancreas]. Pathologe. 2011;32 Suppl 2:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2324] [Article Influence: 129.1] [Reference Citation Analysis (0)] |
| 16. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 17. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2951] [Article Influence: 368.9] [Reference Citation Analysis (35)] |
| 18. | Matsumura T, Arai M, Yonemitsu Y, Maruoka D, Tanaka T, Suzuki T, Yoshikawa M, Imazeki F, Yokosuka O. The traditional Japanese medicine Rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol. 2010;45:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Kono H, Fujii H, Suzuki-Inoue K, Inoue O, Furuya S, Hirayama K, Akazawa Y, Nakata Y, Sun C, Tsukiji N, Shirai T, Ozaki Y. The platelet-activating receptor C-type lectin receptor-2 plays an essential role in liver regeneration after partial hepatectomy in mice. J Thromb Haemost. 2017;15:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | van der Loos CM, de Boer OJ, Mackaaij C, Hoekstra LT, van Gulik TM, Verheij J. Accurate quantitation of Ki67-positive proliferating hepatocytes in rabbit liver by a multicolor immunohistochemical (IHC) approach analyzed with automated tissue and cell segmentation software. J Histochem Cytochem. 2013;61:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13252] [Article Influence: 1104.3] [Reference Citation Analysis (0)] |
| 23. | Micic D, Polovina S, Micic D, Macut D. OBESITY AND GUT-BRAIN AXIS. Acta Endocrinol (Buchar). 2023;19:234-240. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Fava S. Glucagon-like peptide 1 and the cardiovascular system. Curr Diabetes Rev. 2014;10:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Schirra J, Leicht P, Hildebrand P, Beglinger C, Arnold R, Göke B, Katschinski M. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7-36)amide in non-insulin dependent diabetes mellitus. J Endocrinol. 1998;156:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 387] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Ansari MA, Chauhan W, Shoaib S, Alyahya SA, Ali M, Ashraf H, Alomary MN, Al-Suhaimi EA. Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions. Int J Obes (Lond). 2023;47:1179-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Suh HW, Lee KB, Kim KS, Yang HJ, Choi EK, Shin MH, Park YS, Na YC, Ahn KS, Jang YP, Um JY, Jang HJ. A bitter herbal medicine Gentiana scabra root extract stimulates glucagon-like peptide-1 secretion and regulates blood glucose in db/db mouse. J Ethnopharmacol. 2015;172:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Tanaka K, Urita Y, Nara K, Miura O, Sugimoto M. Effects of the traditional Japanese medicine Rikkunshito on postprandial glucose and lipid metabolism. Hepatogastroenterology. 2011;58:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Gasbjerg LS, Rosenkilde MM, Meier JJ, Holst JJ, Knop FK. The importance of glucose-dependent insulinotropic polypeptide receptor activation for the effects of tirzepatide. Diabetes Obes Metab. 2023;25:3079-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |