Published online Sep 20, 2023. doi: 10.5662/wjm.v13.i4.323
Peer-review started: May 1, 2023
First decision: June 20, 2023
Revised: August 2, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 20, 2023
Processing time: 141 Days and 13.5 Hours
The respiratory infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved into a multi-organ disorder, with long-term effects known as post-acute sequelae of SARS-CoV-2 infection or long coronavirus disease (COVID).
To examine the current knowledge and outcomes of long-term neurological and gastrointestinal (GI) symptoms in adult cohorts, including United States minority populations.
PubMed and Google Scholar were searched using relevant terms, and data from five studies were analyzed, comprising 27383 patients with persistent neurological and GI sequelae.
The main symptoms included anxiety, depression, dysphagia, headache, vomiting, nausea, gastroesophageal reflux, fatigue, and abdominal pain. Patients with comorbidities and metabolic syndromes were at higher risk for long COVID. While most patients were European Americans, there was a need for further study on African Americans.
The underlying causes of these symptoms remain unclear, warranting more investigation into the long-term impact of the SARS-CoV-2 on different populations.
Core Tip: Long coronavirus disease (COVID) or post-acute sequelae of severe acute respiratory syndrome coronavirus 2 infection (PASC) can lead to prolonged and debilitating symptoms beyond 30 d after infection. Neurological manifestations are prevalent, with encephalopathy, myalgia, headache, and anosmia being common symptoms. Females seem to be more susceptible to long COVID, and severe disease is associated with longer or more frequent neurological symptoms. Gastrointestinal (GI) sequelae are also reported, with symptoms like difficulty swallowing, nausea, vomiting, and abdominal pain being common. Anxiety, depression, dysphagia, headache, and fatigue are among the top symptoms observed, with potential neurological and GI associations. However, there is a need for further research to explore the underlying causes and potential discrepancies in symptom reporting among different populations affected by long COVID/PASC.
- Citation: Sherif ZA, Deverapalli M, Challa SR, Martirosyan Z, Whitesell P, Pizuorno AM, Naqvi Z, Tulloch IK, Oskrochi G, Brim H, Ashktorab H. Potential long-term neurological and gastrointestinal effects of COVID-19: A review of adult cohorts. World J Methodol 2023; 13(4): 323-336
- URL: https://www.wjgnet.com/2222-0682/full/v13/i4/323.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i4.323
Coronavirus disease 2019 (COVID-19) is not only a respiratory illness; it can lead to multi-organ complications. Studying the long-term effects helps identify the post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) or long COVID (LC), which may involve neurological and gastrointestinal (GI) symptoms. Recognizing these sequelae is vital for developing targeted interventions and treatments. Among the wide range of COVID-19 patients who experience mild or severe symptoms, there is a subset that sustains a prolonged residual illness that lasts beyond 4 wk. Barring the lack of accurate diagnostic or reporting methods and normalized data for age and sex across countries, global estimates of LC or PASC patients vary widely between 15% to 76% depending on the preexisting medical conditions and geographical locations. Irrespective of acute COVID-19 severity and age, reports from the globally affected populations show that neurological and GI[1] dysfunctions remain as the most significant alterations. Post-infectious fatigue syndrome linked to brain fog and post-exertional malaise is not rare. Most well-studied viral or bacterial pathogens have been connected to the development of chronic symptoms in a subset of infected patients such as myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome[2-5].
LC or PASC is a multifactorial condition that lingers in many COVID-19 patients for more than 30 d following the initial viral infection that precipitates COVID-19. This residual illness displays over 200 symptoms and affects multiple tissues, organs, and biological systems including neuropsychiatric and GI systems to varying degrees[6-8]. The major symptoms of PASC are fatigue, brain fog (cognitive dysfunction), post-exertional malaise, and shortness of breath (dyspnea) that can last for months and can debilitate normal daily activities. There are several hypotheses that attempt to explain the underlying mechanisms that propel these multifaceted symptoms. Some of the leading theories include viral persistence, chronic inflammation, autoimmune dysfunction, and microclot formation[9]. In most of the tissues and organs affected by LC/PASC including the neurological and GI disorders, there is evidence of endothelial dysregulation. This has prompted some scientists to declare that PASC is a vascular disease.
There are no universal case definitions of PASC. The National Institute of Health (NIH) defines PASC as “the failure to recover from acute COVID-19, or those persistently symptomatic for > 30 d from onset of infection, with any pattern of tissue injury that remains evolving including the nervous system” (www.NIH.gov). The Centers for Disease Control defines PASC as a condition marked by the continuation of COVID-19 symptoms for four or more weeks after infection with SARS-CoV-2 (www.CDC.gov). The SARS-CoV-2 has been detected in brain tissue and the GI tract. Early research indicates that the virus enters the brain through the nose, and via the olfactory bulb invades the brain cells (neurons) where it prowls unchecked, conceivably leading to lasting neurological symptoms, such as cognitive impairment and brain fog[2,10-15]. The virus can also invade the GI system through angiotensin-converting enzyme 2 (ACE2). Understandably, the GI infiltration of the virus begins in the oral route and may precede the brain invasion. It is not clear, however, how the virus precipitates the neurological or GI symptoms that are common in LC patients. The postulation, although not universally accepted by researchers or scientists, is that neuronal inflammation resulting from the viral invasion and persistence in these biological systems may be the trigger for the GI and neurological symptoms[2,10-13,16].
Understanding the full spectrum of COVID-19 is essential to provide appropriate medical care and support to patients as the disease is relatively new and its long-term effects are still being discovered. By reviewing the existing literature, scientists can gain a comprehensive understanding of the various neurological and GI symptoms that can persist after the acute phase of the infection. In the general COVID-19 population of the United States, those with neurological and GI symptoms experience viral invasion in the central nervous system through vascular and lymphatic systems or the vagal nerve[17]. SARS-CoV-2 or its viral particles including the RNA or the associated proteins can infect leukocytes and migrate into the brain or can be directly transported across the blood-brain barrier (BBB) to the brain. Furthermore, ongoing research reports that the virus can invade the peripheral lymphatic vessels that connects the glymphatic system of the brain[18]. The sequential correlation between neurological and GI symptoms or disorders lends credence to the vascular system as being the primary culprit. But the lymph vessels around the GI tract, or the gut-brain axis or the enteric nervous system may also facilitate entry for SARS-CoV-2 to the brain[19,20].
Minority patients including African Americans and Hispanic Americans have been disproportionally affected by COVID-19. These two population groups share common symptoms with most of COVID-19 patients. However, there is a dire need to characterize the long-term effects and impact of these neuropsychiatric and GI symptoms as a significant proportion of these populations usually are socially and economically poor, and medically underserved. African Americans and Hispanic Americans have a higher disease burden and fatality rates from COVID-19 than the general population. Studies regarding LC sufferers in these communities are rare and the long-term outcomes are difficult to gauge based on the available public data. It is even rarer to assess the neurological and GI tract symptoms in such patients because their asymptomatic and mild cases are scarcely documented. Therefore, although the neurological and GI symptoms may be shared by most SARS-CoV-2-infected long haulers, there must be particular attention afforded to those disproportionally affected patients to truly understand the underlying biological and pathophysiological mechanisms of PASC under a different social construct.
In the United States, there is a high frequency of neurologic involvement in 82% of hospitalized COVID-19 patients[21]. However, most neuro-COVID-19 clinic population consists of individuals who were never hospitalized for respiratory complications resulting from COVID-19, and this includes primarily minority populations. Studying the enduring neurological and GI symptoms in minority LC patients who suffered disproportional affliction during the pandemic, which received scant research in the literature, is crucial. Although other combinations of symptoms such as neurological and pulmonary symptoms or their variations are also apparently important to study, there are numerous reviews on this subject. Comprehensive research in various symptom domains contributes to a holistic understanding of COVID-19 and its effects on human health. In this retrospective review, we will characterize the cardinal symptoms shared or exhibited by PASC patients particularly from communities that are underserved and disproportionally affected by the coronavirus pandemic.
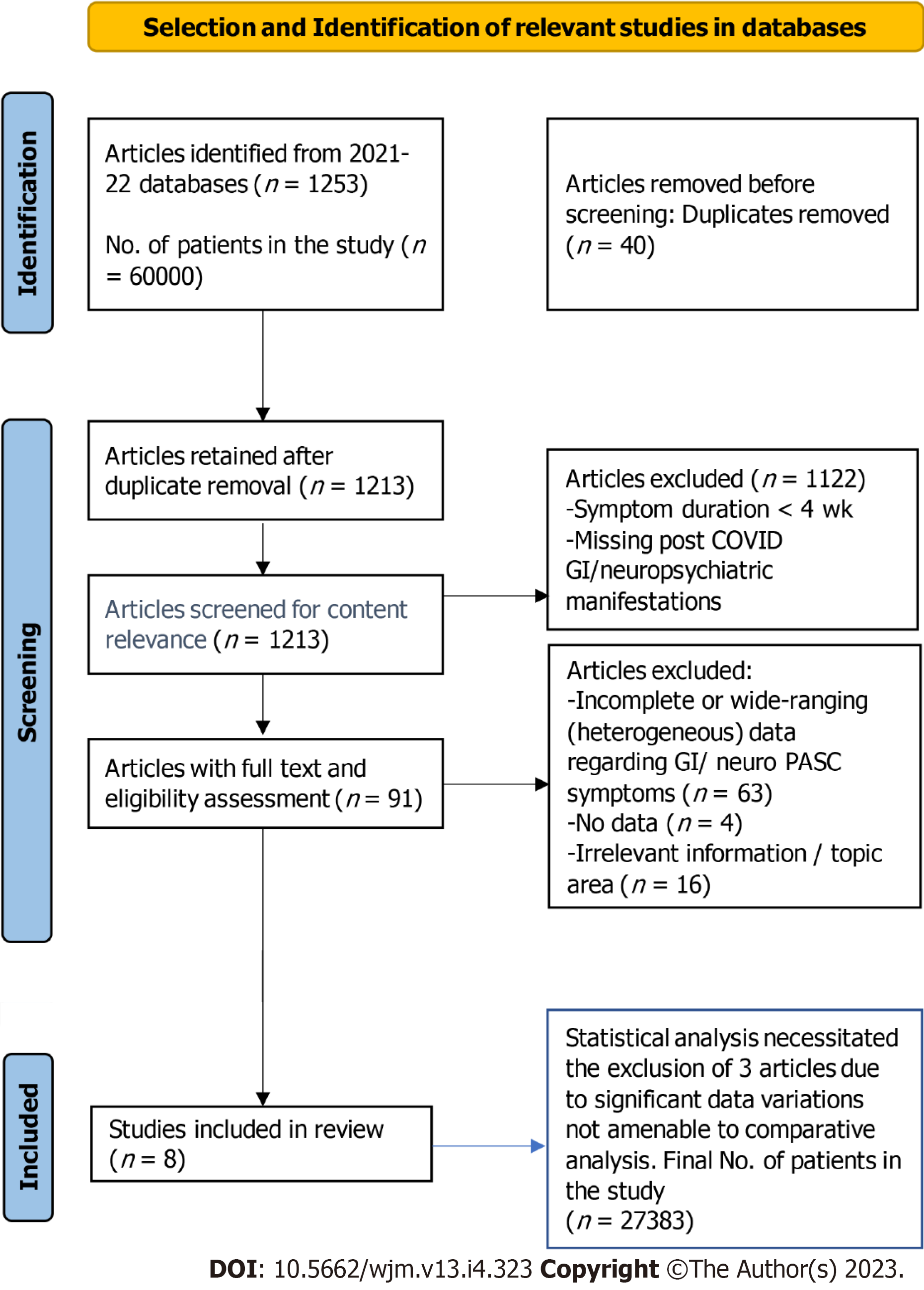
We conducted a systematic literature search of published articles using PubMed and Google Scholar databases from December 2019 to September 30, 2022. We used the following search terms: COVID-19, ACE2, angiotensin converting enzyme, and SARS-CoV-2, long COVID or PASC (post-acute sequalae of SARS-CoV-2 infection), neurological, GI, gastric sequelae of SARS-CoV-2, COVID-19, and post-viral syndromes in the United States. The protocol of this systematic review and analysis of COVID-19 patients’ data is in accordance with the PRISMA Statement guidelines (https://prisma-statement.org) (Figure 1).
We first sorted the LC/PASC studies by title and abstract; then, we compiled the papers by relevance and conducted a new selection process by thoroughly reviewing the data. We incorporated studies that reported GI and neuropsychiatric findings in COVID-19 patients and long-haulers. From the selected papers, tables were generated for each data set in Microsoft Excel. These tables included the following information for each study (when available): General information about the study (year, location, hospital or city, state and country, and publication date), confirmed cases, GI/neuropsychiatric manifestations, symptoms involving other organ systems, comorbidities, hospitalization, outcome measurements, and PASC duration. We compared clinical manifestations, comorbidities, prevalence, and significant outcomes of the long-term neurological and GI sequelae of COVID-19 symptoms. We particularly attempted to include United States minority populations where available.
The following inclusion criteria were adopted to validate article selection: Any study including patients with confirmed previous COVID-19 diagnosis with specified post-COVID neurological and GI sequelae of COVID-19 findings; any study with five or more patients; any study with all or most patients from Western countries including United States minority populations with no distinction regarding sex, age, severity of disease, inpatient or outpatient management, treatment, and outcome. Data on neurological and GI symptoms after 4 wk of SARS-CoV-2 infection were collected and presented.
The following exclusion criteria were adopted to filter out incomplete data: Studies whose duration of symptoms following SARS-CoV-2 infection lasted less than 4 wk were excluded. In addition, studies which did not include post-COVID GI/neuropsychiatric manifestations and studies from Eastern countries were also excluded to have a more homogeneous data source.
The potential studies were assessed for data homogeneity including demographics and LC symptoms. Finally, five studies were included in the analysis of this review. The statistical analysis was performed, as appropriate, by weighted analysis where the weights were associated with the size of the study and the inverse of the variance of the main outcome. Descriptive, parametric, and non-parametric correlation, Chi-square tests, and t-tests, were performed using SPSS and Excel as appropriate. The primary limitation of this review, as our biostatistician has determined, is that the data are aggregated and confined to a small number of papers. Therefore, correlation between GI and neurological symptoms may not be robust.
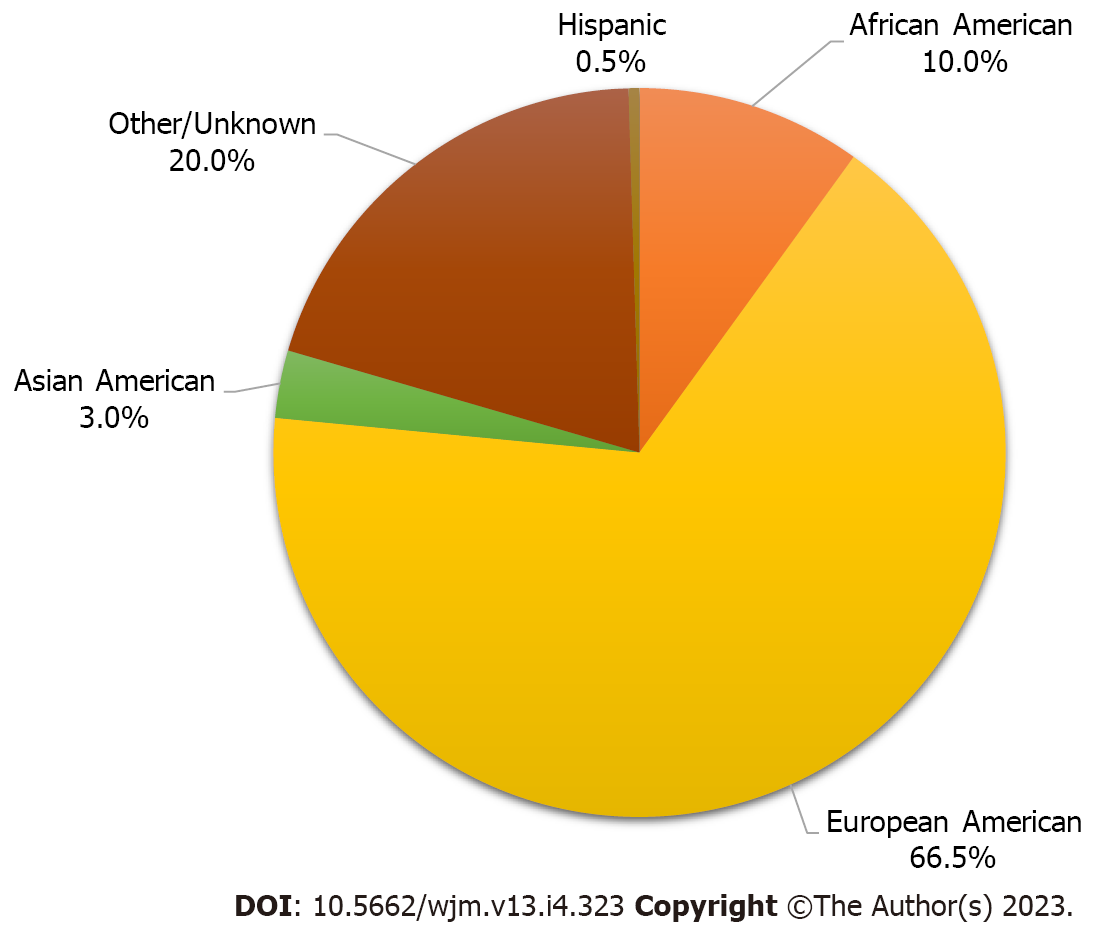
More than 60000 COVID-19 patients of aggregate studies with various COVID and LC symptoms were initially screened for this review. Dozens of papers were sorted out for specific data on LC or PASC symptoms lasting more than 30 d. After excluding duplicates and unrelated studies by screening the title, abstract, or main text, a total of five published studies were included in this review containing a total of 27383 patients with long-term neurological and GI sequelae of COVID-19 symptoms. The mean age of these cohorts is 55.76 years. Females outnumber males by 1.6 to 1.0 as shown in Table 1 and Figure 2.
| Ref. | [22] | [23] | [24] | [25] | [26] | Aggregate | % |
| Female | 236 | 174 | 16177 | 104 | 71 | 16762 | 61.21% |
| Male | 294 | 190 | 9940 | 85 | 112 | 10621 | 38.79% |
| Mean age (SD), year | 59.2 | 61.0 | 51.6 | 50.0 | 57.0 | 54.3 | - |
| African American | 62 | 76 | 2551 | 20 | 16 | 2725 | 9.97% |
| European American | 155 | 74 | 17752 | 148 | 99 | 18228 | 66.54% |
| Asian American | 79 | 6 | 704 | 14 | 16 | 819 | 3.00% |
| Hispanic | 132 | 0 | 0 | 0 | 0 | 132 | 0.48% |
| Unknown | 102 | 208 | 5110 | 7 | 52 | 5479 | 19.98% |
The baseline demographics of the aggregate study cohorts include European Americans (66.5%), African Americans (9.9%), Asian Americans (3.0%), Hispanic Americans (0.5%), and persons with undeclared (or unrecorded) racial or ethnic identities (20.0%), which made up the second largest group (Figure 2).
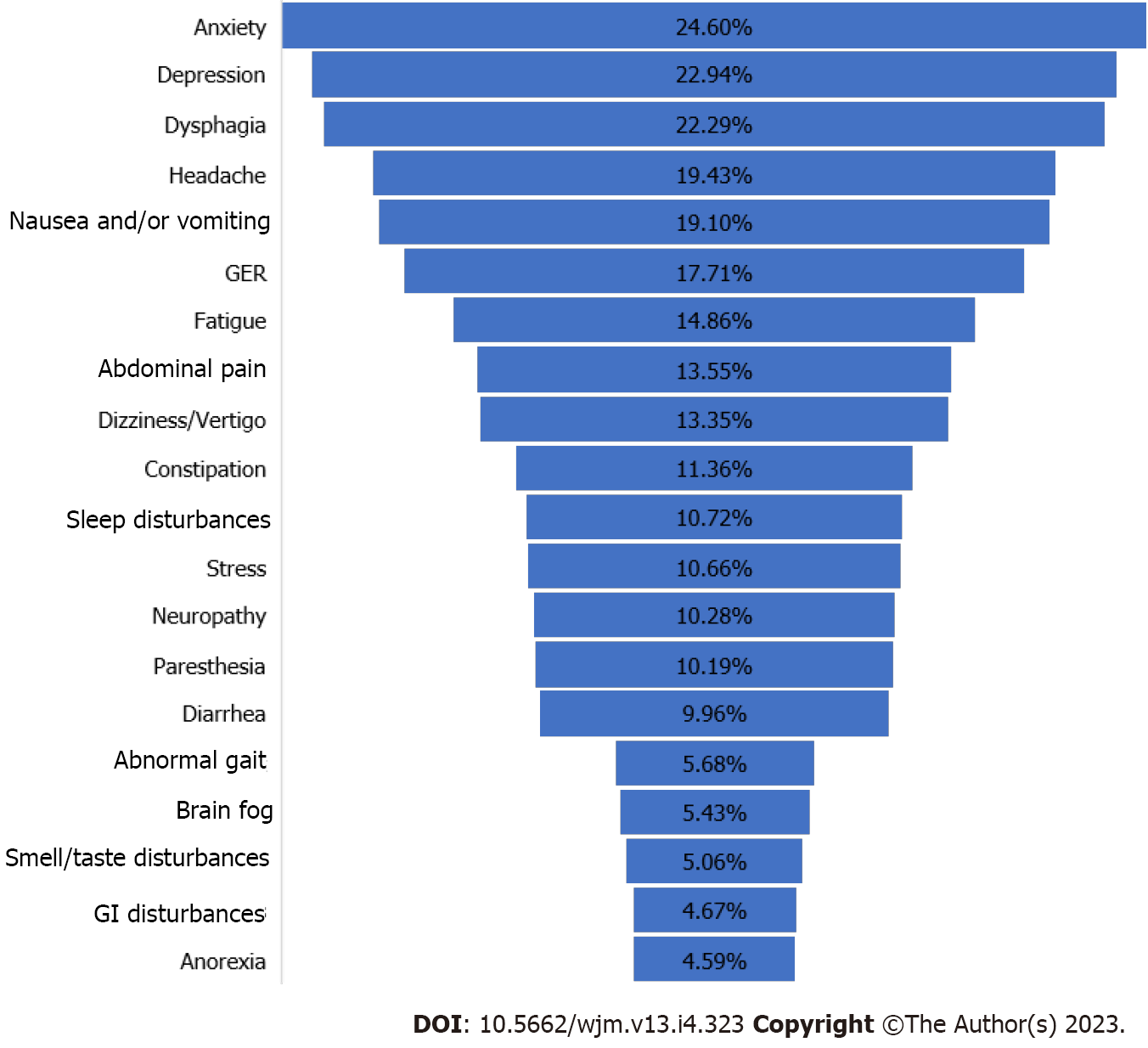
Table 2 and Figure 3 show PASC patients harboring GI or neurological symptoms, or both as documented in the five articles selected for this systematic review.
| Symptom | Number of studies | Minimum number | Maximum number | Total of 5 studies |
| Anxiety | 2 | 11 | 6738 | 6749 |
| Depression | 3 | 6 | 6268 | 6292 |
| Dysphagia | 2 | 3 | 6111 | 6114 |
| Headache | 5 | 6 | 5223 | 5295 |
| Nausea and/or vomiting | 3 | 3 | 5197 | 5223 |
| Myalgia | 4 | 11 | 4962 | 5076 |
| Gastroesophageal reflux | 1 | 4858 | 4858 | 4858 |
| Fatigue | 5 | 29 | 3839 | 4038 |
| Abdominal pain | 3 | 2 | 3682 | 3718 |
| Dizziness or vertigo | 2 | 7 | 3656 | 3663 |
| Constipation | 2 | 8 | 3108 | 3116 |
| Stress | 1 | 2925 | 2925 | 2925 |
| Neuropathy | 2 | 27 | 2794 | 2821 |
| Paresthesia | 2 | 2 | 2794 | 2796 |
| Diarrhea | 4 | 2 | 2690 | 2711 |
| Abnormal gait | 2 | 18 | 1540 | 1558 |
| Brain fog | 5 | 16 | 1410 | 1489 |
| Smell & taste problems | 4 | 8 | 1305 | 1356 |
| General GI | 1 | 1280 | 1280 | 1280 |
| Anorexia | 2 | 5 | 1254 | 1259 |
| Dyspepsia | 1 | 5 | 5 | 5 |
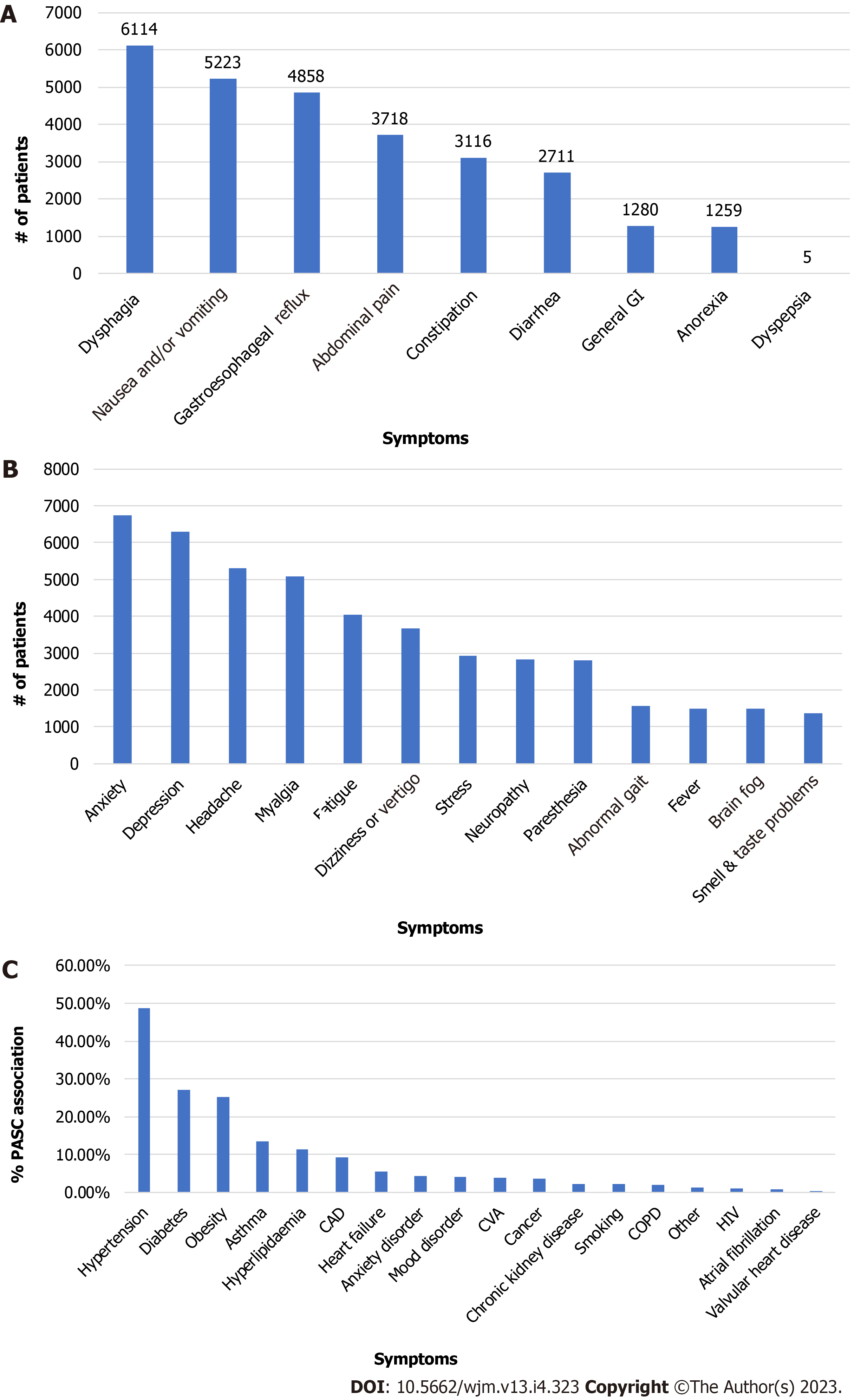
The papers did not single out PASC patients with overlapping GI and neuropsychiatry symptoms. Table 2 also displays the minimum and maximum numbers of each symptom reported among the five studies. Sometimes, only one or two papers reported certain symptoms. For example, among the GI symptoms, dysphagia (i.e., difficulty swallowing) was the number one cited symptom (self-reported by 6114 PASC patients) in one of the studies (Table 2, Figure 3). However, this symptom was cited in only two of the three papers. Similarly, anxiety, the highest-ranked neurologic symptom by 6749 PASC patients, was cited by only two of the papers (Table 2). The separation of GI and neurologic symptoms into Figure 4A and B, respectively provides a clearer picture of the most common symptoms associated with each disorder.
Various GI symptoms to a different degree were reported in the five studies that are reviewed (Table 3, Figure 4A).
| Symptom1 | Number of studies | Minimum number | Maximum number | Total of 5 studies |
| Dysphagia | 2 | 3 | 6111 | 6114 |
| Nausea and/or vomiting | 3 | 3 | 5197 | 5223 |
| Gastroesophageal reflux | 1 | 4858 | 4858 | 4858 |
| Abdominal pain | 3 | 2 | 3682 | 3718 |
| Constipation | 2 | 8 | 3108 | 3116 |
| Diarrhea | 4 | 2 | 2690 | 2711 |
| General GI | 1 | 1280 | 1280 | 1280 |
| Anorexia | 2 | 5 | 1254 | 1259 |
| Dyspepsia | 1 | 5 | 5 | 5 |
These common symptoms in order of descending rank in the aggregated data included abdominal pain (3718, 3 studies)[22-24], dysphagia (6114, 2 studies)[24-27], nausea/vomiting (5223, 3 studies), gastroesophageal reflux (4858, 1 study)[24], and constipation (3116, 2 studies)[23,24]. The remaining GI symptoms were diarrhea (2711, 4 studies)[22-24,26], general GI (1290, 1 study)[24], anorexia (1259, 2 studies), and dyspepsia (5, 1 study).
There are more neurologic symptoms reported than GI symptoms. The five most common neurologic symptoms in the aggregated data of the studies are: Anxiety (6749, 2 studies), depression (6292, 3 studies), headache (5295, 5 studies), myalgia (5076, 4 studies), and fatigue (4038, 5 studies). These top five and the remaining neurologic symptoms are shown in Table 4 and depicted in Figure 4B.
| Symptom1 | Number of studies | Minimum number | Maximum number | Total of 5 studies |
| Anxiety | 2 | 11 | 6738 | 6749 |
| Depression | 3 | 6 | 6268 | 6292 |
| Headache | 5 | 6 | 5223 | 5295 |
| Myalgia | 4 | 11 | 4962 | 5076 |
| Fatigue | 5 | 29 | 3839 | 4038 |
| Dizziness or vertigo | 2 | 7 | 3656 | 3663 |
| Stress | 1 | 2925 | 2925 | 2925 |
| Neuropathy | 2 | 27 | 2794 | 2821 |
| Paresthesia | 2 | 2 | 2794 | 2796 |
| Abnormal gait | 2 | 18 | 1540 | 1558 |
| Fever | 3 | 2 | 1463 | 1478 |
| Brain fog | 5 | 16 | 1410 | 1489 |
| Smell & taste problems | 4 | 8 | 1305 | 1356 |
The five most reported pre-existing conditions or comorbidities by 1266 PASC patients in the study cohorts were documented by four of the five papers reviewed (Table 5). Females constituted about 61% of COVID-19 patients more likely to experience PASC than males. In the cumulative study cohort, those reporting PASC symptoms consisted of White/Caucasians at 66.54%, African Americans at 9.97%, and Asians at 3.0% (Table 1). The leading comorbidities are hypertension (617, 4 studies), diabetes (343, 4 studies), obesity (320, 3 studies), asthma (172, 4 studies), and hyperlipidemia (145, 2 studies)[23,26]. The remaining comorbidities are shown in Table 5 and Figure 4C.
| Ref.1 | [22] | [23] | [25] | [26] | Aggregate sum |
| Total (n) | 530 | 364 | 189 | 183 | 1266 |
| Hypertension | 266 | 225 | 39 | 87 | 617 |
| Diabetes | 146 | 134 | 11 | 52 | 343 |
| Obesity | 158 | - | 72 | 90 | 320 |
| Asthma | 55 | 74 | 24 | 19 | 172 |
| Hyperlipidemia | - | 125 | - | 20 | 145 |
| CAD | 52 | 41 | 3 | 21 | 117 |
| Heart failure | 23 | 41 | - | 5 | 69 |
| Anxiety disorder | - | - | 54 | - | 54 |
| Mood disorder | - | - | 43 | 8 | 51 |
| CVA | 28 | 21 | - | - | 49 |
| Cancer | 15 | 32 | - | - | 47 |
| Chronic kidney disease | - | 29 | - | - | 29 |
| Smoking | 18 | - | 9 | - | 27 |
| COPD | 19 | - | - | 7 | 26 |
| Other | - | - | 15 | - | 15 |
| HIV | 10 | - | 4 | - | 14 |
| Atrial fibrillation | - | - | 2 | 9 | 11 |
| Valvular heart disease | - | - | 3 | - | 3 |
In Table 6, various symptoms other than GI and neurologic were reported by all five studies to varying levels. Furthermore, in the same five study populations, a total of 57 clinical manifestations were also assessed. We divided these clinical manifestations into separate categories of organ systems such as neurologic/psychiatry, mental health, respiratory, cardiovascular, GI, dermatologic, and ear, nose, and throat. The highest ranked general PASC-associated symptom by far was weight gain at 11256 followed by dyspnea at 5638. The rest of these general symptoms are side effects of SARS-CoV-2 infection and are listed in Table 6.
| Ref. | [22] | [23] | [24] | [25] | [26] | Aggregate sum |
| Total number | 530 | 364 | 26117 | 189 | 183 | 27383 |
| Weight gain | - | - | 11256 | - | - | 11256 |
| Dyspnea | 55 | 58 | 5432 | 35 | 58 | 5638 |
| Joint pain | - | - | 5484 | 6 | 29 | 5519 |
| Cough | 10 | 37 | 4570 | - | 46 | 4663 |
| Chills | - | - | 3839 | - | - | 3839 |
| Edema | - | - | 3839 | - | - | 3839 |
| Bleeding | - | - | 3708 | - | - | 3708 |
| Tachycardia | - | - | 3264 | 16 | - | 3280 |
| Pain | - | - | 3212 | - | - | 3212 |
| Wheezing | - | - | 3108 | - | - | 3108 |
| High BP | - | - | 3108 | - | - | 3108 |
| Skin lesion | - | - | 2977 | - | - | 2977 |
| Sleep disturbances | - | - | 2925 | 17 | - | 2942 |
| Swelling | - | - | 2742 | - | - | 2742 |
| Chest pain | 7 | 30 | 2690 | 10 | - | 2737 |
| Rash | - | - | 2455 | - | - | 2455 |
| Erythema | - | - | 2403 | - | - | 2403 |
| Urinary tract symptoms | - | - | 2272 | - | - | 2272 |
| Weakness | - | 34 | 2167 | 1 | - | 2202 |
| Peripheral edema | - | 22 | 2063 | - | - | 2085 |
| Weight loss | - | - | 1906 | - | - | 1906 |
| Erectile dysfunction | - | - | 1854 | - | - | 1854 |
| Sinonasal congestion | - | - | 1671 | - | - | 1671 |
| Respiratory distress | - | - | 1515 | - | - | 1515 |
| Sleep apnea | - | - | 1410 | - | - | 1410 |
| Throat pain | - | - | - | 11 | - | 11 |
| Tinnitus | - | - | 5.2 | - | - | 5.2 |
| Nasal congestion | - | - | - | 3 | - | 3 |
| Hearing loss | - | - | - | 2 | - | 2 |
| Sore throat | - | - | - | - | - | 0 |
| Alopecia | - | - | - | - | - | 0 |
COVID-19 patients experience a multitude of symptoms including respiratory, digestive, and neurological disorders. A subset of these patients experiences LC or PASC that prolongs the duration and the debilitating conditions of these symptoms beyond 30 d after infection. As there is no standard case definition for PASC, there are disparate PASC explanations ranging from a minimum of 30 d duration of symptoms to a maximum of 6 mo or 180 d in the study cohorts. Certain distinctive symptoms such as anosmia indicate a potential neurotropism of this virus. There are several pathways for the virus to enter the nervous system. One of these pathways proposed by Llorens et al[28], is a route to the brain from the infection of the gut via Toll-like receptor 4 and zonulin brain receptor. Other researchers proposed that the evolutionary similarity of SARS-CoV-2 with SARS-CoV makes it more likely that SARS-CoV-2 can invade the olfactory bulb and GI system through ACE2[18]. However, when the GI tract is invaded, the virus may enter the central nervous system through vascular and lymphatic systems or the vagal nerve. SARS-CoV-2 can infect leukocytes and migrate with them into the brain, or the viral particles can be directly transported across the BBB to the brain.
At the beginning of the pandemic in 2019, there have been frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. In fact, more than 80% of hospitalized COVID-19 patients had neurologic symptoms during their disease course, and 82.3% at any time during the disease course according to a retrospective Chicago-area study[21]. In this study, in order of decreasing percentages of neurological symptoms, myalgia (44.8%), headache (37.7%), encephalopathy (31.8%), dizziness (29.7%), dysgeusia (15.9%), and anosmia (11.4%) were the most frequent neurologic manifestations[21]. These neurological symptoms were more prominent in the Chicago-area cohort than the COVID-19 symptoms observed in the adult cohort populations studied in the five papers reviewed in our study demonstrating that anxiety (Table 2) was the most prevalent GI-neuro symptom. In another cohort of LC sufferers reported by Nakhli et al[29], depression (65%), but not anxiety (48%), was significantly more common in those with post-COVID-19 disorders of gut-brain interaction.
The five studies in our report did not categorize PASC patients based on the timing of neurologic manifestations by COVID-19 severity. The mean age was 55 years included female cohort of 62% compared to 38% of the male PASC patients raising the question as to why females seem to be more susceptible to LC. Four of our five study cohorts also comprised 645 hospitalized COVID-19 patients who later developed PASC. The literature is replete with data of PASC patients who developed their various residual illnesses following a lengthy stay in intensive care units connected to ventilators.
As Table 4 summarizes, the neurologic manifestations that occurred at onset and any time during COVID-19 show that patients with severe disease had a longer duration or frequency of neurologic manifestations including encephalopathy than those with milder or no symptoms[21]. The severity of COVID-19 symptoms can be influenced by genetic factors such as variations in the expression of the ACE2 receptor in the body, as well as differences in the virulence and transmissibility of the virus strains[30]. It is also evident in the evolving PASC literature that among the most reported neurological symptoms is fatigue. However, in our limited systematic review, this common condition was not among the top five neurological symptoms. Fatigue is also a major symptom among COVID-19 patients regardless of whether the disease progresses to LC or not. A notable factor is that the most frequently observed LC symptoms in our meta-analysis are anxiety and depression, which are also features of post-traumatic stress disorder (PTSD).
The GI sequelae of SARS-CoV-2 can affect any part of the digestive system, not only in the acute infection phase but also in the post-acute phase, leaving long-term sequelae to manifest frequently or sporadically. The main long-term symptoms that are reported, regardless of the presence of chronic diseases, are diarrhea, nausea, vomiting, abdominal pain, accompanied by increased liver enzymes[31]. In our retrospective study of 27383 PASC patients, the main GI symptoms experienced in decreasing order are difficulty swallowing, nauseas and/or vomiting, abdominal/or visceral pain, GI reflux, diarrhea, and constipation.
Wang et al[24] developed a broad post-acute sequelae of SARS-CoV-2 symptoms lexicon called PASCLex based on physician clinical notes and reviews to facilitate PASC symptom identification and research. There were multiple symptoms identified by the natural language processing tool that Wang et al[24] utilized to validate previously identified post-acute COVID-19 results recorded in meta-analysis studies of observational and survey data. Lopez-Leon et al[32] conducted a systematic literature review and identified more than 50 Long-term effects of COVID-19, with the most common being fatigue, headache, attention disorder, hair loss, and dyspnea. Halpin et al[33] identified fatigue, breathlessness, anxiety/depression, concentration problems, and pain among the five most common post-discharge symptoms in 100 patients hospitalized with COVID-19 (ward and ICU). A recent analysis of new ICD-coded outpatient symptoms among non-hospitalized COVID-19 patients 28-108 d post COVID-19 diagnosis lists that long-term symptoms such as throat and chest pain, shortness of breath, headache, malaise, and fatigue are the most common issues experienced by COVID-19 patients even after they have recovered from the initial infection[24]. We identified the most common ten GI and neurologic symptoms as anxiety, depression, dysphagia, headache, nausea and/or vomiting, myalgia, gastroesophageal reflux, fatigue, abdominal pain, and vertigo, highlighting symptoms common to both prior inpatient and outpatient-based studies and raising additional symptoms for consideration. Among the top 50 symptoms identified in our study, some have not been previously reported, or only reported in small series or single case studies. These include patient-level symptoms that may have previously been obscured in diagnoses or groups of symptoms, such as “cutaneous signs” like skin rash or lesion while our study captured individual symptom descriptors such as “erythema”, “itching”, and “rash”. Similarly, our findings of “visual changes” and “abnormal gait”[34] have not been duly reported in most studies that we screened for this review. It is also interesting that in the general symptom category, both weight gain (11256) and weigh loss (1906) were reported in the same and only one paper among the five reviewed. It is therefore possible that obscure and uncommon symptoms are picked up by the questionnaires or survey questions posed by the investigators that are holistically conducting their research as opposed to selecting the most common symptoms to show in the analysis of their collected data.
The main limitation of our review is the small number of published papers that were used in collating the various common symptoms experienced by LC/PASC patients and the limited sources of data available exclusively in the GI and neurological category of symptoms. Based on our descriptive analysis of the symptoms and the varying degree of sample sizes in each cohort study from the five papers, there is a positive correlation between GI and neurological symptoms regardless of the significance level. It is important to point out that contrary to the published reports, fatigue was not a major symptom among those PASC patients experiencing the most GI or neurologic symptoms. Further work is needed in this area to clearly understand why such a discrepancy exists. It is important that future studies bring light and clarity to the underlying causes of the disproportionate infection, duration of symptoms, or mortality in minority populations. There may also be similarities in symptoms, in patients of other countries that could shed light on the long-term effects of COVID-19.
LC/PASC leads to prolonged and debilitating symptoms beyond 30 d after SARS-CoV-2 infection. Neurological symptoms, such as encephalopathy, myalgia, and anosmia, are common in LC/PASC patients. GI sequelae, including difficulty swallowing and abdominal pain, are frequently observed. Females may be more susceptible to LC/PASC. Anxiety and depression are prevalent, resembling features of PTSD. Fatigue’s discrepancy in symptom reporting requires further investigation. More research is needed to understand the long-term effects and potential treatments for LC/PASC patients.
The respiratory infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for a global epidemic, extends beyond pulmonary issues. It induces multi-organ disorders, affecting cognition (neurological) and gastrointestinal (GI) function. Long-term repercussions of this infection are termed post-acute sequelae of SARS-CoV-2 infection (PASC) or long coronavirus disease 2019 (COVID) (LC). This review aims to analyze current knowledge and outcomes of long-term neurological and GI effects in adult cohorts, encompassing United States minority populations.
This research is motivated by the need to explore and understand the long-term neurological and gastrointestinal effects of COVID-19 in adult cohorts, with a particular focus on United States minority populations. By investigating these long-term sequelae, the study aims to contribute to the existing knowledge, provide insights into potential health impacts, and potentially lead to better management and care for individuals affected by PASC or LC.
To identify and document the neurological symptoms and cognitive impairments experienced by individuals who have recovered from COVID-19, as well as to examine and characterize the gastrointestinal symptoms and disorders observed in post-COVID-19 patients. The research seeks to determine the prevalence and severity of these long-term effects, considering the disproportionate affliction in United States minority populations. Furthermore, the study aims to investigate contributing factors, risk factors, comorbidities, and demographic variables associated with the development of long-term neurological and GI sequelae in post-COVID-19 individuals. We also wish to explore the underlying mechanisms and pathophysiological processes that may lead to these long-term effects. Future investigations might include a comparison with control groups to discern the specific impact of the virus on neurological and GI systems. Moreover, future research will analyze potential disparities in the prevalence and outcomes of long-term effects among different racial and ethnic groups. Ultimately, the study’s findings will provide valuable clinical insights and contribute to public health knowledge, offering evidence-based information for improved assessment, management, and care of individuals experiencing long-term neurological and GI effects following COVID-19. The researchers will suggest recommendations for healthcare providers, policymakers, and future researchers to address the challenges posed by LC in diverse populations.
PubMed and Google Scholar were searched using relevant terms, and data from five studies were analyzed, comprising 27383 patients with persistent neurological and GI sequelae.
The study revealed several prominent symptoms, such as anxiety, depression, dysphagia, headache, vomiting, nausea, gastroesophageal reflux, fatigue, and abdominal pain, among LC patients. Notably, individuals with comorbidities and metabolic syndromes faced an increased risk. While most patients were of European American descent, the impact on African American individuals requires more extensive investigation. The underlying reasons for these symptoms remain uncertain, emphasizing the necessity for further research into the long-term effects of the SARS-CoV-2 on diverse populations.
The study concludes that LC is associated with a range of significant symptoms, including anxiety, depression, dysphagia, headache, vomiting, nausea, gastroesophageal reflux, fatigue, and abdominal pain. Patients with comorbidities and metabolic syndromes are at higher risk of experiencing these long-term effects. The research highlights the need for further investigation into the impact of LC on African American populations and emphasizes the uncertainty surrounding the underlying causes of these symptoms. Overall, the study underscores the importance of understanding the long-term consequences of SARS-CoV-2 infection in different populations.
The study on LC symptoms reveals the importance of considering research perspectives that can enhance our under
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E, E
P-Reviewer: Osatakul S, Thailand; Paparoupa M, Germany; Pitton Rissardo J, Brazil; Taghizadeh-Hesary F, Iran S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, Haroon S, Price G, Davies EH, Nirantharakumar K, Sapey E, Calvert MJ; TLC Study Group. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114:428-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 531] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 2. | Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. 2021;27:895-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 3. | Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol. 2021;12:698169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 539] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 4. | Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 334] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 5. | Varanasi S, Sathyamoorthy M, Chamakura S, Shah SA. Management of Long-COVID Postural Orthostatic Tachycardia Syndrome With Enhanced External Counterpulsation. Cureus. 2021;13:e18398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 258] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 7. | Thurnher MM, Reith W, Thurnher AP, Rommer P. [Long COVID: long-term symptoms and morphological/radiological correlates]. Radiologe. 2021;61:915-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 386] [Article Influence: 128.7] [Reference Citation Analysis (0)] |
| 9. | Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479:537-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 147] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, Scheibenbogen C; European Network on ME/CFS (EUROMENE). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome - Evidence for an autoimmune disease. Autoimmun Rev. 2018;17:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286:438-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 12. | Heming M, Li X, Räuber S, Mausberg AK, Börsch AL, Hartlehnert M, Singhal A, Lu IN, Fleischer M, Szepanowski F, Witzke O, Brenner T, Dittmer U, Yosef N, Kleinschnitz C, Wiendl H, Stettner M, Meyer Zu Hörste G. Neurological Manifestations of COVID-19 Feature T Cell Exhaustion and Dedifferentiated Monocytes in Cerebrospinal Fluid. Immunity. 2021;54:164-175.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 13. | Visvabharathy L, Hanson BA, Orban ZS, Lim PH, Palacio NM, Jimenez M, Clark JR, Graham EL, Liotta EM, Tachas G, Penaloza-MacMaster P, Koralnik IJ. T cell responses to SARS-CoV-2 in people with and without neurologic symptoms of long COVID. medRxiv. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Moghimi N, Di Napoli M, Biller J, Siegler JE, Shekhar R, McCullough LD, Harkins MS, Hong E, Alaouieh DA, Mansueto G, Divani AA. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep. 2021;21:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Che Mohd Nassir CMN, Zolkefley MKI, Ramli MD, Norman HH, Abdul Hamid H, Mustapha M. Neuroinflammation and COVID-19 Ischemic Stroke Recovery-Evolving Evidence for the Mediating Roles of the ACE2/Angiotensin-(1-7)/Mas Receptor Axis and NLRP3 Inflammasome. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Gyebi GA, Batiha GE. Covid-19-Induced Dysautonomia: A Menace of Sympathetic Storm. ASN Neuro. 2021;13:17590914211057635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Cavallieri F, Sellner J, Zedde M, Moro E. Neurologic complications of coronavirus and other respiratory viral infections. Handb Clin Neurol. 2022;189:331-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 18. | Bostancıklıoğlu M. Temporal Correlation Between Neurological and Gastrointestinal Symptoms of SARS-CoV-2. Inflamm Bowel Dis. 2020;26:e89-e91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Shi Y, Li Z, Yang C, Liu C. The role of gut-brain axis in SARA-CoV-2 neuroinvasion: Culprit or innocent bystander? Brain Behav Immun. 2021;94:476-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Tanne JH. Covid-19: US studies show racial and ethnic disparities in long covid. BMJ. 2023;380:535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 21. | Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7:2221-2230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 340] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 22. | Kingery JR, Safford MM, Martin P, Lau JD, Rajan M, Wehmeyer GT, Li HA, Alshak MN, Jabri A, Kofman A, Babu CS, Benitez EK, Palacardo F, Das IG, Kaylor K, Woo KM, Roberts NL, Rahiel S, Gali V, Han L, Lee J, Roszkowska N, Kim YE, Bakshi S, Hogan C, McNairy M, Pinheiro LC, Goyal P. Health Status, Persistent Symptoms, and Effort Intolerance One Year After Acute COVID-19 Infection. J Gen Intern Med. 2022;37:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Shoucri SM, Purpura L, DeLaurentis C, Adan MA, Theodore DA, Irace AL, Robbins-Juarez SY, Khedagi AM, Letchford D, Harb AA, Zerihun LM, Lee KE, Gambina K, Lauring MC, Chen N, Sperring CP, Mehta SS, Myers EL, Shih H, Argenziano MG, Bruce SL, Slater CL, Tiao JR, Natarajan K, Hripcsak G, Chen R, Yin MT, Sobieszczyk ME, Castor D, Zucker JE. Characterising the long-term clinical outcomes of 1190 hospitalised patients with COVID-19 in New York City: a retrospective case series. BMJ Open. 2021;11:e049488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Wang L, Foer D, MacPhaul E, Lo YC, Bates DW, Zhou L. PASCLex: A comprehensive post-acute sequelae of COVID-19 (PASC) symptom lexicon derived from electronic health record clinical notes. J Biomed Inform. 2022;125:103951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, Raza H, Okeke O, Dewar RL, Higgins BP, Tolstenko K, Kwan RW, Gittens KR, Seamon CA, McCormack G, Shaw JS, Okpali GM, Law M, Trihemasava K, Kennedy BD, Shi V, Justement JS, Buckner CM, Blazkova J, Moir S, Chun TW, Lane HC. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann Intern Med. 2022;175:969-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 26. | Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, Nyirenda T, Friedman T, Gupta A, Rasouli L, Zetkulic M, Balani B, Ogedegbe C, Bawa H, Berrol L, Qureshi N, Aschner JL. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15:e0243882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 27. | Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, Burtin C, Posthuma R, Franssen FME, van Loon N, Hajian B, Spies Y, Vijlbrief H, van 't Hul AJ, Janssen DJA, Spruit MA. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 463] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 28. | Llorens S, Nava E, Muñoz-López M, Sánchez-Larsen Á, Segura T. Neurological Symptoms of COVID-19: The Zonulin Hypothesis. Front Immunol. 2021;12:665300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Ebrahim Nakhli R, Shanker A, Sarosiek I, Boschman J, Espino K, Sigaroodi S, Al Bayati I, Elhanafi S, Sadeghi A, Sarosiek J, Zuckerman MJ, Rezaie A, McCallum RW, Schmulson MJ, Bashashati A, Bashashati M. Gastrointestinal symptoms and the severity of COVID-19: Disorders of gut-brain interaction are an outcome. Neurogastroenterol Motil. 2022;34:e14368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L, Zhou Z, Yang J, Zhong J, Yang D, Guo L, Zhang G, Li H, Xu Y, Chen M, Gao Z, Wang J, Ren L, Li M. Corrigendum to: Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin Infect Dis. 2021;73:2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 31. | Bogariu AM, Dumitrascu DL. Digestive involvement in the Long-COVID syndrome. Med Pharm Rep. 2022;95:5-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 1285] [Article Influence: 321.3] [Reference Citation Analysis (0)] |
| 33. | Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T, O'Connor RJ, Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 820] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 34. | Klein S, Davis F, Berman A, Koti S, D'Angelo J, Kwon N. A Case Report of Coronavirus Disease 2019 Presenting with Tremors and Gait Disturbance. Clin Pract Cases Emerg Med. 2020;4:324-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |