Published online Jun 20, 2023. doi: 10.5662/wjm.v13.i3.67
Peer-review started: March 24, 2023
First decision: April 3, 2023
Revised: April 16, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 20, 2023
Processing time: 88 Days and 9.7 Hours
The relationship between IgA nephropathy (IgAN) and Crohn’s disease was reported. IgAN is the most common primary glomerulonephritis and one of the leading causes of chronic kidney disease and end-stage renal failure, and up to 50% of cases progressed to end-stage renal disease within 25 years after IgAN diagnosis. However, specific and effective therapeutic strategies are still lacking. In this review, we discuss the possibility of the mechanism involved in IgAN associated with Crohn’s disease based on the findings of basic and clinical studies. Although the etiology of IgAN associated with Crohn’s disease is not permanent and various factors are thought to be involved, the stabilization of the disease condition of Crohn’s disease is believed to help treat IgAN.
Core Tip: The relationship between IgA nephropathy (IgAN) and Crohn’s disease was reported. Crohn's disease (CD) immunological abnormalities may promote and activate the IgAN inflammatory process. Although the etiology of CD-IgAN is not fixed and various factors are thought to be involved, the stabilization of the disease condition of CD is believed to help treat IgAN.
- Citation: Tamura H. IgA nephropathy associated with Crohn's disease. World J Methodol 2023; 13(3): 67-78
- URL: https://www.wjgnet.com/2222-0682/full/v13/i3/67.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i3.67
Intestinal diseases include chronic nonspecific inflammatory diseases of the gastrointestinal tract. Inflammatory bowel disease (IBD) is a chronic and recurrent inflammatory disease of the gastrointestinal tract that includes crohn’s disease (CD) and ulcerative colitis (UC).
In addition to intestinal inflammation, CD has numerous extraintestinal manifestations of IBD. Extraintestinal complications of renal and urological diseases in CD include internal fistula lesions such as enterovesical fistula and enterovaginal fistula, urolithiasis, and renal disease. Urolithiasis is relatively common in 19% of patients with IBD; however, comorbid renal disease is relatively rare. Interstitial nephritis and secondary renal amyloidosis caused by 5-aminosalicylic acid (5-ASA) are renal diseases that show a clear causal relationship with CD treatment or CD itself; however, glomerulonephritis has been reported in few cases, and the lack of a causal relationship is difficult to prove. Among them, the relationship between IgA nephropathy (IgAN) and CD was reported[1,2].
IgAN is the most common primary glomerulonephritis and one of the leading causes of chronic kidney disease and end-stage renal failure. Up to 50% of cases progressed to end-stage renal disease (ESRD) within 25 years after the diagnosis of IgAN. However, at present, specific and effective therapeutic strategies are still lacking[3,4]. Thus, this paper discusses the possibility of the mechanism involved in IgAN associated with CD (CD-IgAN) based on the findings of basic and clinical studies.
Because IgAN exacerbates nephropathy after upper respiratory tract infection, it has been hypothesized that mucosal immunity is involved in the pathogenesis of IgAN. IgA is an immunoglobulin that mainly acts on the mucosa, and renal glomerular IgA is a mucosal multimeric IgA1 containing a secretory component[5-8], and multimeric IgA1 is also increased in the serum[9,10]. These have also supported these hypotheses.
Conversely, which mucosal-associated lymphoid tissue (MALT) is the main responsible site for IgAN is unclear. The involvement of nasopharynx-associated lymphoid tissue (NALT) is assumed to be mainly responsible for IgAN because it worsens after upper respiratory inflammation. Gut-associated lymphoid tissue (GALT) has a huge mucosal area and is the main production site of mucosal IgA; however, its involvement in the pathology of IgAN is controversial. In addition, the exacerbation of IgAN after a mucosal infection suggests the involvement of foreign antigens; however, many points are unclear about how infection is involved.
Recently, the activation of the innate immune system triggered by infection is involved in the onset and progression of various types of nephritis, along with mechanisms such as molecular mimicry and epitope spreading[11]. However, the specific mechanism of IgAN has not been elucidated.
IgAN leads to ESRD, IgAN recurrence in approximately half of the patients who underwent kidney transplantation, and vice versa. IgAN disappears when the kidney is transplanted as a donor, suggesting that the main cause of IgAN is not kidney-specific cells but the systemic IgA immune system[5].
The facts suggesting the involvement of mucosal immunity are as described above; however, bone marrow abnormalities have also been pointed out in patients with IgAN. van den Wall Bake et al[12] reported that the ratio of IgA-producing plasma cells (IgA + PC) increased in the bone marrow of patients with IgAN, and IgA1 production was enhanced. These suggest that glomerular IgA1 is derived from the bone marrow[13].
Moreover, in patients with IgAN, IgA1 was significantly higher with the production of peripheral blood mononuclear cell IgA[12]. By contrast, Harper et al[14] reported that J-chain mRNA-positive mucosal-type IgA + PC increased in the bone marrow of patients with IgAN. Another study reported that when leukemia develops in a patient with IgAN and the patient undergoes bone marrow transplantation, not only leukemia but also IgAN improves[15]. In other words, in patients with IgAN, mucosal-type IgA + PC, which is involved in the pathology, increased in the extramucosal bone marrow, suggesting the excessive production of mucosal-type multimeric IgA1[5].
Approximately 30 years ago, van den Wall Bake et al[16] hypothesized the existence of “mucosa–bone marrow axis” abnormalities in IgAN[5]. Moreover, the homing mechanism of immunocompetent cells to the effective tissue was clarified. This hypothesis became more realistic because mucosa-derived effector cells were found to be translocated and stored in the lymphatic tissue.
Studies using mouse models of spontaneous IgAN have also shown that cells responsible for the abnormal IgA production related to renal onset are present in the bone marrow and spleen[17-19]. Active cell migration and immune information exchange take place between the mucosal tissue and bone marrow. However, whether the cells responsible for IgAN migrate between the MALT and bone marrow or whether only relatively localized mucosal tissue is involved is unclear.
Moreover, we discuss the possible involvement of GALT and NALT in pathologies with IgAN. Serum IgA is mainly produced in the bone marrow, and intestinal mucosa-derived IgA is thought to be scarce. Conversely, in GALT, large amounts of IgA are produced and secreted in the mucosa, which contribute to intestinal immunity. Unlike mucosal IgA, the physiological role of serum IgA is largely unknown. Although there are IgA1 and IgA2 subclasses in humans, 80%-85% of serum IgA is IgA1[20].
As shown in the IgA1 and IgA2 ratios of IgA + PC in the mucosal execution phase, IgA2 + PC was high (30%-65%) in the intestinal mucosa, whereas it was low (7%-25%) in the peripheral lymph nodes and respiratory tract mucosa[20]. In particular, IgA2 + PC is dominant in the ileum and colon[21-24].
Human IgA2 Lacks amino acids, which are the recognition sites of hinge-specific proteases derived from intestinal bacteria, and is protease resistant, which is thought to work favorably in intestinal immunity[24]. The organogenesis mechanisms of Peyer’s patch (PP) and NALT are significantly different.
In the PP, the number of CD3−CD4+CD45+ inducer cells increased from the embryonic period and decreased until 3 wk after birth. In NALT, the number of inducer cells increased because of postnatal stimulation with foreign antigens and peaks at 3 wk after birth[25]. The molecular mechanisms involved in the organogenesis of the two are also different[26]. These facts suggest the difference in the basic roles and needs of both in the mucosa.
The polymeric immunoglobulin receptor (pIgR) is a protein expressed on the basolateral side of mucosal secretory epithelial cells that carry IgA and IgM produced in the mucosa to the mucosal surface.
In GALT, IgA and IgM produced from the PC of the lamina propria (LP) form dimeric IgA and pentameric IgM by the J-chain produced at the same time. pIgR easily binds to multimeric IgA and IgM containing this J-chain and efficiently transports them to the mucosal surface. The transported multimeric IgA and IgM trap antigens in the mucosa and act as a non-inflammatory mucosal immune defense mechanism[27,28].
In IBD, IgA is actively produced in the LP. Conversely, inflammatory pIgR expression and dysfunction occur, inhibiting its secretion on the mucosal surface. Therefore, mucosal IgA, which increased in the LP, was also thought to increase in the blood[29]. Mucosal IgA in the blood is increased in patients with IBD such as UC and CD[30].
The lymphotoxin-β receptor (LTβR) is essential for the formation of mucosal lymphoid tissue[31] and plays an important role in IgA production in the small intestine[32].
LIGHT is a ligand for LTβR and is expressed on activated T cells[33]. In LIGHT transgenic mice (LIGHT Tg), sustained stimulation of LIGHT via LTβR on T cells causes the over-induction of IgA + PC in the LP, and increased expression of MAdCAM-1 by stimulation from LTβR leads to the activation of inflammatory cells, causing severe enteritis[30].
Impaired pIgR expression and excessive IgA production caused by this intestinal inflammation induce mucosal multimeric IgA, which is not transported to the mucosa, to enter and increase their presence in the blood circulation. Because these multimeric IgAs have high affinity to the kidney, they are deposited in the kidney and cause IgAN[30].
On the contrary, the bias of Th2 cytokines may be involved in the glycosylation of IgA in the intestinal tract. Even a small amount of IgA released into the blood from the intestinal tract may cause nephropathy[34-36]. Thus, induction of glomerular deposition of intestinal IgAN is theoretically possible if conditions such as inflammation-associated pIgR dysfunction are met.
However, clinically, most patients with IgAN do not have IBD or gastrointestinal symptoms, and the so-called secondary IgAN associated with intestinal abnormalities occurs in a small proportion of patients. The involvement of IBD in IgAN is considered limited, at least in terms of IgAN in Japan.
Virchows Archiv investigated the clinical and pathological differences CD-IgAN and common IgANs (NOS-IgAN) associated with upper airway inflammation such as tonsillitis[37]. The PCs of the upper airway mucosa mainly produce IgA1[38]. However, the intestinal mucosa, especially PP, is thought to predominantly secrete IgA2 (approximately 60% in mucosal cells) rather than IgA1[39]. For IgAN with CD, intestinal IgA2 is deposited in the glomerular mesangium and may be involved in IgAN induction and progression. However, among the deposited IgA subclasses, the IgA1 subclass was predominant in both the CD-IgAN and NOS-IgAN groups, and no significant difference in the staining intensity of IgA2 was found between the two groups. Then, they examined the deposition of galactose-deficient IgA1 (Gd-IgA1) in the glomerulus of primary IgAN[40].
Gd-IgA1 is an abnormal IgA1 of the IgA1 subclass, exhibiting a structure in which the o-linked glycans in the hinge lack galactose and expose N-acetylgalactosamine (GalNAC)[41,42]. They found no difference in the extent of Gd-IgA1 deposition in the glomeruli, irrespective of CD complications, and a significant difference in Gd-IgA1 deposition was found the between CD-IgANs and NOS-IgAN. CD-IgANs were suggested to share the same pathogenesis as primary IgANs. Furthermore, negative views have emerged regarding the disease specificity of Gd-IgA1[43]. In their renal histological findings, patients with CD-IgAN were found to have significantly more severe glomerulosclerosis, arteriolar hyalinosis grade, tubular atrophy, and interstitial fibrosis than those with NOS-IgAN.
A comparison of the Oxford Classification MEST-C scores revealed that the T-scores representing tubular atrophy and interstitial fibrosis were significantly higher in CD-IgANs than in NOS-IgANs. To confirm this trend, they performed a meta-analysis comparing MEST-C scores using a large cohort of patients with IgAN reported in previous studies[44,45]. The incidence of T1 and T2 was higher in CD-IgANs than in IgANs. These histological differences were speculated to be related to the following factors: (1) CD pathophysiology (i.e., diarrhea and dehydration); (2) Therapeutic agents for CD; and (3) Systemic inflammation, including the gut.
First, in the course of CD, dehydration due to diarrhea, reduced fluid intake, and surgery can reduce the circulating blood volume, which can lead to tubulointerstitial damage and glomerulosclerosis. In addition, undernutrition and hypokalemia have been reported to cause chronic tubulointerstitial injury[46]. The possible reason is that the reduced effective circulating blood volume stimulates the renin–angiotensin–aldosterone system, which in turn enhances the activation of angiotensin II, which can lead to arteriolar contraction, glomerular ischemia, and interstitial fibrosis[47,48]. In addition, evidence showed that hyperuricemia, common in CD, may exacerbate glomerulosclerosis[49].
Second, this factor is related to the effect of treatment. 5-ASA remains the main treatment for CD, and its adverse renal effects are collectively called mesalamine-associated kidney disease[50]. The mechanism by which mesalamine promotes renal injury appears to be through the salicylic acid inhibition of intrarenal prostaglandin synthesis, which is a vasoactive mediator of intrarenal blood flow and uncouples mitochondrial oxidative phosphorylation[46,51].
Several studies have also reported that mesalamine promotes histologically renal injury through interstitial nephritis[52], and similar findings have been reported in patients with CD, not on 5-ASA[53]. Therefore, distinguishing whether interstitial nephritis is due to drugs or CD is challenging.
Third, in CD pathogenesis, immune abnormalities may be involved. Macrophages and T cells produce large amounts of interleukin-23 and tumor necrosis factor (TNF)-α in immune diseases such as CD and are thought to play a central role in CD pathophysiology[54]. These cytokines also contribute to the exacerbation of IgAN tubulointerstitial lesions[54]. A study has also reported a mechanism by which dysfunctional macrophages promote intestinal fibrosis[55].
In recent years, the mechanisms and systemic responses of B cell immune abnormalities in CD have been elucidated[56-58]. Interstitial inflammation in chronic kidney disease involving IgAN was reported to involve B cell-mediated immune dysfunction[59], and immune dysfunction in CD was found to be associated with IgAN in terms of immune dysfunction, which may act as an aggravating factor for renal function. Moreover, in IgAN, complement activity was thought to promote glomerulosclerosis and interstitial fibrosis[60,61].
Previous studies have shown that in patients with CD, activated complement (predominantly C3b) stains strongly in the intestinal mucosa[62] and that resected ileocecal specimens show increased expression of complement C3 mRNA[63]. The effects of CD-associated complement activation may influence glomerular and tubulointerstitial inflammations in IgAN.
Several common genetic predispositions to CD and IgAN have been reported. HLA-DR1 in IgAN[64] and HLA-DR1 DQw5 in CD[65] are characteristic genetic predispositions. Moreover, HLA-DR1-positive patients may have an increased risk of IgAN and CD[66]. A genome-wide association study found that CARD9, HORMAD2, and HLA-DQB1susceptibility genes for IgAN were also associated with IBD. These IgAN loci encode a protein involved in maintaining the intestinal barrier and regulating mucosal immune responses to pathogens[67-70]. Yuan et al[71] reported that the gene for CXCL2 is critically linked to immune infiltration during CD and IgAN. CXCL2, also known as macrophage inflammatory protein-2, belongs to the CXC subfamily. It is secreted by many cell types, including monocytes, macrophages, endothelial cells, and hepatocytes, in response to infection and injury. It primarily affects the recruitment of polymorphonuclear leukocytes to sites of injury or infection, thereby modulating immune and inflammatory responses. The analysis of the relationship between CXCL2 and immune cell infiltration in CD and IgAN diseases suggested that CXCL2 is involved in immune infiltration, thereby contributing to the pathogenesis of the two diseases.
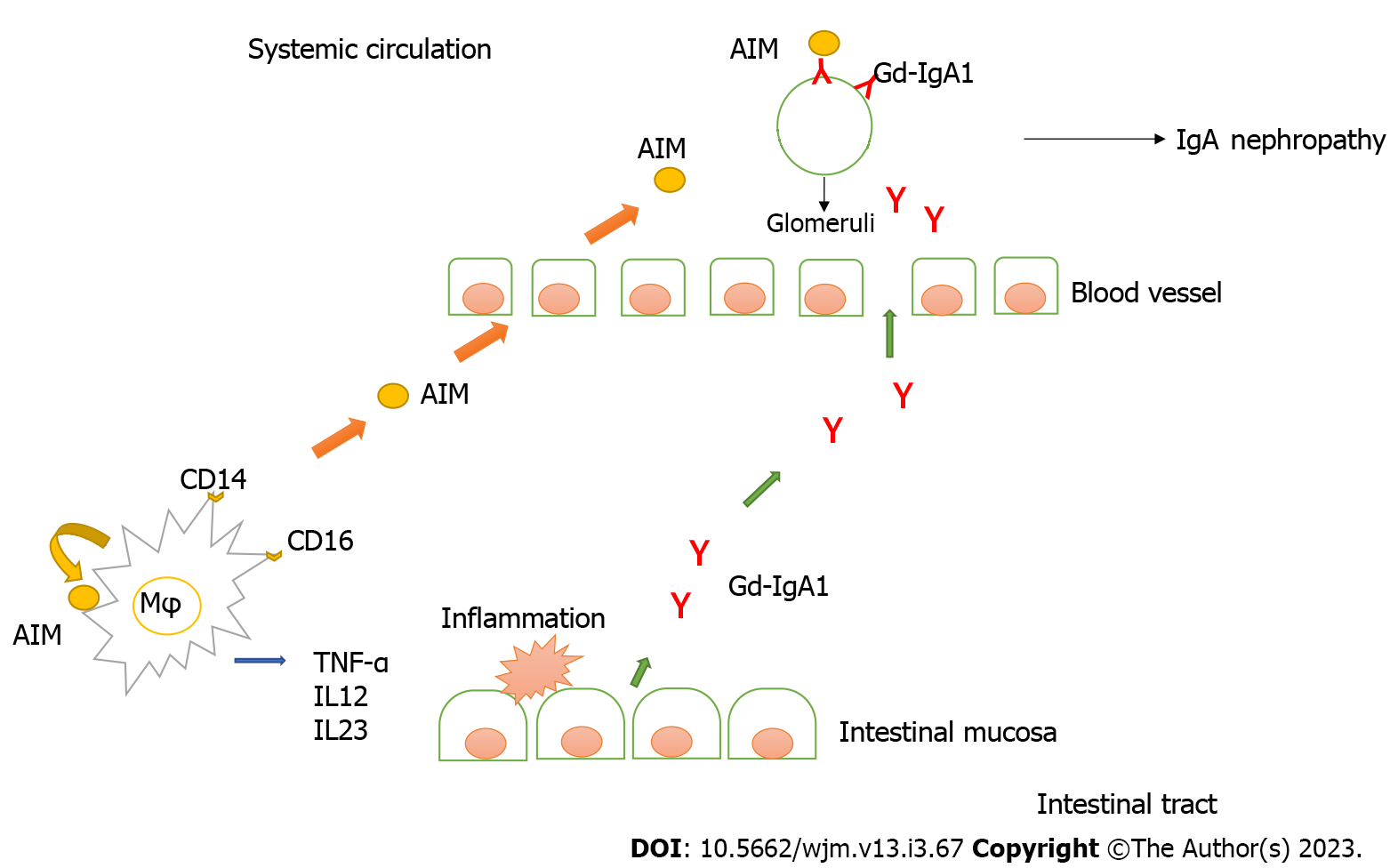
Recent research has revealed that the apoptosis inhibitor of macrophages (AIM) is involved in the pathogenesis of renal failure through the function of macrophages, a type of leukocytes. AIM deposited in the same sites as IgA in the glomeruli in human IgAN and a spontaneous mouse model of IgAN. As a result, AIM deficiency in the spontaneous IgAN mouse model, although IgA deposition in the glomeruli was observed, did not develop IgAN. Subsequently, when AIM was administered to mice, IgG and IgM were co-deposited in the glomeruli, proteinuria and occult blood appeared, and nephropathy was confirmed. From this, IgA deposition in the glomerulus alone does not progress to nephropathy. In addition, glomerular deposition of AIM, IgG, and IgM was not induced in IgA-deficient mice. These results demonstrate that IgAN involves the deposition of IgA into the glomeruli as the first step and that co-deposition of IgG and IgM via AIM leads to inflammation as the second step. Furthermore, in human IgAN, AIM was co-deposited with glomerular IgA, IgG, IgM, and complement (C3). Therefore, AIM is a key molecule that initiates inflammation in IgAN[72].
Ono et al[73] showed that serum AIM levels were higher in patients with CD than in patients with UC, patients with intestinal BD, and healthy controls. Furthermore, AIM is expressed in CD14- and CD16-positive macrophages in the intestinal tissue.
AIM produced by resident macrophages in the intestinal tissue was believed to contribute to intestinal inflammation and that active macrophage-derived AIM in the intestine results in elevated serum AIM levels. AIM is taken up by the adipocytes via CD36-mediated endocytosis, which subsequently induces lipolysis[74,75]. Therefore, it may be involved in the recruitment of inflammatory macrophages and induce TNF-α in mesenteric adipose tissue in CD.
AIM may affect the pathogenesis of CD by not only inhibiting the apoptosis of active intestinal macrophages but also enhancing TNF-α expression in these mesenteric adipose tissues.
Inoue et al[76] reported that glycosylated IgA is produced in the gastrointestinal mucosa of CD and that glycosylated IgA correlates with CD disease activity. These results are crucial because they not only indicate that CD induced IgAN but also serve as evidence of the correlation between CD and IgAN.
We hypothesized the pathogenesis of IgAN associated with Crohn’s disease (Figure 1).
We searched PubMed for studies on CD with IgAN (Supplementary Figure 1). Twenty-five cases have been reported[77-92], including a self-case, with an average age of 27.5 (9–39) years, male-to-female ratio of 20:5 (male 80%), seven cases with underlying IgAN, and seven cases with underlying CD. Moreover, 17 patients had comorbidities, and one case had a simultaneous onset (Table 1).
| Age (yr) | Sex | Clinical course | CD disease type | IgAN MEST-C grade | CD treatment | IgAN treatment | CD and IgAN worsened simultaneously |
| 20 | Female | CD(y)→IgAN(y) | Ileocolitis | NR | SASP | nothing | + |
| 25 | Male | CD(4y)→IgAN(12y) | Ileocolitis | NR | ED only | NR | + |
| 18 | Male | IgAN(NR)→CD(2y) | colitis | NR | 5-ASA | ACE-I | NR |
| 24 | Male | CD(NR)→IgAN(3y) | NR | NR | 5-ASA | NR | - |
| 38 | Male | CD(NR)→IgAN(6y) | NR | NR | PSL+5-ASA →IFX | ACE-I | + |
| 40 | Female | CD(NR)→IgAN(5y) | colitis | NR | 5-ASA | ARB | + |
| 38 | Male | CD(NR)→IgAN(16y) | Ileocolitis | NR | 5-ASA | NR | NR |
| 40 | Male | CD(NR)→IgAN(12y) | NR | NR | PSL+5-ASA | PSL | + |
| 34 | Female | IgAN(4y)→CD(5y) | Ileocolitis | NR | ED only | PSL | + |
| 25 | Male | CD(25y)→IgAN(33y) | NR | M1E1S1T1C1 | ADA | MPT→PSL, ACE-I | - |
| 24 | Male | CD(22y)→IgAN(24y) | Ileitis | M1E0S1T1C2 | IFX →UST | MPT→PSL | - |
| 10 | Male | CD(1y)→IgAN(7y) | colitis | M1E0S0T0C0 | IFX | IFX→ADA, PSL | + |
| 40 | Male | CD(NR)→IgAN(39y) | NR | M1E0S1T0C1 | AZA | PSL | - |
| 31 | Male | CD(16)→IgAN(18y) | Ileocolitis | M1E0S1T1C0 | PSL+5-ASA | PSL | + |
| 46 | Male | IgAN(17y)→CD(40y)→IgAN(46y) | NR | M1E0S0T0C1 | 5-ASA, AZA, IFX | PSL | + |
| 15 | Female | CD(16)→IgAN(18y) | NR | NR | 5-ASA, PSL | IFX | + |
| 13 | Male | IgAN(10y)→CD(13y) | NR | M1E0S0T0C1 | PSL, SASP | PSL, CPA, ACE-I | + |
| 9 | Male | CD(9)→IgAN(11y) | Ileitis | M1E0S0T1C0 | ADA | ADA →AZA; MPT, PSL, ACE-I | - |
| 39 | Female | CD(32)→IgAN(39y) | NR | M1E0S1T0C0 | ADA, AZA | ARB, ADA→IFX | + |
| 27 | Male | IgAN(22y)→CD(27y) | NR | M1E0S1T2C1 | 5-ASA, IFX | MPT, MMF, CsA | + |
| 18 | Male | CD(9)→IgAN(11y) | NR | M1E1S1T1C2 | 5-ASA | PSL, CPA | + |
| 22 | Male | IgAN(8y)→CD(22y) | NR | M1E1S1T1C0 | SASP, AZA | PSL, 5-ASA | + |
| 36 | Male | CD(30)→IgAN(36y) | NR | M1E0S0T2C0 | 5-ASA, AZA | ACE-I | - |
| 29 | Male | IgAN(24y)→CD(29y) | colitis | NR | PSL | non | + |
| 10 | Male | CD(30)→IgAN(36y) | Ileocolitis | M1E0S0T0C0 | ADA, AZA, budesonide | PSL | + |
However, certain cases had urinalysis abnormalities such as proteinuria and microscopic hematuria, which have been pointed out before the definitive diagnosis of IgAN. Because CD also progresses preclinically, identifying the time of onset of CD and IgAN is very difficult.
In the literature, the pathological findings of CD-complicated nephritis and IgAN with CD also tended to have a higher T-score, which represents tubular atrophy and interstitial fibrosis, than IgAN without CD. Five patients developed IgAN during biologic therapy, and CD and proteinuria improved after biologics were discontinued or changed.
Simultaneous exacerbation of both diseases was observed in 17 of 23 patients, and many studies have reported that the disease activity of both diseases was related, suggesting that IgAN is an extraintestinal complication of CD. This tendency was also observed in our case (Figure 2). Considering that the disease progression of both diseases is the same, controlling the disease progression of CD is also important to prevent the progression of renal failure.
The use of immunomodulators to maintain remission in both diseases is an option. In addition, because TNF-α induces glomerular inflammation and enhances permeability[93], anti-TNF-α antibodies are theoretically expected to improve proteinuria.
However, TNF-α inhibitors were also reported to induce systemic vasculitis and several types of glomerulonephritis, such as minimal-change disease group, membranous nephropathy, IgAN, and lupus nephritis[94-98].
Although the following hypotheses have been suggested and the following possibilities have been proposed, the mechanism of vasculitis associated with anti-TNFα therapy is unclear: (1) TNFα/anti-TNFα immune complexes may deposit in small blood vessels and induce local complementary activation[94]; (2) A cytokine imbalance with a shift from a Th1 profile to a Th2 profile could induce symptoms associated with antibody-mediated immunity[95]; and (3) In glomerulonephritis, immune complexes are thought to form by cross-reactivity of Gd-IgA1 and anti-drug antibodies to the glycan structures of TNFα inhibitors. They are deposited in the mesangium and can induce IgAN[96].
The reported detection rate of antinuclear antibodies in patients treated with anti-TNFα ranged from 29% to 76.7%. Immunological abnormalities can lead to glomerulonephritis, including membranous glomerulonephritis and lupus nephritis[97,98].
In many cases, proteinuria progressed simultaneously with CD; moreover, it is possible that IgANand Crohn’s disease occur in parallel. The factors governing the simultaneous occurrence of IgAN and Crohn’s disease is still unknown; however, the involvement of biologics has been pointed out. Although the etiology of CD-IgAN is not fixed and various factors are thought to be involved, the stabilization of the disease condition of CD is believed to help treat IgAN.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Perse M, Slovenia; Triantafillidis J, Greece S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1806] [Article Influence: 225.8] [Reference Citation Analysis (111)] |
| 3. | Rodrigues JC, Haas M, Reich HN. IgA Nephropathy. Clin J Am Soc Nephrol. 2017;12:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 387] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 4. | Huang L, Guo FL, Zhou J, Zhao YJ. IgA nephropathy factors that predict and accelerate progression to end-stage renal disease. Cell Biochem Biophys. 2014;68:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Tomino Y, Endoh M, Nomoto Y, Sakai H. Immunoglobulin A1 and IgA nephropathy. N Engl J Med. 1981;305:1159-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Tomino Y, Sakai H, Miura M, Endoh M, Nomoto Y. Detection of polymeric IgA in glomeruli from patients with IgA nephropathy. Clin Exp Immunol. 1982;49:419-425. [PubMed] |
| 7. | Monteiro RC, Halbwachs-Mecarelli L, Roque-Barreira MC, Noel LH, Berger J, Lesavre P. Charge and size of mesangial IgA in IgA nephropathy. Kidney Int. 1985;28:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Bene MC, Faure G, Duheille J. IgA nephropathy: characterization of the polymeric nature of mesangial deposits by in vitro binding of free secretory component. Clin Exp Immunol. 1982;47:527-534. [PubMed] |
| 9. | Suzuki Y, Tomino Y. The mucosa-bone-marrow axis in IgA nephropathy. Contrib Nephrol. 2007;157:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Harper SJ, Feehally J. The pathogenic role of immunoglobulin A polymers in immunoglobulin A nephropathy. Nephron. 1993;65:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014;86:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | van den Wall Bake AW, Daha MR, Evers-Schouten J, van Es LA. Serum IgA and the production of IgA by peripheral blood and bone marrow lymphocytes in patients with primary IgA nephropathy: evidence for the bone marrow as the source of mesangial IgA. Am J Kidney Dis. 1988;12:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | van den Wall Bake AW, Daha MR, Radl J, Haaijman JJ, Van der Ark A, Valentijn RM, Van Es LA. The bone marrow as production site of the IgA deposited in the kidneys of patients with IgA nephropathy. Clin Exp Immunol. 1988;72:321-325. [PubMed] |
| 14. | Harper SJ, Allen AC, Pringle JH, Feehally J. Increased dimeric IgA producing B cells in the bone marrow in IgA nephropathy determined by in situ hybridisation for J chain mRNA. J Clin Pathol. 1996;49:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Sakai O. IgA nephropathy: current concepts and feature trends. Nephrology. 1997;3:2-3. |
| 16. | van den Wall Bake AW, Daha MR, van Es LA. Immunopathogenetic aspects of IgA nephropathy. Nephrologie. 1989;10:141-145. [PubMed] |
| 17. | Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, Pang H, Horikoshi S, Tomino Y. Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int. 2007;72:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Aizawa M, Suzuki Y, Suzuki H, Pang H, Kihara M, Nakata J, Yamaji K, Horikoshi S, Tomino Y. Uncoupling of glomerular IgA deposition and disease progression in alymphoplasia mice with IgA nephropathy. PLoS One. 2014;9:e95365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, Horikoshi S, Novak J, Tomino Y. Experimental evidence of cell dissemination playing a role in pathogenesis of IgA nephropathy in multiple lymphoid organs. Nephrol Dial Transplant. 2013;28:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Crago SS, Kutteh WH, Moro I, Allansmith MR, Radl J, Haaijman JJ, Mestecky J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J Immunol. 1984;132:16-18. [PubMed] |
| 22. | Jonard PP, Rambaud JC, Dive C, Vaerman JP, Galian A, Delacroix DL. Secretion of immunoglobulins and plasma proteins from the jejunal mucosa. Transport rate and origin of polymeric immunoglobulin A. J Clin Invest. 1984;74:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Kett K, Brandtzaeg P, Radl J, Haaijman JJ. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631-3635. [PubMed] |
| 24. | Brandtzaeg P, Baekkevold ES, Farstad IN, Jahnsen FL, Johansen FE, Nilsen EM, Yamanaka T. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today. 1999;20:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, Terawaki S, Shikina T, Yamamoto M, Kurono Y, Kiyono H. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(-)CD4(+)CD45(+) cells. Immunity. 2002;17:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Kiyono H, Fukuyama S. NALT- vs Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 562] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 27. | Brandtzaeg P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am J Respir Crit Care Med. 2011;183:1595-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Brandtzaeg P. Secretory IgA: Designed for Anti-Microbial Defense. Front Immunol. 2013;4:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 29. | Arsenescu R, Bruno ME, Rogier EW, Stefka AT, McMahan AE, Wright TB, Nasser MS, de Villiers WJ, Kaetzel CS. Signature biomarkers in Crohn's disease: toward a molecular classification. Mucosal Immunol. 2008;1:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Wang J, Anders RA, Wu Q, Peng D, Cho JH, Sun Y, Karaliukas R, Kang HS, Turner JR, Fu YX. Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest. 2004;113:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 588] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 32. | Kang HS, Chin RK, Wang Y, Yu P, Wang J, Newell KA, Fu YX. Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol. 2002;3:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 620] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 34. | Chintalacharuvu SR, Yamashita M, Bagheri N, Blanchard TG, Nedrud JG, Lamm ME, Tomino Y, Emancipator SN. T cell cytokine polarity as a determinant of immunoglobulin A (IgA) glycosylation and the severity of experimental IgA nephropathy. Clin Exp Immunol. 2008;153:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Gesualdo L, Lamm ME, Emancipator SN. Defective oral tolerance promotes nephritogenesis in experimental IgA nephropathy induced by oral immunization. J Immunol. 1990;145:3684-3691. [PubMed] |
| 36. | Yamanaka T, Tamauchi H, Suzuki Y, Suzuki H, Horikoshi S, Terashima M, Iwabuchi K, Habu S, Okumura K, Tomino Y. Release from Th1-type immune tolerance in spleen and enhanced production of IL-5 in Peyer's patch by cholera toxin B induce the glomerular deposition of IgA. Immunobiology. 2016;221:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Akiyama M, Shimomura K, Yoshimoto H, Sako M, Kodama M, Abe K, Gunji M, Kang D, Takaki T, Wada Y, Iyoda M, Honda K. Crohn's disease may promote inflammation in IgA nephropathy: a case-control study of patients undergoing kidney biopsy. Virchows Arch. 2022;481:553-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Perše M, Večerić-Haler Ž. The Role of IgA in the Pathogenesis of IgA Nephropathy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Chiba M, Ohta H, Yagisawa H, Masamune O. IgA1 & IgA2 distribution in the intestine. Gastroenterol Jpn. 1987;22:18-23. [PubMed] |
| 40. | Martín-Penagos L, Fernández-Fresnedo G, Benito-Hernández A, Mazón J, de Cos M, Oviedo MV, San Segundo D, López-Hoyos M, Gómez-Román J, Ruiz JC, Rodrigo E. Measurement of galactosyl-deficient IgA1 by the monoclonal antibody KM55 contributes to predicting patients with IgA nephropathy with high risk of long-term progression. Nefrologia (Engl Ed). 2021;41:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 232] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 43. | Yuzawa Y, Yamamoto R, Takahashi K, Katafuchi R, Tomita M, Fujigaki Y, Kitamura H, Goto M, Yasuda T, Sato M, Urushihara M, Kondo S, Kagami S, Yasuda Y, Komatsu H, Takahara M, Harabuchi Y, Kimura K, Matsuo S. Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin Exp Nephrol. 2016;20:511-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts IS, Feehally J, Herzenberg AM, Cattran DC; Oxford Derivation, North American Validation and VALIGA Consortia; Oxford Derivation North American Validation and VALIGA Consortia. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int. 2016;89:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 45. | Kamano C, Shimizu A, Joh K, Hashiguchi A, Hisano S, Katafuchi R, Kawamura T; Japan IgA nephropathy prospective cohort Study Group. A cross-sectional study in patients with IgA nephropathy of correlations between clinical data and pathological findings at the time of renal biopsy: a Japanese prospective cohort study. Clin Exp Nephrol. 2021;25:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Thuluvath PJ, Ninkovic M, Calam J, Anderson M. Mesalazine induced interstitial nephritis. Gut. 1994;35:1493-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Feehally J, Khosravi M. Effects of acute and chronic hypohydration on kidney health and function. Nutr Rev. 2015;73 Suppl 2:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Wijkström J, González-Quiroz M, Hernandez M, Trujillo Z, Hultenby K, Ring A, Söderberg M, Aragón A, Elinder CG, Wernerson A. Renal Morphology, Clinical Findings, and Progression Rate in Mesoamerican Nephropathy. Am J Kidney Dis. 2017;69:626-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 49. | Momoki K, Kataoka H, Moriyama T, Mochizuki T, Nitta K. Hyperuricemia as a Predictive Marker for Progression of Nephrosclerosis: Clinical Assessment of Prognostic Factors in Biopsy-Proven Arterial/Arteriolar Nephrosclerosis. J Atheroscler Thromb. 2017;24:630-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Heap GA, So K, Weedon M, Edney N, Bewshea C, Singh A, Annese V, Beckly J, Buurman D, Chaudhary R, Cole AT, Cooper SC, Creed T, Cummings F, de Boer NK, D'Inca R, D'Souza R, Daneshmend TK, Delaney M, Dhar A, Direkze N, Dunckley P, Gaya DR, Gearry R, Gore S, Halfvarson J, Hart A, Hawkey CJ, Hoentjen F, Iqbal T, Irving P, Lal S, Lawrance I, Lees CW, Lockett M, Mann S, Mansfield J, Mowat C, Mulgrew CJ, Muller F, Murray C, Oram R, Orchard T, Parkes M, Phillips R, Pollok R, Radford-Smith G, Sebastian S, Sen S, Shirazi T, Silverberg M, Solomon L, Sturniolo GC, Thomas M, Tremelling M, Tsianos EV, Watts D, Weaver S, Weersma RK, Wesley E, Holden A, Ahmad T. Clinical Features and HLA Association of 5-Aminosalicylate (5-ASA)-induced Nephrotoxicity in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Oikonomou KA, Kapsoritakis AN, Stefanidis I, Potamianos SP. Drug-induced nephrotoxicity in inflammatory bowel disease. Nephron Clin Pract. 2011;119:c89-94; discussion c96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Waters AM, Zachos M, Herzenberg AM, Harvey E, Rosenblum ND. Tubulointerstitial nephritis as an extraintestinal manifestation of Crohn's disease. Nat Clin Pract Nephrol. 2008;4:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Izzedine H, Simon J, Piette AM, Lucsko M, Baumelou A, Charitanski D, Kernaonet E, Baglin AC, Deray G, Beaufils H. Primary chronic interstitial nephritis in Crohn's disease. Gastroenterology. 2002;123:1436-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Li G, Wu W, Zhang X, Huang Y, Wen Y, Li X, Gao R. Serum levels of tumor necrosis factor alpha in patients with IgA nephropathy are closely associated with disease severity. BMC Nephrol. 2018;19:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Salvador P, Macías-Ceja DC, Gisbert-Ferrándiz L, Hernández C, Bernardo D, Alós R, Navarro-Vicente F, Esplugues JV, Ortiz-Masiá D, Barrachina MD, Calatayud S. CD16+ Macrophages Mediate Fibrosis in Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Sieber G, Herrmann F, Zeitz M, Teichmann H, Rühl H. Abnormalities of B-cell activation and immunoregulation in patients with Crohn's disease. Gut. 1984;25:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Uzzan M, Colombel JF, Cerutti A, Treton X, Mehandru S. B Cell-Activating Factor (BAFF)-Targeted B Cell Therapies in Inflammatory Bowel Diseases. Dig Dis Sci. 2016;61:3407-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Brandtzaeg P, Carlsen HS, Halstensen TS. The B-cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, Kretzler M, Anders HJ, Sitter T, Mosberger I, Kerjaschki D, Regele H, Schlöndorff D, Segerer S. The contribution of B cells to renal interstitial inflammation. Am J Pathol. 2007;170:457-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Xavier S, Sahu RK, Landes SG, Yu J, Taylor RP, Ayyadevara S, Megyesi J, Stallcup WB, Duffield JS, Reis ES, Lambris JD, Portilla D. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2017;312:F516-F532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 61. | Liu Y, Wang K, Liang X, Li Y, Zhang Y, Zhang C, Wei H, Luo R, Ge S, Xu G. Complement C3 Produced by Macrophages Promotes Renal Fibrosis via IL-17A Secretion. Front Immunol. 2018;9:2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Surface epithelium related activation of complement differs in Crohn's disease and ulcerative colitis. Gut. 1992;33:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Sugihara T, Kobori A, Imaeda H, Tsujikawa T, Amagase K, Takeuchi K, Fujiyama Y, Andoh A. The increased mucosal mRNA expressions of complement C3 and interleukin-17 in inflammatory bowel disease. Clin Exp Immunol. 2010;160:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Freedman BI, Spray BJ, Heise ER. HLA associations in IgA nephropathy and focal and segmental glomerulosclerosis. Am J Kidney Dis. 1994;23:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Toyoda H, Wang SJ, Yang HY, Redford A, Magalong D, Tyan D, McElree CK, Pressman SR, Shanahan F, Targan SR. Distinct associations of HLA class II genes with inflammatory bowel disease. Gastroenterology. 1993;104:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 153] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Hubert D, Beaufils M, Meyrier A. [Immunoglobulin A glomerular nephropathy associated with inflammatory colitis. Apropos of 2 cases]. Presse Med. 1984;13:1083-1085. [PubMed] |
| 67. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2007] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 68. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3603] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 69. | McGovern DP, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagacé C, Li C, Green T, Stevens CR, Beauchamp C, Fleshner PR, Carlson M, D'Amato M, Halfvarson J, Hibberd ML, Lördal M, Padyukov L, Andriulli A, Colombo E, Latiano A, Palmieri O, Bernard EJ, Deslandres C, Hommes DW, de Jong DJ, Stokkers PC, Weersma RK; NIDDK IBD Genetics Consortium, Sharma Y, Silverberg MS, Cho JH, Wu J, Roeder K, Brant SR, Schumm LP, Duerr RH, Dubinsky MC, Glazer NL, Haritunians T, Ippoliti A, Melmed GY, Siscovick DS, Vasiliauskas EA, Targan SR, Annese V, Wijmenga C, Pettersson S, Rotter JI, Xavier RJ, Daly MJ, Rioux JD, Seielstad M. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 70. | Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, Fasel D, Lata S, Prakash S, Shapiro S, Fischman C, Snyder HJ, Appel G, Izzi C, Viola BF, Dallera N, Del Vecchio L, Barlassina C, Salvi E, Bertinetto FE, Amoroso A, Savoldi S, Rocchietti M, Amore A, Peruzzi L, Coppo R, Salvadori M, Ravani P, Magistroni R, Ghiggeri GM, Caridi G, Bodria M, Lugani F, Allegri L, Delsante M, Maiorana M, Magnano A, Frasca G, Boer E, Boscutti G, Ponticelli C, Mignani R, Marcantoni C, Di Landro D, Santoro D, Pani A, Polci R, Feriozzi S, Chicca S, Galliani M, Gigante M, Gesualdo L, Zamboli P, Battaglia GG, Garozzo M, Maixnerová D, Tesar V, Eitner F, Rauen T, Floege J, Kovacs T, Nagy J, Mucha K, Pączek L, Zaniew M, Mizerska-Wasiak M, Roszkowska-Blaim M, Pawlaczyk K, Gale D, Barratt J, Thibaudin L, Berthoux F, Canaud G, Boland A, Metzger M, Panzer U, Suzuki H, Goto S, Narita I, Caliskan Y, Xie J, Hou P, Chen N, Zhang H, Wyatt RJ, Novak J, Julian BA, Feehally J, Stengel B, Cusi D, Lifton RP, Gharavi AG. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 71. | Yuan J, Wang Z, Wang YP. Identification of common key genes associated with Crohn's Disease and IgA nephropathy. Eur Rev Med Pharmacol Sci. 2022;26:3607-3620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | Takahata A, Arai S, Hiramoto E, Kitada K, Kato R, Makita Y, Suzuki H, Nakata J, Araki K, Miyazaki T, Suzuki Y. Crucial Role of AIM/CD5L in the Development of Glomerular Inflammation in IgA Nephropathy. J Am Soc Nephrol. 2020;31:2013-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 73. | Ono Y, Kanmura S, Morinaga Y, Oda K, Kawabata K, Arima S, Sasaki F, Nasu Y, Tanoue S, Hashimoto S, Taguchi H, Uto H, Tsubouchi H, Ido A. The utility of apoptosis inhibitor of macrophages as a possible diagnostic marker in patients with Crohn's disease. BMC Gastroenterol. 2017;17:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Kurokawa J, Nagano H, Ohara O, Kubota N, Kadowaki T, Arai S, Miyazaki T. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011;108:12072-12077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Fink C, Karagiannides I, Bakirtzi K, Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2012;18:1550-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Inoue T, Iigima T, Shiraisi E, Hiyama S, Mukai A, Nakashima S, Shinzaki S, Nishida T, Miyoshi H, Tujii M, Takehara T. Diagnosis and prognosis of inflammatory bowel disease based on IgA glycan structure. Nihon Shokakibyo Gakkai Zasshi 2011; 108: A837. |
| 77. | Forshaw MJ, Guirguis O, Hennigan TW. IgA nephropathy in association with Crohn's disease. Int J Colorectal Dis. 2005;20:463-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Lee JM, Lee KM, Kim HW, Chung WC, Paik CN, Lee JR, Choi YJ, Yang JM. [Crohn's disease in association with IgA nephropathy]. Korean J Gastroenterol. 2008;52:115-119. [PubMed] |
| 79. | Angeletti A, Arrigo S, Madeo A, Molteni M, Vietti E, Arcuri L, Coccia MC, Gandullia P, Ghiggeri GM. Different renal manifestations associated with very early onset pediatric inflammatory bowel disease: case report and review of literature. BMC Nephrol. 2021;22:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Graziano F, Busè M, Cassata N, Lentini VL, Citrano M. IgA nephropathy in a child: Crohn's disease-associated or adalimumab induced? Curr Med Res Opin. 2022;38:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Terasaka T, Uchida HA, Umebayashi R, Tsukamoto K, Tanaka K, Kitagawa M, Sugiyama H, Tanioka H, Wada J. The possible involvement of intestine-derived IgA1: a case of IgA nephropathy associated with Crohn's disease. BMC Nephrol. 2016;17:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Ueno Y, Tanaka S, Onitake T, Hanaoka R, Yoshioka K, Ito M, Chayama K. Infliximab treatment for Crohn's disease in a patient with IgA nephropathy. Clin J Gastroenterol. 2009;2:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Filiopoulos V, Trompouki S, Hadjiyannakos D, Paraskevakou H, Kamperoglou D, Vlassopoulos D. IgA nephropathy in association with Crohn's disease: a case report and brief review of the literature. Ren Fail. 2010;32:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Choi JY, Yu CH, Jung HY, Jung MK, Kim YJ, Cho JH, Kim CD, Kim YL, Park SH. A case of rapidly progressive IgA nephropathy in a patient with exacerbation of Crohn's disease. BMC Nephrol. 2012;13:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Kanazawa N, Wada Y, Akiyama M, Shikida Y, Sugiyama M, Abe M, Iyoda M, Honda K, Shibata T. Crescentic IgA nephropathy after administration of human monoclonal interleukin-12/23p40 antibody in a patient with Crohn's disease: a case report. CEN Case Rep. 2020;9:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Youm JY, Lee OY, Park MH, Yang SY, Han SH, Baek YH, Park SR, Lee HL, Yoon BC, Choi HS, Hahm JS, Lee MH, Lee DH, Kee CS. [Crohn's disease associated with IgA nephropathy]. Korean J Gastroenterol. 2006;47:324-328. [PubMed] |
| 87. | Mertelj T, Smrekar N, Kojc N, Lindič J, Kovač D. IgA Nephropathy in a Patient Treated with Adalimumab. Case Rep Nephrol Dial. 2021;11:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Arai O, Shibata N, Kuboki M, Ikeda H, Omoto K. [A case of Crohn's disease concomitant with IgA nephropathy]. Nihon Shokakibyo Gakkai Zasshi. 2013;110:1265-1271. [PubMed] |
| 89. | Bhagat Singh AK, Jeyaruban AS, Wilson GJ, Ranganathan D. Adalimumab-induced IgA nephropathy. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Zhang Q, Li H, Huang WF. When Crohn's disease meets IgA nephropathy: What do you think? Am J Med Sci. 2023;365:e78-e79. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 91. | Takemura T, Okada M, Yagi K, Kuwajima H, Yanagida H. An adolescent with IgA nephropathy and Crohn disease: pathogenetic implications. Pediatr Nephrol. 2002;17:863-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Hasegawa M, Sasaki H, Takahashi K, Hayashi H, Koide S, Tomita M, Takeda A, Hoshinaga K, Yuzawa Y. Recurrent IgA nephropathy complicated with Crohn's disease after renal transplantation. CEN Case Rep. 2014;3:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 93. | McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998;9:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Marques I, Lagos A, Reis J, Pinto A, Neves B. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Saint Marcoux B, De Bandt M; CRI (Club Rhumatismes et Inflammation). Vasculitides induced by TNFalpha antagonists: a study in 39 patients in France. Joint Bone Spine. 2006;73:710-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 96. | Di Lernia V. IgA nephropathy during treatment with TNF-alpha blockers: Could it be predicted? Med Hypotheses. 2017;107:12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Stokes MB, Foster K, Markowitz GS, Ebrahimi F, Hines W, Kaufman D, Moore B, Wolde D, D'Agati VD. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant. 2005;20:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 98. | Atzeni F, Sarzi-Puttini P. Autoantibody production in patients treated with anti-TNF-alpha. Expert Rev Clin Immunol. 2008;4:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |