Published online Sep 20, 2022. doi: 10.5662/wjm.v12.i5.448
Peer-review started: October 28, 2021
First decision: December 27, 2021
Revised: December 30, 2021
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: September 20, 2022
Processing time: 323 Days and 1.4 Hours
Microvessel density (MVD) has been proposed as a direct quantification method of tumor neovascularization. However, the current literature regarding the role of MVD in differentiated thyroid carcinoma (DTC) remains inconclusive.
To appraise the effect of tumoral MVD on the survival of patients with DTC.
This meta-analysis was based on the PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. The electronic databases Medline, Web of Science, and Scopus were systematically screened. A fixed-effects or random-effects model was used, according to the Cochran Q test. The data were then extracted and assessed on the basis of the Reference Citation Analysis (https://www.referencecitationanalysis.com/).
A total of nine studies were included in the present study. Superiority of low MVD tumors in terms of 10-year disease free survival (OR: 0.21, 95%CI: 0.08–0.53) was recorded. Lowly vascularized thyroid cancers had a lower recurrence rate (OR: 13.66, 95%CI: 3.03–61.48). Moreover, relapsing tumors [weighed mean difference (WMD): 11.92, 95%CI: 6.32–17.52] or malignancies with regional lymph node involvement (WMD: 8.53, 95%CI: 0.04–17.02) presented with higher tumoral MVD values.
MVD significantly correlates with the survival outcomes of thyroid cancer patients. However, considering several study limitations, further prospective studies of higher methodological and quality level are required.
Core Tip: This systematic review is the first meta-analysis investigating the effect of tumoral vascularity, through microvessel density (MVD) assessment, on the survival of patients with differentiated thyroid carcinoma. Higher intratumoral MVD values were associated with inferior disease-free survival outcomes.
- Citation: Perivoliotis K, Samara AA, Koutoukoglou P, Ntellas P, Dadouli K, Sotiriou S, Ioannou M, Tepetes K. Microvessel density in differentiated thyroid carcinoma: A systematic review and meta-analysis. World J Methodol 2022; 12(5): 448-458
- URL: https://www.wjgnet.com/2222-0682/full/v12/i5/448.htm
- DOI: https://dx.doi.org/10.5662/wjm.v12.i5.448
Thyroid cancer is the most common endocrine tumor and includes several subtypes with different histologic, epidemiologic, and prognostic characteristics. Although they display a stable mortality rate, thyroid carcinomas are characterized by a rising trend of overall incidence of nearly 5.5% annually[1-3]. The above-mentioned increase is primarily attributed to a steady increment of new papillary thyroid cancer cases[1-3]. This is translated to an average of 56000 new cases and 2000 deaths per year in the United States alone[4]. Therefore, an attempt to identify survival-prognostic indicators for thyroid cancer has been implemented[5,6]. More specifically, extrathyroidal infiltration, aggressive histological pattern, vascular invasion, lymph node involvement, distant metastases, and BRAF mutations have been linked to a poorer survival outcome[7,8]. Various other serological, genetic, molecular, and immu
Angiogenesis represents a pivotal part of tumor expansion and metastasis development. It includes a cascade of processes such as degradation of the basal membrane, remodeling of the extracellular matrix, migration of the endothelial cells, and maturation of the newly formed capillaries, which are regulated by several angiogenic and angioinhibitory factors[12-14]. Activation of the “angiogenic switch” due to alterations in the concentration of agents, such as vascular endothelial growth factor (VEGF) and thrombospondin-1 (TSP-1), as well as the subsequent upregulation of the de novo formation of blood vessels, has been associated with survival outcomes in thyroid cancer[15,16]. Microvessel density (MVD) as described by Weidner et al[17] has been proposed as a direct quantification method of tumor neovascularization. The methodology of MVD assessment involves the immunohistochemical staining for endothelium specific markers, such as von Willebrand factor (vWF), cluster of differentiation (CD)31, and CD34, for the labeling of microvessels[18]. The correlation between survival outcome and vascularity of a solid tumor has been extensively validated[19-22].
The current literature regarding the role of MVD in differentiated thyroid carcinoma (DTC) remains inconclusive. Initial studies reported that MVD displayed negative prognostic value in terms of survival, and was reversibly associated with the differentiation status of thyroid carcinomas[9,23,24]. However, subsequent trials did not confirm the prognostic role of MVD value or even document a positive correlation with survival endpoints[25,26]. Taking into consideration the above-mentioned evidence, a systematic literature review and meta-analysis was designed and conducted to clarify the effect of tumoral vascularity - through MVD assessment - on the survival of patients with DTC.
This review was performed by applying the guidelines proposed in the PRISMA Statement and the Cochrane Handbook for Systematic Reviews of Interventions[27].
The primary endpoint of the present meta-analysis was the pooled odds ratio (OR) of disease-free survival (DFS) between high and low MVD measurements in patients with DTC[28,29]. Secondary endpoints included the hazard ratio (HR) of DFS and the OR of overall survival (OS) and DFS at specific time endpoints (5 and 10 years). Moreover, the effect of MVD on certain disease outcomes was examined, such as lymph node involvement, extrathyroidal infiltration, and recurrence rates.
All prospective or retrospective studies that included a trial population diagnosed with DTC, reported outcomes of interest in English, and could be retrieved were considered as eligible. The MVD assessment of the primary tumor should have been introduced in the study design. The exclusion criteria for this meta-analysis were studies: (1) Written in a language other than English; (2) With no outcome of interest; (3) With insufficient data; (4) With no human subjects; (5) Including a pediatric study population; (6) Including undifferentiated or medullary thyroid cancer; or (7) In the form of editorials, letters, conference abstracts, or expert opinions.
A systematic literature search was performed in the electronic scholar databases Medline, Scopus, and Web of Science. The last search date was August 31, 2021. The following keywords were used: “Thyroid”, “thyroid cancer”, “thyroid carcinoma”, “papillary”, “follicular”, “Hurthle cell”, “well differentiated”, “MVD”, “microvessel density”, “microvascular density”, and “vessel density”.
The first step of our review was removal of duplicate entries, followed by screening of titles and abstracts for consistency with the eligibility criteria. The remaining articles were submitted to a full text review. Searching of electronic databases, study selection, data extraction, and methodological assessment of the studies were performed blindly and in duplicate by two independent investigators (Perivoliotis K, Koutoukoglou P). If disagreement arose between the two investigators, a mutual revision and discussion process followed. If consensus was not achieved, the opinion of a third researcher was considered (Ntellas P). The methodological and quality evaluation was performed on the basis of the Newcastle-Ottawa Scale (NOS)[30]. This evaluation tool ranks non-RCT trials based on different domains, such as selection and comparability of the study groups and confirmation of the exposure. All eligible studies were rated with a score ranging from 0 to 9. Interrater agreement was estimated based on Cohens k statistic.
The statistical software used for the analyses included the Cochrane Collaboration RevMan version 5.3 and IBM SPSS version 23. All results are presented with the corresponding 95%CI. If the trials included did not directly provide data concerning the HR and OR endpoints, they were then estimated through the implementation of the algorithm proposed by Parmar et al[31] and Tierney et al[32]. By utilizing digitizing software, an accurate reconstruction of the primary data from the Kaplan-Meier (KM) curves was performed[33,34]. Furthermore, if the mean and standard deviation (SD) of the continuous variables were not reported, they were estimated from the respective median, range, or interquartile range (IQR)[35,36].
The statistical methods applied were the Maentel-Haenszel (MH) and inverse variance (IV), for OR and HR, respectively. If a statistically significant heterogeneity was present (Cochran Q test P < 0.1), a random-effects (RE) model was used. Otherwise, the pooled result estimation was based on a fixed-effects (FE) model. Overall heterogeneity was also quantified through the calculation of I2. Statistical significance was considered at the level of P < 0.05.
Visual inspection of the primary outcome funnel plot was applied, to identify possible outliers. Moreover, Egger’s statistical test was calculated.
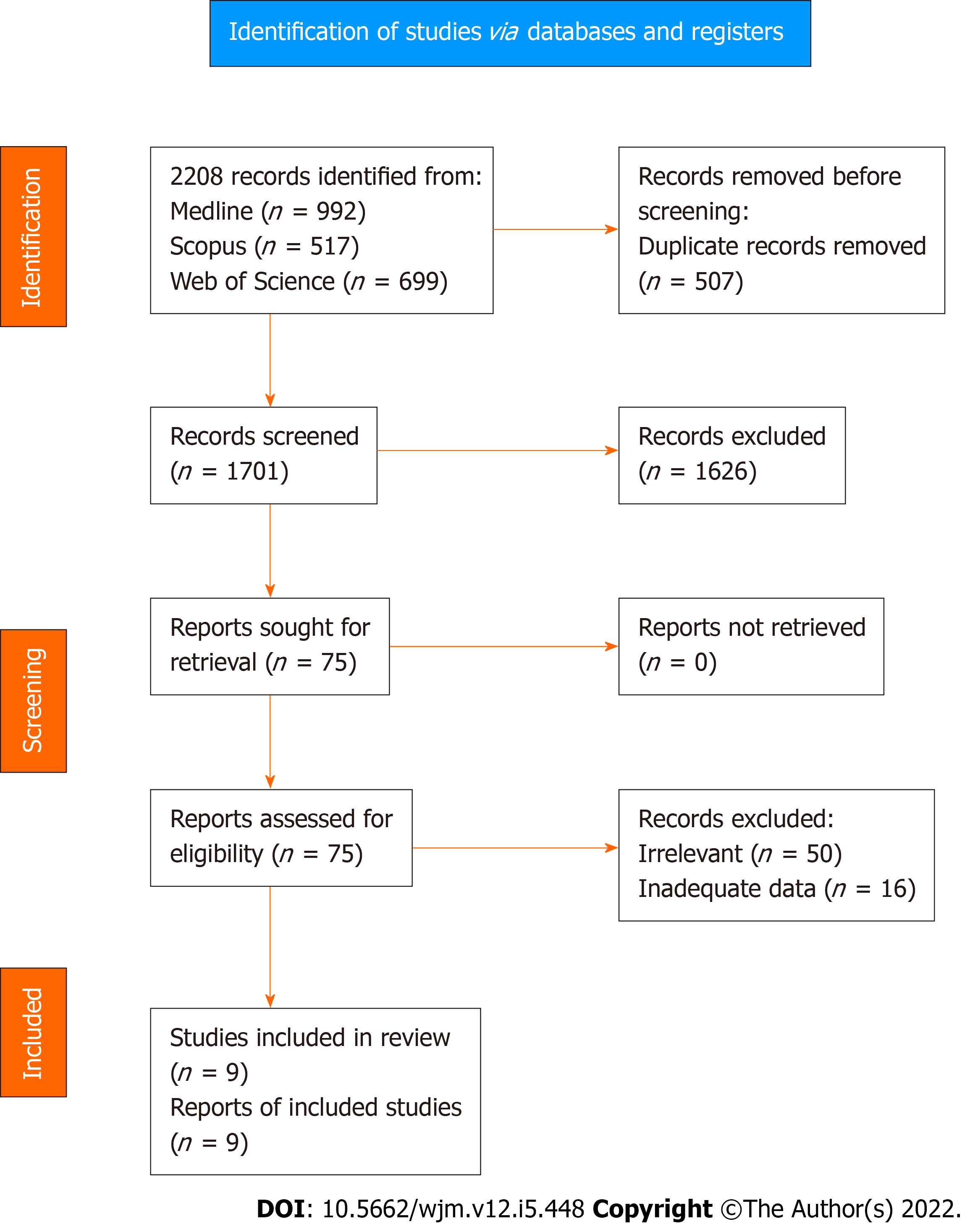
Application of the search algorithm resulted in the retrieval of 2208 citations (Figure 1). More specifically, the number of studies identified through Medline, Scopus, and Web of Science were 992, 517, and 699, respectively. After removal of 507 duplicate records, 1701 titles and abstracts were reviewed. In this phase of literature screening, 1626 studies (125 non-human studies, 130 reviews or meta-analyses, and 1371 irrelevant trials) were excluded. Full text review was applied to 75 articles to assess consistency with the inclusion criteria. After the exclusion of 50 irrelevant records and 16 studies with inadequate survival data, a total of nine trials[23,25,37-43] were introduced in the qualitative and quantitative synthesis of the present systematic review.
Table 1 summarizes the characteristics of studies included in the systematic review. Concerning the study design, all trials were retrospective and single centered, with publication years ranging from 1998 to 2017. In total, 738 patients were included in this meta-analysis. Mean age and gender allocation are also presented in Table 1. Mean follow up extended from 61.7 mo to 180 mo.
| Ref. | Type of study | Year | Country | Center | Sample (patients) | Age | Gender (male/female) | Follow-up |
| Lee et al[36] | Retrospective | 2017 | Korea | Single center | 202 | 43.4 (13.6) | 43/159 | NA |
| Liu et al[37] | Retrospective | 2017 | China | Single center | 42 | 49.1 (13.5) | 9/33 | NA |
| Hakala et al[40] | Retrospective | 2014 | Finland | Single center | 51 | 52 | 19/32 | NA |
| Lee et al[41] | Retrospective | 2012 | Korea | Single center | 47 | > 45: 24 | 11/36 | NA |
| Yasuoka et al[38] | Retrospective | 2005 | Japan | Single center | 49 | 48.8 (15) | 7/42 | NA |
| Kilicarslan et al[39] | Retrospective | 2003 | Turkey | Single center | 48 | 39.8 | 21/27 | 61.7 (29.7) |
| Akslen et al[25] | Retrospective | 2000 | Norway | Single center | 128 | 45.1 | 36/89 | 145 (35.8) |
| Dhar et al[35] | Retrospective | 1998 | Japan | Single center | 71 | 50 (9.8) | 11/60 | 180 mo |
| Ishiwata et al[23] | Retrospective | 1998 | Japan | Single center | 100 | 48 (9.6) | 5/95 | 101 mo |
Supplementary Table 1 provides information regarding the tumor characteristics. The most frequent malignancy was papillary thyroid carcinoma (PTC) (708 cases), followed by follicular thyroid carcinoma (FTC) (27 cases). Although data regarding the tumor stage and the TNM classification were scarce and inconsistent, the respective allocations are also displayed.
Regarding MVD assessment method (Supplementary Table 2), in the majority of the articles[23,25,37,39,40], the technique proposed by Weidner et al[17] was implemented. In the remaining studies[38,41-43], variations of the hot spot method, such as the methodology described by Bono et al[44], were applied. The antibodies used for the immunochemical staining of the microvessels included the anti-CD34[35-39], anti-CD31[42,43], and anti-VIII antibodies[23,25]. The initial magnification applied spanned from 4 × to 40 ×, whereas the final magnification included values ranging from 200 × to 400 ×. The number of pathologists and hot spots examined varied among studies, thereby increasing the methodological heterogeneity. Blinding of the MVD estimator was applied in four trials[23,37,40,43]. Assessment of both intra- and peri-tumoral vessels was performed in only two studies[25,40]. Furthermore, the MVD cut off values are included in Supplementary Table 2. Overall, 324 total or subtotal thyroidectomies and 71 lobectomies were performed Supplementary Table 3). Lymph node dissection was reported in 574 cases. Data regarding the adjuvant chemotherapy or radiotherapy mode were not systematically provided.
Supplementary Table 4 provides a detailed report on the quality and methodological evaluation of the included trials. Although the number of stars awarded ranged from 3[39] to 7[42], the majority of trials received a 5 star grade. A satisfying rate of interrater agreement was identified (Cohen’s k: 72.1%, P < 0.001).
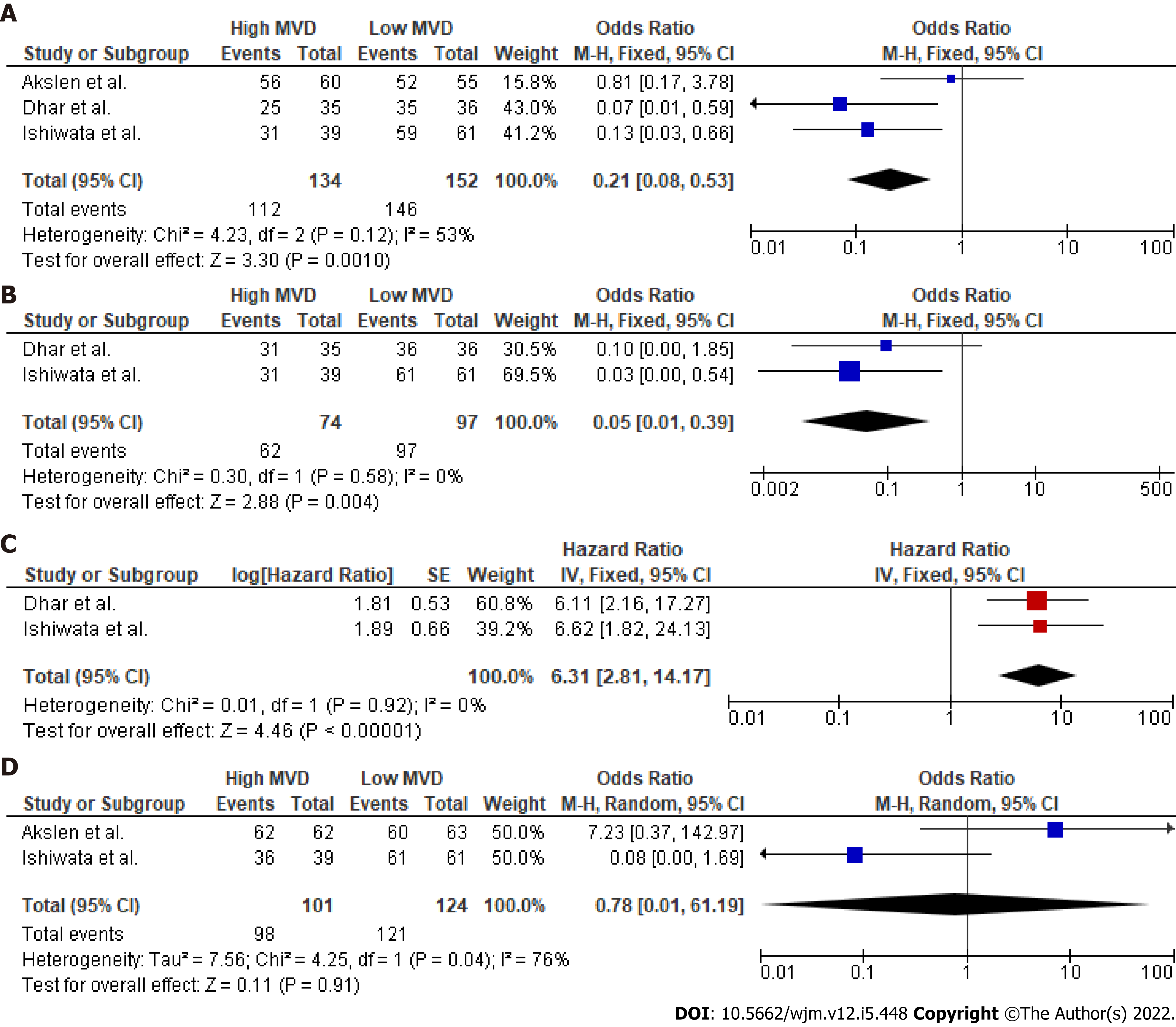
Data regarding the primary outcome were extracted from three studies (Figure 2). Pooled analysis of these data showed a statistically significant OR (P < 0.001) for DFS between high and low MVD groups (OR: 0.21, 95%CI: 0.08–0.53). Heterogeneity levels were not significant (Q test P = 0.12, I2=53%) and as a result, a FE model was applied. Due to the small number of studies reporting on the primary outcome and the moderate heterogeneity, further sub-analyses included only sensitivity analysis Supple
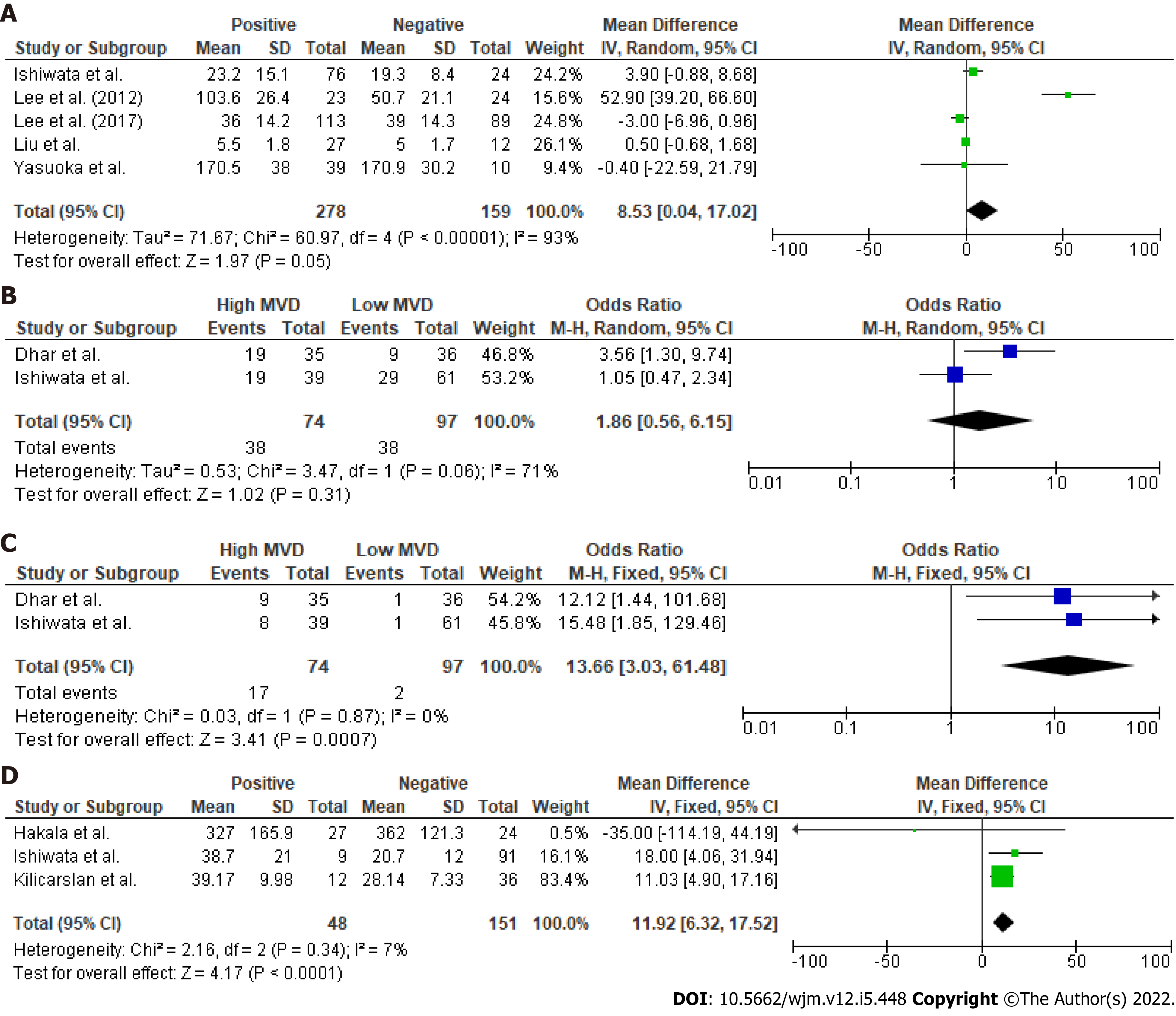
In accordance with the primary outcome, a statistically significant OR for DFS at 5 years (Figure 1) was identified (P = 0.004). Therefore, overall HR (P < 0.001) for DFS was in favor of the low vascularity group (HR: 6.31, 95%CI: 2.81–14.17). However, meta-analysis of the raw data at 10 years postoperatively did not show a significant difference in survival terms (10-year OS: OR: 0.78, 95%CI: 0.01–61.19, P = 0.91). In total, five studies (Figure 3) provided data regarding mean MVD measurements between tumors with positive and negative lymph nodes. Although heterogeneity was high (Q test P < 0.001, I2 = 93%), tumors that involved lymph nodes had higher mean MVD measurements (weighed mean difference [WMD]: 8.53, 95%CI: 0.04–17.02, P = 0.05) when compared to DTCs with negative nodes. Despite this, extrathyroidal infiltration was not associated with tumoral vascularity (OR: 1.86, 95%CI: 0.56–6.15, P = 0.31). Finally, recurrence rates in DTCs were significantly higher in the highly vascularized tumors (OR: 13.66, 95%CI: 3.03–61.48, P = 0.0007). Besides this, the thyroid malignancies that relapsed had significantly higher mean vascularization values (WMD: 11.92, 95%CI: 6.32–17.52, P < 0.001) than those that did not recur.
Concerning the funnel plot of the primary outcome Supplementary Figure 2), eligible trials were symmetrically distributed on both sides of the combined effect size line. Moreover, Egger’s test did not confirm the presence of a significant publication bias (P = 0.585).
Our study validated a negative linkage between the intratumoral vascularity and the survival outcomes in DTC. Specifically, higher MVD values translated to a lower HR of DFS. In a similar manner, the DFS probabilities at 5 and 10 years after diagnosis increased when the DTC was hypovascularized. Furthermore, lymph node metastases were associated with a denser microvessel plexus in the primary tumor. In terms of recurrence, higher MVD measurements were correlated to superior relapse rates and vice versa. The rate of extrathyroidal invasion, however, did not appear to be affected by the tumor vascularization pattern. The effect of microvessel quantity in thyroid carcinoma is still a matter of controversy[16]. In 1994, Herrmann and his colleagues reported that reduced vascularization was found in less differentiated tumors[9]. Similarly, according to Kavantzas et al[45], FTCs and medullary thyroid cancers (MTCs) were characterized by different mean MVD values. Diversity in the neovascularization pattern was also found among the differentiated carcinomas. Giatromanolaki et al[46] showed that FTCs displayed a higher vascular density, whereas subsequent research by Gulubova et al[26] suggested higher CD31 MVD in PTCs. However, several successive studies which applied either a CD34 or CD31 immunohistochemical marker for staining of the endothelium, could not identify a correlation between MVD and histology[10,11].
The fact that most of our quantitative comparisons were statistically significant suggests a possibly strong overall correlation between MVD and prognosis in DTCs. In a retrospective analysis of 71 DTCs, Dhar et al[37] correlated a lower recurrence free survival rate with a hypervascularized tumor. Correspondingly, using VIII-related immunohistochemical stain, Ishiwata et al[23] identified mean microvessel count as an independent prognostic factor for DFS. A denser angiogenetic pattern was also reported in tumors with a higher metastatic potential[47]. Additionally, MVD has been found significantly higher in malignancies with high risk characteristics, such as extrathyroidal and vascular invasion[48].
In contrast to the above-mentioned statements, a considerable number of studies do not recognize the prognostic character of MVD in thyroid carcinomas. Goldenberg et al[11] showed that although mean vessel density in the tumor was higher when compared to the healthy surrounding tissues, MVD lacked a significant correlation with histology or recurrence rates. Furthermore, in the study by Gulubova et al[26], postoperative survival rates in PTC patients were not associated with MVD values. Lee et al[43] also suggested that lymph node status was not linked to the MVD value of the primary malignancy. Moreover, in a study by Akslen et al[25], higher MVD was associated with improved OS rates in PTC patients.
The process of angiogenesis and the corresponding modulators have been extensively studied and related to MVD in thyroid carcinoma. VEGF was directly linked to the number of microvessels, and was characterized as a negative prognostic index for lymph node metastasis as well as local and distant recurrence[26,40,41,43]. A higher rate of immunoreactive cells for metalloproteinase-9, an enzyme necessary for collagen degradation and subsequent angiogenesis, were present in advanced stages of FTCs[14]. Increased values of circulating and tumoral angiopoietins (Ang) have also been linked to poorer outcomes in thyroid cancer[49-51]. Based on the work of Tanaka et al, the levels of TSP-1 have been inversely correlated with the infiltration status of the tumor and MVD[52]. As a result, ratios representing the balance of angiogenic and inhibitory factors VEGF/TSP-1, VEGF-C/TSP-1, and Ang-2/TSP-1 have been significantly associated with the number of microvessels[52].
In addition to prognosis, tumor vascularization has also been proposed as a diagnostic tool in thyroid carcinomas. Using color flow Doppler sonographic analysis with a cut off value at 70% of microvessels, differential diagnosis between PTCs and adenomas or adenomatous nodules demonstrated a sensitivity of 92% and specificity of 89%[53]. The administration of contrast agents further validated the correlation of tumoral MVD and ultrasonographic assessment of vascularity, and increased the accuracy of PTC detection at the level of 95.9%[54,55]. In addition, the application of a shear elastography model by Gu et al[56] linked tumor stiffness with MVD values. Therefore, subsequent studies examined the role of the relationship between ultrasound estimation of vascularity and MVD, as a potential prognostic and risk assessment factor[38,39].
Before assessing the results of our meta-analysis, several limitations should be appraised. First, only a limited number of studies with a small sample size were introduced in each comparison, thus compromising the validity of our estimations. Moreover, all eligible studies had a retrospective study design, with a moderate-to-low methodological evaluation. Although significant heterogeneity was identified in only two endpoints, bias could be introduced from the non-homogeneous stratification of factors such as the histopathological subtype, the stage, and the TNM status. Another source of potential bias could be the heterogeneous allocation in operative and adjuvant treatment modules. Inconsistency was further identified in technical characteristics of the MVD assessment process. Finally, the estimation of survival endpoints required the reconstruction of raw information from the KM curves; therefore, a small amount of bias was inherent in our data extraction methodology, although this process has been reported and applied in several studies[31,32,57].
The present systematic review and meta-analysis is the first study that attempts to provide a pooled correlation between MVD and survival endpoints in DTC. Higher intratumoral MVD values were associated with inferior DFS outcomes. Moreover, the thyroid malignancies presenting with lymph node infiltration displayed a higher vascularization pattern. Similarly, relapsing thyroid cancers when compared to non-recurring tumors were characterized by a denser microvascular plexus. Our study concludes that there are significant primary indications of a negative relationship between intratumoral MVD and survival outcomes. However, to clarify the exact effect of MVD on thyroid cancer and due to several study limitations, further prospective studies with a larger sample size as well as a higher methodological and quality level are required.
MVD significantly correlates with the survival outcomes of thyroid cancer patients. However, considering several study limitations, further prospective studies of higher methodological and quality level are required.
An attempt to identify survival-prognostic indicators for thyroid cancer has been implemented
Microvessel density (MVD) has been used as a direct quantification method of tumor neovascularization
This meta-analysis attempted to clarify the effect of tumoral vascularity - through MVD assessment - on the survival of patients with differentiated thyroid carcinoma (DTC).
The present meta-analysis was based on the PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions.
Lowly vascularized thyroid cancers had a lower recurrence rate. Moreover, relapsing tumors or malignancies with regional lymph node involvement presented with higher tumoral MVD values.
MVD significantly correlates with the survival outcomes of DTC patients
Further prospective studies and randomized controlled trials have to be conducted in order to elucidate the correlation between MVD and prognosis in DTC.
Special thanks to Anagnostopoulos L for language editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report”s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fang L, China; Lee KS, South Korea S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017;317:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1532] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 2. | Shah JP. Thyroid carcinoma: epidemiology, histology, and diagnosis. Clin Adv Hematol Oncol. 2015;13:3-6. [PubMed] |
| 3. | Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 702] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 5. | Passler C, Scheuba C, Prager G, Kaczirek K, Kaserer K, Zettinig G, Niederle B. Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. Endocr Relat Cancer. 2004;11:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Duntas L, Grab-Duntas BM. Risk and prognostic factors for differentiated thyroid cancer. Hell J Nucl Med. 2006;9:156-162. [PubMed] |
| 7. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9647] [Article Influence: 1071.9] [Reference Citation Analysis (1)] |
| 8. | Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1021] [Article Influence: 113.4] [Reference Citation Analysis (1)] |
| 9. | Herrmann G, Schumm-Draeger PM, Müller C, Atai E, Wenzel B, Fabian T, Usadel KH, Hübner K. T lymphocytes, CD68-positive cells and vascularisation in thyroid carcinomas. J Cancer Res Clin Oncol. 1994;120:651-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Rydlova M, Ludvikova M, Stankova I. Potential diagnostic markers in nodular lesions of the thyroid gland: an immunohistochemical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Goldenberg JD, Portugal LG, Wenig BL, Ferrer K, Wu JC, Sabnani J. Well-differentiated thyroid carcinomas: p53 mutation status and microvessel density. Head Neck. 1998;20:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 1014] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 13. | Rzeszutko M, Rzeszutko W, Dziegiel P. The morphological analysis of vasculature in thyroid tumours: immunoexpression of CD34 antigen. Folia Histochem Cytobiol. 2004;42:235-240. [PubMed] |
| 14. | FrigugliettI CU, Mello ES, Castro IV, Filho GB, Alves VA. Metalloproteinase-9 immunoexpression and angiogenesis in thyroid follicular neoplasms: relation to clinical and histopathologic features. Head Neck. 2000;22:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Rotondi M, Coperchini F, Latrofa F, Chiovato L. Role of Chemokines in Thyroid Cancer Microenvironment: Is CXCL8 the Main Player? Front Endocrinol (Lausanne). 2018;9:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Sprindzuk MV. Angiogenesis in Malignant Thyroid Tumors. World J Oncol. 2010;1:221-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4087] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 18. | Marien KM, Croons V, Waumans Y, Sluydts E, De Schepper S, Andries L, Waelput W, Fransen E, Vermeulen PB, Kockx MM, De Meyer GR. Development and Validation of a Histological Method to Measure Microvessel Density in Whole-Slide Images of Cancer Tissue. PLoS One. 2016;11:e0161496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94 1823-1832 [PMID:16773076 DOI: 10.1038/sj.bjc.6603176. |
| 20. | Li Y, Ma X, Zhang J, Liu X, Liu L. Prognostic value of microvessel density in hepatocellular carcinoma patients: a meta-analysis. Int J Biol Markers. 2014;29:e279-e287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hong WG, Ko YS, Pyo JS. Clinicopathological significance and prognostic role of microvessel density in gastric cancer: A meta-analysis. Pathol Res Pract. 2017;213:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ma G, Zhang J, Jiang H, Zhang N, Zhu Y, Deng Y, Zhou Q. Microvessel density as a prognostic factor in esophageal squamous cell cancer patients: A meta-analysis. Medicine (Baltimore). 2017;96:e7600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ishiwata T, Iino Y, Takei H, Oyama T, Morishita Y. Tumor angiogenesis as an independent prognostic indicator in human papillary thyroid carcinoma. Oncol Rep. 1998;5:1343-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Fontanini G, Vignati S, Pacini F, Pollina L, Basolo F. Microvessel count: an indicator of poor outcome in medullary thyroid carcinoma but not in other types of thyroid carcinoma. Mod Pathol. 1996;9:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Akslen LA, Livolsi VA. Increased angiogenesis in papillary thyroid carcinoma but lack of prognostic importance. Hum Pathol. 2000;31:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Gulubova M, Ivanova K, Ananiev J, Gerenova J, Zdraveski A, Stoyanov H, Vlaykova T. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol Biotechnol Equip. 2014;28:508-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1162] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 28. | Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, Papotti MG, Berruti A; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1856-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 698] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 29. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47090] [Article Influence: 2943.1] [Reference Citation Analysis (0)] |
| 30. | Wells G, Shea B, O Connell D. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. |
| 31. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 32. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4948] [Article Influence: 274.9] [Reference Citation Analysis (0)] |
| 33. | Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 1705] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 34. | Bormann I. DigitizeIt 2.2. Digitizer Software—Digitize a Scanned Graph or Chart Into (x, y) Data. 2016. |
| 35. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 6976] [Article Influence: 634.2] [Reference Citation Analysis (0)] |
| 36. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6875] [Article Influence: 343.8] [Reference Citation Analysis (0)] |
| 37. | Dhar DK, Kubota H, Kotoh T, Tabara H, Watanabe R, Tachibana M, Kohno H, Nagasue N. Tumor vascularity predicts recurrence in differentiated thyroid carcinoma. Am J Surg. 1998;176:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Lee JH, Shin HJ, Yoon JH, Kim EK, Moon HJ, Lee HS, Kwon HJ, Kwak JY. Predicting lymph node metastasis in patients with papillary thyroid carcinoma by vascular index on power Doppler ultrasound. Head Neck. 2017;39:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Liu Y, Zhou H, Yang P, Zhou Y, Wu J, Chen C, Ye M, Luo J. Contrast-enhanced ultrasonography features of papillary thyroid carcinoma for predicting cervical lymph node metastasis. Exp Ther Med. 2017;14:4321-4327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Yasuoka H, Nakamura Y, Zuo H, Tang W, Takamura Y, Miyauchi A, Nakamura M, Mori I, Kakudo K. VEGF-D expression and lymph vessels play an important role for lymph node metastasis in papillary thyroid carcinoma. Mod Pathol. 2005;18:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Kilicarslan AB, Ogus M, Arici C, Pestereli HE, Cakir M, Karpuzoglu G. Clinical importance of vascular endothelial growth factor (VEGF) for papillary thyroid carcinomas. APMIS. 2003;111:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Hakala T, Sand J, Kellokumpu-Lehtinen PL, Huhtala H, Leinonen R, Kholová I. Recurrent thyroid cancers have more peritumoural lymphatic vasculature than nonrecurrent thyroid cancers. Eur J Clin Invest. 2014;44:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Lee SH, Lee SJ, Jin SM, Lee NH, Kim DH, Chae SW, Sohn JH, Kim WS. Relationships between Lymph Node Metastasis and Expression of CD31, D2-40, and Vascular Endothelial Growth Factors A and C in Papillary Thyroid Cancer. Clin Exp Otorhinolaryngol. 2012;5:150-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Bono P, Wasenius VM, Heikkilä P, Lundin J, Jackson DG, Joensuu H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. 2004;10:7144-7149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Kavantzas N, Tseleni-Balafouta S, Davaris P. Computerized nuclear morphometry and quantitation of angiogenesis in thyroid neoplasms. J Exp Clin Cancer Res. 2002;21:247-254. [PubMed] |
| 46. | Giatromanolaki A, Lyberakidis G, Lyratzopoulos N, Koukourakis MI, Sivridis E, Manolas C. Angiogenesis and angiogenic factor expression in thyroid cancer. J BUON. 2010;15:357-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Stabenow E, Tavares MR, Ab”Saber AM, Parra-Cuentas ER, de Matos LL, Eher EM, Capelozzi VL, Ferraz AR. Angiogenesis as an indicator of metastatic potential in papillary thyroid carcinoma. Clinics (Sao Paulo). 2005;60:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Skuletic V, Radosavljevic GD, Pantic J, Markovic BS, Jovanovic I, Jankovic N, Petrovic D, Jevtovic A, Dzodic R, Arsenijevic N. Angiogenic and lymphangiogenic profiles in histological variants of papillary thyroid carcinoma. Pol Arch Intern Med. 2017;127:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Niedźwiecki S, Stepień T, Kopeć K, Kuzdak K, Komorowski J, Krupiński R, Stepień H. Angiopoietin 1 (Ang-1), angiopoietin 2 (Ang-2) and Tie-2 (a receptor tyrosine kinase) concentrations in peripheral blood of patients with thyroid cancers. Cytokine. 2006;36:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Hsueh C, Lin JD, Wu IC, Chao TC, Yu JS, Liou MJ, Yeh CJ. Vascular endothelial growth factors and angiopoietins in presentations and prognosis of papillary thyroid carcinoma. J Surg Oncol. 2011;103:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Kang YE, Kim KS, Park SJ, Jung SN, Chang JW, Yi S, Jung MG, Kim JM, Koo BS. High Expression of Angiopoietin-1 is Associated with Lymph Node Metastasis and Invasiveness of Papillary Thyroid Carcinoma. World J Surg. 2017;41:3128-3138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Tanaka K, Sonoo H, Kurebayashi J, Nomura T, Ohkubo S, Yamamoto Y, Yamamoto S. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma. Clin Cancer Res. 2002;8:1125-1131. [PubMed] |
| 53. | Sancak S, Hardt A, Gärtner R, Eszlinger M, Aslan A, Eren FT, Güllüoglu BM, Sen LS, Sever Z, Akalin NS, Paschke R. Comparison of Color Flow Doppler Sonography (CFDS) and immunohistologic detection of microvessels for the assessment of the malignancy of thyroid nodules. Horm Metab Res. 2010;42:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Jiang J, Shang X, Zhang H, Ma W, Xu Y, Zhou Q, Gao Y, Yu S, Qi Y. Correlation between maximum intensity and microvessel density for differentiation of malignant from benign thyroid nodules on contrast-enhanced sonography. J Ultrasound Med. 2014;33:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Zhou Q, Jiang J, Shang X, Zhang HL, Ma WQ, Xu YB, Wang H, Li M. Correlation of contrast-enhanced ultrasonographic features with microvessel density in papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2014;15:7449-7452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Gu J, Zhang H, Li F. Relationship of shear wave elastography findings with pathology in papillary thyroid carcinomas model. Int J Clin Exp Med. 2017;10:8110-8117. |
| 57. | Perivoliotis K, Ntellas P, Dadouli K, Koutoukoglou P, Ioannou M, Tepetes K. Microvessel Density in Patients with Cutaneous Melanoma: An Up-to-Date Systematic Review and Meta-Analysis. J Skin Cancer. 2017;2017:2049140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |