Published online Jul 20, 2021. doi: 10.5662/wjm.v11.i4.199
Peer-review started: December 31, 2020
First decision: April 6, 2021
Revised: April 9, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: July 20, 2021
Processing time: 199 Days and 16.3 Hours
Flow cytometry is widely used for lymphocyte immunophenotyping in clinical settings. However, few studies have applied it for analyzing lymphocytes of the gastric mucosa. This review offers an overview of methodologies for isolating lymphocytes from the human stomach. Previously reported articles were review
Core Tip: This review provides an overview of methodologies used to analyze lym
- Citation: Iwamuro M, Takahashi T, Watanabe N, Okada H. Isolation of lymphocytes from the human gastric mucosa. World J Methodol 2021; 11(4): 199-207
- URL: https://www.wjgnet.com/2222-0682/full/v11/i4/199.htm
- DOI: https://dx.doi.org/10.5662/wjm.v11.i4.199
Helicobacter pylori (H. pylori) infection is responsible for most peptic ulcers and chronic inflammation in the stomach. Such prolonged inflammation causes mucosal damage and regeneration, in turn leading to carcinogenesis of the gastric epithelium and lymphomagenesis. Additionally, recent advances in cancer immunology and immunology-based anticancer therapies highlight the contribution of tumor-infiltrating lymphocytes in gastric cancer treatment. Thus, chronic inflammation with lymphocyte infiltration in the stomach may be involved in various gastric diseases[1,2].
Flow cytometry is widely used for lymphocyte immunophenotyping. An advantage of flow cytometry over immunohistochemistry is its multicolor analysis, providing an accurate characterization of the surface antigen profile of specific cells. Despite its superiority, few studies have assessed gastric mucosal lymphocytes by flow cytome
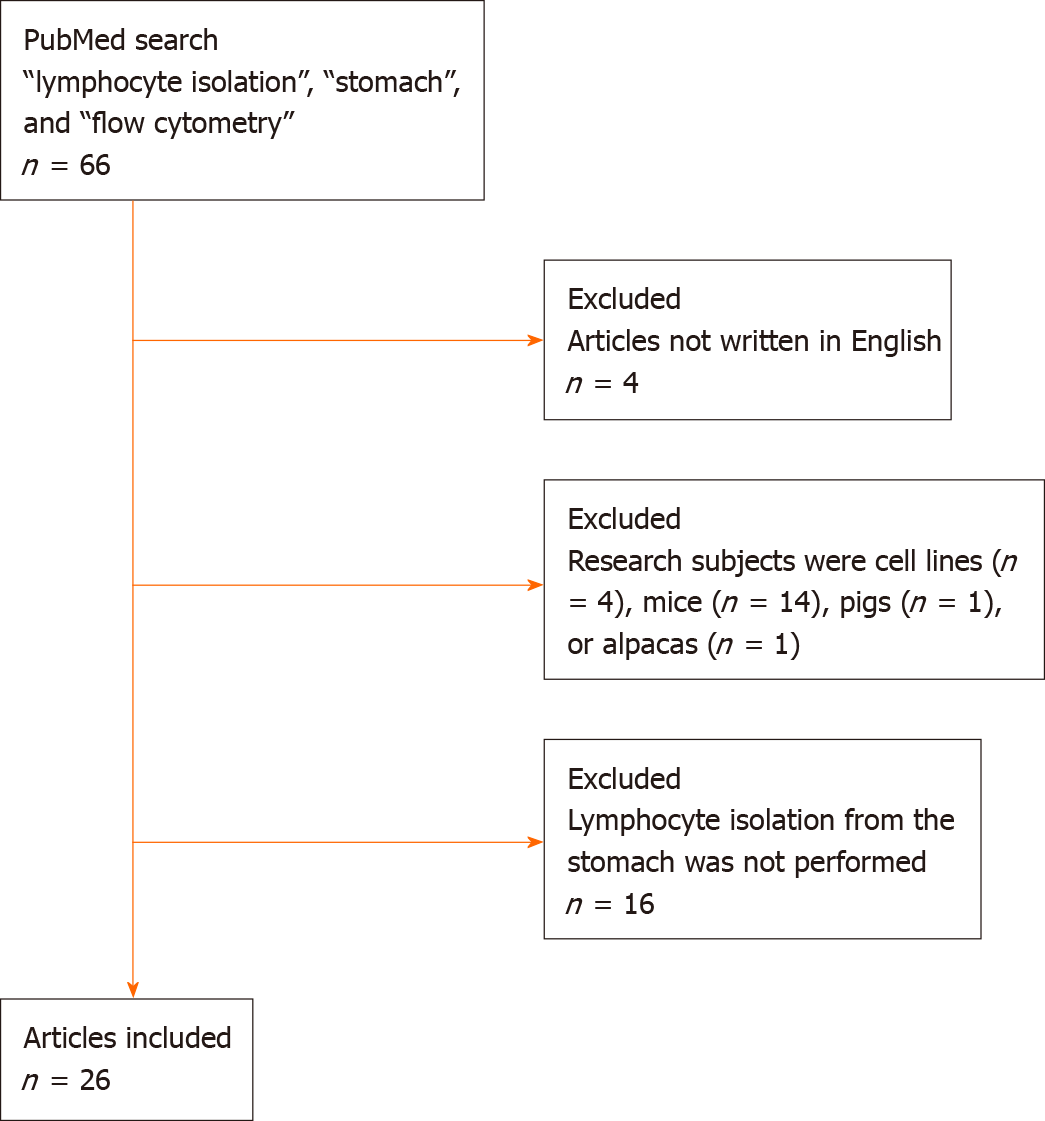
We performed a literature search on October 13, 2020, in the PubMed database. The search terms used were “lymphocyte isolation”, “stomach”, and “flow cytometry”. We retrieved 66 titles[1-66], and no previous systematic review of lymphocyte isolation from the gastric mucosa was identified (Figure 1). Articles not written in English (n = 4) were excluded from this review. A further 20 articles were excluded as the research subjects were cell lines (n = 4) or non-human mammals, including mice (n = 14), pigs (n = 1), or alpacas (n = 1). Among the remaining 42 articles, lymphocyte isolation from the stomach was not performed in 16 articles. Finally, we reviewed 26 articles and summarized the methodologies for lymphocyte isolation from the human gastric mucosa.
Lymphocyte isolation was performed using endoscopic biopsy specimens (n = 15), surgically resected specimens (n = 10), or both (n = 1). The number of endoscopically biopsied specimens was 1 (n = 2), 1–2 (n = 1), 2 (n = 3), 4 (n = 5), 5 (n = 1), or 6 (n = 1). The number used for lymphocyte isolation was not specified in the remaining three articles. Gastric mucosal lymphocyte analysis was performed in association with H. pylori-related peptic diseases (n = 12), gastric cancer (n = 9), or other diseases (n = 5).
During lymphocyte isolation, enzymatic degradation and/or mechanical shredding and grinding are performed to remove the epithelial cells and connective tissue of the stomach. Collagenase is commonly used for enzymatic processing, while mesh strainers or glass slides are used for mechanical processing. Hereafter, we review representative methods for lymphocyte isolation from the stomach.
In previously reported studies, sequential processing with enzymatic and mechanical dissociation is most frequently used for lymphocyte isolation from the gastric mucosa. In one protocol[2,6,16], fresh tissues were obtained from the surgically resected stomach and washed three times with Hank’s solution containing 1% fetal calf serum. The specimen was cut into small pieces and collected in RPMI 1640 medium con
In another protocol[10], two specimens were obtained from the gastric mucosa by endoscopic biopsy. The specimens were placed on ice in RPMI 1640 medium containing 10% fetal calf serum, glutamine, and penicillin/streptomycin. The biopsy specimens were transferred to a dithiothreitol/EDTA solution and incubated for 15 min at 37 °C. The samples were finely sliced and digested in a collagenase A solution for 1 h at 37 °C. The digested cell suspensions were filtered through a 70 μm cell filter and collected in RPMI 1640 medium. After centrifugation, the pellet was resuspended in phosphate-buffered saline.
For enzymatic dissociation, other reagent combinations are also used, such as deoxyribonuclease 1 (0.02 mg/mL), collagenase (0.25 mg/mL), and hyaluronidase (0.1 mg/mL)[28]; collagenase (300 μg/mL) and Dispase II (500 U/mL)[33]; or collagenase (5 mg/mL), DNase (0.1 mg/mL), and protease (2 U/mL)[43,49,58,62].
Isolation of lymphocytes can be performed by enzymatic dissociation alone. For instance[60], fresh gastric tissue can be digested in a rotating chamber (60 rpm, 37 °C, 12 h) with NaCl (8 mg/mL), KCl (0.4 mg/mL), CaCl2 (0.56 mg/mL), NaHPO4 (60 μg/mL), Na2HPO4-12H2O (151 μg/mL), HEPES (2.3 mg/mL), Clostridium hystolyticum collagenase (0.5 mg/mL), and soybean trypsin inhibitor (5 μg/mL). The lymphocytes are then dispersed and enriched on Ficoll-Paque density gradients.
Without enzymes, lymphocytes can be obtained by mechanical mincing of surgically resected or endoscopically biopsied specimens, followed by passing through a 40-100 μm filter to exclude tissue fragments[23,24,52] or by pressing and grinding in a coarse glass grinder[48].
The predominance, heterogeneity, and distribution of lymphocytes are diverse at different locations within the gastric mucosa[22]. Therefore, intra-epithelial lympho
We recently introduced a simplified, one-step procedure for lymphocyte isolation from an endoscopic biopsy sample[67]. To isolate lymphocytes, we used a porcelain bowl with a spout and a wire mesh tea strainer. First, the porcelain bowl and wire mesh strainer were sterilized by autoclaving. During esophagogastroduodenoscopy, enteroscopy, or colonoscopy, a single tissue sample was collected from the gastro
As far as we know, this is the easiest procedure reported to date; lymphocyte isolation by this method is achieved within 2 min. Besides, laboratory wares and apparatus are not necessary for this method. Therefore, our approach for lymphocyte separation can be completed in an endoscopy unit right after taking a biopsy sample. This method would allow widespread evaluation of lymphocytes in the field of gastroenterology.
During the early development stages of flow cytometry for gastric disorders (1995-1999), the association between peptic ulcers and inflammation induced by mucosal lymphocytes was investigated in patients with H. pylori infection or duodenal ulcers[52,54,55,58,61,62]. Activation markers such as CD25 [interleukin (IL)-2 receptor alpha chain], CD69 (activation inducer molecule), CD71 (transferrin receptor protein 1), HLA-DR, and adhesion and emigration-related molecules, such as CD11a/CD18 (lymphocyte function-associated antigen 1: LFA-1), CD11b (integrin alpha-M), CD54 (intercellular adhesion molecule-1: ICAM-1), CD106 (vascular cell adhesion molecule-1: VCAM-1), and CD49d (very late antigen-4: VLA-4), were analyzed by flow cytometry in these patients. Also analyzed were the pan T cell markers CD2 and CD3, T-helper cell marker CD4, and T-cytotoxic cell marker CD8. Cytokines, such as interferon-gamma, tumor necrosis factor beta (TNF-β), IL-2, IL-4, and IL-5, were also examined by flow cytometry[52,55].
In the year 2000, research was focused on CD95, also known as Fas or TNF receptor superfamily member 6[47,48]. CD95 is a death receptor localized on the surface of cells that triggers a signal transduction pathway upon binding its ligand, leading to programmed cell death (apoptosis). In H. pylori-associated gastritis, epithelial cell damage is mediated through Fas/Fas ligand interactions[47]. Simultaneously, apop
To our knowledge, lymphocyte infiltration in gastric cancer was first assessed in 1996 in patients with lymphocyte-rich, Epstein-Barr virus-associated gastric carcinoma[60]. In 2006, tumor-infiltrating lymphocytes were investigated in two major types of gastric adenocarcinoma (Lauren classification): intestinal type or diffuse type[28]. The number of B cells was significantly higher, while the number of T cells was significantly lower in intestinal type compared with diffuse type tumors. Furthermore, cloning and characterization of tumor-infiltrating T cells isolated from gastric cancers revealed a specific type-1 T cell response to gastric cancer antigens[21]. Analysis of CD8+ cells that produce IL-17 (Tc17 cells) showed that the percentage of Tc17 cells increased with tumor progression and was associated with overall survival time[15]. Tc17 cells induce CXCR4-dependent chemotaxis of myeloid-derived suppressor cells and impair cytotoxic functions of anti-tumor CD8+ cells, promoting tumor progression.
Since 2008, regulatory T (Treg) cells have gained attention in association with gastric disorders owing to their involvement in immune regulation[9,23,24]. In these studies, Tregs were defined as CD4+CD25high[24], CD4+CD25+CD127low/-[23], or CD4+FOXP3+ cells[9]. Studies show that local Treg cells in gastric cancer express a suppressive cytokine profile characterized by high IL-10, low transforming growth factor-beta (TGF-β), and interferon-gamma production[9]. Thus, it is believed that Tregs suppress effector T cell proliferation and contribute to gastric cancer progression[23]. Furthermore, increase of IL-10 secretion by Tregs was confirmed in H. pylori-infected gastric mucosa[24]. IL-10 inhibits IL-8 expression, activates nuclear factor kappa B in the gastric epithelium, and enhances H. pylori growth in vitro, suggesting the participation of Tregs in gastric ulcer formation and persistent H. pylori infection.
In one study, T cells expressing natural killer cell receptors, defined as CD3+CD56+, CD3+CD161+, or CD3+CD94+ cells, were quantified in H. pylori-positive and -negative patients[22]. CD3+CD161+ cells were higher in the epithelium of H. pylori-infected gastric mucosa, whereas CD3+CD56+ cells were lower in the lamina propria, indicating a site-specific distribution of T cells bearing natural killer receptors. In another study, mast cells, defined as CD45+CD117+FcεRI+ cells, were investigated in patients with gastric cancer[2]. A significantly higher number of mast cells exist in gastric cancer tissues, and the mast cell levels increase with tumor progression and independently predict a reduced survival. Besides lymphocytes, tumor-infiltrating neutrophils were examined in gastric cancers by cell sorting against CD66b, which allows for the enrichment of mature neutrophils[6].
Using flow cytometry, specific lymphocyte subsets are defined based on their lineage-, developmental stage-, and function-specific cell surface markers. In addition, fluorescence-activated cell sorting technology enables diversion of individual cells from the fluid stream and collection into viable, homogeneous fractions. Efficient isolation of a lymphocyte population enables characterization of the specific fractions in in vitro and animal studies[6,9,15]. More recently, mass cytometry and single-cell RNA sequencing are available. These cutting-edge technologies may reveal distinct immune cell signatures of gastric disorders, such as gastric cancers and H. pylori-related peptic diseases.
In addition to the aforementioned technologies of evaluating gastric diseases, flow cytometry has been used for the diagnosis of lymphoma of the stomach. Flow cytometry is a rapid and practical diagnostic tool for B-cell lymphoma. Analysis of the distribution of surface immunoglobulin light chain kappa and lambda using flow cytometry offers evidence for the monoclonality of B-cell neoplasms because these lymphomas that typically arise from an expansion of a B-cell clone expressing only one class of immunoglobulin light chain, either a kappa or lambda chain. Thus, isolation of lymphocytes from the gastric mucosa and detection of monoclonality using flow cytometry lead to the prompt diagnosis of lymphoma of the stomach. This approach may be particularly useful for the detection and therapeutic monitoring of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), which is the most common non-Hodgkin lymphoma subtype arising in the stomach[68-70].
We have reported the utility of a single-step lymphocyte isolation procedure from an endoscopic biopsy specimen, which is described above, for the diagnosis of gastrointestinal lymphoma[67,71]. Our previous study included patients with gastric extranodal marginal zone MALT lymphoma (n = 8), duodenal follicular lymphoma (grade 1; n = 5), and benign lymphoid hyperplasia (ileum, n = 1, and rectum, n = 1). Lymphocytes were successfully isolated from 14 (93.3%) patients. The sensitivity and specificity of flow cytometric analysis of immunoglobulin light chain expression for the diagnosis of B-cell lymphoma were 83.3% and 100%, respectively. These results suggest that a single endoscopic biopsy specimen contains enough lymphocytes for flow cytometric analysis and can be used for the diagnosis of gastrointestinal lymphoma.
In this review, we provided methodologies for lymphocyte isolation from the gastric mucosa that are reported in the literature. We also described the history and current trends of lymphocyte analysis in the stomach. Owing to the multicolor analysis that accurately defines the surface antigen profile of specific lymphocyte populations, flow cytometry will continue to be a powerful tool for revealing the pathogenesis of gastric disorders. We believe that the methodologies described herein will provide a better understanding of the application of flow cytometry.
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Mario F S-Editor: Gao CC L-Editor: A P-Editor: Li X
| 1. | Fenton TM, Jørgensen PB, Niss K, Rubin SJS, Mörbe UM, Riis LB, Da Silva C, Plumb A, Vandamme J, Jakobsen HL, Brunak S, Habtezion A, Nielsen OH, Johansson-Lindbom B, Agace WW. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity. 2020;52:557-570.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Lv Y, Zhao Y, Wang X, Chen N, Mao F, Teng Y, Wang T, Peng L, Zhang J, Cheng P, Liu Y, Kong H, Chen W, Hao C, Han B, Ma Q, Zou Q, Chen J, Zhuang Y. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway. J Immunother Cancer. 2019;7:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Bagheri V, Abbaszadegan MR, Memar B, Motie MR, Asadi M, Mahmoudian RA, Gholamin M. Induction of T cell-mediated immune response by dendritic cells pulsed with mRNA of sphere-forming cells isolated from patients with gastric cancer. Life Sci. 2019;219:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Martins MR, Santos RLD, Jatahy KDN, Matta MCD, Batista TP, Júnior JIC, Begnami MDFS, Torres LC. Could OX40 agonist antibody promote activation of the anti-tumor immune response in gastric cancer? J Surg Oncol. 2018;117:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Zhou Y, Guo F. A selective sphingosine-1-phosphate receptor 1 agonist SEW-2871 aggravates gastric cancer by recruiting myeloid-derived suppressor cells. J Biochem. 2018;163:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, Fu XL, Yu PW, Guo G, Luo P, Zhuang Y, Zou QM. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 7. | Topliff CL, Alkheraif AA, Kuszynski CA, Davis WC, Steffen DJ, Schmitz JA, Eskridge KM, Charleston B, Henningson JN, Kelling CL. Experimental acute infection of alpacas with Bovine viral diarrhea virus 1 subgenotype b alters peripheral blood and GALT leukocyte subsets. J Vet Diagn Invest. 2017;29:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hou M, Zhou NB, Li H, Wang BS, Wang XQ, Wang XW, Wang KG, Xue FS. Morphine and ketamine inhibit immune function of gastric cancer patients by increasing percentage of CD4(+)CD25(+)Foxp3(+) regulatory T cells in vitro. J Surg Res. 2016;203:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Kindlund B, Sjöling Å, Yakkala C, Adamsson J, Janzon A, Hansson LE, Hermansson M, Janson P, Winqvist O, Lundin SB. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer. 2017;20:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Bashir M, Prietl B, Tauschmann M, Mautner SI, Kump PK, Treiber G, Wurm P, Gorkiewicz G, Högenauer C, Pieber TR. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr. 2016;55:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Chen J, Yang J, Jiang J, Zhuang Y, He W. Function and subsets of dendritic cells and natural killer cells were decreased in gastric cancer. Int J Clin Exp Pathol. 2014;7:8304-8311. [PubMed] |
| 12. | Sparks D, Bhalla A, Dodge J, Saldinger P. Isolated gastric amyloidoma in the setting of marginal zone MALT lymphoma: case report and review of the literature. Conn Med. 2014;78:277-280. [PubMed] |
| 13. | Odiere MR, Scott ME, Leroux LP, Dzierszinski FS, Koski KG. Maternal protein deficiency during a gastrointestinal nematode infection alters developmental profile of lymphocyte populations and selected cytokines in neonatal mice. J Nutr. 2013;143:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ruiz VE, Sachdev M, Zhang S, Wen S, Moss SF. Isolating, immunophenotyping and ex vivo stimulation of CD4+ and CD8+ gastric lymphocytes during murine Helicobacter pylori infection. J Immunol Methods. 2012;384:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, Zeng H, Liu KY, Guo G, Tong WD, Tang B, Li N, Yu S, Luo P, Zhang WJ, Lu DS, Yu PW, Zou QM. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951-62.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Yoneda A, Ito S, Susumu S, Matsuo M, Taniguchi K, Tajima Y, Eguchi S, Kanematsu T, Nagata Y. Immunological milieu in the peritoneal cavity at laparotomy for gastric cancer. World J Gastroenterol. 2012;18:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Nishimoto T, Satoh T, Takeuchi T, Ikeda Y, Kuwana M. Critical role of CD4(+)CD25(+) regulatory T cells in preventing murine autoantibody-mediated thrombocytopenia. Exp Hematol. 2012;40:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Lulla P, Bandeali S, Baker K. Fatal paraneoplastic systemic leukocytoclastic vasculitis as a presenting feature of chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11 Suppl 1:S14-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Modi N, Gulati N, Solomon K, Monaghan T, Robins A, Sewell HF, Mahida YR. Differential binding and internalization of Clostridium difficile toxin A by human peripheral blood monocytes, neutrophils and lymphocytes. Scand J Immunol. 2011;74:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Becher D, Deutscher ME, Simpfendorfer KR, Wijburg OL, Pederson JS, Lew AM, Strugnell RA, Walduck AK. Local recall responses in the stomach involving reduced regulation and expanded help mediate vaccine-induced protection against Helicobacter pylori in mice. Eur J Immunol. 2010;40:2778-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Amedei A, Niccolai E, Della Bella C, Cianchi F, Trallori G, Benagiano M, Bencini L, Bernini M, Farsi M, Moretti R, Del Prete G, D'Elios MM. Characterization of tumor antigen peptide-specific T cells isolated from the neoplastic tissue of patients with gastric adenocarcinoma. Cancer Immunol Immunother. 2009;58:1819-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | O'Keeffe J, Gately CM, O'Donoghue Y, Zulquernain SA, Stevens FM, Moran AP. Natural killer cell receptor T-lymphocytes in normal and Helicobacter pylori-infected human gastric mucosa. Helicobacter. 2008;13:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, Zaitoun AM, Atherton JC. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57:1375-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Xu HY, Xu L, Gao JH, Li KZ, Dou KF. [T lymphocytes with chimeric receptor induce carcinoembryonic antigen-positive specific gastric carcinoma cells apoptosis]. Zhonghua Yi Xue Za Zhi. 2007;87:1053-1057. [PubMed] |
| 26. | Wang LY, Zeng Y, Pan ZZ, Zhu ZH. [Detection of intracellular and extracellular cytokines of CD4+CD25+ regulatory T cells in gastric cancer patients]. Ai Zheng. 2007;26:270-273. [PubMed] |
| 27. | Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, Reindl W, Dossumbekova A, Friedrich M, Saur D, Wagner H, Schmid RM, Prinz C. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | van den Engel NK, Winter H, Rüttinger D, Shau I, Schiller M, Mayer B, Moudgil T, Meimarakis G, Stolte M, Jauch KW, Fox BA, Hatz RA. Characterization of immune responses in gastric cancer patients: a possible impact of H. pylori to polarize a tumor-specific type 1 response? Clin Immunol. 2006;120:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Hatzifoti C, Roussel Y, Harris AG, Wren BW, Morrow JW, Bajaj-Elliott M. Mucosal immunization with a urease B DNA vaccine induces innate and cellular immune responses against Helicobacter pylori. Helicobacter. 2006;11:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Dutta N, Gupta A, Mazumder DN, Banerjee S. Down-regulation of locus-specific human lymphocyte antigen class I expression in Epstein-Barr virus-associated gastric cancer: implication for viral-induced immune evasion. Cancer. 2006;106:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Hase K, Murakami T, Takatsu H, Shimaoka T, Iimura M, Hamura K, Kawano K, Ohshima S, Chihara R, Itoh K, Yonehara S, Ohno H. The membrane-bound chemokine CXCL16 expressed on follicle-associated epithelium and M cells mediates lympho-epithelial interaction in GALT. J Immunol. 2006;176:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Li ZY, Chen FB, Chen J. [CD4+ and CD8+ T cells in gastric mucosa in children infected with Helicobacter pylori]. Zhonghua Er Ke Za Zhi. 2005;43:453-456. [PubMed] |
| 33. | Itoh T, Seno H, Kita T, Chiba T, Wakatsuki Y. Th response to Helicobacter pylori differs between patients with gastric ulcer and duodenal ulcer. Scand J Gastroenterol. 2005;40:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Velin D, Bachmann D, Bouzourene H, Michetti P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology. 2005;129:142-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Johansson C, Ahlstedt I, Furubacka S, Johnsson E, Agace WW, Quiding-Järbrink M. Differential expression of chemokine receptors on human IgA+ and IgG+ B cells. Clin Exp Immunol. 2005;141:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Wu YY, Tsai HF, Lin WC, Chou AH, Chen HT, Yang JC, Hsu PI, Hsu PN. Helicobacter pylori enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in human gastric epithelial cells. World J Gastroenterol. 2004;10:2334-2339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Valeri AP, Pérez-Blas M, Gutiérrez A, López-Santalla M, Aguilera N, Rodríguez-Juan C, Sala-Silveira L, Martín J, Lasa I, Mugüerza JM, López A, García-Sancho L, Granell J, Martín-Villa JM. Intrinsic defects explain altered proliferative responses of T lymphocytes and HVS-derived T-cell lines in gastric adenocarcinoma. Cancer Immunol Immunother. 2003;52:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Bontems P, Robert F, Van Gossum A, Cadranel S, Mascart F. Helicobacter pylori modulation of gastric and duodenal mucosal T cell cytokine secretions in children compared with adults. Helicobacter. 2003;8:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606-612. [PubMed] |
| 40. | Kudo T, Iwai T, Kubota T, Iwasaki H, Takayma Y, Hiruma T, Inaba N, Zhang Y, Gotoh M, Togayachi A, Narimatsu H. Molecular cloning and characterization of a novel UDP-Gal:GalNAc(alpha) peptide beta 1,3-galactosyltransferase (C1Gal-T2), an enzyme synthesizing a core 1 structure of O-glycan. J Biol Chem. 2002;277:47724-47731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Zavros Y, Rieder G, Ferguson A, Merchant JL. Gastritis and hypergastrinemia due to Acinetobacter lwoffii in mice. Infect Immun. 2002;70:2630-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Li X, Guo M, Mori E, Mori T. Active roles of caspase-3 in human gastric carcinoma cell death by apoptosis inducing nucleosides from CD57+HLA-DRbright natural suppressor cell line. Int J Oncol. 2001;18:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 43. | Ihan A, Tepes B, Gubina M. Diminished Th1-type cytokine production in gastric mucosa T-lymphocytes after H. pylori eradication in duodenal ulcer patients. Pflugers Arch. 2000;440:R89-R90. [PubMed] |
| 44. | Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Michetti M, Kelly CP, Kraehenbuhl JP, Bouzourene H, Michetti P. Gastric mucosal alpha(4)beta(7)-integrin-positive CD4 T lymphocytes and immune protection against helicobacter infection in mice. Gastroenterology. 2000;119:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Zavros Y, Van Antwerp M, Merchant JL. Use of flow cytometry to quantify mouse gastric epithelial cell populations. Dig Dis Sci. 2000;45:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Wang J, Fan X, Lindholm C, Bennett M, O'Connoll J, Shanahan F, Brooks EG, Reyes VE, Ernst PB. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect Immun. 2000;68:4303-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Koyama S. Apoptotic depletion of infiltrating mucosal lymphocytes associated with Fas ligand expression by Helicobacter pylori-infected gastric mucosal epithelium: human glandular stomach as a site of immune privilege. Dig Dis Sci. 2000;45:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Ihan A, Tepez B, Gubina M, Malovrh T, Kopitar A. Diminished interferon-gamma production in gastric mucosa T lymphocytes after H. pylori eradication in duodenal ulcer patients. Hepatogastroenterology. 1999;46:1740-1745. [PubMed] |
| 50. | Wieckiewicz J, Krzeszowiak A, Ruggiero I, Pituch-Noworolska A, Zembala M. Detection of cytokine gene expression in human monocytes and lymphocytes by fluorescent in situ hybridization in cell suspension and flow cytometry. Int J Mol Med. 1998;1:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 51. | Stallmach A, Schäfer F, Hoffmann S, Weber S, Müller-Molaian I, Schneider T, Köhne G, Ecker KW, Feifel G, Zeitz M. Increased state of activation of CD4 positive T cells and elevated interferon gamma production in pouchitis. Gut. 1998;43:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Sommer F, Faller G, Konturek P, Kirchner T, Hahn EG, Zeus J, Röllinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543-5546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Sikora J, Dworacki G, Trybus M, Batura-Gabryel H, Zeromski J. Correlation between DNA content, expression of Ki-67 antigen of tumor cells and immunophenotype of lymphocytes from malignant pleural effusions. Tumour Biol. 1998;19:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Terrés AM, Pajares JM. An increased number of follicles containing activated CD69+ helper T cells and proliferating CD71+ B cells are found in H. pylori-infected gastric mucosa. Am J Gastroenterol. 1998;93:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 424] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 56. | Ermak TH, Ding R, Ekstein B, Hill J, Myers GA, Lee CK, Pappo J, Kleanthous HK, Monath TP. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology. 1997;113:1118-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Shiyan SD, Bovin NV. Carbohydrate composition and immunomodulatory activity of different glycoforms of alpha1-acid glycoprotein. Glycoconj J. 1997;14:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Ihan A, Tepez B, Kavcic I, Gubina M. Il-2 receptor expression on gastric mucosa T lymphocytes is enhanced in duodenal ulcer patients compared with non-ulcer dyspeptic patients. Hepatogastroenterology. 1996;43:1665-1670. [PubMed] |
| 59. | Krakowka S, Ringler SS, Eaton KA, Green WB, Leunk R. Manifestations of the local gastric immune response in gnotobiotic piglets infected with Helicobacter pylori. Vet Immunol Immunopathol. 1996;52:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Saiki Y, Ohtani H, Naito Y, Miyazawa M, Nagura H. Immunophenotypic characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996;75:67-76. [PubMed] |
| 61. | Fan X, Long A, Fan X, Keeling PW, Kelleher D. Adhesion molecule expression on gastric intra-epithelial lymphocytes of patients with Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1995;7:541-546. [PubMed] |
| 62. | Ihan A, Krizman I, Ferlan-Marolt V, Tepez B, Gubina M. HLA-DR expression on CD8 lymphocytes from gastric mucosa in urease-positive and urease-negative gastritis. FEMS Immunol Med Microbiol. 1995;10:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Alderuccio F, Toh BH, Gleeson PA, van Driel IR. A novel method for isolating mononuclear cells from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity. 1995;21:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Yamaguchi Y, Takashima I, Funakoshi M, Kawami H, Toge T. Defective natural killer activity in gastric cancer patients: possible involvement of suppressor factor receptor. In Vivo. 1994;8:279-283. [PubMed] |
| 65. | Ebihara T, Sakai N, Koyama S. Suppression by sorted CD8+CD11b- cells from T-cell growth factor-activated peripheral blood lymphocytes on cytolytic activity against tumour in patients with gastric carcinoma. Eur J Cancer. 1991;27:1654-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Yamaue H, Tanimura H, Tsunoda T, Iwahashi M, Tani M, Inoue M, Tamai M. [Clinical application of adoptive immunotherapy by cytotoxic T lymphocytes induced from tumor-infiltrating lymphocytes]. Nihon Gan Chiryo Gakkai Shi. 1990;25:978-989. [PubMed] |
| 67. | Iwamuro M, Takahashi T, Watanabe N, Omote S, Matsueda K, Tanaka T, Ennishi D, Otsuka F, Yoshino T, Okada H. Technique for single-step lymphocyte isolation from an endoscopic biopsy specimen for the diagnosis of gastrointestinal lymphoma. MethodsX. 2020;7:101095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Oka S, Muroi K, Sato K, Uskudar Teke H, Sahin Mutlu F, Gulbas Z. Flow cytometric evaluation of endoscopic biopsy specimens from patients with gastrointestinal tract B-cell lymphoma: a preliminary report. Jichi Med Univ J. 2007;30:129-135. |
| 69. | Oka S, Muroi K, Sato K, Fujiwara S, Oh I, Matsuyama T, Ohmine K, Suzuki T, Ozaki K, Mori M, Nagai T, Fukushima N, Tanaka A, Ozawa K. Flow cytometric analysis of kappa and lambda light chain expression in endoscopic biopsy specimens before the diagnosis of B-cell lymphoma. J Clin Exp Hematop. 2012;52:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Almasri NM, Zaer FS, Iturraspe JA, Braylan RC. Contribution of flow cytometry to the diagnosis of gastric lymphomas in endoscopic biopsy specimens. Mod Pathol. 1997;10:650-656. [PubMed] |
| 71. | Iwamuro M, Matsueda K, Takahashi T, Omote S, Tanaka T, Ennishi D, Otsuka F, Yoshino T, Okada H. An Endoscopic Biopsy Specimen Contains Adequate Lymphocytes for Flow Cytometric Analysis of Light Chain Expression in the Gastrointestinal Mucosa. Ann Clin Lab Sci. 2020;50:348-353. [PubMed] |