Published online Dec 20, 2023. doi: 10.5662/wjm.v13.i5.426
Peer-review started: October 17, 2023
First decision: November 9, 2023
Revised: November 21, 2023
Accepted: December 11, 2023
Article in press: December 11, 2023
Published online: December 20, 2023
Processing time: 63 Days and 20.3 Hours
The results of years of dental study serve as the foundation for the practise of medicine and, for that matter, dentistry. Doctors may have their own preferences for techniques and materials, but whether directly or indirectly, their decisions are influenced by systematic reviews and meta-analyses. However, due to poorly conducted or presented research, this very basic foundation may not be reliable. Bias in research is one of several factors that might make study results or research itself unreliable. Bias can be introduced into research at many stages, deliberately or unknowingly. Bias can appear at any point during the research process, even before the study itself begins. There are many biases in research, but some of them are more relevant to dentistry research than others. Because it is said that “eyes see what the mind knows”, it is essential to have a complete understanding of the different types of bias, how and when they get entrenched, and what steps may be taken to prevent or lessen them if they do occur. This comprehensive summary of bias in dentistry research is provided by this synoptic review. The goal is to identify gaps and measures that have been taken-or that should have been taken-by providing both descriptive and evaluative summaries, as well as examples from the literature, when needed.
Core Tip: Be it clinical or in-vitro, bias may arise at any point in the course of research. Always make efforts to minimise, if not completely eradicate, any potential bias that could show up in a study. However, how can a researcher take preventative or remedial actions if they are oblivious that bias is being introduced into their study? This article lists and summarises every potential bias that could arise during a study so that the researcher is aware of the possibilities and can take the necessary precautions to contribute reliable scientific data to the literature.
- Citation: Agrawal AA, Prakash N, Almagbol M, Alobaid M, Alqarni A, Altamni H. Synoptic review on existing and potential sources for bias in dental research methodology with methods on their prevention and remedies. World J Methodol 2023; 13(5): 426-438
- URL: https://www.wjgnet.com/2222-0682/full/v13/i5/426.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i5.426
The practice of medicine, or for that matter dentistry, is primarily based on outcomes generated from years of relevant research. Although doctors do have their own preference with regard to procedures and materials, the choice, either directly or indirectly, is based on systematic reviews and meta-analysis. However, this very fundamental base may not be reliable owing to improperly executed or improperly presented research. Of the various reasons that make the research outcome or the research itself unreliable, bias is an important aspect that is either knowingly or unknowingly introduced at various steps.
By definition, bias is any tendency that prevents unprejudiced consideration of a question[1]. Bias can occur at any phase of research, even before starting the study itself. Bias is not just a yes or no variable, and its interpretation cannot be restricted to whether bias is present. Instead, a critical reader should consider the efforts taken in a study to decrease the degree of bias and also critically evaluate the influence of the bias on the conclusion[2]. There is a long list of biases in research, but some of them are more applicable to dental research. The “eyes see what the mind knows”; hence, a thorough knowledge of types of biases, how and at which stage they are introduced, and the ways to avoid or reduce them are crucial for justifying the results of the research. Awareness of these biases is imperative to promote rigorous and unbiased dental research. Researchers, peer reviewers, journal editors, and funding agencies should collaborate to establish robust methodologies, transparent reporting standards, and effective peer-review processes to minimize the impact of bias and enhance the reliability and validity of dental research. Additionally, promoting open access to research findings and fostering a culture of transparency and reproducibility can ensure a comprehensive and unbiased body of dental literature.
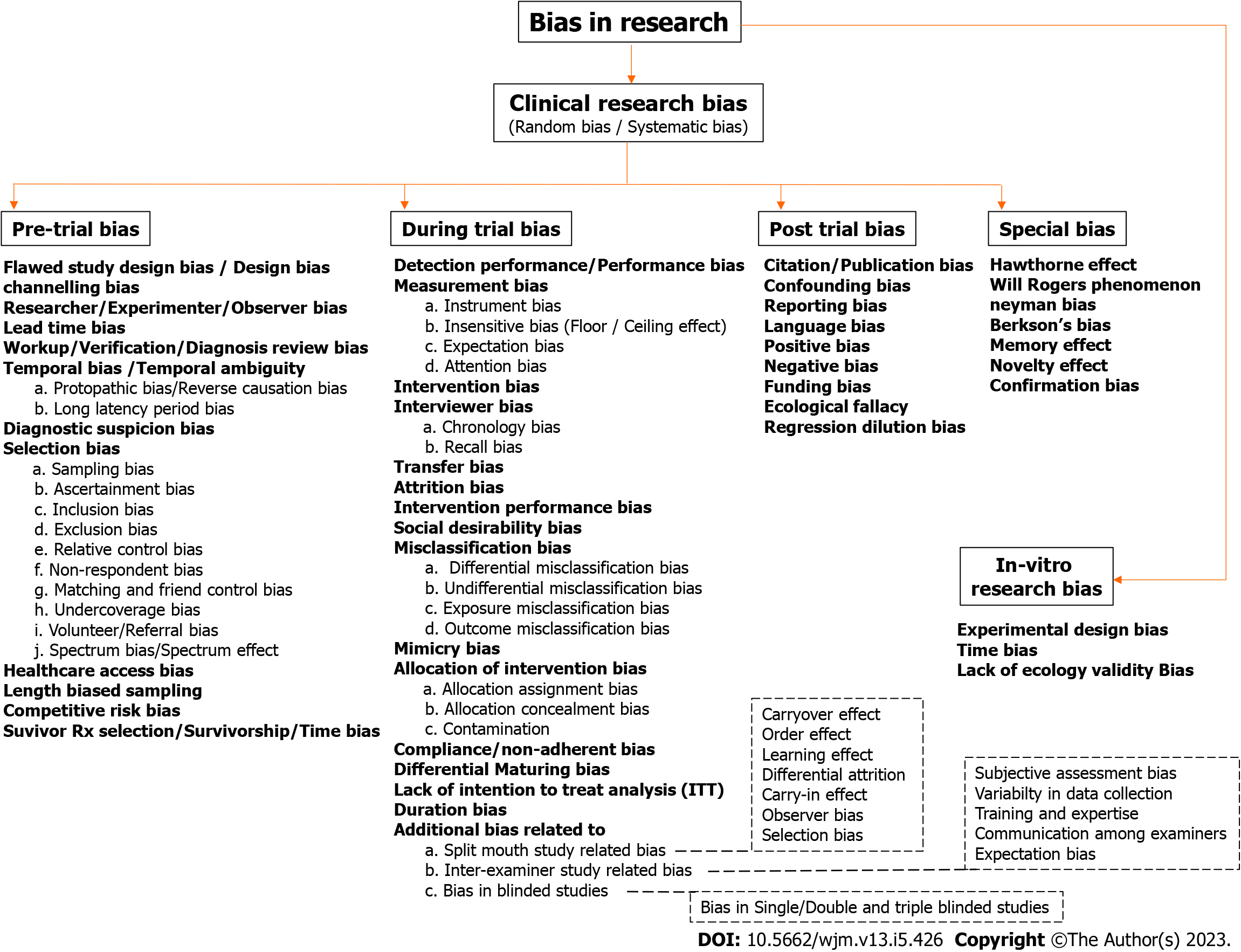
This synoptic review provides a concise but accurate overview of all materials related to bias in dental research. The aim is to provide both descriptive and evaluative summary along with examples from literature to identify gaps and measures taken or should have been taken. In dental research, like any other field, various types of biases may affect the validity and reliability of the results. A detailed and well-categorized flowchart listing all possible biases that can occur in clinical and in vitro research is depicted in Figure 1. A special emphasis is given to certain categories of biases, such as those related to split-mouth study, inter-examiner studies, and blinded studies. Finally, a brief description of various biases that are difficult to categorize under a single category are mentioned in detail under the special bias category. The following detailed discussion about various biases adheres to the headings and subheadings with reference to Figure 1 for ease of understanding and clarification.
It refers to the unavoidable fluctuation in data that results from chance. There is no way to completely prevent or control this type of inaccuracy. Measurement precision and accuracy, as well as the outcomes of statistical analysis, can be impacted by random error. To lessen the effects of random bias, researchers often use statistical techniques and large sample sizes. In addition, if investigators are themselves involved in sample collection, it would further reduce the chances of random bias.
It is a type of prejudice that is present throughout the planning, execution, or analysis of research investigations. This bias is systematic or persistent, which means that it does not result from chance but rather from certain defects or problems in the study process. Systematic bias has an enormous effect on the reliability and validity of research findings. Typical examples of systemic bias in research include selection bias, sampling bias, measurement bias, and funding bias. The various types of systemic bias have been discussed under different subheadings along with the measures to be taken to avoid or at least reduce them, wherever relevant.
The pretrial stage ranges from selecting a topic for the research to literature search, preparing a research proposal, and designing a study and even screening tests and patient recruitment. Bias at the stage of study design and patient recruitment can cause fatal flaws in the resultant data, which are almost impossible to compensate during analysis. The following is the list of possible biases that can occur in the pretrial phase of a research.
It refers to a type of systematic bias that arises from errors or inadequacies in the planning, structure, or methodology of a research study. This bias can significantly impact the validity and reliability of the findings as it introduces systematic errors that are not due to random chance. Almost none of the studies with this bias get published in reputed journals as they get rejected either at the editor or reviewer level.
As the name suggests, channeling bias occurs at the stage when patients are channeled to a specific group intentionally. This bias occurs in nonrandomized trials wherein patients’ prognostic factors and degree of illness determine the study cohort in which they are placed[4].
It occurs when researchers’ personal beliefs, expectations, or preferences influence the study design, data collection, analysis, or interpretation. This bias can introduce systematic errors and affect the objectivity and integrity of dental research.
It occurs when a new diagnostic test or screening method is introduced, which leads to the early detection of a disease without improving the patient’s overall health or survival. As the new diagnostic/screening test is more sensitive, the disease is detected earlier. The patients appear to have a longer survival time from the time of diagnosis compared with those diagnosed later using the older methods. This time difference creates the illusion that the new test or method improves patient outcomes.
It is a potential source of bias in diagnostic studies. Study participants may be subjected to different levels of diagnostic work-up based on their initial test results or other factors. For example, individuals with positive initial test results may receive additional more definitive tests or clinical evaluations, whereas those with negative results may not undergo further assessment. Those selected for verification may not be representative of the entire study population, and this differential verification process can lead to an overestimation or underestimation of the test accuracy. These issues can result in biased estimates of the test sensitivity, specificity, positive predictive value, and negative predictive value.
It refers to a situation wherein it is unclear or ambiguous whether an exposure (such as a treatment or risk factor) occurred before or after the outcome of interest (such as a disease or health event). This ambiguity can introduce a bias and make it challenging to establish a causal relationship between the exposure and the outcome. In retrospective studies, if the timings of the exposure and outcome are not clearly established, it can lead to temporal ambiguity. Even participants or medical records may not provide accurate or detailed information about the exposure and outcome timings, thus making it difficult to determine which occurred first.
Protopathic bias: Also known as reverse causation bias, it occurs in certain epidemiological studies when the symptoms or early manifestations of a disease/condition may lead individuals to modify their behavior or seek medical attention. This behavioral change or medical intervention may include exposure to a particular treatment, medication, or lifestyle modification. Researchers may mistakenly attribute the exposure (e.g., a specific treatment or medication) to be the cause of the outcome (e.g., the development of the disease) when, in fact, the exposure occurred after the initial symptoms or early signs of the disease developed. For example, in a hypothetical study, an association is sought between amoxicillin 500 mg administered to patients experiencing fever (cause) and gastric upset (outcome as a side effect). Some patients having fever may also have undiagnosed or yet-to-manifest gastric upset. After taking amoxicillin for fever, if gastric upset occurs, although it was already present subclinically and only manifested later on, it would be assumed that the drug caused the side effect.
Long latency period bias: Some diseases or health events have long latency periods, meaning there is a substantial delay between the exposure to a risk factor and the development of the outcome. During this extended period, other factors or interventions may come into play, adding complexity to the temporal relationship.
Healthcare providers or researchers might have preconceived beliefs or suspicions about the likelihood of a particular diagnosis based on the patient’s symptoms, medical history, or other factors. These prior beliefs can influence the decisions made during the diagnostic process. Healthcare providers may be more likely to order specific tests related to the suspected diagnosis and may interpret the results with a bias toward confirming that diagnosis.
It occurs during the identification of a study population wherein participants or subjects are not randomly selected, resulting in a nonrepresentative sample. For instance, if certain demographic groups are overrepresented or underrepresented in the study, it can affect the generalizability of the findings. Selection bias arises when participants or study subjects are not adequately representative of the target population. This bias can occur because of inappropriate sampling methods or exclusion criteria, which may limit the generalizability of the study findings to the broader dental patient population. This type of bias is commonly observed in case–control and retrospective cohort studies wherein exposure and outcome would have already occurred at the time of selection[5]. This bias can be reduced by incorporating strict randomization and blinding with best possible efforts. Allocation concealment should be carefully designed and also reported at the time of publishing the findings.
Sampling bias: It is a type of selection bias wherein the sample is not representative of the target population. For example, if the study only includes patients from a specific dental clinic and not from other clinics in the area, the findings may not reflect the characteristics of the broader population.
Ascertainment bias: This bias arises as a result of disproportionate statement of the eligible population. Here, the category of patients examined does not correspond to the incidents in the population.
Inclusion bias: Inclusion bias occurs when study participants are selected to represent the research population, and groups with different experiences are ignored. This bias is especially common in quantitative research. For example, an online oral health survey is supposed to be conducted using available internet sources. One may choose a study group based on possible confounding factors but might skip the fact that some people have access to the internet while others do not.
Exclusion bias: Exclusion bias occurs when some subgroups are intentionally excluded from the sample population before randomizing them into groups. For example, in a periodontal surgery in which patients should be followed up for 4-6 mo, intentionally excluding those patients who stay very far from the research site to avoid later dropouts can result in exclusion bias.
Relative control bias: This type of bias occurs when patients’ relatives are included in the study as a control group. This inclusion may or may not have an effect on the actual outcome of the study depending on the parameters to be checked. If it is a placebo-controlled trial, patients and their relatives can easily discuss the intervention received by them and might use each other’s medicines.
Nonrespondent bias: This type of bias usually occurs in a large-scale survey, prospective study, or longitudinal study. Many participants may not respond to a survey; however, their actual opinion might be significantly different and would have affected the results of the study if all of them had responded. Maintaining a patient record (email ID/phone number/address) might help in reaching out to non-responders and reducing this bias.
Matching and friend control bias: Friend controls are useful because researchers can quickly locate them via the instances. Friends are expected to be more motivated and have higher response rates than general population controls who are unaware of the case. Introverted people will appear on fewer or even no friend rosters, and sociable people will appear on more of those rosters. Extroverts are therefore more prone than introverts to develop into friend controllers. Based on the degree to which the exposures of interest are linked to sociability or personality, selection bias will alter the results[6].
Under-coverage bias: Under-coverage bias arises when a representative sample is drawn from a small proportion of the target population. Online surveys are especially vulnerable to under-coverage bias. For instance, if a survey on oral precancerous lesions is planned and the researcher includes patients visiting a dental hospital as the study population, the vast majority of people who may be at a greater risk but not motivated enough to visit a hospital will be left out. The results of such research cannot be reliably applied to the wider population.
Volunteer/referral bias: Part of the study population who either volunteered for the study or were referred for the specific disease would logically be more motivated for the treatment and follow-ups than non-volunteers. The results of such a study would have very low external validity. For instance, in a study on the prevalence of oral precancerous lesions in young adults, if the study population comprises patients visiting/referred to a dental hospital for consultation, the results would not apply to the general population.
Spectrum bias: Also known as spectrum effect or spectrum of disease bias, it is a type of bias in clinical research and diagnostic testing. This bias occurs when the characteristics of the study population do not accurately represent the broader population of individuals who may be affected by the condition of interest. In other words, the study sample does not adequately capture the entire range or spectrum of disease severity or characteristics seen in the real-world population.
It describes a situation in which the accessibility and availability of healthcare services and resources might have an impact on the demographic characteristics or results of the study. This bias can appear when specific groups of people have unequal access to healthcare services, thereby resulting in the unequal representation of those groups in research studies or in divergent health outcomes.
It occurs when the selection of study participants is more likely to include individuals who have experienced a longer duration of disease or exposure. This bias can occur because individuals with longer durations of disease/exposure are more likely to be detected or referred for testing. This bias can result in the overestimation of the sensitivity of a diagnostic test because it is more likely to identify cases in later stages of the disease, which are easier to detect. Conversely, it may result in the underestimation of specificity because individuals with shorter disease durations or milder conditions may not be included.
Competitive risk may cause bias in epidemiology and survival studies. This bias occurs when researchers study time-to-event data without properly considering competing hazards. These are circumstances that could prevent the relevant event from occurring or alter the likelihood of its occurrence. To prevent skewed findings and incorrect conclusions, competitive risk should be considered in the study. Other occurrences that could precede or compete with the main event are referred to as competitive risks. For instance, while evaluating cancer-specific mortality in a cancer study, death from causes unrelated to the disease is regarded as a competitive risk.
A type of bias that can occur in observational studies, particularly in those assessing the efficacy of therapies or interventions, is the survivorship bias or immortal time bias. The bias in survivor treatment selection occurs when individuals who suffer the relevant event (such as death) soon after the start date of the study or before they are eligible to receive the therapy are not included in the analysis. Short-term non-survivors are not included in the study because only people who have survived long enough to receive the treatment are included, which can give the impression that the treatment is more beneficial than it actually is. This bias may cause the advantages of the treatment to be overestimated. The phrase “immortal time” describes the timeframe during which people are regarded as “immortal” because they have not yet gone through the event (such as death) or have not yet received the therapy of interest.
This type of bias occurs when the measurement or assessment of outcomes is influenced by the researcher’s knowledge of the participants’ exposure status. This bias happens when there are differences in the care or treatment provided to study groups owing to the knowledge or expectations of healthcare providers or participants. For example, in a clinical trial assessing the effectiveness of a new drug, if the healthcare providers are aware of the treatment assignment (e.g., via differences in appearance or side effects), they may provide varying levels of care to participants in the treatment and control groups, thereby leading to performance bias.
Instrument bias: In many research investigations, data are gathered with the aid of measurements tools, such as questionnaires, scales, sensors, lab equipment, and diagnostic testing. These tools measure or evaluate specific variables of interest. When an instrument consistently overestimates or underestimates the true value of a variable being measured, bias is introduced, which produces unreliable results. The proper selection of a good-quality instrument can reduce this bias.
Insensitive measure bias: Also known as “floor effect” or “ceiling effect”, it is a type of measurement bias that can occur in research when a measurement instrument or scale is not sensitive enough to capture the complete range of values or responses within a sample. This bias can lead to inaccurate or incomplete data, thereby affecting the validity and reliability of the study findings. “Floor effect” occurs when a measurement instrument is unable to detect or distinguish values at the lower end of the range. Hence, a substantial portion of participants score at the lowest possible value, creating a “floor” for the data. Conversely, the “ceiling effect” happens when the instrument cannot differentiate values at the upper end of the range. Several participants achieve the highest possible scores, creating a “ceiling” for the data.
Expectation bias: Also known as observer-expectancy bias or experimenter-expectancy effect, it is a type of cognitive bias that can impact the outcomes of studies, particularly experimental and observational research. This bias occurs when researchers, either knowingly or unknowingly, have certain expectations about the results of the study, which can influence their observations and potentially affect participant behavior or data collection. In a study by Nevins et al[7], the questionnaire given to the test group was forwarding or positive-answer provoking, which could have obvious answers.
Attention bias: It is type of cognitive bias arising from selectively focusing on certain stimuli or information while ignoring or downplaying others. Individual cognitive processes and experiences may contribute to this bias. Attention bias can influence how participants perceive and react to intervention or stimuli in clinical research. For instance, owing to ingrained beliefs, concerns, or expectations, participants may pay more attention to specific symptoms, treatments, or results. Participants’ reports of their experiences may be impacted by attention bias, which could result in inaccurate self-reporting.
Interviewer bias: It refers to the systematic difference in the manner in which information is solicited, recorded, and interpreted[5,8]. This bias can be minimized or eliminated if interviewers are blinded to the outcome of interest or if the outcome of interest has not yet occurred, as in prospective studies.
Chronology bias: Comparing the intervention with historic findings creates chronology bias. Historic controls are past data rather than present data. Hence, comparing current interventions with such data may affect the results because the two data are collected in different timeframes. Over the years, the methods of diagnosis, investigations, and treatment change, which might improve the results and decrease the rate of complications. For example, a study to identify the prevalence of the MB2 canal (mesio-buccal canal) in upper first molar using cone beam computed tomography (CBCT) and comparing the results with data published decades earlier when CBCT was not available will yield biased results. Such bias can be minimized by conducting prospective studies, or if retrospective, the data should be compared only with findings in the recent past.
Recall bias: In studies that rely on participants’ memory or self-reporting, there is a risk of recall bias. Participants may not accurately recall past dental experience or behaviors, thus leading to inaccurate data. This bias occurs when participants in a study inaccurately remember or report past events or experiences. In dental research, this bias can affect studies that rely on self-reported data, such as surveys or questionnaires, leading to distorted associations between exposures and outcomes. The simplest way to reduce this bias is to frame questions in such a way that it is easy for participants to recall and/or questions from the recent past.
Attrition bias: Also known as dropout bias or nonresponsive bias, it is a type of bias than can occur in research studies when there is a differential loss of participants or data during the course of the study. This bias arises when the characteristics of individuals who dropout or are lost to follow-up are different from those who remain in the study. This can distort the findings and compromise the validity of the results. Attrition bias can manifest in various types of research, including longitudinal studies, clinical trials, surveys, and observational studies. Some common reasons for attrition include participant withdrawal, loss to follow-up, nonresponse to surveys, and incomplete data collection.
Intervention performance bias: It arises when there are differences in the care or treatments provided to different study groups, which can affect the outcomes. For example, if one group receives better dental care than the other, it could influence the results. Similarly, if a particular treatment in a study, such as periodontal surgery or disimpaction, is performed by two or three different doctors, it can result in bias. The variations in the performance of different clinicians might affect the outcomes.
Social desirability bias: Social desirability bias is a form of response bias that is related to systematic errors in the way participants respond to survey questions, interviews, or assessments. To win others’ praise or to escape criticism from others, people may deliberately or unconsciously alter their responses to conform to cultural standards, values, or expectations. The responses may not accurately reflect the participants’ actual experiences or behaviors owing to social desirability bias. This bias might have an impact on survey results, patient-reported outcomes, self-reported data, etc. Aggarwal et al[9], in their study on an oral questionnaire about tobacco smoking status, validated the social desirability bias by identifying that a whopping 30% of the participants underreported their smoking status.
Differential misclassification bias: Misclassification bias occurs when the exposure or outcome status of the study participants is incorrectly classified. This can happen for various reasons, including measurement errors, inaccurate data collection methods, or imperfect diagnostic tests. Differential misclassification occurs when the probability of misclassification differs between comparison groups in a systematic manner. In this scenario, the bias introduced can lead to incorrect associations that favor one group over another. This type of bias can either exaggerate or attenuate the true effect.
Nondifferential misclassification bias: This bias occurs when misclassification occurs randomly or with equal probability in all study groups. In such instances, the bias introduced may be toward the null (i.e., it tends to make associations appear weaker than they truly are), but it does not systematically favor one group over another.
Exposure misclassification bias: It occurs if the exposure itself is poorly defined or if proxies of exposure are utilized.
Outcome misclassification bias: Here, misclassification of the outcome can occur if nonobjective measures are used to define the outcome. For example, using change in color of the gingival margin to determine the presence of gingivitis instead of a more objective method, such as gingival index or sulcular bleeding index.
It occurs during trial when the investigator is examining how exposures are related to a disease. It is important to ensure that the outcome being investigated is the true disease and not a condition mimicking the disease, which could lead to false conclusions about the causes of the disease of interest.
It typically refers to a type of bias that can occur during the process of assigning the study participants to different intervention groups in experimental research settings. This bias, if not properly managed, can undermine the validity of the study findings and often arises in randomized controlled trials (RCTs) and other experimental designs.
Allocation assignment bias: This occurs when the investigator either consciously or unconsciously assigns certain participants to a specific intervention group based on their characteristics or other factors. For example, if participants who are more likely to benefit from the intervention are placed in the treatment group and those less likely to benefit in the control group, it introduces selection bias.
Allocation concealment bias: This type of bias occurs when the process of randomization itself is not adequately concealed from the researchers or study staff who are responsible for assigning participants to groups. If researchers are aware of upcoming assignments, they may either inadvertently or intentionally influence the allocation process.
Contamination bias: This bias occurs when participants in different intervention groups interact or share information with each other, leading to the exchange of elements of the interventions. This exchange can blur the distinction between groups and undermine the study’s ability to determine the true effects of the interventions.
The degree to which study participants follow the recommended therapy or intervention protocols is referred to as compliance. For instance, participants in clinical trials might be told to take a certain drug, adhere to a certain diet, or make a certain behavioral change. When the participants do not adhere to these guidelines diligently, noncompliance happens. When there are systematically different compliance levels across treatment groups (such as intervention groups) and control groups (such as placebo groups) or between subgroups within the same study group, compliance bias is present. Such discrepancies in compliance may skew the study findings.
This bias is observed in group randomized trials and reflects uneven secular (long-term) trends among the groups in the trial, favoring one condition over another[10].
Clinical trials and other intervention studies sometimes employ intention to treat (ITT) analysis, wherein all participants are evaluated according to their initial treatment assignment regardless of what transpired later, such as noncompliance, dropouts, or protocol violations. ITT analysis ensures that the groups being compared remain comparable even if some participants did not adhere to their assigned therapy, thus maintaining the advantages of randomization. In the absence of ITT analysis, the research may be systematically different from those who discontinue or do not fully adhere to the treatment, thereby resulting in exaggerated or underexaggerated treatment effects that could mislead clinical or policy decisions.
The length of the study or follow-up period is a crucial factor in clinical research. This period should be selected in accordance with the study question and the desired results. Long-term effects or late-occurring events may not be captured if the study duration is too short. On the contrary, very protracted follow-up times can be expensive and may result in participant attrition, making it difficult to sustain the validity of the study. These issues cause duration bias in either situation. Additional bias related to: Split-mouth study-related bias, inter-examiner-related study bias, bias in blinded studies, questionnaire/survey-related bias.
The split-mouth study design is a type of within-subject design often used in clinical research, especially in dentistry and some medical fields. In this design, each subject serves as their control, with one side of the mouth (e.g., left side) receiving one treatment or intervention and the other side (e.g., right side) receiving a different treatment or intervention. Some potential biases related to the split-mouth study design are as follows: (1) Carryover effect: There may be a carryover effect from one treatment to the other, particularly if there is a washout period between interventions. This effect could impact the results of the study as the treatments may not be entirely independent of each other; (2) Order effect: The order in which the treatments are administered can introduce bias. For example, if one treatment is more effective, it might influence the response to the subsequent treatment on the other side of the mouth; (3) Learning effect: Participants may become more accustomed to the study procedures or interventions over time, resulting in different responses for later treatments compared with earlier ones; (4) Differential attrition: There might be differences in dropout rates between the two sides of the mouth, which could introduce bias in the analysis; (5) Carry-in effect: The baseline characteristics of the two sides of the mouth may not be entirely equal, leading to potential confounding; (6) Observer bias: If the outcome measures are assessed subjectively or by different observers, biases may arise because of variations in interpretation or assessment; and (7) Selection bias: The choice of the split-mouth design could introduce bias if certain participants are selected for the study based on specific criteria or characteristics.
To mitigate these biases, researchers employing a split-mouth study design should carefully plan the study, randomize the order of treatments, and ensure standardized and blinded assessments of outcomes. Furthermore, it is essential to consider the potential for bias when interpreting the results of such studies and to be cautious while making generalizations beyond the study population.
Also known as inter-rater or inter-observer bias, it refers to a type of bias that can occur in research studies when different examiners or observers assess the same subjects or data points differently. This bias can influence the results and conclusions of the study, potentially leading to inaccurate or misleading findings. Understanding and addressing this bias is important to ensure the validity and reliability of research outcomes. Some common factors contributing to inter-examiner bias are as follows: (1) Subjectivity in assessments: When examiners employ subjective criteria for evaluating subjects or data, their interpretations and judgments may vary. For instance, in dental research, different doctors may diagnose the same condition differently based on their experiences and personal biases; (2) Variability in data collection: Different examiners may use slightly different methods or instruments to collect data, leading to inconsistencies in the collected data. This variation can affect the reliability of the results; (3) Training and expertise: The level of training and expertise may differ among examiners, leading to differences in their ability to interpret and analyze the data accurately; (4) Communication among examiners: If there is a lack of standardized communication or guidelines for examiners, they may inadvertently influence each other’s judgments, leading to biased outcomes; and (5) Expectation bias: Examiners might be influenced by their expectations or prior beliefs about the study outcomes, consciously or unconsciously affecting their observations and assessments.
Several strategies can be employed to mitigate inter-examiner bias and enhance the reliability of the findings. Implementing clear and standardized protocols for data collection and assessment can reduce variability between examiners. Inter-rater reliability can be calculated to assess the level of agreement among examiners, which can help identify potential bias and guide improvements in the assessment process. Moreover, consistent training can be provided to all examiners to ensure that they have a shared understanding of the assessment criteria and methodologies. In some cases, blinding the examiners to certain aspects of the study (e.g., treatment groups) can help prevent their expectations from influencing the results. In addition, having multiple independent examiners evaluate the same subjects or data can help identify discrepancies and potential biases. A review process can be implemented in which an experienced and impartial researcher checks and validates the assessments of different examiners. By recognizing the potential for inter-examiner bias and taking steps to minimize its impact, researchers can enhance the credibility and accuracy of their findings. One of the best examples of measures taken to reduce inter-examiner bias has been presented in our previous study (Agrawal[11], 2011) on the reliability and reproducibility of a sign grading system for plaque and calculus.
Bias in research can occur in various forms, and single, double, or triple-blind studies are not entirely immune to these biases. Although these study designs are adopted to reduce certain types of biases, they may still be affected by other sources of bias. The ways in which each study design can be impacted have been discussed below: (1) Single-blind studies: In a single-blind study, the participants are unaware of their group assignment (e.g., treatment or control), but the researchers are aware of the groups. Bias can still arise in this design if the researchers inadvertently influence the participants’ behavior or the study outcome. For example, unintentional cues or communication from researchers to participants may subtly affect the way in which participants respond or behave during the study; (2) Double-blind studies: In a double-blind study, both the participants and the researchers directly involved in the study are unaware of the group assignment. This design helps reduce potential bias caused by participants’ expectations or researchers’ conscious or unconscious influence. However, bias can still occur if those administering the treatments or interventions (e.g., nurses or doctors) are aware of the group assignment and unconsciously treat participants differently based on that knowledge; and (3) Triple-blind studies: Triple-blind studies go one step further by also keeping the data analysts or statisticians blinded to the group assignment. This design aims to minimize bias during data analysis. However, researchers might still inadvertently introduce bias during the conduct of the study even if the analysts remain blinded to the groups.
Bias in research after the conclusion of the trial could occur during data analysis or publication or interpretation of the results. Some possible biases that can occur in the post-trail stage are discussed below.
This bias occurs when studies with positive or significant results are more likely to be published and those with null or negative findings are less likely to be published. In dental journals, 82% of the published articles report positive results[12]. This bias can create an overrepresentation of certain results and lead to an inaccurate overall picture. It occurs when research findings are selectively published based on their statistical significance or favoring positive outcomes. This bias can lead to an overrepresentation of studies with favorable results, potentially distorting the overall understanding of the effectiveness or safety of dental interventions. Despite the measures adopted by the International Committee of Medical Journal Editors[13,14], citation bias continues to exist. Although centralized documentation of all trials provides information about unpublished trials, the results of such studies can only be speculated. Locating unpublished studies via trial registers, search engines, and other databases can reduce this bias.
It occurs when the observed association between variables are attributed to three possible causes: The exposure itself, the outcome of interest, and an independent factor[5]. One such example is the association between obesity and dental caries (confounded by frequent eating or a high-sugar diet). After the completion of the study, the identified confounders can be controlled by analyzing the association only in those cohorts that are similar in terms of the identified confounding factors. Stratified analysis or multivariable regression analysis can be used to control the identified confounders; however, the role of unidentified confounders cannot be controlled. Unknown confounders can, however, be controlled with randomization.
It involves the selective reporting of outcomes within a study. Researchers may emphasize certain findings while downplaying or omitting others, which can skew the overall conclusions.
This type of bias may occur when research studies published in a particular language are favored over those published in other languages, resulting in an incomplete representation of the available evidence.
The value, effect, or data observed are greater than the actual or casual data, which is away from null. Here, the perceived value is closer to 1.0 than the actual value.
In this case, the value, effect, or data observed are less than the actual/casual data and can be termed null. The perceived value is lower to 1.0 than the actual value.
If the research is funded by a specific dental product manufacturer or entity with vested interests, there might be a tendency to favorably report outcomes related to their products. The bias refers to the potential influence of financial or nonfinancial conflicts of interest on the study outcomes. Dental research studies funded by industry sources might have a higher likelihood of reporting results favorable to the sponsor’s products or interests, which can compromise the impartiality and independence of the research.
Data are collected and analyzed at the population or group level in some investigations. For instance, researchers may examine health results, risk factor exposure, or other aspects for entire populations or geographical areas. When researchers base their conclusions or judgements about individuals within such groups only on the observed group-level data, they are committing an ecological fallacy. Problems can arise as individual-level traits or connections might be different from those observed in groups.
When the values of the independent variable (exposure) are measured incorrectly, measurement error arises. This inaccuracy may be the result of inaccurate data acquisition techniques, tools, or participant self-reporting. When performing regression analysis, measurement error in the exposure variable tends to weaken the relationship between the exposure and the result. Thus, the expected impact of exposure on the result is less significant than the actual impact. Regression dilution bias can cause researchers to draw wrong conclusions about how strong and important a relationship between two variables is. They might overestimate the genuine effect of an exposure on the result, which affect policy, clinical practice, or public health.
The Hawthorne effect is a psychological phenomenon that can significantly influence research outcomes. This effect occurs when participants in a study modify their behavior or performance simply because they are aware of being observed or receiving special attention. In an RCT evaluating a novel manual toothbrush design for orthodontic patients, the methodology necessitated that patients brush in front of the investigator[15]. This could have led to the Hawthorne effect as the performance of patients in home brushing may not be same as that in brushing in front of the dentist.
The Hawthorne effect can significantly influence research outcomes in various fields. For instance: (1) Bias in participant responses: Participants may alter their responses so that these are aligned with what they believe the researchers desire rather than providing genuine or accurate information; (2) Enhanced performance: Participants might perform better than they typically would because of the extra attention received during the study, leading to inflated results; (3) Social desirability bias: Participants may alter their behavior to appear more socially acceptable or desirable, resulting in responses that do not accurately reflect their usual behavior; and (4) Temporary changes: The effect is often short-lived, and once the observation or special treatment ends, participants may revert to their typical behavior.
Of these, the naturalistic observation strategy might be useful in mitigating the Hawthorne effect. It means that researchers can conduct observations unobtrusively without the participants being aware of it, thereby enhancing the authenticity of the results.
In clinical research and epidemiology, a shift in the staging or classification of people when a new diagnostic test or set of criteria is introduced is referred to as the “Will Rogers phenomenon”, after the American comedian Will Rogers. This modification may appear to improve the outcomes or survival rates for some populations, but there may be no real change in the severity or prognosis of the disease. In essence, this phenomenon illustrates the notion that “moving people across groups can improve the image of both groups”. An interesting study in this regard was published by Oshman et al[16] in which similar patients were presented at two different timepoints to a group of specialists. When the patients’ age was added at one timepoint, the agreement between the diagnosis changed significantly.
Neyman bias, often referred to as “incidence–prevalence bias” or “Neyman’s bias”, is a statistical phenomenon that can affect how epidemiological or clinical research findings are interpreted, especially in cross-sectional and observational studies. This bias, which bears Jerzy Neyman’s name, occurs when the incidence rate of a condition or disease is incorrectly estimated based on its prevalence in the studied population.
This bias develops when researchers estimate the incidence rate of a disorder based on its prevalence, particularly when the length of the condition differs among people. This approach can lead to problems because prevalence is affected by the frequency of new cases as well as the length of the illness in those patients in whom the illness is already present.
Individuals with a prolonged disease duration are more likely to be counted in the prevalent cases, which results in an overestimation of the incidence rate. In contrast, individuals with a shorter disease duration are less likely to be included, which causes the incidence rate to be underestimated.
A statistical phenomenon known as Berkson’s bias, often referred to as Berkson’s paradox or Berkson’s fallacy, can affect how clinical research findings are interpreted, particularly in studies conducted in hospitals or using data from hospitals. When the study subjects are selected from a hospital or clinical context, Berkson’s bias occurs. Owing to the fact that they are seeking medical attention or treatment, participants in these studies often have a higher risk of suffering from one or more health issues. Therefore, the correlation between risk factors and diseases may be inflated. In other words, simply because people who have the condition are more likely to be in a hospital or clinical setting, certain risk variables may seem to be more strongly connected with the disease. In a retrospective study by Jain[17], the author concluded that mandibular molars were the most frequently treated posterior teeth in both sexes and that women constituted the majority of those receiving posterior root canal therapy. However, the data were obtained from patients who reported to their college, and hence, the conclusion may not be applicable to the general population. In the discussion section, the authors compared their findings with a previous study that also had Berkson’s bias. The study by Al-Negrish[18] aimed to determine the incidence and distribution of root canal treatment in a dentition of Jordanian population, but the study sample was from the department of dentistry of a hospital in Jordan.
Also known as recall bias or retrospective bias, it is a prevalent form of bias in research studies that can significantly impact the validity and reliability of the findings. This bias occurs when participants’ memories of past events are flawed or selectively recalled, leading to inaccurate or distorted data.
In research, the memory effect can arise in various scenarios: (1) Retrospective studies: Research designs that rely on participants’ recollection of past experiences, habits, or exposures are particularly vulnerable to the memory effect. Participants may inadvertently misremember or exaggerate certain events or behaviors, which can skew the results; (2) Surveys and questionnaires: When individuals are asked to report past events via surveys or questionnaires, their ability to accurately recall specific details can be influenced by various factors. These factors include the emotional impact of an event, the passage of time, or even external influences, such as media coverage or personal beliefs; (3) Longitudinal studies: The memory effect can occur even in longitudinal studies that track participants over an extended period. Participants might remember events differently or become influenced by subsequent experiences, leading to alterations in their responses; (4) Case–control studies: In case–control studies in which researchers compare individuals with a specific condition to those without it, recall bias can be especially problematic. Patients may be more likely to recall specific exposures or events owing to their current condition, leading to an overestimation of the association between the condition and the exposure; and (5) Self-reported data: Any study relying heavily on self-reported data is susceptible to the memory effect. People may unintentionally misrepresent their experiences or provide socially desirable responses, leading to biased conclusions.
Novelty-related bias refers to the tendency of individuals, organizations, and societies to place greater emphasis and value on novel information, ideas, products, or experiences than on established or conventional ones. This bias can manifest in various aspects of human behaviors, decision-making processes, and cultural preferences. While embracing novelty can pave the way for progress and positive change, an excessive bias toward it has certain drawbacks. In academia and scientific research, there can be a pressure to publish novel findings, which might lead to a focus on flashy but potentially less rigorous or significant studies. As for the actual clinical research studies, evaluation of a novel item (e.g., a fancy toothbrush design or a noncommercially available mouthwash) can also introduce a bias in that the participants use the novel product more seriously and without skipping the recommended dose or duration or both. Over the weeks, as the novelty effect fades off and opinions of clinicians might. In a recent study, comparing the manual toothbrush with a novel sonic toothbrush, the authors unknowingly introduced a potential novelty bias[7]. Had the investigators asked the patients to use the sonic toothbrush for a few weeks before starting the trial, the results might have been different. On the contrary, some individuals may develop a bias against novelty, preferring familiar and established practices owing to a fear of the unknown or a desire for stability. This resistance to change can hinder progress and stifle innovation.
Confirmation bias has been included under special bias category as it exerts its effect at every stage of clinical research right from study design to conducting the trial and publishing the results and even in clinical case scenarios. Researchers might design studies in a way that unconsciously favors the hypothesis they believe to be true, leading to biased data collection or experimental design. During data collection and analysis, researchers might unconsciously focus on aspects of the data that support their hypothesis while overlooking or downplaying those that are against it. After the completion of the research, there may be a tendency to submit and publish studies with positive or confirmatory results, whereas those with negative or contradictory findings might remain unpublished or receive less attention. Furthermore, confirmation bias can affect the peer review process because reviewers might be more critical of studies that challenge established beliefs and more lenient toward those that confirm prevailing notions. Clinicians involved in research or medical practice may be influenced by confirmation bias when interpreting patient data or deciding on treatments. They might give more weightage to data that support their initial diagnosis or treatment plan.
In vitro studies are experiments conducted in a controlled laboratory environment using isolated cells, tissues, or organs outside of their natural environment. While these studies are valuable for investigating specific biological mechanisms and testing hypotheses, they are not free from potential biases. Some possible biases in in vitro studies include the following: (1) Publication bias: In vitro studies that yield positive or significant results are more likely to be published than those with negative or inconclusive findings. This bias can lead to an overrepresentation of positive outcomes in the literature; (2) Selection bias: The choice of cell lines or experimental models may not entirely represent the complexity of human biology. Researchers might select cells that are easier to work with or those that support their hypothesis, potentially overlooking relevant but less convenient models; (3) Sampling bias: In in vitro studies, researchers often use specific cell lines or samples obtained from a specific source, such as a commercial supplier. These samples may not accurately represent the target population or disease being studied; (4) Measurement bias: Errors such as inaccurate calibration of instruments or subjective interpretation of results can occur during the measurement process. These errors can introduce discrepancies between the observed and actual outcomes; (5) Contamination or cross-contamination: In vitro experiments are susceptible to contamination by bacteria, fungi, or other cell lines. If not appropriately controlled, such contamination can affect the validity of the study; (6) Experimental design bias: The design of the in vitro study may introduce a bias. One such example is using higher concentrations of a substance to achieve more significant effects even if these concentrations are not physiologically relevant; (7) Time bias: In vitro studies may be conducted over a relatively short period, whereas biological processes often occur over longer timeframes. This discrepancy can lead to an incomplete understanding of the dynamics involved; and (8) Lack of ecological validity: Findings from in vitro studies might not translate accurately to the complexities of the human body and its interactions in the natural environment.
Hence, in vitro study results must be interpreted cautiously, and their limitations should be considered. To gain a more comprehensive understanding, results from such studies should be complemented with data from other research approaches, such as animal studies or clinical trials.
Researchers should be aware of these biases and adopt measures to minimize their impact to ensure the validity and reliability of their findings. Transparent reporting of study methods and potential sources of bias is crucial for the dental research community to assess the quality of the research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Indian Society of Periodontology; Indian Society of Oral Implantologist; Indian Society of Clinical Research; Society of Oral Laser Applications; Indian Dental Association.
Specialty type: Medical laboratory technology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta L, Indonesia S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Godlee F. Milestones on the long road to knowledge. BMJ. 2007;334 Suppl 1:s2-s3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Gerhard T. Bias: considerations for research practice. Am J Health Syst Pharm. 2008;65:2159-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Jain S, Debbarma S, Jain D. Bias in Dental research/dentistry. Ann Int Med Den Res. 2016;2:DE05-09. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126:619-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 519] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 5. | Hennekens CH, Buring JE. Epidemiology in Medicine. Boston: Little, Brown, and Company, 1987. |
| 6. | Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol. 1992;135:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 389] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Nevins M, Chen CY, Kerr E, Mendoza-Azpur G, Isola G, Soto CP, Stacchi C, Lombardi T, Kim D, Rocchietta I. Comparison of a Novel Sonic Toothbrush to Manual Brushing on Plaque Control and Gingival Inflammation: A Multicenter, Randomized, Controlled Clinical Trial. Int J Periodontics Restorative Dent. 2021;41:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Davis RE, Couper MP, Janz NK, Caldwell CH, Resnicow K. Interviewer effects in public health surveys. Health Educ Res. 2010;25:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Aggarwal P, Varshney S, Kandpal SD, Gupta D. Tobacco Smoking Status as Assessed by Oral Questionnaire Results 30% Under-reporting by Adult Males in Rural India: A Confirmatory Comparison by Exhaled Breath Carbon Monoxide Analysis. J Family Med Prim Care. 2014;3:199-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Murray DM. Statistical models appropriate for designs often used in group-randomized trials. Stat Med. 2001;20:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Agrawal AA. A randomized clinical study to assess the reliability and reproducibility of "Sign Grading System". Indian J Dent Res. 2011;22:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 12. | Yuan JC, Shyamsunder N, Barao VA, Lee DJ, Sukotjo C. Publication bias in five dental implant journals: an observation from 2005 to 2009. Int J Oral Maxillofac Implants. 2011;26:1024-1032. [PubMed] |
| 13. | DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJ, Schroeder TV, Sox HC, Van Der Weyden MB; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA. 2004;292:1363-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, Hébert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van der Weyden MB, Verheugt FW. Clinical trial registration--looking back and moving ahead. N Engl J Med. 2007;356:2734-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Al Shammari A, Farook FF, Fallatah A, Aldosari S, Ababneh KT, Aleissa BM. A Randomized Clinical Study of the Plaque Removal Efficacy of a Novel Manual Toothbrush With Micro-Pulse Bristles on Fixed Orthodontic Patients. Cureus. 2022;14:e28453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Oshman S, El Chaar E, Lee YN, Engebretson S. Effect of patient age awareness on diagnostic agreement of chronic or aggressive periodontitis between clinicians; a pilot study. BMC Oral Health. 2016;17:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Jain AA. Incidence of Root Canal Treatment in Posterior Teeth and its Association with the Gender - A Retrospective Study. J Res Med Dent Sci. 2022;10:41-45. |
| 18. | Al-Negrish AR. Incidence and distribution of root canal treatment in the dentition among a Jordanian sub population. Int Dent J. 2002;52:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |