Published online Sep 20, 2023. doi: 10.5662/wjm.v13.i4.238
Peer-review started: July 2, 2023
First decision: July 5, 2023
Revised: July 16, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: September 20, 2023
Processing time: 79 Days and 12.9 Hours
In 2019, cirrhosis accounted for 2.4% of global deaths. The projection for 2030 is an increase in this index. In recent years, hospitalization costs have escalated by 36% for compensated cirrhosis and 24% for decompensated cirrhosis. Therefore, it is necessary to identify a tool capable of predicting the mortality of these patients according to their clinical condition and consequently extending their survival time. Different studies have shown that the phase angle (PA) can be a feasible method in clinical practice, with the potential to guide assertive patient management in the therapeutic of chronic liver disease.
To evaluate the prognostic role of PA in cirrhotic patients over a 15-year follow-up period.
Retrospective cohort study with 129 cirrhotic patients of both sexes over 18 years old. Diagnosis of cirrhosis by liver biopsy. The first year of data collection was 2007, and data regarding outcomes was collected in 2023. Data were gathered from medical records, such as esophageal varices (EV), EV bleeding, ascites, spontaneous bacterial peritonitis (SBP), encephalopathy, laboratory findings and PA. The cut-off value for the PA was 5.4°, a value described in 2012 by Fernandes et al for 129 patients evaluated in this study and the cut-off points for the Brazilian population presented in percentiles (P), as described by Mattiello et al. The mortality was assessed using the PA percentile through Kaplan-Meier curves and multivariate binary logistic regression models.
Patients were divided into two groups according to the PA 5.4th (PA > 5.4°, n = 40; PA ≤ 5.4°, n = 89) PA percentile (< P50, n = 56; ≥ P50 n = 73). The percentile classification was more accurate in identifying long-term deaths than the 5.4º PA. Patients with < P50 had a higher number of relevant complications such as ascites, SBP, liver encephalopathy and HCC. PA is strongly correlated with serum albumin (P < 0.001), International Normalized Ratio (P = 0.01), total bilirubin (P = 0.02) and direct bilirubin (P = 0.003). PA is correlated with survival time (P < 0.001) and length of stay (P = 0.02). Logistic regression analysis shows that an increase of 1° in PA enlarges the cirrhotic patient's chance of survival by 17.7%.
PA is a good predictor of morbidity and mortality for cirrhotic patients. The PA by percentile showed greater sensitivity in predicting mortality compared to the cut-off point of 5.4º.
Core Tip: Cirrhosis is characterized by the destruction of hepatic cells, resulting in metabolic alterations that compromise the body's homeostasis. This has a detrimental effect on the patient's clinical condition, negatively impacting their prognosis. Identifying a parameter capable of predicting relevant events is essential for a more assertive approach, reducing mortality and promoting higher life quality. This study aimed to assess the prognostic role of Phase Angle (PA) in cirrhotic patients. One hundred and twenty-nine patients were included, concluding that PA is a promising tool in predicting the clinical condition of chronic liver disease.
- Citation: Pinto LP, Marroni CA, Czermainski J, Dahlem MLF, Carteri RB, Fernandes SA. Role of the phase angle in the prognosis of the cirrhotic patient: 15 years of follow-up. World J Methodol 2023; 13(4): 238-247
- URL: https://www.wjgnet.com/2222-0682/full/v13/i4/238.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i4.238
In 2019, cirrhosis was associated with 2.4% of global deaths. The main etiology of cirrhosis during that period was hepatitis C virus infection, followed by alcohol-related liver disease. However, with advancements in the management of viral hepatitis and the increase in obesity levels and alcohol consumption, the epidemiology of cirrhosis is changing, leading to a growing number of cases associated with Metabolic Associated Steatotic Liver Disease (MASLD) and alcohol, an epidemiological situation that results in a reduced mortality rate for other etiologies[1]. It is estimated that mortality from advanced chronic liver disease will more than double between 2016 and 2030[2].
Cirrhosis has a peculiar pathophysiological characteristic, an increase in the body's energy demand, which results in increased depletion of muscle and hepatic glycogen, increased degradation and protein needs, increased lipoperoxidation due to oxidative stress, and activation of the pro-inflammatory cascade, favoring disease progression and development of complications[3].
This disruption of body homeostasis in cirrhosis, regardless of etiology, leads to cellular damage in terms of integrity and functionality, resulting from parenchymal rearrangement, which causes vascular alterations, portal hypertension, and hepatocellular dysfunction. This pathophysiological characteristic substantially impacts the prognosis of cirrhotic patients[4].
Systematically monitoring the progression of chronic liver disease is a complex task for many healthcare services, as it has numerous limitations, such as being an invasive and costly method and discrepancies in the collected data among professionals. Therefore, there is an urgent need to explore new tools, such as a temporal prognostic index of chronic liver disease based on the natural history of the disease.
Currently, it is possible to assess cellular structure through phase angle (PA). PA is derived from the relationship between resistance and reactance, reflecting the conductivity of electric current through body cells. The value of PA expressed in degrees, is measured by the bioelectrical impedance analysis device[5].
Several studies refer to PA as a prognostic indicator because it can measure damage to cellular integrity and func
Therefore, we conducted a cohort study of cirrhotic patients with different etiologies to capture longitudinal trajectories over 15 years. We analyzed the association between PA and the two main outcomes, death and liver transplantation. Additionally, we evaluated the correlation of PA with laboratory parameters of liver function, survival curves of the study population according to PA values, and associations of PA with the main complications of cirrhosis, such as ascites, encephalopathy, spontaneous bacterial peritonitis (SBP), and bleeding esophageal varices.
This study suggests that the segmented and systematic use of PA in routine evaluation of cirrhotic patients can provide important information about the patient's health condition at the time of assessment, enabling early and effective therapeutic intervention to extend the survival time of cirrhotic patients while improving their quality of life.
A retrospective cohort study was conducted, enrolling cirrhotic patients of both sexes, over 18 years old, treated at the gastroenterology clinic of a hospital complex in Porto Alegre, Brazil. The diagnosis of cirrhosis was established through histological analysis of liver biopsy, imaging tests, and biological markers. Patients with intestinal malabsorption, acquired immunodeficiency syndrome, chronic kidney failure, those on enteral diet, upper limb neuromuscular alterations, chronic pancreatitis, chronic diarrhea, and mental and/or cognitive disorders were excluded from the study. For survival analysis, patients who underwent transplantation during the follow-up were included until the moment of transplantation. This study was conducted in accordance with the Helsinki Declaration and was approved by the ethics and research committee of the Federal University of Health Sciences of Porto Alegre, RS, Brazil, under the number 5203619. All participants signed the Informed Consent Form in advance.
Data collection in the first year occurred in January 2007, and data regarding the outcomes were gathered in January 2023. Demographic data such as age and sex were collected. The clinical data on patient outcomes included the number of hospitalizations, ascites, paracentesis, encephalopathy, VE, SBP, and hepatocellular carcinoma (HCC). The staging of cirrhosis was assessed through the Child-Pugh and Model for End-Stage Liver Disease (MELD) scores.
The laboratory tests taken into consideration were those performed up to three months before or after the evaluation, which included: Albumin, aspartate aminotransferase (AST or TGO), alanine aminotransferase (ALT or TGP), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP or FA), bilirubin levels, and International Normalized Ratio (INR). The duration of hospitalization was calculated based on the admission date and discharge date.
The PA data was collected using tetrapolar electrical bioimpedance from the Biodynamics 450 brand. For the test, participants were instructed to remove all metal objects that could interfere with the examination. The patients were positioned in a supine position with the palms of their hands facing down and their arms along their bodies; electrodes were placed on the middle finger and wrist of the right hand and the ankle and middle finger of the right foot. The PA was established using the following formula: PA = tangent arc (Xc/R) × 180/π.
The PA was categorized according to two references for analysis of the outcomes of death and transplantation: Fernandes[7], which establishes the cut-off point for PA at 5.4°. Thus, a PA score of 5.4 points indicates a suboptimal outcome for all statistical evaluations of the study. The additional reference used was Mattiello[11], through their epidemiological study, which establishes cut-off points of PA for the Brazilian population by percentiles (p) p5, p25, p50, p75, p95. The PA value by percentile is distributed considering the age and sex of the studied sample. A PA in the range of the p50 represents the average normality; hence, a p below 50 (< P50) represents a poor prognosis.
A general description of the data gathered is provided through simple and relative frequencies. Data normality was assessed using the Shapiro-Wilk test, and the data were compared using the student's t-test for independent samples. Pearson's correlation was used to evaluate potential interactions between different variables. Survival analysis was performed using the Kalan-Meier method, using PA values. Binary logistic regression was also performed to assess the predictive potential of specific variables with the patient's outcome. Significant values were considered when P < 0.05. All data were analyzed using the statistical program Statistical Package for Social Sciences version 22.0.
The study assessed 129 cirrhotic patients with an average age of 56.32 (SD 56.32 ± 11.25), with a predominance of male sex (56.6%). The prevailing etiology was hepatitis C virus (43.40%). Other patient characteristics included in the study are described in Table 1.
| Variáveis | Total, n = 129 |
| Age (yr), mean ±SD | 56.32 ± 11.25 |
| Gender | |
| Male | 73 (56.6) |
| Female | 56 (43.4) |
| Weight (kg), mean ± SD | 74.46 ± 14.69 |
| Height (m), mean ± SD | 164.43 ± 8.97 |
| Phase angle, mean ± SD | 6.62 ± 2.95 |
| Etiology Cirrhosis | |
| Alcohol | 31 (24) |
| Autoimmune | 5 (3.90) |
| Nash | 5 (3.90) |
| Others | 16 (12.5) |
| Virus C | 56 (43.40) |
| Virus C+ alcohol | 12 (9.30) |
| Virus B | 4 (3.10) |
| Meld, mean ± SD | 10.98 ± 4.69 |
| Classification Child-Turcotte-Pugh | |
| Child A | 85 (65.89) |
| Child B | 33 (25.59) |
| Child C | 11 (8.52) |
The clinical condition of the patients was determined by the Child-Pugh score, where 65.89% were classified as A, 25.59% as B, and 8.52% as C. The MELD score showed an average score of 10.70 (SD 10.70 ± 4.70).
The mortality rate of the non-transplanted patients (n = 111) over the 15-year period showed that 66 died (59.45%), and among these, 41 (62.12%) died within the first to fifth year, 19 (28.78%) from the sixth to the tenth year, and 6 (9.09%) from the eleventh to the fifteenth year. Of the 41 who died in the first five years, 41.46% had a PA < 5.4° and 53.65% had a percentile < P50. Of the 19 who died between the sixth and tenth year, 36.84% had PA< 5.4, and 52.63% had < P50. The 6 patients who died in the last 5 years of the study, 16.66% had PA < 5.4 and 33.33% had < P50 (Table 2).
| Variable | n = 129 | < 5.4, n = 89 | > 5.4, n = 40 | < 50, n = 56 | > 50, n = 73 | mean ± SD, PA |
| Deaths from the 1st year to the 5th year (1-60 mo) | 41 | 17 (41.46) | 24 (58.53) | 22 (53.65) | 18 (46.35) | 5.81 ± 1.87 |
| Deaths from the 6th to the 10th year (61-120 mo) | 19 | 7 (36.84) | 12 (63.15) | 10 (52.63) | 9 (47.36) | 6.63 ± 3.21 |
| Deaths from the 11th to the 15th year (121-180 mo) | 6 | 1 (16.67) | 5 (83.33) | 2 (33.34) | 4 (66.66) | 6.65 ± 1.90 |
| Alive not transplanted | 45 | 6 (13.33) | 39 (86.66) | 11 (24.44) | 34 (75.55) | 7.04 ± 3.07 |
| Alive transplanted | 10 | 8.62 ± 5.48 | ||||
| Dead transplanted | 8 | 4 (50) | 4 (50) | 6 (75) | 2 (25) | 5.75 ± 1.01 |
| Deaths from the 1st year to the 5th year (1-60 mo) | 3 | 1 (33.33) | 2 (66.67) | 1 (33.33) | 2 (66.67) | 6.57 ± 1 |
| Deaths from the 6th to the 10th year (61-120 mo) | 4 | 2 (50) | 2 (50) | 4 (100) | - | 5.28 ± 0.56 |
| Deaths from the 11th to the 15th year (121-180 mo) | 1 | 1 (100) | 0 | 1 (100) | - | 5.19 ± 0 |
Over the 15 years, 18 patients underwent liver transplantation, and 8 (44.44%) died. Of these, 4 (50%) died in the first six years and the remaining 50% between the seventh and fifteenth year. Of the patients who died between the first and sixth year, 2 (50%) had P50 and a PA 5.40, and those who died between the seventh and fifteenth years, 4 (100%) had P50, and 50% had a PA 5.40.
The average age of the transplanted patients was 51 (SD 51 ± 11) years, 61.11% were male. Among the main causes of transplantation, we cite C virus (27.77%) and C virus + alcohol (16.66%). The leading cause of death of the transplanted patients was post-transplant infection (37.5%). When analyzing the cause of death according to the percentile, infection was the predominant cause (50%) in the < P50 group, while in the >P50 group, the unknown cause and pulmonary cause had the same distribution (50%).
Upon analyzing the clinical outcomes, 57.40% of the patients died due to cirrhosis. Among them, 71.4% had a PA in the P < 50. As for cirrhosis complications, 57% of the patients had ascites, 34% developed encephalopathy, and 75% had esophageal varices (EV), of which 10.9% presented with upper gastrointestinal bleeding (Table 3).
| Variables | Total, n = 129 | < P50, n = 56 | > P50, n = 73 |
| Deaths | 57.4 | 71.40 | 46.60 |
| SPB | 18.6 | 23.2 | 15 |
| Ascites | 57 | 66.1 | 50.70 |
| Paracentesis | 30.20 | 32.10 | 28.80 |
| EV | 75 | 82.10 | 69.90 |
| Varicose veins bleeding | 10 | 10.71 | 10.95 |
| Encephalopathy | 34.9 | 46.40 | 26 |
| HCC | 8.50 | 10.70 | 6.80 |
Patients classified between the P25 and P50 had a higher occurrence of EV; however, those classified between the P5 and P25 had a higher occurrence of variceal bleeding as well as encephalopathy.
The serum values of total bilirubin and direct bilirubin were significantly higher in the P < 50 group (P = 0.02) in addition, this same group had significantly lower levels of sodium (P = 0.03) and INR (P = 0.02) as shown in Table 4.
| Variable | P < 50, n = 56 | P > 50, n = 73 | P value |
| GOT (mg/dL) mean ± Dp | 128 ± 428 | 71 ± 47 | 0.27 |
| GPT (mg/dL) mean ± Dp | 79 ± 227 | 58 ± 42 | 0.46 |
| GGT (mg/dL) mean ± Dp | 102 ± 80 | 94 ± 103 | 0.69 |
| ALP (mg/dL) mean ± Dp | 117.4 ± 55.1 | 101.4 ± 54.6 | 0.13 |
| Albumin (g/dL) mean ± Dp | 3.6 ± 1.9 | 3.9 ± 0.6 | 0.34 |
| Total Bilirubin (mg/dL) mean ± Dp | 2.4 ± 2.6 | 1.7 ± 1.2 | 0.02a |
| Direct Bilirubin (mg/dL) mean ± SD | 1 ± 1.1 | 0.7 ± 0.5 | 0.02a |
| Indirect bilirubin (mg/dL) mean ± SD | 1.3 ± 1.5 | 0.9 ± 0.9 | 0.10 |
| Urea (mg/dL) mean ± SD | 36 ± 16 | 36 ± 13 | 0.79 |
| Creatinine (mg/dL) mean ± SD | 1 ± 0.3 | 1 ± 0.2 | 0.64 |
| Sodium (mg/dL) mean ± SD | 137 ± 4 | 139 ± 4 | 0.03a |
| Potassium (mg/dL) mean ± SD | 4.6 ± 2 | 4.9 ± 2.7 | 0.32 |
| INR mean ± SD | 1.36 ± 0.38 | 1.45 ± 0.51 | 0.02a |
Upon verifying the relationship between the PA and the duration of hospitalization, the interval in days between hospitalizations, and the survival time, it was observed that the phase angle is significantly correlated with survival time (R2 = 0.242; P = 0.006), and the length of hospital stay (Rho = -0.201; P = 0.02).
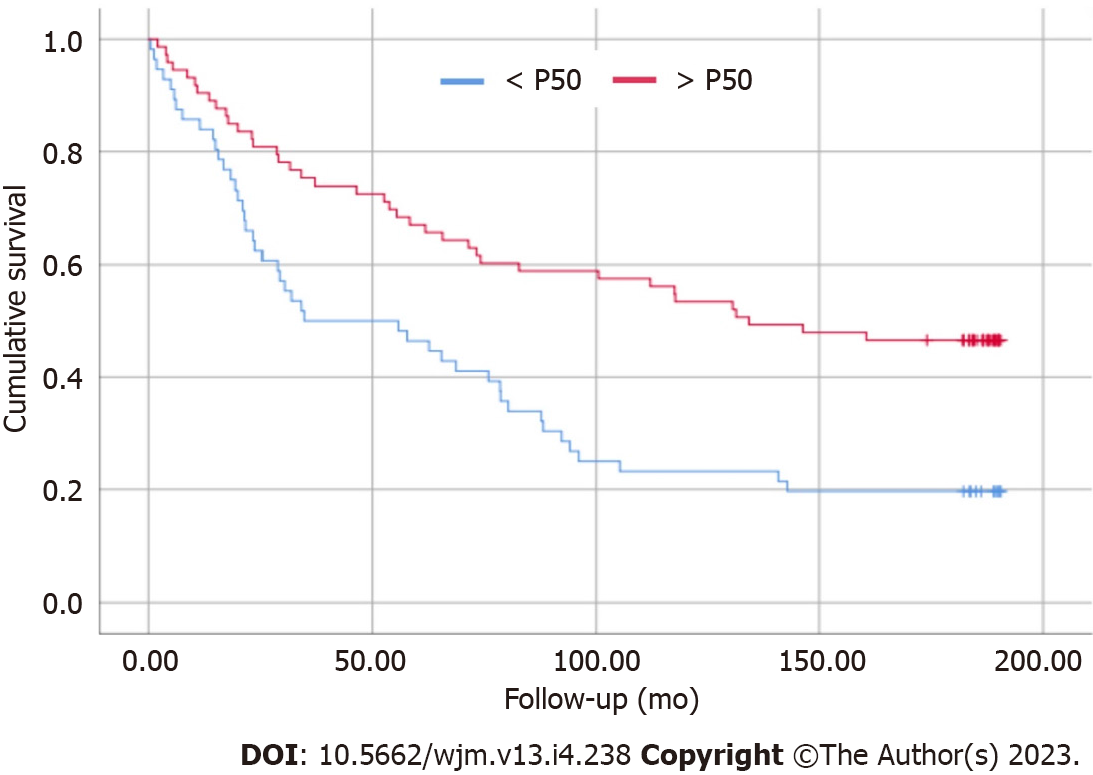
The survival curve is a point that deserves attention regarding the PA values reflecting the staging of cirrhosis. Patients with PA < P50 presented higher mortality when compared to patients in the > P50 group (Figure 1). Through logistic regression, for each 1° increase in PA, the patient increases their chance of survival by 17.7%.
The natural history of cirrhotic patients indicates an average survival duration of approximately 10 years. However, depending on their clinical conditions, some patients may exhibit distinctive cirrhosis complications that negatively impact morbidity and mortality[12-14].
Early diagnosis or treatment of these complications prolongs survival time, improves the quality of life for cirrhotic patients, and reduces the mortality rate of patients on the waitlist for liver transplantation.
Accordingly, this study evaluated 129 cirrhotic patients over a 15-year follow-up to identify a non-invasive, practical, observer-independent, and easily reproducible parameter capable of predicting cellular impairment in the patho
The demographic characteristics of the population in this study align with the findings of Belarmino's study, which followed 134 cirrhotic patients with an average age of 54.3 (SD 54.3 ± 10.10), predominantly male[4]. Similar demographic findings were noted in Ruiz et al's cohort[15], which assessed 136 cirrhotic patients, again predominantly male, with an average age of 54.5 (SD 54.5 ± 10.0). However, it is noteworthy that the population of this study presented compensated cirrhosis, as opposed to the findings by Belarmino et al[4], where only 18% were classified as Child-Pugh A, 55% as B, and 27% as C, and the average MELD score was 14.11 (SD14.11 ± 4,95). Ruiz et al's study included a homogeneous sample according to the Child-Pugh classification, with 34.1% B, 33.3% C, and a MELD score of 14 (14 ± 6)[15].
Regarding mortality, the increased percentage of deaths is related to a reduction in PA in degrees and percentile. These findings align with Belarmino et al[4] study, which identified that patients with PA less than 4.9°, even with compensated cirrhosis, had a higher mortality rate. A similar mortality outcome associated with PA was observed in Saueressig et al[16] study, evaluating 97 hospitalized cirrhotic patients with decompensated disease throughout 11.2 mo (2.4-21). However, it is worth highlighting that the same study established 5.52° as the cut-off point for PA, resembling the 5.4° value utilized in this study, but with divergent disease staging characteristics as per the Child-Pugh score, being 9.3% A, 60.8% B, and 29.9% C. This strengthens the hypothesis that PA reflects systemic cellular damage, not solely linked to the parameters used for score calculations.
Regarding liver transplantation, the etiological presence of hepatitis C virus was observed, recalling that data collection began in 2007. Until 2014, hepatitis C cirrhosis was the leading cause of liver transplantation. However, a study conducted between 2014 and 2019, including 51329 patients listed for transplantation, demonstrated a change in this scenario due to an increased incidence of MASLD and alcoholic cirrhosis, justified by rising obesity levels and an increase in alcoholic individuals[17].
Serrano et al[18] study, evaluating the outcome of 15998 liver transplants over 10 years, sustains the findings of this study, showing an exponential increase in the mortality rate. On the other hand, the authors observed a difference in survival between men and women, as in the first year, male mortality was higher, which did not occur in subsequent years. This difference in findings between the two studies can be justified by the size of the population studied by Serrano et al[18].
Nitski et al[19] evaluated 42146 Liver transplant recipients from the Scientific Registry of Transplant Recipients in the US (UHN) and data from the University Health Network (UHN) in Canada, with average follow-ups of 8 and 5 years, respectively, and found that among the leading causes of death in post-liver transplant patients were various types of cancer, infection, organ rejection, and cardiovascular causes, including stroke. These findings align with the results found in this study, which shows a predominance of deaths due to infection followed by HCC and stroke. Patients who died due to infection had < P50, demonstrating the potential of PA as a predictive value of cellular impairment related to the inflammatory process[20,21].
Ascites, encephalopathy, and EV are commonly observed complications in the cirrhosis picture. Román et al[22], evaluating 100 cirrhotic patients, described that 63% of these had ascites, 12% had encephalopathy, and 31% had EV, unlike the findings of this study, which showed a prevalence of encephalopathy and EV. The difference in disease staging could explain the difference between the studies, as in Román et al's study, 81% of patients were classified as Child-Pugh A[23].
Shi et al[24] evaluated 248 cirrhotic patients over 4 years, with the variables analyzed including serum levels of AST, GGT, AP, bilirubin, albumin, and PA. The authors presented a statistically significant correlation of patients with PA < 5.1° (the cut-off point for this patient sample) with increased levels of albumin, total and direct bilirubin, which corroborates this study.
Hospitalization costs for cirrhotic patients grew by 30.2% from 2008 to 2014. Concurrently, there was a 36% increase in hospitalizations of compensated cirrhotic patients and a 24% gain in those of decompensated patients. Hospitalizations of patients with decompensated cirrhosis represented 58.6% of the total hospitalizations of cirrhotic patients in 2014. The main operators of rising costs are the costlier procedures, escalating from 15% to 152%, and the presence of clinical complications[25].
Román et al[22] observed that PA is also related to the occurrence of clinically relevant events. The same was observed in this study, where a significant correlation was found between PA, the number of hospitalizations (P = 0.01), length of hospital stay (P = 0.003), and mortality (P = 0.001).
The survival curve presented by various studies[7,26-29] evaluating PA in cirrhotic patients reinforces the results found in this study, demonstrating that a reduction in PA, regardless of the cut-off point, is associated with a growth in morbidity and mortality of cirrhotic patients.
Among the study's limitations, we mention the manual recording of collected data in the patient's medical record, which makes detailing some information impracticable, such as the degree of cirrhosis complications. However, the outcome of the 129 patients included in the study was described.
This study is a pioneer on PA as a predictor of mortality in cirrhotic patients, with PA values per percentile for the Brazilian population. The PA by percentile showed greater sensitivity in predicting mortality compared to the cut-off point of 5.4º.
The PA, measured through electrical bioimpedance, can be measured in a segmented way, where the patient can be evaluated daily. In more specific cases, such as in the case of ascites, the measurement of the PA can be performed before and after the paracentesis, informing the cellular condition after a procedure. Thus, the PA becomes a guiding tool in the clinical management of cirrhotic patients by significantly reducing the number of events due to complications characteristic of chronic liver disease. The use of PA can bring as positive outcomes: a lower number and length of hospitalizations, improved quality of life, better results in liver transplantation and, consequently, increased survival time.
The number of new cases of cirrhotic patients is growing worldwide and, as a consequence, an increase in the demand for specialized care to treat the disease per se and the complications inherent in cirrhosis, in addition to the increase in patients on the waiting list for orthotopic transplantation of liver. Given this scenario, it is necessary to identify a tool to predict the mortality of these patients linked to their clinical condition. Within this perspective, the phase angle (PA) becomes a good alternative because it is a viable method in clinical practice, with the potential to guide the clinical management of the patient and extend their survival time and better quality of life.
Identifying a tool capable of predicting mortality and severity of chronic liver disease in real time as a clinical practice brings numerous benefits to this population and to the professionals who treat them.
To the best of our knowledge, no study has evaluated the role of PA as a predictor of mortality in cirrhotic patients with a 15-year follow-up.
Retrospective cohort study with 129 cirrhotic patients of both genders aged over 18 years. Diagnosis of cirrhosis by liver biopsy. The cut-off value for the PA was 5.4°, a value described in 2012 by Fernandes et al for 129 patients evaluated in this study and the cut-off points for the Brazilian population presented in percentiles (P), as described by Mattiello et al. Mortality was assessed using the PA percentile using Kaplan-Meier curves and multivariate binary logistic regression models.
The percentile ranking was more accurate in identifying long-term deaths than the 5.4th PA. Patients with < P50 had a higher number of relevant complications, such as ascites, SBP, liver encephalopathy and HCC. PA is strongly correlated with serum albumin (P < 0.001), INR (P = 0.01), total bilirubin (P = 0.02) and direct bilirubin (p = 0.003). PA is correlated with survival time (P < 0.001) and length of stay (P = 0.02). Logistic regression analysis shows that a 1° increase in PA increases the chance of survival of cirrhotic patients by 17.7%.
PA is a good predictor of morbidity and mortality for cirrhotic patients.
Identifying clinical factors that enhance a poor prognosis for cirrhotic patients, such as ascites, encephalopathy, length of stay, is relevant. With this information, it is possible to act early in the clinical management of these patients and increase the effectiveness of the therapeutic response, with consequent improvement in the prognosis and quality of life of this population.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Krygier R, Poland; Tsoulfas G, Greece S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 347] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 2. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1295] [Article Influence: 185.0] [Reference Citation Analysis (0)] |
| 3. | Bittencourt PL, Zollinger CC. Manual de cuidados intensivos em hepatologia. Online 2017. [acesso em: maio de 2012]; Available from: sbhepatologia.org.br/wpcontent/uploads/2017/10/Manual-de-Cuidados-Intensivos-em-Hepatologia-1.pdf. |
| 4. | Belarmino G, Gonzalez MC, Torrinhas RS, Sala P, Andraus W, D'Albuquerque LA, Pereira RM, Caparbo VF, Ravacci GR, Damiani L, Heymsfield SB, Waitzberg DL. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J Hepatol. 2017;9:401-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Ramos L, Eickemberg M, Moreira P, de Oliveira CC. Bioimpedância elétrica. 2012. [DOI] [Full Text] |
| 6. | Nunes G, Santos CA, Barosa R, Fonseca C, Barata AT, Fonseca J. Outcome and nutritional assessment of chronic liver disease patients using anthropometry and subjective global assessment. Arq Gastroenterol. 2017;54:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Fernandes SA, Bassani L, Nunes FF, Aydos ME, Alves AV, Marroni CA. Nutritional assessment in patients with cirrhosis. Arq Gastroenterol. 2012;49:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Fernandes SA. O ângulo de Fase como marcador prognóstico associado ao estado nutricional do cirrótico e à gravidade da doença: do modelo clínico ao experimental. Porto Alegre. Dissertação [Mestrado em Medicina: Hepatologia] – Universidade Federal de Ciências da Saúde de Porto Alegre; 2013. |
| 9. | Marroni CA, Miranda D, Boemeke L, Fernandes SA. Phase angle bioelectrical impedance analysis (BIA) as a biomarker tool for liver disease.Biomarkers in Liver Disease: Biomarkers in Disease: Methods, Discoveries and Applications. Berlim: Springer Science, 2017; 735-751. Available from: https://link.springer.com/referenceworkentry/10.1007/978-94-007-7742-2_43-1. |
| 10. | Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 672] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 11. | Mattiello R, Mundstock E, Ziegelmann PK. Brazilian Reference Percentiles for Bioimpedance Phase Angle of Healthy Individuals. Front Nutr. 2022;9:912840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2124] [Article Influence: 111.8] [Reference Citation Analysis (2)] |
| 13. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1309] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 14. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 831] [Article Influence: 207.8] [Reference Citation Analysis (1)] |
| 15. | Ruiz-Margáin A, Xie JJ, Román-Calleja BM, Pauly M, White MG, Chapa-Ibargüengoitia M, Campos-Murguía A, González-Regueiro JA, Macias-Rodríguez RU, Duarte-Rojo A. Phase Angle From Bioelectrical Impedance for the Assessment of Sarcopenia in Cirrhosis With or Without Ascites. Clin Gastroenterol Hepatol. 2021;19:1941-1949.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Saueressig C, Glasenapp JH, Luft VC, Alves FD, Ferreira PK, Hammes TO, Dall'Alba V. Phase Angle Is an Independent Predictor of 6-Month Mortality in Patients With Decompensated Cirrhosis: A Prospective Cohort Study. Nutr Clin Pract. 2020;35:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Wong RJ, Singal AK. Trends in Liver Disease Etiology Among Adults Awaiting Liver Transplantation in the United States, 2014-2019. JAMA Netw Open. 2020;3:e1920294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 18. | Serrano MT, Sabroso S, Esteban LM, Berenguer M, Fondevila C, Lorente S, Cortés L, Sanchez-Antolin G, Nuño J, De la Rosa G, Salcedo M. Mortality and Causes of Death After Liver Transplantation: Analysis of Sex Differences in a Large Nationwide Cohort. Transpl Int. 2022;35:10263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Nitski O, Azhie A, Qazi-Arisar FA, Wang X, Ma S, Lilly L, Watt KD, Levitsky J, Asrani SK, Lee DS, Rubin BB, Bhat M, Wang B. Long-term mortality risk stratification of liver transplant recipients: real-time application of deep learning algorithms on longitudinal data. Lancet Digit Health. 2021;3:e295-e305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Lee GR, Kim EY. Usefulness of phase angle on bioelectrical impedance analysis as a surveillance tool for postoperative infection in critically ill patients. Front Med (Lausanne). 2023;10:1111727. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ceolin J, de Borba EL, Mundstock E, de Oliveira JR, Mattiello R, Bodanese LC. Phase angle of bioimpedance as a marker of inflammation in cardiovascular diseases: A systematic review. Nutrition. 2023;112:112064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Román E, Poca M, Amorós-Figueras G, Rosell-Ferrer J, Gely C, Nieto JC, Vidal S, Urgell E, Ferrero-Gregori A, Alvarado-Tapias E, Cuyàs B, Hernández E, Santesmases R, Guarner C, Escorsell À, Soriano G. Phase angle by electrical bioimpedance is a predictive factor of hospitalisation, falls and mortality in patients with cirrhosis. Sci Rep. 2021;11:20415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Liu YB, Chen MK. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J Gastroenterol. 2022;28:5910-5930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (21)] |
| 24. | Shi JY, Yang G, Liu B, Shang X Cui GZ Huang JX, Wang WT, Chen KY, Wang NY. Low Phase Angle Predicts Poor Survival in Patients with Hepatocellular Carcinoma: A Retrospective Study. Journal of Nutritional Oncology. 2022;7:75-84. [DOI] [Full Text] |
| 25. | Desai AP, Mohan P, Nokes B, Sheth D, Knapp S, Boustani M, Chalasani N, Fallon MB, Calhoun EA. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019;10:e00062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Ruiz-Margáin A, Macías-Rodríguez RU, Duarte-Rojo A, Ríos-Torres SL, Espinosa-Cuevas Á, Torre A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis. 2015;47:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 311] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Peres WA, Lento DF, Baluz K, Ramalho A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr Hosp. 2012;27:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Bosy-Westphal A, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr. 2006;30:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 391] [Article Influence: 48.9] [Reference Citation Analysis (0)] |