Published online Dec 20, 2023. doi: 10.5662/wjm.v13.i5.446
Peer-review started: June 26, 2023
First decision: August 24, 2023
Revised: August 30, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: December 20, 2023
Processing time: 177 Days and 7.7 Hours
Reports of a decrease in hospital admissions during the coronavirus disease 2019 (COVID-19) lockdown period have raised concerns about delayed or missed diagnoses and treatments for non-COVID-19-related illnesses.
To investigate the impact of the COVID-19 pandemic-induced lockdown and its end on hospital admissions of patients with epistaxis in Germany.
A retrospective analysis based on the national database of the Hospital Remuneration System was used to compare hospital admissions during defined time periods between 2019 and 2022 with the lockdown period as the reference period. This was done on a weekly basis before, during, and after the lockdown. An Interrupted Time Series was used as the analysis method.
In our analysis, we included 26183 patients. The implementation of the lockdown led to a substantial reduction in the overall occurrence of epistaxis among patients (P < 0.05). This effect was most pronounced in the age group of 0-39 years, where the decrease was highly significant (P < 0.001). However, there was no change observed in patients aged 80 years and older (not significant). With the end of the lockdown period, the overall number of patients, especially in the youngest age group, increased abruptly and significantly (P < 0.01).
During the lockdown period, there was a decrease in hospital admissions for younger patients with epistaxis, possibly due to the fear of COVID-19 exposure. We also conclude that the severity of epistaxis was not underestimated in the elderly during the pandemic.
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic has had a significant impact on healthcare systems worldwide. In an effort to reduce the spread of the virus, many countries have implemented lockdown measures that restrict movement and social interaction. While these measures have been effective in reducing the transmission of COVID-19, they have also had unintended consequences on healthcare delivery and hospital admissions. Several studies have reported a decrease in hospital admissions during the COVID-19 lockdown period. We showed that the pandemic-induced lockdown resulted in a direct decrease in hospitalizations especially for young patients with epistaxis and an immediate increase in hospitalizations with its end. This might be caused by fear of exposure to COVID-19, unintended consequences of public health recommendations to minimize non-urgent healthcare, or stay at home orders. These findings match with results from previous studies. Conversely, these measures did not lead to any change in older patients, which suggests that at least in this age group, the symptoms of epistaxis should not be underestimated, even with regard to a possible exposure to the coronavirus.
- Citation: Hoenle A, Wagner M, Lorenz S, Steinhart H. Impact of COVID-19 lockdown on hospital admissions for epistaxis in Germany. World J Methodol 2023; 13(5): 446-455
- URL: https://www.wjgnet.com/2222-0682/full/v13/i5/446.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i5.446
Epistaxis is a common emergency in the field of ear, nose, and throat, with varying degrees of severity. Roughly 60% of the population are expected to encounter it at least once during their lifetime[1]. The causes of epistaxis can range from idiopathic to cancerous lesions, with only about 6% of cases requiring medical or surgical attention[2,3]. Idiopathic or spontaneous factors are the primary cause of epistaxis, constituting the cause in at least 70% of cases. These occurrences are frequently associated with conditions such as hypertension, atherosclerotic disease, smoking, or the use of oral anticoagulation medications[4]. Age has also been shown to be a factor in the incidence of epistaxis, with the risk increasing as individuals get older[5].
The Little area, located along the anterior septum, is the origin of approximately 90% of epistaxis cases[6]. Blood supply to this region is provided by the Kiesselbach plexus, composed of second-order branches from both the external and internal carotid arteries[7]. Hemorrhage in this area is commonly referred to as anterior epistaxis and can often be managed using conservative approaches like nostril pressure, topical vasoconstrictors or hemostatic agents, cryotherapy, electrocautery, or anterior nasal packing[8].
In contrast, posterior epistaxis, accounting for only 5% to 10% of cases, originates from the more posterior regions of the nasal cavity[9]. Managing posterior-based nasal bleeding with anterior and posterior nasal packs is less successful, with success rates ranging from 48% to 83%[10-12]. In some cases, nasal hemorrhage persists despite packing or recurs upon pack removal. Posterior epistaxis can be effectively treated through endoscopic or open surgical approaches involving direct ligation or cauterization of the affected artery, with a reported success rate of 97%[13,14].
Endovascular embolization is another viable option to halt nasal bleeding, with reported success rates ranging from 71% to 100%. However, this approach may entail minor complications, such as septal perforation, sinusitis, headache, facial or jaw pain, and facial edema[2,13]. More serious complications, including stroke, facial nerve paresis, soft-tissue necrosis, and even blindness, can occur as a result of inadvertent embolization[14-16].
Recent research indicates that epistaxis may serve as an initial symptom of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. This virus can affect the nasal epithelium, potentially increasing the risk of developing epistaxis[17,18].
The global healthcare systems have been profoundly affected by the coronavirus disease 2019 (COVID-19) pandemic. To mitigate the virus's spread, numerous nations have enacted lockdown measures, curtailing mobility and social interactions. While these steps have effectively curbed COVID-19 transmission, they have also led to unintended repercussions for healthcare provision and hospital admissions. Several studies have reported a decrease in hospital admissions during the COVID-19 lockdown period[19,20]. This decrease has been attributed to several factors, including the cancellation of elective surgeries, reduced emergency department visits, and a decrease in the incidence of some illnesses due to lifestyle changes[21-23].
While the decrease in hospital admissions during the COVID-19 lockdown period may seem like a positive outcome, it has raised concerns about delayed or missed diagnoses and treatments for non-COVID-19-related illnesses. This could potentially result in long-term health consequences for patients[24].
The aim of this study was to examine whether there was a decline in hospital admissions for epistaxis in Germany during the COVID-19 lockdown. Given the potential impact of the COVID-19 pandemic on healthcare delivery and the need for timely treatment of epistaxis to prevent complications, it is important to investigate whether there has been a decline in hospital admissions for this condition during the lockdown period. By examining hospital admission rates for epistaxis during the COVID-19 lockdown period and comparing them to pre-lockdown rates, we can determine whether there was a significant decrease in hospital admissions for this condition.
Motivated by the pressing need to understand how the COVID-19 pandemic might have influenced the healthcare-seeking behavior of individuals, the authors of this study meticulously collected and analyzed nationwide data. The authors recognized that fear of potential coronavirus exposure could have deterred patients from seeking necessary medical attention, even for severe conditions such as epistaxis. This study not only highlights the authors' commitment to addressing a critical gap in medical research during a global crisis but also showcases their dedication to ensuring comprehensive and accurate data collection.
This approach is positioned in the temporal course prior to the outcome following the inpatient admission and can therefore be considered as a complement to other recently published studies that have dealt with patient outcomes after coronavirus infection. In this domain, models using machine learning approaches have been introduced to, for example, estimate the mortality risk of patients with pre-existing diabetes based on various input parameters or to perform early classification of COVID-19 patients through deep learning techniques[25,26].
The findings of this study will provide valuable insights into the impact of the COVID-19 pandemic on the management of epistaxis and may inform the development of strategies to ensure timely access to care for patients with this condition. Furthermore, the authors' contributions extend beyond the immediate scope of the research by shedding light on the broader challenges of maintaining regular healthcare services during times of crisis. Through this study, the authors aim to support healthcare systems in adapting to unforeseen disruptions and guaranteeing that patients receive the essential care that they require, irrespective of external circumstances.
This epidemiological retrospective observational study was performed by using quasi-anonymous open-access population data from the Institute for the Hospital Remuneration System in Germany[27]. This database was used to access the weekly number of hospital admissions for patients with epistaxis (ICD R04.0), with the ICD-10 diagnosis not differentiating between the cause and type of epistaxis (for example anterior or posterior). All patients of all ages and gender who were admitted within Germany with the diagnosis of epistaxis during the specified period were included.
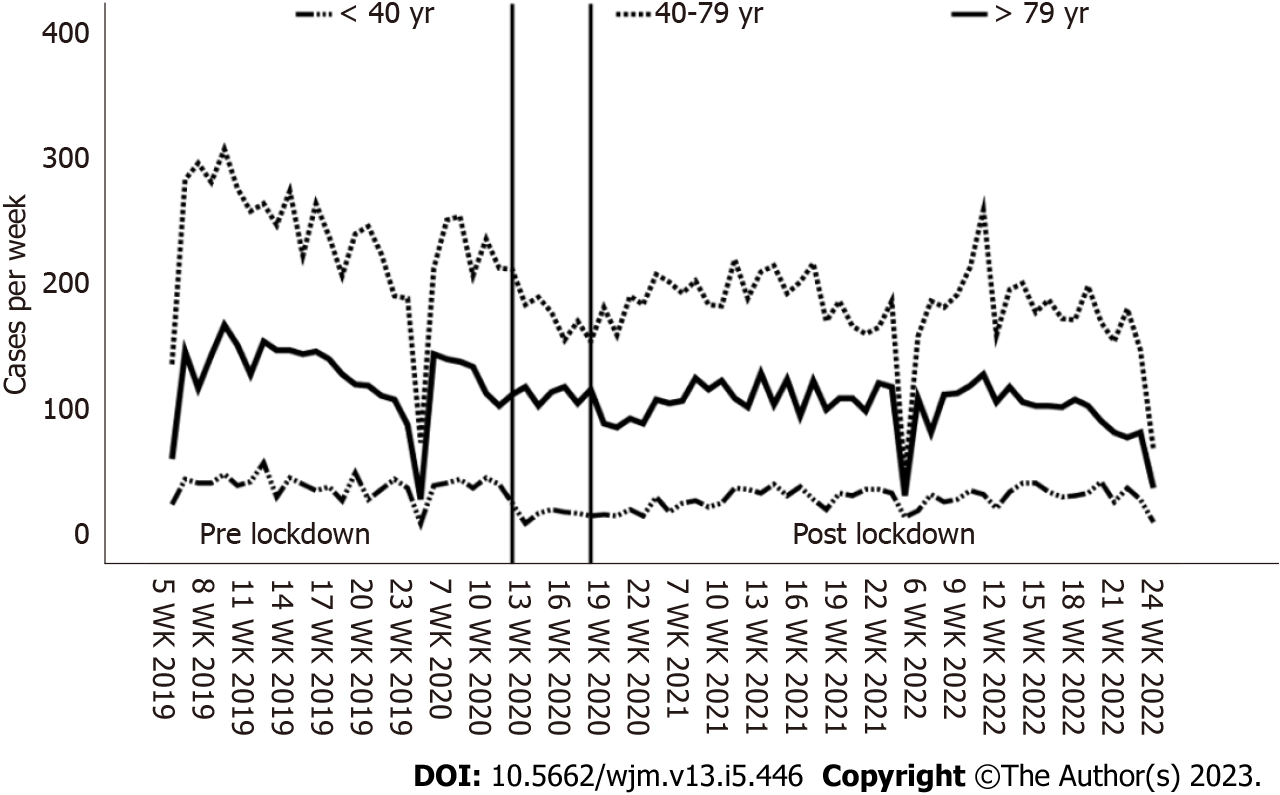
The study period extended from February 1st to June 8th of the years 2019 to 2022. This period was divided into 6-wk blocks, with the government-initiated coronavirus lockdown from March 15, 2020 to April 26, 2020 forming a separate group (lockdown). This resulted in a uniform study period of 18 wk per year to rule out the seasonal incidence of epistaxis[28-30]. Cases before March 15, 2020 (2020 wk 12) were classified into the pre-lockdown group, and cases after April 26, 2020 (2020 wk 18) were classified into the post-lockdown group. These respective time periods of inpatient admission were compared to investigate whether there have been time-dependent changes in patient numbers as the outcome of interest.
The average number of hospital admissions for epistaxis per week was calculated according to time period, gender, and age. Depending on age, patients were grouped into 0-39 years, 40-79 years, or 80 years and older. To evaluate the data, weekly case numbers were presented using an interrupted time series (ITS) analysis. This is a quasi-experimental design suitable for measuring the population-level impact of healthcare interventions[31,32]. The ITS was presented in tabular form. An AutoRegressive Integrated Moving Average (ARIMA) forecast model without seasonal effects was used as a counterfactual in order to provide a more accurate estimate of what would have happened in the absence of the intervention than linear regression[33]. The counterfactual was calculated from the pre-lockdown group as well as from the lockdown group. The results are presented as a percentage deviation from the predicted value, with the respective time boundaries of the Interrupted Time Series corresponding to the start and end of the COVID-19 lockdown.
Values are reported as absolute numbers (n), mean, standard deviation (SD), and 95% confidence interval (95%CI). Due to the metric scaling, analysis of variance was performed to test for mean differences. A t-test for independent samples was used as a post-hoc test with Bonferroni correction to avoid alpha error accumulation[34].
A P value of P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS, version 29.0.0.0 (IBM Corporation, Armonk, NY, United States) and Microsoft Excel, version 2019 (Microsoft Corporation, Redmond, WA, United States).
The recommendations for good practice in secondary data analysis established by the German Working Group on the Collection and Use of Secondary Data were taken into full account. According to the Professional Code of Conduct of the Regional Medical Association, the study did not require ethical approval since it did not involve the use of identifiable patient data.
Overall, 26183 inpatient cases were included in the analysis with a male-to-female ratio of 1.51. The largest age group was the 40-79-year-old group (n = 15145; 58%), followed by those over 80 years of age (n = 8526; 33%). The smallest group was made up of patients aged up to 39 years old (n = 2512; 9%).
It was found that regardless of age and gender, most weekly admissions occurred during the 2019 study period. In contrast, 2020 marked the lowest weekly case numbers in the group up to 39 years compared to the other study periods (P < 0.001). In the age group of 40-79 years, the highest case numbers were also observed in 2019 (P < 0.001), with a statistically constant value in the following years. Only in the group of those over 80 years old, a statistically constant weekly case number was observed for each study year.
Fragmented into 12 equally sized time periods (Table 1), the weekly caseloads for epistaxis were significantly different compared to the restriction period (P < 0.001).
| Category | All | Gender | Age group (yr) | ||||
| Female | Male | 0-39 | 40-79 | 80+ | |||
| February 1, 2019- March 14, 2019 | N | 2620 | 1075 | 1545 | 249 | 1580 | 791 |
| Cases per week | 179.3 (36.8) | 257.5 (70.0) | 41.5 (8.2) | 263.3 (62.9) | 131.8 (37.8) | ||
| March 15, 2019-April 26, 2019 | N | 3082 | 1201 | 1881 | 301 | 1768 | 1013 |
| Cases per week | 171.4 (16.3) | 268.7 (20.6) | 43.0 (8.6) | 252.6 (17.6) | 144.7 (8.1) | ||
| April 27, 2019-June 8, 2019 | N | 2210 | 920 | 1290 | 233 | 1297 | 680 |
| Cases per week | 153.3 (14.6) | 215.0 (24.5) | 38.8 (8.7) | 216.2 (24.4) | 113.3 (13.9) | ||
| February 1, 2020-March 14, 2020 | N | 2526 | 955 | 1571 | 269 | 1449 | 808 |
| Cases per week | 136.4 (42.3) | 224.4 (69.7) | 38.4 (12.4) | 207.0 (61.2) | 115.4 (40.6) | ||
| March 15, 2020-April 26, 2020 (lockdown) | N | 1885 | 735 | 1150 | 118 | 1091 | 676 |
| Cases per week | 122.5 (8.1) | 191.7 (18.7) | 19.7 (5.2) | 181.8 (18.8) | 112.7 (6.4) | ||
| April 27, 2020-June 8, 2020 | N | 1790 | 718 | 1072 | 123 | 2090 | 587 |
| Cases per week | 119.7 (20.8) | 178.7 (15.4) | 20.5 (6.0) | 180.0 (19.7) | 97.8 (12.2) | ||
| February 1, 2021-March 14, 2021 | N | 2042 | 853 | 1189 | 166 | 1185 | 691 |
| Cases per week | 142.2 (9.2) | 198.2 (24.2) | 27.7 (6.4) | 197.5 (13.9) | 115.2 (8.5) | ||
| March 15, 2021-April 26, 2021 | N | 2127 | 889 | 1238 | 218 | 1226 | 683 |
| Cases per week | 148.2 (12.2) | 206.3 (7.6) | 36.3 (8.6) | 204.3 (11.6) | 113.8 (14.2) | ||
| April 27, 2021-June 8, 2021 | N | 1998 | 808 | 1190 | 217 | 1086 | 695 |
| Cases per week | 115.4 (32.5) | 170.0 (54.3) | 31.0 (8.6) | 155.1 (48.8) | 99.3 (30.4) | ||
| February 1, 2022-March 14, 2022 | N | 2046 | 812 | 1234 | 184 | 1193 | 669 |
| Cases per week | 135.3 (19.5) | 205.7 (35.0) | 30.7 (5.7) | 198.8 (34.4) | 111.5 (15.5) | ||
| March 15, 2022-April 26, 2022 | N | 1953 | 753 | 1200 | 213 | 1096 | 644 |
| Cases per week | 125.5 (11.7) | 200.0 (22.3) | 35.5 (7.5) | 182.7 (15.3) | 107.3 (6.0) | ||
| April 27, 2022-June 8, 2022 | N | 1904 | 743 | 1161 | 221 | 1094 | 589 |
| Cases per week | 106.2 (27.0) | 165.9 (46.3) | 31.6 (10.1) | 156.3 (42.0) | 84.1 (22.9) | ||
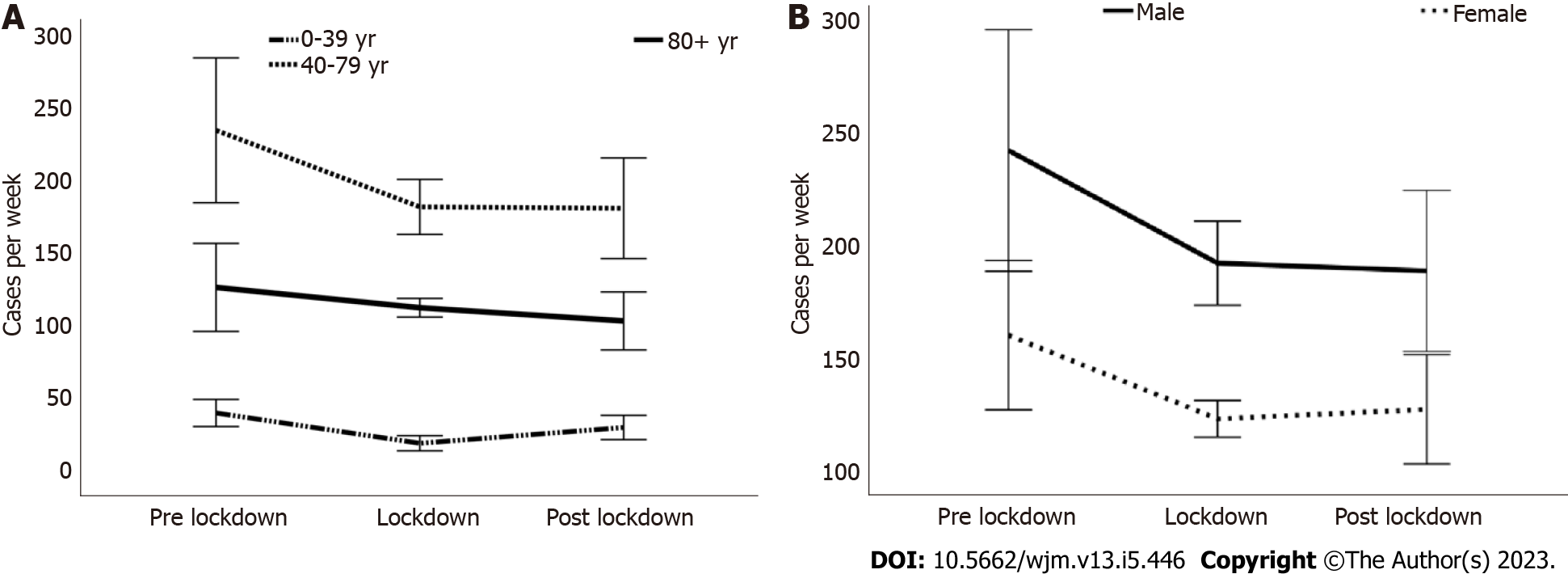
Cases had decreased by 22% from 401.5 (SD 84.2) in the pre-lockdown period to 314.2 (SD 20.6) during the lockdown period, irrespective of age and gender (P < 0.05). Subsequently, there was a marginal, non-significant increase of 0.3% to 315.0 (SD 56.2). Notably, Figure 1 illustrates that the majority of patients were middle-aged and male.
When considering gender, a notable decrease of 29% (P < 0.01) was observed during the lockdown period for female patients, while male patients experienced a decrease of 21%. In the post-lockdown period, cases increased by 11% among females, whereas male patients showed a slight additional decrease of 2% (Table 2, Figure 1B).
| Category | Pre-lockdown | Lockdown | Post-lockdown | |||||
| Cases per week | 95%CI | P value | Cases per week | 95%CI | Cases per week | 95%CI | P value | |
| Total | 401.5 (84.2) | 367.5-435.5 | 0.05 | 314.2 (20.6) | 292.5-335.8 | 315.0 (56.2) | 297.9-332.1 | NS |
| Female | 159.7 (33.1) | 146.3-173.0 | 0.01 | 112.5 (8.1) | 113.9-131.0 | 126.7 (24.3) | 119.3-134.1 | NS |
| Male | 241.8 (53.7) | 220.1-263.5 | NS | 191.7 (18.7) | 172.1-211.2 | 188.3 (35.8) | 117.4-199.2 | a |
| MFR | 1.52 (0.18) | 1.45-1.59 | NS | 1.57 (0.18) | 1.38-1.76 | 1.50 (0.22) | 1.43-1.56 | NS |
| 0-39 | 40.4 (9.3) | 36.7-44.2 | a | 19.7 (5.2) | 14.2-25.1 | 30.5 (8.3) | 27.7-33.0 | 0.01 |
| 40-79 | 234.4 (49.7) | 214.3-254.5 | 0.01 | 181.8 (18.8) | 162.0-201.6 | 180.9 (34.5) | 170.4-191.4 | NS |
| 80+ | 126.6 (30.2) | 114.4-138.8 | NS | 112.7 (6.4) | 105.9-119.4 | 103.6 (19.9) | 97.5-109.6 | NS |
The male-to-female ratio remained approximately constant during the individual study periods. During the lockdown period, there was an increase of 3.4%, and during the subsequent post-lockdown period, there was a decrease of 4.8% (not significant; Table 2).
The lockdown period led to a significant decrease in epistaxis cases, especially among young people (0-39 years of age), with a reduction of 51% (P < 0.001). After the end of the restriction period, there was a clear and continuing statistically significant, increase in patient numbers by 50% (P < 0.01). In the age group of 40-79 years, there was also a significant decrease in the number of cases by 22% (P < 0.01) with the start of the lockdown period, which remained constant thereafter. Only in the group of the oldest patients (80+ years of age), a statistically constant weekly number of cases was observed. This number decreased by 11% at the beginning of the lockdown period and then by an additional 8% (Table 2, Figure 1A).
These observations are also consistent with the results of the ITS. Compared to the actual case numbers, the counterfactual for the lockdown resulted in a significantly higher number, which was also most pronounced in the youngest age group. With the end of the lockdown period, as described above, there was again an increase in patient numbers. Based on the estimated value from the counterfactual calculated from the lockdown period, there were also significant deviations from the actual case numbers during the post-lockdown period. Here too, the effect was strongest in the youngest age group (Table 3, Figure 2).
| Category | Lockdown | Post-lockdown | ||||
| Cases per week | 95%CI | Δpred-rep (%)a | Cases per week | 95%CI | Δpred-rep (%)b | |
| Total | ||||||
| Reported | 314.2 (20.6) | 292.5-335.8 | 315.0 (56.2) | 297.9-332.1 | ||
| Predicted | 364.7 | 152.8-576.9 | +13.9 | 253.3 | 214.9-291.7 | -24.5 |
| Female | ||||||
| Reported | 112.5 (8.1) | 113.9-131.0 | 126.7 (24.3) | 119.3-134.1 | ||
| Predicted | 126.3 | 67.5-185.1 | +12.3 | 123.0 | 102.0-143.0 | -3.0 |
| Male | ||||||
| Reported | 191.7 (18.7) | 172.1-211.2 | 188.3 (35.8) | 117.4-199.2 | ||
| Predicted | 242.0 | 131.0-352.0 | +26.3 | 139.1 | 100.0-178.1 | -35.4 |
| 0-39 | ||||||
| Reported | 19.7 (5.2) | 14.2-25.1 | 30.5 (8.3) | 27.7-33.0 | ||
| Predicted | 41.3 | 23.9-59.3 | +52.3 | 20.0 | 6.0-33.0 | -52.5 |
| 40-79 | ||||||
| Reported | 181.8 (18.8) | 162.0-201.6 | 180.9 (34.5) | 170.4-191.4 | ||
| Predicted | 212.1 | 92.3-332.5 | +14.3 | 127.4 | 89.4-165.1 | -42.0 |
| 80+ | ||||||
| Reported | 112.7 (6.4) | 105.9-119.4 | 103.6 (19.9) | 97.5-109.6 | ||
| Predicted | 127.0 | 64.0-189.0 | +11.3 | 113.0 | 86.0-129.0 | +8.3 |
This study aimed to investigate the influence of the nationwide Corona lockdown on hospital admissions pertaining to epistaxis. Therefore, in addition to the 6-wk lockdown, hospital admissions from 2019 to 2022 were also investigated nationwide. This was done over a total period of 18 wk per year to compensate for seasonal differences in the incidence of epistaxis. From our point of view, epistaxis is a clear and serious symptomatology, while other diseases with ambiguous symptoms such as stroke could be underestimated in terms of possible exposure to the coronavirus[35-38]. The decrease in patients with various diagnoses during the Corona lockdown has already been reported in numerous studies[39-44].
The majority of patients in this study were between 40 and 79 years old and male. This is consistent with previous publications that have associated older age and male gender with a higher incidence of epistaxis[4,45-47].
It was shown that the introduction of the COVID-19 lockdown was associated with a decrease in nationwide hospital admissions for epistaxis. This was not dependent on gender. However, a strongly significant decrease was observed in the youngest age group of 0-39 years. In the oldest age group of 79 years and older, there was no significant decrease in the number of patients. With the end of the COVID-19 lockdown 6 wk later, the numbers significantly increased again in the age group up to 39 years, while no significant changes were observed in patients aged 40 years and older.
Using ITS analysis, a clear association could be demonstrated both with the start and the end of the COVID-19 lockdown. The difference to the counterfactual was most pronounced in the youngest patient group, in line with the significance described above. It can be inferred from this that both the start and end of the COVID-19 lockdown had a direct influence on the number of hospital admissions.
The significant decrease in patients with epistaxis in the youngest age group could be due, on the one hand, to a greater fear of exposure to the coronavirus in this group. Another reason could be the less severe symptomatology on average in younger patients[48-50]. This is supported by the subsequent significant increase in patient numbers in this age group after the lockdown, which was also shown in the ITS and was associated with it.
First, the hospital data analyzed represent a comprehensive inquiry into inpatient admissions for epistaxis in Germany. However, no data on outpatient visits to emergency departments are available in the dataset. Second, adjustments for comorbidities, socio-economic factors, or place of residence were not feasible due to the absence of these patient-level data. Third, while coding issues might have arisen for different diagnoses, we determined that these were unlikely to be a significant confounding factor, given the size of the population.
Our study demonstrated that, based on actual case numbers and simulated calculations using ARIMA forecast along with ITS analysis, the pandemic-induced lockdown led to a direct reduction in hospitalizations, particularly among young patients with epistaxis, and an immediate surge in hospitalizations upon its termination. This could be attributed to concerns about COVID-19 exposure, unanticipated outcomes of public health advice to reduce non-urgent healthcare visits, or adherence to stay-at-home orders, aligning with findings from prior research[39-44].
Conversely, these measures did not lead to any change in older patients, which implies that at least in this age group, the symptoms of epistaxis should not be underestimated, even with regard to a possible exposure to the coronavirus.
These findings highlight how a nationwide intervention, such as the COVID-19 lockdown in this instance, can directly influence hospital admissions within specific segments of the population.
The COVID-19 pandemic has had a profound impact on global healthcare systems, leading many nations to enforce lockdowns that restrict movement and social interactions in an effort to curb virus transmission. Although effective against COVID-19, these measures have inadvertently affected healthcare delivery and hospital admissions. Numerous studies have noted a decline in hospital admissions during the lockdown, attributed to factors such as postponed elective surgeries, decreased Emergency Room visits, and lifestyle-related illness reductions. Despite the apparent benefits of reduced admissions, apprehensions arise over potential long-term health implications due to delayed diagnoses and treatments for non-COVID-19 conditions.
Concerns have arisen due to reports of reduced hospital admissions during the COVID-19 lockdown, suggesting potential delays or omissions in diagnosing and treating non-COVID-19-related illnesses. The decrease in hospital visits during this period has sparked worries about the impact on timely medical interventions. The lockdown's effect on hospital admissions has prompted discussions regarding possible disruptions to the identification and management of non-COVID-19-related medical conditions.
To examine how the COVID-19 pandemic-induced lockdown and its conclusion affected hospital admissions among patients with epistaxis in Germany.
Utilizing quasi-anonymous open-access data from Germany's Institute for the Hospital Remuneration System, this retrospective observational study analyzed hospital admissions for epistaxis, considering patient age and gender. The study covered February 1 to June 8 from 2019 to 2022, segmented into six-week periods, with a distinct lockdown group from March 15 to April 26, 2020. Statistical analysis employed Interrupted Time Series and AutoRegressive Integrated Moving Average models, presenting deviations from predicted values. Ethical approval was not necessary due to the absence of identifiable patient data, aligned with ethical guidelines.
In total, 26183 inpatient cases were analyzed, with a male-to-female ratio of 1.51. The 40-79-year-old group (n = 15145; 58%) had the most cases, followed by those over 80 years (n = 8526; 33%), and the smallest group was aged up to 39 years (n = 2512; 9%). Weekly admissions peaked in 2019 across age and gender groups, while the 2020 lockdown period saw the lowest weekly case numbers for those under 39 years (P < 0.001). In the age group of 40-79 years, 2019 had the highest case numbers (P < 0.001), remaining constant in subsequent years; those over 80 years showed consistent weekly case numbers. Fragmented into 12 time periods, weekly epistaxis cases significantly differed during the restriction (P < 0.001). There was a 22% decrease in cases during the lockdown (P < 0.05), followed by a slight increase of 0.3%, particularly affecting middle-aged males. Lockdown caused a notable 29% decrease in female cases (P < 0.01) and 21% in males, with an 11% post-lockdown increase in females and 2% decrease in males. The male-to-female ratio remained stable. Lockdown led to a significant 51% decrease in young patients (0-39 years, P < 0.001) and a subsequent 50% increase (P < 0.01), while the 40-79 age group had a 22% decrease (P < 0.01) and the oldest group remained constant. These trends were consistent with ITS results, showcasing the impact on different age groups.
Our study demonstrated that the pandemic-induced lockdown led to a direct decrease in hospitalizations, particularly among young patients with epistaxis. This was followed by a rapid increase after the lockdown was ended. Possible factors contributing to this trend include COVID-19-related fears, unintended consequences of healthcare recommendations, or stay-at-home orders – findings that align with previous research. Notably, older patients were not similarly affected, highlighting the importance of addressing epistaxis symptoms, even in the context of potential COVID-19 exposure. These results emphasize the significant impact of a nationwide intervention like the COVID-19 lockdown on hospital admissions in specific demographic groups.
Further and more comprehensive research based on larger datasets is necessary to obtain insights into lockdown-induced changes in hospital admissions for other diagnoses as well.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Otorhinolaryngology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Alsamhi SH, Ireland; Hariyanto TI, Indonesia S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Turowski B, Zanella FE. Interventional neuroradiology of the head and neck. Neuroimaging Clin N Am. 2003;13:619-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Christensen NP, Smith DS, Barnwell SL, Wax MK. Arterial embolization in the management of posterior epistaxis. Otolaryngol Head Neck Surg. 2005;133:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Andersen PJ, Kjeldsen AD, Nepper-Rasmussen J. Selective embolization in the treatment of intractable epistaxis. Acta Otolaryngol. 2005;125:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Pallin DJ, Chng YM, McKay MP, Emond JA, Pelletier AJ, Camargo CA Jr. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Tomkinson A, Roblin DG, Flanagan P, Quine SM, Backhouse S. Patterns of hospital attendance with epistaxis. Rhinology. 1997;35:129-131. [PubMed] |
| 6. | Villwock JA, Jones K. Recent trends in epistaxis management in the United States: 2008-2010. JAMA Otolaryngol Head Neck Surg. 2013;139:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Koh E, Frazzini VI, Kagetsu NJ. Epistaxis: vascular anatomy, origins, and endovascular treatment. AJR Am J Roentgenol. 2000;174:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Tan LK, Calhoun KH. Epistaxis. Med Clin North Am. 1999;83:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Cullen MM, Tami TA. Comparison of internal maxillary artery ligation versus embolization for refractory posterior epistaxis. Otolaryngol Head Neck Surg. 1998;118:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Pollice PA, Yoder MG. Epistaxis: a retrospective review of hospitalized patients. Otolaryngol Head Neck Surg. 1997;117:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 11. | Klotz DA, Winkle MR, Richmon J, Hengerer AS. Surgical management of posterior epistaxis: a changing paradigm. Laryngoscope. 2002;112:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Schaitkin B, Strauss M, Houck JR. Epistaxis: medical versus surgical therapy: a comparison of efficacy, complications, and economic considerations. Laryngoscope. 1987;97:1392-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Jindal G, Gemmete J, Gandhi D. Interventional neuroradiology applications in otolaryngology, head and neck surgery. Otolaryngol Clin North Am. 2012;45:1423-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Soyka MB, Nikolaou G, Rufibach K, Holzmann D. On the effectiveness of treatment options in epistaxis: an analysis of 678 interventions. Rhinology. 2011;49:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Moreau S, De Rugy MG, Babin E, Courtheoux P, Valdazo A. Supraselective embolization in intractable epistaxis: review of 45 cases. Laryngoscope. 1998;108:887-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Barlow DW, Deleyiannis WB, Pinczower EF. Effectiveness of surgical management of epistaxis at a tertiary care center. Laryngoscope. 1997;107:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Hussain MH, Mair M, Rea P. Epistaxis as a marker for severe acute respiratory syndrome coronavirus-2 status - a prospective study. J Laryngol Otol. 2020;134:717-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1729] [Cited by in RCA: 1736] [Article Influence: 347.2] [Reference Citation Analysis (0)] |
| 19. | Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2061] [Cited by in RCA: 2062] [Article Influence: 412.4] [Reference Citation Analysis (0)] |
| 20. | Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The Impact Of The COVID-19 Pandemic On Hospital Admissions In The United States. Health Aff (Millwood). 2020;39:2010-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 21. | Wang Q, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol. 2021;7:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 293] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 22. | Lippi G, Henry BM, Bovo C, Sanchis-Gomar F. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID-19). Diagnosis (Berl). 2020;7:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 23. | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1236] [Article Influence: 247.2] [Reference Citation Analysis (0)] |
| 24. | Ding YY, Ramakrishna S, Long AH, Phillips CA, Montiel-Esparza R, Diorio CJ, Bailey LC, Maude SL, Aplenc R, Batra V, Reilly AF, Rheingold SR, Lacayo NJ, Sakamoto KM, Hunger SP. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr Blood Cancer. 2020;67:e28427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Aggarwal A, Chakradar M, Bhatia MS, Kumar M, Stephan T, Gupta SK, Alsamhi SH, Al-Dois H. COVID-19 Risk Prediction for Diabetic Patients Using Fuzzy Inference System and Machine Learning Approaches. J Healthc Eng. 2022;2022:4096950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Kumar S, Chaube MK, Alsamhi SH, Gupta SK, Guizani M, Gravina R, Fortino G. A novel multimodal fusion framework for early diagnosis and accurate classification of COVID-19 patients using X-ray images and speech signal processing techniques. Comput Methods Programs Biomed. 2022;226:107109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Institut für das Entgeltsystem im Krankenhaus. InEK Daten-Browser—Unterjährige Datenlieferung DRG. Accessed February 20, 2023. Available from: https:// www.g-drg.de/Datenlieferung_gem._21_KHEntgG/InEK_DatenBrowser. |
| 28. | Purkey MR, Seeskin Z, Chandra R. Seasonal variation and predictors of epistaxis. Laryngoscope. 2014;124:2028-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Bray D, Giddings CE, Monnery P, Eze N, Lo S, Toma AG. Epistaxis: are temperature and seasonal variations true factors in incidence? J Laryngol Otol. 2005;119:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Manfredini R, Gallerani M, Portaluppi F. Seasonal variation in the occurrence of epistaxis. Am J Med. 2000;108:759-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 1160] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 32. | Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13:S38-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 762] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 33. | Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2271] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 34. | Miller R. Simultaneous statistical inference. 1981. NY: McGraw-Hill [Google Scholar]. 1967. [DOI] [Full Text] |
| 35. | Sheth K. Hospital admissions for strokes appear to have plummeted, a doctor says, a possible sign people are afraid to seek critical help. The Washington Post. Health & Science Perspective. 2020. [DOI] [Full Text] |
| 36. | Bres Bullrich M, Fridman S, Mandzia JL, Mai LM, Khaw A, Vargas Gonzalez JC, Bagur R, Sposato LA. COVID-19: Stroke Admissions, Emergency Department Visits, and Prevention Clinic Referrals. Can J Neurol Sci. 2020;47:693-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 37. | McManus M, Liebeskind DS. Blood Pressure in Acute Ischemic Stroke. J Clin Neurol. 2016;12:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Lange SJ, Ritchey MD, Goodman AB, Dias T, Twentyman E, Fuld J, Schieve LA, Imperatore G, Benoit SR, Kite-Powell A, Stein Z, Peacock G, Dowling NF, Briss PA, Hacker K, Gundlapalli AV, Yang Q. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions - United States, January-May 2020. Am J Transplant. 2020;20:2612-2617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 39. | Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2871-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 754] [Cited by in RCA: 908] [Article Influence: 181.6] [Reference Citation Analysis (0)] |
| 40. | Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N Engl J Med. 2020;383:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 501] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 41. | Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, Januzzi JL, Butler J, Adler DS, Solomon SD, Vaduganathan M. Fewer Hospitalizations for Acute Cardiovascular Conditions During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;76:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 42. | Gogia S, Newton-Dame R, Boudourakis L. Covid-19 X-Curves: Illness Hidden, Illness Deferred. [DOI] [Full Text] |
| 43. | Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 447] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 44. | Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral Effect of Covid-19 on Stroke Evaluation in the United States. N Engl J Med. 2020;383:400-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 45. | Smith J, Siddiq S, Dyer C, Rainsbury J, Kim D. Epistaxis in patients taking oral anticoagulant and antiplatelet medication: prospective cohort study. J Laryngol Otol. 2011;125:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Chaaban MR, Zhang D, Resto V, Goodwin JS. Factors influencing recurrent emergency department visits for epistaxis in the elderly. Auris Nasus Larynx. 2018;45:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Reis LR, Correia F, Castelhano L, Escada P. Epidemiology of epistaxis in the emergency department of a southern European tertiary care hospital. Acta Otorrinolaringol Esp (Engl Ed). 2018;69:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Dal Secchi MM, Indolfo MLP, Rabesquine MM, de Castro FB. Epistaxis: prevailing factors and treatment. Int Arch Otorhinolaryngol. 2009;13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Mangussi-Gomes J, Enout MJ, Castro TC, de Andrade JS, Penido NO, Kosugi EM. Is the occurrence of spontaneous epistaxis related to climatic variables? A retrospective clinical, epidemiological and meteorological study. Acta Otolaryngol. 2016;136:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Page C, Biet A, Liabeuf S, Strunski V, Fournier A. Serious spontaneous epistaxis and hypertension in hospitalized patients. Eur Arch Otorhinolaryngol. 2011;268:1749-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |