Published online May 20, 2021. doi: 10.5662/wjm.v11.i3.95
Peer-review started: November 1, 2020
First decision: November 30, 2020
Revised: December 2, 2020
Accepted: March 28, 2021
Article in press: March 28, 2021
Published online: May 20, 2021
Processing time: 192 Days and 0.7 Hours
The majority of patients with coronavirus disease 2019 (COVID-19) have good prognoses, but some develop a critical illness that can lead to death. Evidence shows severe acute respiratory syndrome is closely related to the induced cytokine storm. Interleukin-6 is a key player; its role in systemic inflammation is well known.
To evaluate the effect of tocilizumab (TCZ), an interleukin-6 receptor antagonist, on the outcomes for patients with COVID-19 pneumonia.
PubMed, EMBASE, SCOPUS, Web of Science, MedRxiv, Science Direct, and the Cochrane Library were searched from inception to 9th June 2020 for observational or prospective studies reporting results of hospitalized adult patients with COVID-19 infection treated with TCZ. Effect sizes were reported as odds ratios (ORs) with 95% confidence intervals (CIs), and an OR less than 1 was associated with a better outcome in those treated with TCZ.
Overall 13476 patients (33 studies; n = 3264 received TCZ) with COVID-19 pneumonia and various degree of severity were included. Outcome was improved with TCZ. In the primary analysis (n = 19 studies reporting data), mortality was reduced in patients treated with TCZ (OR = 0.64, 95%CI: 0.47-0.87; P < 0.01). In 9 studies where risk of death with TCZ use was controlled for other variables mortality was reduced by 57% (OR = 0.43, 95%CI: 0.27-0.7; P < 0.01). Intensive care need (mechanical ventilation) was also reduced (OR = 0.36, 95%CI: 0.14-0.89; P = 0.02).
In COVID-19-infected patients treated with TCZ, outcome may be improved compared to those not treated with TCZ.
Core Tip: Coronavirus disease 2019 (COVID-19) infection is associated with a citokine storm during acute phase. Interleukin-6 is a key player in this systemic inflammation. We evaluated the effect of tocilizumab (TCZ) on the outcomes of COVID-19 pneumonia. Mortality was reduced in patients treated with TCZ (Odds ratio =0.64, 95% confidence intervals: 0.47-0.87; P < 0.01). We conclude that TCZ may improve outcome of COVID-19 infected patients.
- Citation: Petrelli F, Cherri S, Ghidini M, Perego G, Ghidini A, Zaniboni A. Tocilizumab as treatment for COVID-19: A systematic review and meta-analysis. World J Methodol 2021; 11(3): 95-109
- URL: https://www.wjgnet.com/2222-0682/full/v11/i3/95.htm
- DOI: https://dx.doi.org/10.5662/wjm.v11.i3.95
Severe acute respiratory syndrome coronavirus 2 emerged in Wuhan, China in December 2019 and a pandemic was declared by the World Health Organization on March 11, 2020. The pandemic rapidly became a major global health concern. The vast majority of patients with coronavirus disease 2019 (COVID-19) have good prognoses, but some develop a critical illness that can lead to death. The data show that approximately 20% become severe or critical and require hospitalization[1]. Evidence shows that severe deterioration following severe acute respiratory syndrome coronavirus 2 infection is closely related to the associated cytokine storm[2]. Tocilizumab (TCZ) is an immunomodulatory therapeutic, an interleukin (IL)-6 receptor antagonist approved by the United States Food and Drug Administration and the European Medicine Agency for treating cytokine release syndrome. One of the key cytokines described in the cytokine storm induced by COVID-19 is IL-6, and its role in systemic inflammation is well known. Following an intriguing biological rationale, several institutions have proposed using TCZ off-label to treat COVID-19[3]. Thus far, randomized controlled trials have not been reported in the literature, but observational studies and case reports describe the compassionate use of TCZ. Results leave the efficacy of TCZ controversial. We performed a meta-analysis of the studies available to date.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed for evaluating records identified during the literature search[4].
The search included MEDLINE, EMBASE, Scopus, the medRxiv preprint server, Science Direct, Web of Science, and the Cochrane Controlled Register of Trials for articles published up to June 9, 2020 describing trials or observational series about the efficacy of TCZ in patients with COVID-19 pneumonia. Search terms were tocilizumab and COVID-19. The inclusion criteria were: (1) Randomized or single-arm prospective studies, observational or retrospective case series of patients with COVID-19 and treated with TCZ outside of clinical trials; (2) written in the English language; (3) reporting patient clinical characteristics; and (4) including at least 5 patients. Animal studies, case reports, editorials, commentaries, and clinical or pharmacological reviews were excluded. If multiple studies reported on the same population and met the inclusion criteria, the newest study was selected unless different endpoints or subgroup analyses were performed or updated.
Two authors (Ghidini A, Petrelli F) determined article eligibility based on the abstracts. A third (Zaniboni A) independently read the articles, and agreement for trial inclusion was reached. Two authors (Petrelli F, Ghidini A) independently extracted data to a standard form constructed using Microsoft Word and compared results for agreement. Extracted data were author, publication year, number of participants treated, study design, patient group demographics and clinical characteristics (e.g., median age, sex, country, comorbidities), median follow-up, laboratory and clinical parameters (symptoms) of participants, rate of admission to the intensive care unit (ICU) before and after TCZ use, associated drugs, imaging (baseline and improve
Eligible studies were critically appraised by two independent reviewers at the study level for methodological and reporting bias by adapting the ROBIN-I tool[5] for assessing risk of bias in selected observational studies. By definition, single-arm or observational trials have a high risk of bias due to the absence of a control group and randomization. Otherwise, the Nottingham-Ottawa-Scale was used as a quality check for retrospective studies.
The primary endpoints were mortality (%) and ventilatory improvement (defined as the proportion of participants relieved from ICU admission or from non-invasive ventilation defined at the time from initiation of the study treatment) among those treated with TCZ. The outcome data extracted for each study were analyzed using random-effects models and were reported as weighted measures of any event. Event rates reported in individual studies were aggregated into pooled rates. All other continuous variables were analyzed using descriptive statistics. We used the procedures of the comprehensive meta-analysis (CMA) software to calculate the effect size using dichotomous outcomes; and if these were not available either, we used other statistics (such as t-value or P value) to calculate the effect size. A random-effects meta-analysis of odds ratios (ORs) was used to aggregate efficacy outcomes reported across trials. A meta-analysis of adjusted ORs attained from multivariate analysis only was also provided.
Heterogeneity was assessed using the χ2 test. Statistical significance and the magnitude of I2 were considered. When I2 was less than 50%, low to moderate heterogeneity was assigned; otherwise, substantial heterogeneity was assigned. A significance threshold of P < 0.05 was adopted. All analyses were performed using CMA software version 2.2 (Biostat).
We tested publication bias by inspecting the funnel plot on primary outcome measures and by Duval and Tweedie’s trim and fill procedure yields an estimated effect size after publication bias has been taken into account (as implemented in CMA). We also conducted Egger’s test of the intercept to quantify the bias captured by the funnel plot and to test whether it was significant.
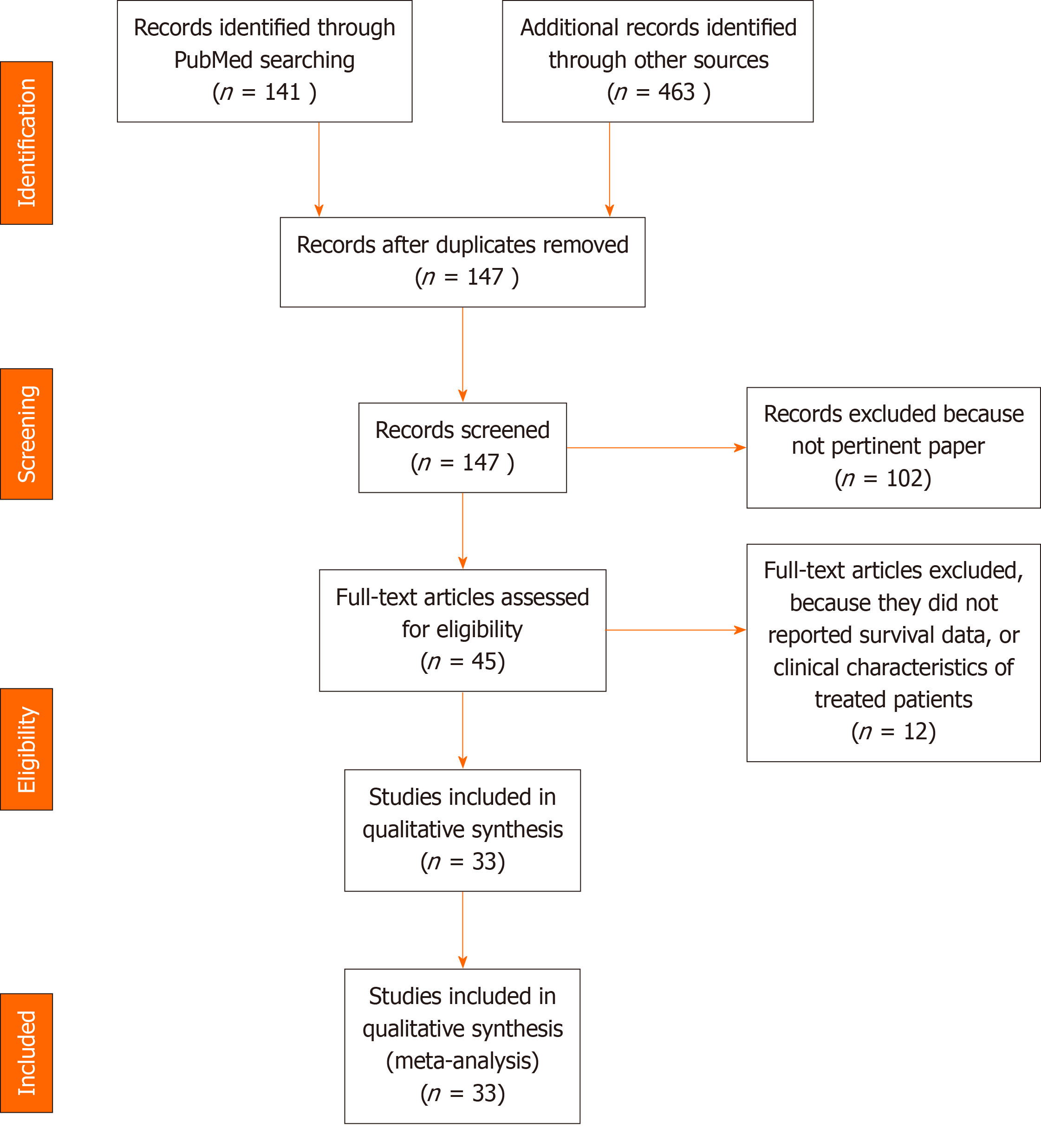
Thirty-three studies met inclusion criteria among 604 retrieved (Figure 1). The demographic and clinical characteristics of included studies are reported in Tables 1-3 (references reported in Supplementary material). Overall 13476 patients (n = 3264 received TCZ) with COVID-19 pneumonia and various degree of severity were included. The median age was 62 years. Almost all received treatments consisting of antibiotics (e.g., azithromycin), antivirals, steroids plus or minus hydroxychloroquine. Mortality was 22.4% [95% confidence intervals (CIs): 17.9%-26.8%]. Ventilatory status improved in 63.9% (95%CI: 50.4%-75.6%).
| Ref. | Country | Type of study | No. of pts | Median follow up (d) | Male/Female, % | Median age (yr) | CV Comorbities, % | Respiratory/diabetes % | Other/cancer, % | Other medications, % | Ventilatory status (Baseline to end of follow up, %) | ICU admission %/time to ICU admission (d) |
| Alattar et al[11], 2020 | Quatar | Retrospective | 25 | 14 | 92/8 | 58 | 12 HTN | -/48 | CKD 16/4 | HCQ (100), AZITRO (96), lopinavir/ritonavir (96), ribavirin (88), and INF 1-α2a (60) | 56 (invasive) | 100/1 |
| Alberici et al[12], 2020 | Italy | Retrospective | 6 | 4 | - | - | - | -/- | -/- | Steroids, antivirals, HCQ | 33 (16 worsened) | -/- |
| Capra et al[13], 2020 | Italy | Retrospective (with ctr arm1) | 82 (n = 62 TCZ) | 9 | 73/27 | 63 | 63 HTN | -/16 | -/- | HCQ (100), lopinavir/ritonavir (100) | 35.2 (27% worsened) | 4.8/- |
| Colaneri et al[14], 2020 | Italy | Retrospective with prop. score | 112 (n = 21 TCZ) | 7 | 90/10 | 62.3 | 47.6 HTN | 0/9.5 | 19/4.7 | HCQ, AZITRO, steroids (100) | - | 14/- |
| Hassoun et al[15], 2020 | United States | Retrospective | 9 | - | 66/33 | 60 | 55 HTN | 11/11 | 66/- | HCQ, AZITRO (100) steroids (33), antibiotics (66) | - | 89/- |
| Klopfenstein et al[16], 2020 | France | Case control | 45 (n = 20 TCZ) | - | - | 76.8 | 55 HTN/70 CVS disease | 20/25 | -/35 | HCQ or lopinavir/ritonavir + antibiotics ± steroids (100) | - | 0/- |
| Luo et al[17], 2020 | China | Retrospctive | 15 | - | 80/20 | 73 | 66 HTN | -/26.6 | -/- | Steroids (53) | 6.6 (33.3% worsened) | -/- |
| Quartuccio et al[18], 2020 | Italy | Retrospective (with ctr arm1) | 111 (n = 42 TCZ) | 17.8 | 78.6/21.4 | 62.4 | 47.6 HTN | -/- | -/- | Antivirals (100), HCq (92.9) steroids (40); antibiotics (28.6) | 65 (invasive) | 57/- |
| Sciascia et al[19], 2020 | Italy | Prospective | 63 | - | 89/11 | 62.6 | 45 | 4.7/9.5 | - | Lopinavir/ritonavir (71), darunavir/cobicistat (29) | 95 | 7.9/- |
| Toniati et al[20], 2020 | Italy | Prospective | 100 | 10 | 88/12 | 62 | 62 | 9/17 | 11/6 | HCQ, lopinavir/ritonavir or remdesivir, antibiotic, steroids | 69 (n = 23 worsened) | 43/- |
| Xu et al[21], 2019 | China | Retrospective | 21 | - | 86/14 | 56.8 | 57.2 | 9.6/23.8 | CKD 4.8/- | Lopinavir/ritonavir, IFN-α, ribavirin, steroids (100) | 100 | -/- |
| Ramaswamy et al[22], 2020 | United States | Case control | 86 (n = 21 TCZ) | - | 61.9/38.1 | 63.2 | 14.3 HTN/heart disease, AF or stroke 19.1 | 28.6/14.3 | -/0 | HCQ (81), AZITRO (23.8), steroids (42.9) | - | 47.6/- |
| Rimland et al[23], 2020 | United States | Retrospective | 11 | 17 | 82/18 | 59 | 73 HTN/18 CVS | 27/36 | Renal or liver 18/9 | HCQ (36), AZITRO (64) | 54 (10% worsened) | 73/- |
| Sanchez-Montalva et al[24], 2020 | Spain | Prospective | 82 | - | 63/37 | 59.1 | 39 HTN/6.1 heart failure/12.2 AF | 23.5/19.5 | Liver 1.2/- | HCQ (98.9), lopinavir/ritonavir (76.8), AZITRO (96.3), darunavir/cobicistat (25) | 53 (52% worsened) | 2.9/- |
| Wadud et al[25], 2020 | United States | Case control | 94 (n = 44 TCZ) | - | -/- | 55.5 | - | -/- | -/- | - | - | - |
| Campochiaro et al[26], 2020 | Italy | Retrospective | 65 (n = 32 TCZ) | 28 | 91/9 | 64 | 37 HTN/12 CAD | 3/12 | CKD 9/6 | HCQ, AZITRO, lopinavir/ritonavir (100) | 91 | 0/- |
| Morena et al[27], 2020 | Italy | Prospective | 51 | 30 | 78.4/21.6 | 60 | 29.4 HTN/49 CVS disease | 9.8/11.8 | 5.9/5.9 | HCQ (98), antibiotics (76), lopinavir/ritonavir (82), remdesivir (42) | 66.6 (33% worsened) | 11.8/- |
| Kimmig et al[28], 2020 | United States | Retrospective (with ctr arm) | 60 (n = 28 TCZ) | - | 46.8/53.2 | 63.8 | 53.6 HTN/43 other | 35.7/14.3 | 14/14.3 | - | - | - |
| Roumier et al[29], 2020 | France | Compassionate use | 59 (n = 30 TCZ) | 8 | 80/20 | 50 | 20 HTN/13 CVS | 13/23 | 33/- | HCQ (6.6), steroids (6.6) | - | 23.3/- |
| Ip et al[30], 2020 | United States | Retrospective | 547 (n = 134 TCZ) | 30 | 78/22 | 62 | 71.6 HTN and coronary arthery disease | 15/35 | 15/9 | HCQ + AZITRO (92), steroids (66) | - | 100/- |
| Perrone et al[31], 2020 | Italy | Phase 2 and expansion cohort | 1221 (n = 708 TCZ3) | 30 | 82/18 | 61% > 60 | 68 heart disease or HTN | -/15 | -/- | HCQ (75), anti-retroviral (65), antibiotics (50), steroids (28) | - | 16 invasive ventilation/- |
| Perez-Tanoira et al[32], 2020 | Spain | Cohort study | 562 (n = 36 TCZ) | - | -/- | - | - | -/- | -/- | - | - | - |
| Somers et al[33], 2020 | United States | Observational | 154 (n = 78 TCZ) | 47 | 68/32 | 55 | 85 HTN or heart failure | 54/13 | CKD 35/- | HCQ (26), steroids (29), remdesivir (3) | 56 (18 and worsened) | 100/41 < 24 h, 36 > 48 h |
| Heili-Frades et al[34], 2020 | Spain | Cohort study | 4712 (n = 366 TCZ)2 | - | -/- | - | - | -/- | -/- | - | - | 40.7/- |
| Issa et al[35], 2020 | France | Retrospective | 10 | - | 100/0 | 66 | 60 HTN | -/30 | -/- | HCQ (100), steroids (30) | 50 | 70/7 d |
| Garcia et al[36], 2020 | Spain | Retrospective | 171 (n = 77 TCZ) | - | 58.8/51.2 | 61.5 | 61 HTN or heart disease | 10.3/15.6 | -/- | Antivirals (100, steroids (50) | 90 | 10.3/- |
| Ayerbe et al[37], 2020 | United Kingdom | Retrospective | 2075 (n = 421 TCZ) | 8 | -/- | - | - | -/- | -/- | - | - | -/- |
| Borku Uysal et al[38], 2020 | Turkey | Retrospective | 12 | 22 | 50/50 | 65.8 | 58 HTN | 16/58 | CKD 8/16 | HCQ and antivirals (100), AZITRO (50), antibiotics (58) | 82 | 17/- |
| Fernandez-Cruz et al[39], 2020 | Spain | Retrospective | 463 (n = 189 TCZ) | - | -/- | - | - | -/- | -/- | Steroids (100), other not available | - | -/- |
| Garibaldi et al[40], 2020 | United States | Cohort study | 832 (n = 39 TCZ) | - | -/- | - | - | -/- | -/- | - | - | -/- |
| Martínez-Sanz et al[41], 2020 | Spain | Cohort study | 1229 (n = 260 TCZ) | - | 73/27 | 65 | 17 HTN, 8 CAD, 2 heart failure | 18/15 | CKD 4/- | - | - | 19/6 d |
| Petrak et al[42], 2020 | United States | Retrospective | 145 | - | 64/36 | 58.1 | - | - | - | Corticosteroids (60), HCQ + AZITRO (98.6) | - | -/- |
| Rossi et al[43], 2020 | France | Case control | 246 (n = 106 TCZ) | 28 | 66/34 | 64 | 60 HTN, 23.6 CVS | 16/45 | -/5.7 | Antibiotics (100), HCQ (83), steroids (40), lopinavir/ritonavir (0.9) | - | -/- |
| Ref. | Fever (baseline) °C/% | O2 sat. % | Cough % | Dyspnea % | Leucocytes 109/L | Lymphocites/Neutrophil 109/L | PLT 109/L | Hb g/dL | LDH | Liver tests IU/L | CRP mg/L | PCT ng/L | D-dimer | IL6 ng/L | Imaging % |
| Alattar et al[11], 2020 | 38/92 | - | 84 | 72 | 6.0 | 0.9/5.0 | 208 | - | - | 46/30 | 95.2 | 0.38 | - | - | Infiltrates and ground glass opacities 100 |
| Alberici et al[12], 2020 | -/- | - | - | - | - | -/- | - | - | - | - | - | - | - | - | - |
| Capra et al[13], 2020 | 38/- | - | - | - | - | -/- | - | - | - | - | 123 | 0.6 | - | - | Bilateral pulmonary opacities 100 |
| Colaneri et al[14], 2020 | -/- | - | - | - | - | 0.6/8.4 | 303 | - | 445 | 38/72 | 21.3 | 0.24 | - | - | Interstitial lung disease 100 |
| Hassoun et al[15], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | - | - | - | - | - |
| Klopfenstein et al[16], 2020 | -/- | 90 | - | - | - | 0.67/- | - | - | - | -/- | 158 | - | - | - | ≥ 50% lung involvement 60 |
| Luo et al[17], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | 96 | - | - | 71 | - |
| Quartuccio et al[18], 2020 | -/- | - | - | - | 5540 | 0.68/4.5 | 157 | - | 625 | -/- | 79.05 | - | 835 | 63.5 | - |
| Sciascia et al[19], 2020 | < 38/39.7 | - | - | - | - | - | - | - | - | - | - | - | - | - | Bilateral pulmonary infiltrates |
| Toniati et al[20], 2020 | > 37.5/85 | - | 55 | 73 | 6 | 0.78 | 177 | 13.6 | 413 | 55/39 | 97 | - | 525 | 41 | Ground glass opacities and consolidation, bilateral pulmonary infiltration |
| Xu et al[21], 2019 | -/100 | - | 66.7 | - | 6.3 | 0.97 | 170 | - | 370 | 31/29 | 75 | 0.33 | 0.8 | 153 | Ground glass opacities and focal consolidation, peripheral and subpleural |
| Ramaswamy et al[22], 2020 | -/- | - | - | - | - | 1.1/6.7 | 200 | - | - | 60/43.5 | 15.9 | 2.2 | 2900 | 371 | - |
| Rimland et al[23], 2020 | -/- | - | - | - | 8.5 | -/0.8 | 230 | - | 1203 | 51/35 | 197.3 | - | 343.5 | 30.65 | - |
| Sanchez-Montalva et al[24], 2020 | 37.7/91.5 | 94 | 86.6 | 65.9 | 9.2 | 0.86/ | 199 | 13.3 | 446 | 53/41 | 17.98 | - | 295 | 74.8 | - |
| Wadud et al[25], 2020 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Campochiaro et al[26], 2020 | 37.6/- | - | - | - | - | -/- | - | - | 469 | -/- | 156 | - | - | - | - |
| Morena et al[27], 2020 | 74.5/- | - | 62.7 | 54.9 | 9.1 | 0.8/7.3 | 230 | - | 470 | 48/39 | 189 | - | 1706 | 116 | Bilateral pulmonary opacities 100 |
| Kimmig et al[28], 2020 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Roumier et al[29], 2020 | - | - | - | - | - | - | - | - | - | - | 189 | - | 3712 | - | - |
| Ip et al[30], 2020 | 80 | - | 78 | 80 | - | -/- | - | - | - | -/- | - | - | - | - | |
| Perrone et al[31], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | 30 | - | - | - | - |
| Perez-Tanoira et al[32], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | - | - | - | - | - |
| Somers et al[33], 2020 | -/- | - | - | - | 12.1 | 0.9/- | - | - | 627 | 50/76 | 185 | - | 2400 | - | - |
| Heili-Frades et al[34], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | - | - | - | - | - |
| Issa et al[35], 2020 | -/100 | - | - | - | - | -/- | - | - | - | -/- | 246 | - | 1354 | - | Ground glass opacities |
| Garcia et al[36], 2020 | -/98.7 | - | 83 | 43 | - | 0.87/- | - | - | - | -/- | 97 | - | 918 | - | - |
| Ayerbe et al[37], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | - | - | - | - | - |
| Borku Uysal et al[38], 2020 | -/92 | 92 | 100 | 67 | 6.1 | 1.09/4.3 | 180 | 13.8 | 259 | 33/39 | 54 | - | 599 | - | Ground glass opacities |
| Fernandez-Cruz et al[39], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | - | - | - | - | - |
| Garibaldi et al[40], 2020 | -/- | - | - | - | - | -/- | - | - | - | -/- | - | - | - | - | - |
| Martínez-Sanz et al[41], 2020 | 36.8/- | 91 | - | - | - | 0.89/5.4 | - | - | 669 | -/32 | 113 | - | 809 | 70 | - |
| Petrak et al[42], 2020 | - | - | - | - | - | - | - | - | 538 | - | 53.3 | - | 1.3 | - | - |
| Rossi et al[43], 2020 | 37.5/- | 94 | - | - | - | 1.128/- | - | - | - | - | 168 | - | - | - | - |
| Ref. | N° TCZ administered (median doses) | Death % | Dismissed % | Median hospitalization (d) | TCZ AEs % | Comparison with other medications or no TCZ | NOS Scale | ROBIN risk |
| Alattar et al[11], 2020 | 1 | 12 | 36 (from ICU) | - | Anemia 64; ALT ↑ 44 | HR for discharge from ICU 0.64 (0.37-1.11) | 8 | Low |
| Alberici et al[12], 2020 | 1 | 33 | 16 | - | - | - | 6 | Moderate |
| Capra et al[13], 2020 | 1 | 8 | 92 | 12.5 | - | OR for OS 0.036 (0.07-0.18)° | 7 | Low |
| Colaneri et al[14], 2020 | 2 | 23.8 | 85.7 (from ICU) | 2 | 0 | OR for OS 0.78 (0.06-9.34); OR for ICU 0.11 (0-3.38) | 7 | Low |
| Hassoun et al[15], 2020 | 1 | 22 | 55 | 13.5 (n = 7) | - | - | 5 | Low |
| Klopfenstein et al[16], 2020 | 1 or 2 | 25 | 55 | 13 | - | OR for OS and ICU admission 0.36 (0.1-1.3) and 0.03 (0.002-0.56); OR for mechanical vent 0.05 (0.003-0.93) | 5 | Low |
| Luo et al[17], 2020 | 1 | 20 | - | - | - | - | 5 | High |
| Quartuccio et al[18], 2020 | 1 | 9.5 | 28.5 | - | - | OR for OS 14.5 (0.76-278.3); OR for ICU admission 220.9 (12.7-3826.1) | 8 | Moderate |
| Sciascia et al[19], 2020 | 1 (2 in 82.5%) | 11 | - | - | - | - | 6 | Moderate |
| Toniati et al[20], 2020 | 1 (2 in 87%) | 20 | 15 | - | Septic shock (n = 2), GI perforation (n = 1) | - | 8 | Low |
| Xu et al[21], 2019 | 1 (2 in 14.3%) | 0 | 100 | 15.1 | - | - | 5 | Moderate |
| Ramaswamy et al[22], 2020 | 1 (2 in 38%) | 14.3 | - | - | - | HR for OS 0.25 (0.07-0.9) | 5 | Moderate |
| Rimland et al[23], 2020 | 1 | 27 | 18 | 18 | - | - | 7 | Low |
| Sanchez-Montalva et al[24], 2020 | 1 | 26.8 | 41.5 | - | - | - | 6 | Low |
| Wadud et al[25], 2020 | - | 38.6 | - | - | - | OR for OS 0.58 (0.25-1.32) | 6 | Moderate |
| Campochiaro et al[26], 2020 | 1 (2 in 28%) | 15 | 63 | 13.5 | SAEs (25) | OR for OS 0.38 (0.11-1.27); OR for ICU admission 0.33 (0.13-8.5) | 8 | Low |
| Morena et al[27], 2020 | - | 27 | 61 | - | AST/ALT ↑ 29, PLT 14, neutropenia 6, rash 2 | - | 8 | Low |
| Kimmig et al[28], 2020 | 1 (2 in 10.7%) | 42.9 | 25 | - | Infections 71.4 | OR for OS 2.25 (0.75-2.24) | 6 | Moderate |
| Roumier et al[29], 2020 | 1 | 10 | 20 | - | - | OR for OS 0.25 (0.05-1.03); OR for ICU 0.17 (0.06-0.48) | 7 | Low |
| Ip et al[30], 2020 | 1 (78%) | 46 | - | - | Bacteriemia (13), secondary pneumonia (9) | OR for OS 0.66 (045-0.99) | 8 | Low |
| Perrone et al[31], 2020 | 1 (59.8), 2 (54.5) | 20 | - | - | 26.4 G3-5; 14.4 G1-2 | OR for 30-d OS 0.7 (0.41-1.22) and 1.22 (0.86-1.92) in phase 2 and validation cohort | 8 | Low |
| Perez-Tanoira et al[32], 2020 | - | 27.7 | - | - | - | OR for OS 1.015 (0.47-2.18) | 5 | Moderate |
| Somers et al[33], 2020 | 1 | 18 | 56 | 20.4 | Superinfection (54) | OR 0.39 (0.18-0.82) | 8 | Low |
| Heili-Frades et al[34], 2020 | - | 22.4 | - | - | - | - | 6 | Moderate |
| Issa et al[35], 2020 | 1 | 10 | - | 11 (ICU) | - | - | 5 | High |
| Moreno-Garcia et al[36], 2020 | - | 10.3 | 84.4 | - | - | OR for ICU 0.3 (0.12-0.71) and OR for OS 0.52 (0.21-1.29) | 5 | Moderate |
| Ayerbe et al[37], 2020 | - | 21.1 | - | - | - | OR for OS 1.9 (1.44-2.51) | 5 | High |
| Borku Uysal et al[38], 2020 | 2 | 0 | 100 | - | - | - | 6 | Moderate |
| Fernandez-Cruz et al[39], 2020 | - | - | - | - | - | OR for OS 0.69 (0.41-1.19) | 5 | High |
| Garibaldi et al[40], 2020 | - | 5 | - | - | - | OR for OS 1.14 (0.46-2.81) | 5 | Moderate |
| Martínez-Sanz et al[41], 2020 | 1 | 23 | - | 13 | - | OR for OS 2.19 (1.54-3.1) | 5 | Low |
| Petrak et al[42], 2020 | 1 (84.8), 2 (15.2) | 28.3 | 48.3 | - | - | - | 5 | Moderate |
| Rossi et al[43], 2020 | 1 | 28.9 | - | - | - | HR for OS 0.29 (0.17-0.49) | 8 | Low |
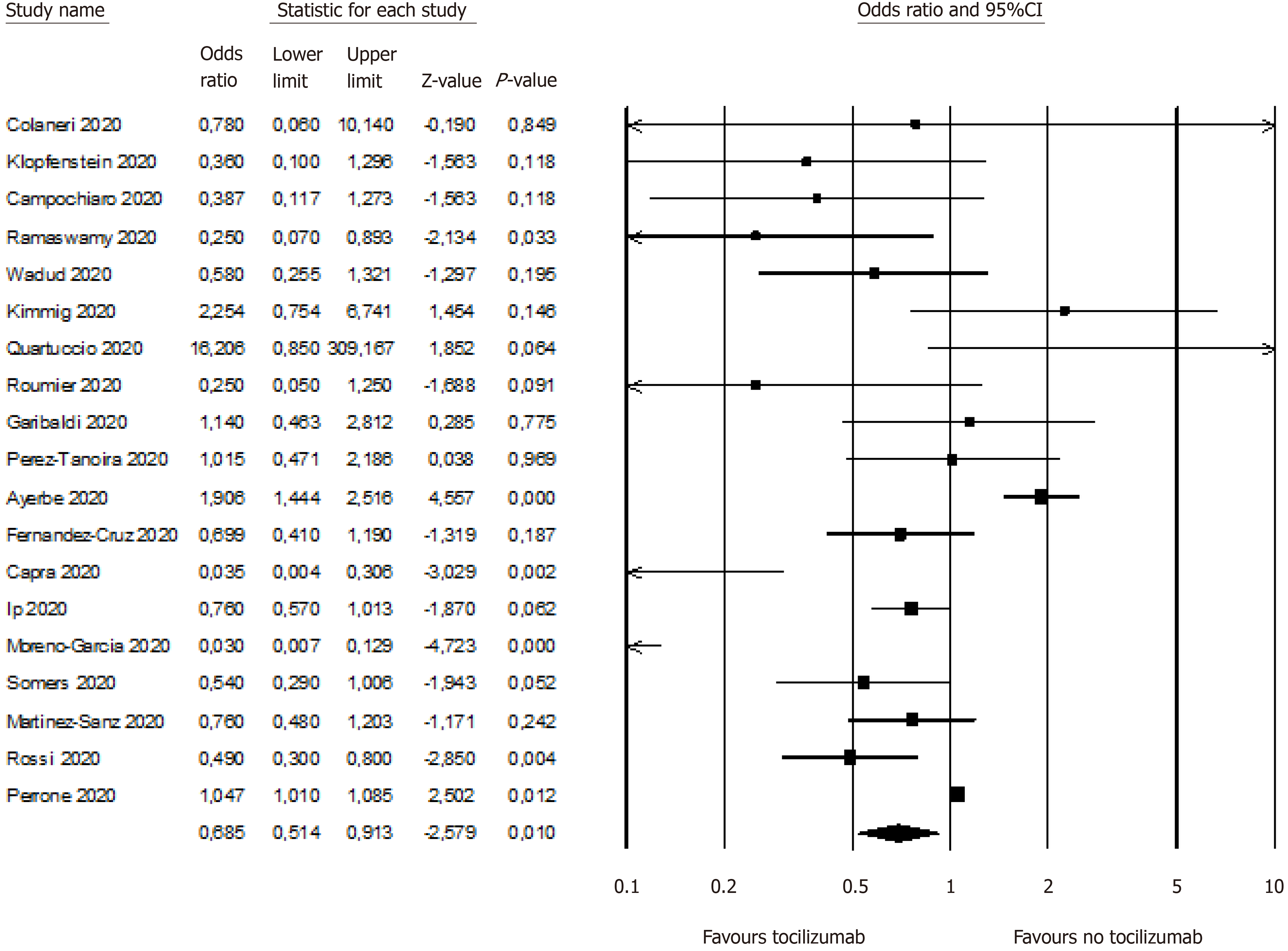
Outcome was improved with TCZ. In the primary analysis (n = 19 studies reporting data), mortality was reduced in patients treated with TCZ (OR = 0.64, 95%CI: 0.47-0.87; P < 0.01; Figure 2). In 9 studies where risk of death with TCZ use was controlled for other variables mortality was reduced by 57% (OR = 0.43, 95%CI: 0.27-0.7; P < 0.01). Intensive care need (mechanical ventilation) was also reduced (OR = 0.36, 95%CI: 0.14-0.89; P = 0.02). In all cases, a random effect model was used.
Egger’s test indicated a significant publication bias (P = 0.01). Duvall and Tweedie’s trim and fill procedure indicated 4 missing studies (see the funnel plot with imputed studies in Supplementary material). The adjusted effect size (after imputation of the missing studies) was 0.84 (95%CI: 0.63-1.14).
A large part of the ongoing research into COVID-19 infection is concentrated on finding an immunomodulatory therapy to down-regulate the cytokine storm, usually combining it with antiviral agents[6]. In fact, IL-6 binds either with transmembrane IL-6 receptors or soluble IL-6 receptors, and the resulting complex can combine with the signal-transducing component gp130 to activate the inflammatory response. In an emergent situation where no approved drugs are available and supportive measures are available only for critically ill patients, any new promising agent merits attention. A meta-analysis has correlated IL-6 concentration with COVID-19 severity. Those with severe cases show a 2.9-fold higher concentration than those without complications[7].
Siltuximab, a chimeric monoclonal antibody acting and blocking IL-6, is being tested in the SISCO study, including patients with acute respiratory distress syndrome related to COVID-19 infection (NCT04322188). Preliminary data from 21 patients showed a reduction in the C-reactive protein levels in 16 patients, a clinical improvement in 33% and disease stabilization in 43% of cases[8].
In this pooled analysis of 31 studies including 2898 patients treated with TCZ, we found a strong trend toward improved survival with the use of TCZ (a significant reduction in acute mortality risk by 36%). Tocilizumab administration was also independently associated with a 57% reduced risk of death in multivariable analysis. Tocilizumab reduced also the risk of mechanical ventilation and ICU admission by 64%. Overall mortality rate was 22%.
The limitations of these data are related to the observational nature of the studies, primarily monocentric and non-controlled. The population treated with TCZ was negatively selected for the worst clinical and inflammatory conditions. Also, due to the non-randomized design of all studies, final results might have been biased, and the added value of TCZ might not have been formally proven. However, despite a likely imbalance among clinical and laboratory baseline variables between the 2 groups, the effect of TCZ on clinical outcomes appears sustained. We finally recognize that some papers reported in the primary analysis were pre-printed in MedRxiv archive and not still finally reviewed and published in full.
At this time, 45 trials are underway to explore the contribution of TCZ when added to the standard of care for COVID-19. Four are in phase 3 trials: the COVACTA study (NCT04320615), in which TCZ is compared with placebo, the NCT04361552 study in which the control arm is represented by best practices, the COV-AID study (NCT04330638), a six-arm study including anakinra and the association of anakinra + TCZ, and the RECOVERY study (NCT04381936), also a six-arm study, including hydroxychloroquine, lopinavir/ritonavir, and low doses of steroids.
Recently, the use of hydroxycloroquine or chloroquine with or without a macrolide was associated with decreased survival and increased rate of ventricular arrhythmias in COVID-19 hospitalized patients[9]. Despite this alarming concern, article and data purity were subsequently questioned and article retracted. Similarly, results of a separate study with data attained from a different database, showed that hydroxycloroquine failed to reduce infection risk in people exposed to patients with confirmed COVID-19. Results indicated that the incidence of new illness compatible with COVID-19 did not differ significantly between those who received hydroxycloroquine and those who received placebo[10]. Therefore, new combinations of potentially active drugs need to be tested, and efficacy confirmed in these patients[11-43].
In conclusion, we provide the first evidence that TCZ can improve the respiratory and clinical outcomes of patients with COVID-19 pneumonia in clinical practice, but its use merits further confirmatory trials.
Coronavirus disease 2019 (COVID-19) infection is associated with a cytokine storm during acute phase.
Interleukin-6 is a key player in this systemic inflammation.
We evaluated the effect of tocilizumab (TCZ) on the outcomes of COVID-19 pneumonia.
We performed a systematic review and pooled analysis of published literature.
Mortality was reduced in patients treated with TCZ (Odds ratio = 0.64, 95%CI: 0.47-0.87; P < 0.01).
We conclude that TCZ may improve outcome of COVID-19 infected patients.
Current use of tocilizumab in clinical practice has to be validated further through large randomized trials.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Medical Oncology.
Specialty type: Infectious diseases
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan Y S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30106] [Article Influence: 6021.2] [Reference Citation Analysis (3)] |
| 2. | Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1228] [Cited by in RCA: 1217] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 3. | Alzghari SK, Acuña VS. Supportive Treatment with Tocilizumab for COVID-19: A Systematic Review. J Clin Virol. 2020;127:104380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47144] [Article Influence: 2946.5] [Reference Citation Analysis (0)] |
| 5. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10833] [Article Influence: 1203.7] [Reference Citation Analysis (2)] |
| 6. | Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55:105982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 7. | Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 493] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 8. | Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, Frigeni M, Damiani M, Micò C, Fagiuoli S, Cosentini R, Lorini FL, Gandini L, Novelli L, Morgan JP, Owens BMJ, Kanhai K, Reljanovic GT, Rizzi M, Di Marco F, Rambaldi A. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. 2020 Preprint. [DOI] [Full Text] |
| 9. | Mehra MR, Desai SS, Ruschitzka F, Patel AN. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 479] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 10. | Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383:517-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 923] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 11. | Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the Treatment of Severe COVID-19. J Med Virol. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 12. | Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Zambetti N, Moscato M, Venturini M, Affatato S, Gaggiotti M, Bossini N, Scolari F. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 13. | Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, Cossi S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 14. | Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, Montecucco C, Mojoli F, Giusti EM, Bruno R, The Covid Irccs San Matteo Pavia Task Force. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Hassoun A, Thottacherry ED, Muklewicz J, Aziz QU, Edwards J. Utilizing tocilizumab for the treatment of cytokine release syndrome in COVID-19. J Clin Virol. 2020;128:104443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, Toko L, Mezher C, Kadiane-Oussou NJ, Bossert M, Bozgan AM, Charpentier A, Roux MF, Contreras R, Mazurier I, Dussert P, Gendrin V, Conrozier T; HNF Hospital Tocilizumab multidisciplinary team. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;50:397-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 17. | Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 834] [Cited by in RCA: 876] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 18. | Quartuccio L, Sonaglia A, McGonagle D, Fabris M, Peghin M, Pecori D, De Monte A, Bove T, Curcio F, Bassi F, De Vita S, Tascini C. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129:104444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 19. | Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, Bonora S, Calcagno A, Cecchi I, Cinnirella G, Converso M, Cozzi M, Crosasso P, De Iaco F, Di Perri G, Eandi M, Fenoglio R, Giusti M, Imperiale D, Imperiale G, Livigni S, Manno E, Massara C, Milone V, Natale G, Navarra M, Oddone V, Osella S, Piccioni P, Radin M, Roccatello D, Rossi D. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529-532. [PubMed] |
| 20. | Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Airò P, Bazzani C, Beindorf EA, Berlendis M, Bezzi M, Bossini N, Castellano M, Cattaneo S, Cavazzana I, Contessi GB, Crippa M, Delbarba A, De Peri E, Faletti A, Filippini M, Frassi M, Gaggiotti M, Gorla R, Lanspa M, Lorenzotti S, Marino R, Maroldi R, Metra M, Matteelli A, Modina D, Moioli G, Montani G, Muiesan ML, Odolini S, Peli E, Pesenti S, Pezzoli MC, Pirola I, Pozzi A, Proto A, Rasulo FA, Renisi G, Ricci C, Rizzoni D, Romanelli G, Rossi M, Salvetti M, Scolari F, Signorini L, Taglietti M, Tomasoni G, Tomasoni LR, Turla F, Valsecchi A, Zani D, Zuccalà F, Zunica F, Focà E, Andreoli L, Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 560] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 21. | Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970-10975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1811] [Cited by in RCA: 1748] [Article Influence: 349.6] [Reference Citation Analysis (0)] |
| 22. | Ramaswamy M, Mannam P, Comer R, Sinclair E, McQuaid DB, Schmidt ML. COVID-19 Disease in a Regional Community Health System: A Case-Control Study. medRxiv:20099234. |
| 23. | Rimland CA, Morgan CE, Bell GJ, Kim MK, Hedrick T, Marx A, Bramson B, Swygard H, Napravnik S, Schmitz JL, Carson SS, Fischer WA, Eron JJ, Gay CL, Parr JB. Clinical characteristics and early outcomes in patients with COVID-19 treated with tocilizumab at a United States academic center. medRxiv:20100404. [DOI] [Full Text] |
| 24. | Sanchez-Montalva A, Selares-Nadal J, Espinosa-Pereiro J, Fernandez-Hidalgo N, Perez-Hoyos S, Salvador F, Dura-Miralles X, Miarons M, Anton A, Eremiev S, Sempere-Gonzalez A, Bosch-Nicolau P, Monforte-Pallares A, Augustin S, Sampol J, Guillen-del-Castillo A, Almirante B. Early outcomes of tocilizumab in adults hospitalized with severe COVID19: An initial report from the Vall dHebron COVID19 prospective cohort study. medRxiv:20094599. [DOI] [Full Text] |
| 25. | Wadud N, Ahmed N, Shergil MM, Khan M, Krishna MG, Gilani A, Zarif SE, Galaydick J, Linga K, Koor S, Galea J, Stuczynski L, Osundele MB. Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. medRxiv:20100081. [DOI] [Full Text] |
| 26. | Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F, Zangrillo A, Tresoldi M, Castagna A, Dagna L; TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 27. | Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, Torre A, Cossu MV, Minari C, Ballone E, Perotti A, Mileto D, Niero F, Merli S, Foschi A, Vimercati S, Rizzardini G, Sollima S, Bradanini L, Galimberti L, Colombo R, Micheli V, Negri C, Ridolfo AL, Meroni L, Galli M, Antinori S, Corbellino M. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 28. | Kimmig LM, Wu D, Gold M, Pettit NN, Pitrak D, Mueller J, Husain AN, Mutlu EA, Mutlu GM. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front Med (Lausanne). 2020;7:583897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 29. | Roumier M, Paule R, Vallée A, Rohmer J, Ballester M, Brun AL, Cerf C, Chabi ML, Chinet T, Colombier MA, Farfour E, Fourn E, Géri G, Khau D, Marroun I, Ponsoye M, Roux A, Salvator H, Schoindre Y, Si Larbi AG, Tchérakian C, Vasse M, Verrat A, Zuber B, Couderc LJ, Kahn JE, Groh M, Ackermann F; Foch COVID-19 Study Group. Tocilizumab for Severe Worsening COVID-19 Pneumonia: a Propensity Score Analysis. J Clin Immunol. 2021;41:303-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Ip A, Berry DA, Hansen E, Goy AH, Pecora AL, Sinclaire BA, Bednarz U, Marafelias M, Berry SM, Berry NS, Mathura S, Sawczuk IS, Biran N, Go RC, Sperber S, Piwoz JA, Balani B, Cicogna C, Sebti R, Zuckerman J, Rose KM, Tank L, Jacobs LG, Korcak J, Timmapuri SL, Underwood JP, Sugalski G, Barsky C, Varga DW, Asif A, Landolfi JC, Goldberg SL. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study. PLoS One. 2020;15:e0237693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 31. | Perrone F, Piccirillo MC, Ascierto PA, Salvarani C, Parrella R, Marata AM, Popoli P, Ferraris L, Marrocco-Trischitta MM, Ripamonti D, Binda F, Bonfanti P, Squillace N, Castelli F, Muiesan ML, Lichtner M, Calzetti C, Salerno ND, Atripaldi L, Cascella M, Costantini M, Dolci G, Facciolongo NC, Fraganza F, Massari M, Montesarchio V, Mussini C, Negri EA, Botti G, Cardone C, Gargiulo P, Gravina A, Schettino C, Arenare L, Chiodini P, Gallo C; TOCIVID-19 investigators; Italy. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J Transl Med. 2020;18:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 32. | Perez-Tanoira R, Garcia FP, Romanyk J, Gomez-Herruz P, Arroyo T, Gonzalez R, Garcia LL, Exposito CV, Moreno JS, Gutierrez I, Mathews AU, Ramos EL, Garcia LM, Troncoso D, Cuadros J. Prevalence and risk factors for mortality related to COVID-19 in a severely affected area of Madrid, Spain. medRxiv:20112912. [DOI] [Full Text] |
| 33. | Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, Zhou N, Petty LA, Baang JH, Dillman NO, Frame D, Gregg KS, Kaul DR, Nagel J, Patel TS, Zhou S, Lauring AS, Hanauer DA, Martin E, Sharma P, Fung CM, Pogue JM. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 34. | Heili-Frades S, Minguez P, Mahillo-Fernandez I, Prieto-Rumeau T, Gonzalez AH, de la Fuente L, Nieto MJR, Peces-Barba Romero G, Peces-Barba M, de Miguel MPC, Ormaechea IF, Prieto AN, de Blas FE, Hiscock LJ, Calvo CP, Santos A, Alameda LEM, Bueno FR, Hernandez-Mora MG, Ubeda AC, Alvarez BA, Petkova E, Carrasco N, Martin Rios D, Mangado NG, Pernaute OS. COVID-19 Outcomes in 4712 consecutively confirmed SARS-CoV2 cases in the city of Madrid. medRxiv:20109850. [DOI] [Full Text] |
| 35. | Issa N, Dumery M, Guisset O, Mourissoux G, Bonnet F, Camou F. Feasibility of tocilizumab in ICU patients with COVID-19. J Med Virol. 2021;93:46-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Garcia EM, Caballero VR, Albiach L, Aguero D, Ambrosioni J, Bodro M, Cardozo C, Chumbita M, De la Mora L, Pouton NG, Vidal CG, GonzalezCordon A, Meneses MH, Inciarte A, Laguno M, Leal L, Linares L, Macaya I, Meira F, Mensa J, Moreno A, Morata L, Alcalde PP, Rojas J, Sola M, Torres B, Torres M, Tome A, Castro P, Fernandez S, Nicolas JM, Riera AA, Munoz J, Fernandez MJ, Marcos MA, Soy D, Martinez JA, Garcia F, Soriano A. Ocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv:20113738. [DOI] [Full Text] |
| 37. | Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. 2020;50:298-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 38. | Borku Uysal B, Ikitimur H, Yavuzer S, Ikitimur B, Uysal H, Islamoglu MS, Ozcan E, Aktepe E, Yavuzer H, Cengiz M. Tocilizumab challenge: A series of cytokine storm therapy experiences in hospitalized COVID-19 pneumonia patients. J Med Virol. 2020;92:2648-2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Ana Fernández-Cruz, Belén Ruiz-Antorán, Ana Muñoz-Gómez, Aránzazu Sancho-López, Patricia Mills-Sánchez, Gustavo Adolfo Centeno-Soto, Silvia Blanco-Alonso, Laura Javaloyes-Garachana, Amy Galán-Gómez, Ángela Valencia-Alijo, Javier Gómez-Irusta, Concepción Payares-Herrera, Ignacio Morrás-Torre, Enrique Sánchez-Chica, Laura Delgado-Téllez-de-Cepeda, Alejandro Callejas-Díaz, Antonio Ramos-Martínez, Elena Múñez-Rubio. Cristina Avendaño-Solá on behalf of the Puerta de Hierro COVID-19 Study Group. Antimicrobial Agents and Chemotherapy Aug. 2020;64:e01168-20. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 40. | Garibaldi BT, Fiksel J, Muschelli J, Robinson ML, Rouhizadeh M, Perin J, Schumock G, Nagy P, Gray JH, Malapati H, Ghobadi-Krueger M, Niessen TM, Kim BS, Hill PM, Ahmed MS, Dobkin ED, Blanding R, Abele J, Woods B, Harkness K, Thiemann DR, Bowring MG, Shah AB, Wang MC, Bandeen-Roche K, Rosen A, Zeger SL, Gupta A. Patient Trajectories Among Persons Hospitalized for COVID-19 : A Cohort Study. Ann Intern Med. 2021;174:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 41. | Martínez-Sanz J, Muriel A, Ron R, Herrera S, Pérez-Molina JA, Moreno S, Serrano-Villar S. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. Clin Microbiol Infect. 2021;27:238-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Petrak RM, Skorodin NC, Van Hise NW, Fliegelman RM, Pinsky J, Didwania V, Anderson M, Diaz M, Shah K, Chundi VV, Hines DW, Harting BP, Sidwha K, Yu B, Brune P, Owaisi A, Beezhold D, Kent J, Vais D, Han A, Gowda N, Sahgal N, Silverman J, Stake J, Nepomuceno J, Heddurshetti R. Tocilizumab as a Therapeutic Agent for Critically Ill Patients Infected with SARS-CoV-2. Clin Transl Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Rossi B, Nguyen LS, Zimmermann P, Boucenna F, Dubret L, Baucher L, Guillot H, Bouldouyre MA, Allenbach Y, Salem JE, Barsoum P, Oufella A, Gros H. Effect of Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia: A Case-Control Cohort Study. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |