Published online May 20, 2021. doi: 10.5662/wjm.v11.i3.75
Peer-review started: January 2, 2021
First decision: January 25, 2021
Revised: February 5, 2021
Accepted: March 12, 2021
Article in press: March 12, 2021
Published online: May 20, 2021
Processing time: 122 Days and 15.1 Hours
Systematic reviews in orthopaedic literature are frequently criticised for offering inconsistent conclusions. On top of that, high-quality randomized human evidence on crucial orthopaedic topics is more often than not lacking. In this situation, pooling animal literature could offer an excellent insight into unanswered critical clinical questions, thus potentially improving healthcare. In this paper, we sought to present the rationale and basic principles governing meta-analysis of animal research. More specifically, we elaborated on the available evidence-based methods to achieve a scientifically sound animal data synthesis. In addition, we discussed result interpretation, strength of recommen
Core Tip: Relying on the findings of properly conducted meta-analyses of animal research is crucial, particularly in the paucity of human evidence on crucial orthopaedic topics. It is an undeniable fact that authors tend to encounter a great many challenges when conducting this type of research as they have to address several potential sources of bias. For that reason, we advocate that readers should critically appraise the findings of animal syntheses papers.
- Citation: Tsikopoulos K, Sidiropoulos K, Kitridis D, Drago L, Ebnezar R, Lavalette D. Rationalising animal research synthesis in orthopaedic literature. World J Methodol 2021; 11(3): 75-80
- URL: https://www.wjgnet.com/2222-0682/full/v11/i3/75.htm
- DOI: https://dx.doi.org/10.5662/wjm.v11.i3.75
Given the nature and rarity of many orthopaedic diseases, conducting high-quality double-blind randomized control trials is not always feasible. This is particularly true when it comes to addressing a particular orthopaedic surgical intervention. Hence, crucial research questions remain unanswered due to the fact that safe conclusions cannot be drawn purely based on a few underpowered and low-quality individual studies. In this situation, animal evidence could offer valuable information towards delineating the potential of a prevention and/or therapeutic orthopaedic intervention.
The rationale behind synthesizing animal literature is to avoid the potential bias which is commonly detected in narrative literature reviews. To elaborate, selective presentation of individual study findings and incorrect weighting of conclusions can exert a negative impact on the credibility of a systematic review. Rather, by summarizing the results of multiple individual studies a researcher could potentially produce more valid results provided that guidelines governing meta-analyses of animal papers are respected. In this paper, we sought to present the key elements for conducting a high-quality meta-analysis of animal research which could provide a useful insight into unanswered clinical questions in orthopaedics.
Regardless of the nature of the subjects utilised in an in vivo evidence synthesis, it is strongly advocated that systematic reviews be prospectively registered with a valid database (e.g., PROSPERO). The main reason behind this protocol registration is to increase transparency in reporting and prevent selective outcome reporting issues.
On top of that, abiding by published guidelines for systematic reviews (e.g., Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is of utmost importance given the fact that poor reporting diminishes accuracy and potential usefulness of an animal meta-analysis[1].
From a methodological standpoint, if properly controlled homogenous groups are available, then standard head-to-head meta-analysis can be safely undertaken by using a readily available piece of statistical software [e.g., Review Manager (RevMan)][2]. However, synthesising uncontrolled research represents a different task which can be achieved by means of proportional meta-analysis[3]. It is underlined that although indirect comparisons could be made by comparing overlapping of confidence intervals in the aforementioned type of meta-analysis, safe conclusions on the comparative efficacy of interventions cannot be reached and therefore this approach is not generally recommended.
One frequently encountered methodological issue in pair-wise meta-analyses is the limited statistical power precluding reliable conclusions to be drawn[4]. To address this issue, lumping intervention groups into valid subgroups with respect to literature classifications[5,6] is recommended. By and large, a crucial point authors need to pay attention to when they elect for the subgroup pathway is the trade-off between statistical power and precision in reporting. We advocate that as long as published guidelines have been followed prior to creating subgroups and sensitivity analysis has been conducted to investigate the impact of subgrouping on the data synthesis, the validity of the findings is not severely compromised.
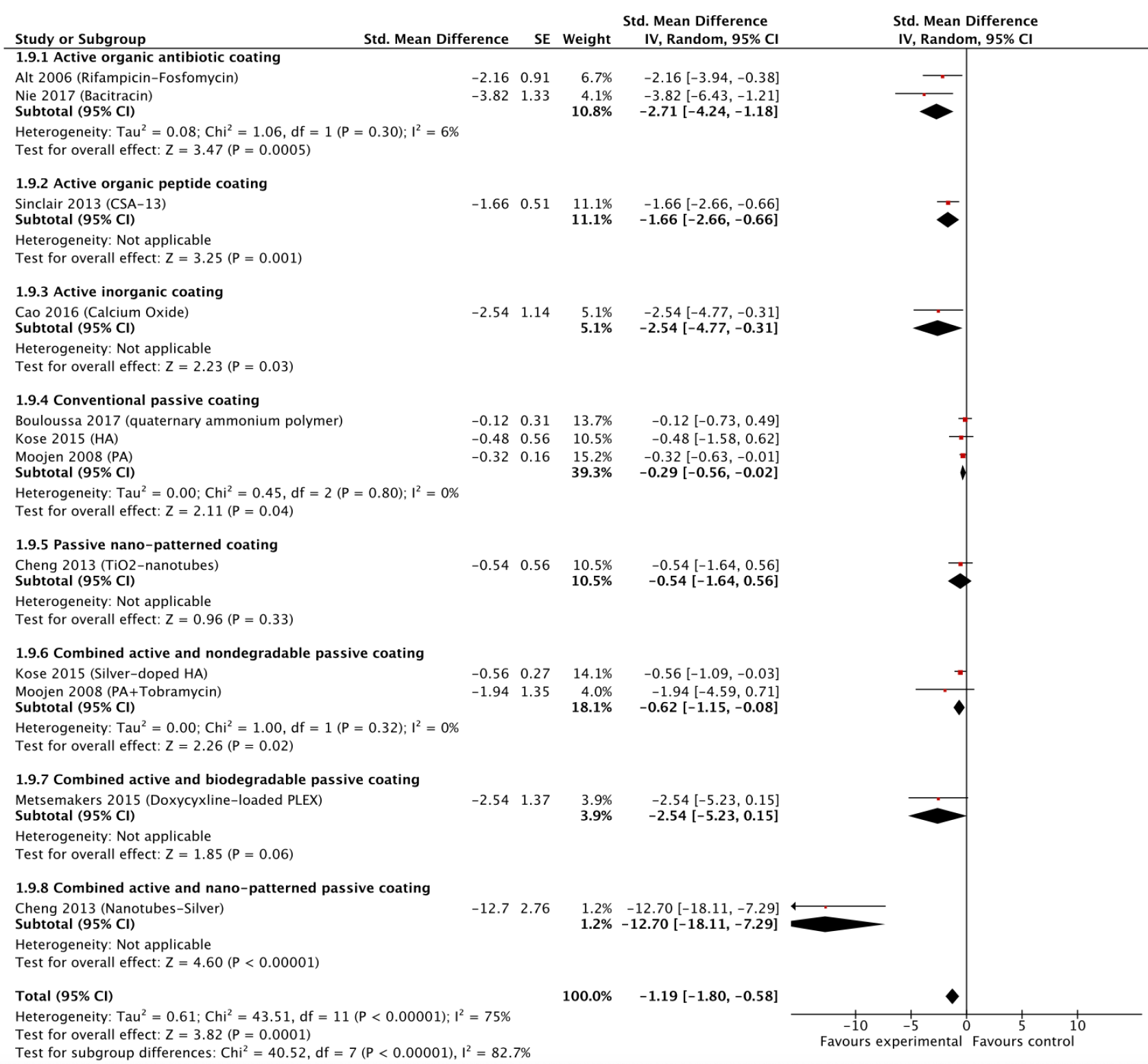
Encountering a situation where information for the same outcome is presented in some studies as dichotomous data and in other papers by means of a continuous variable is a common phenomenon in animal research. To address this issue, re-expressing standardized mean differences to odds ratios (or the vice versa) is recommended[7]. Subsequently the generic inverse variance model in RevMan can be utilised to pool those converted data together[7] (Figure 1). Although we recognise this could be a challenging task for a researcher to accomplish, the problem of missing information which may compromise the validity of the meta-analysis results can be overcome[5].
Meticulous data extraction is a crucial element in performing a satisfactory systematic review and meta-analysis. It is a common phenomenon in original papers published a long time ago to present their findings in a graphical manner with no corresponding numerical data. In this situation, taking advantage of the use of an appropriate software tool (e.g., Plot Digitizer and Getdata Graph Digitizer)[8] which allows for reliable digitization of graphs and/or plots is recommended to abstract and subsequently synthesise the required information.
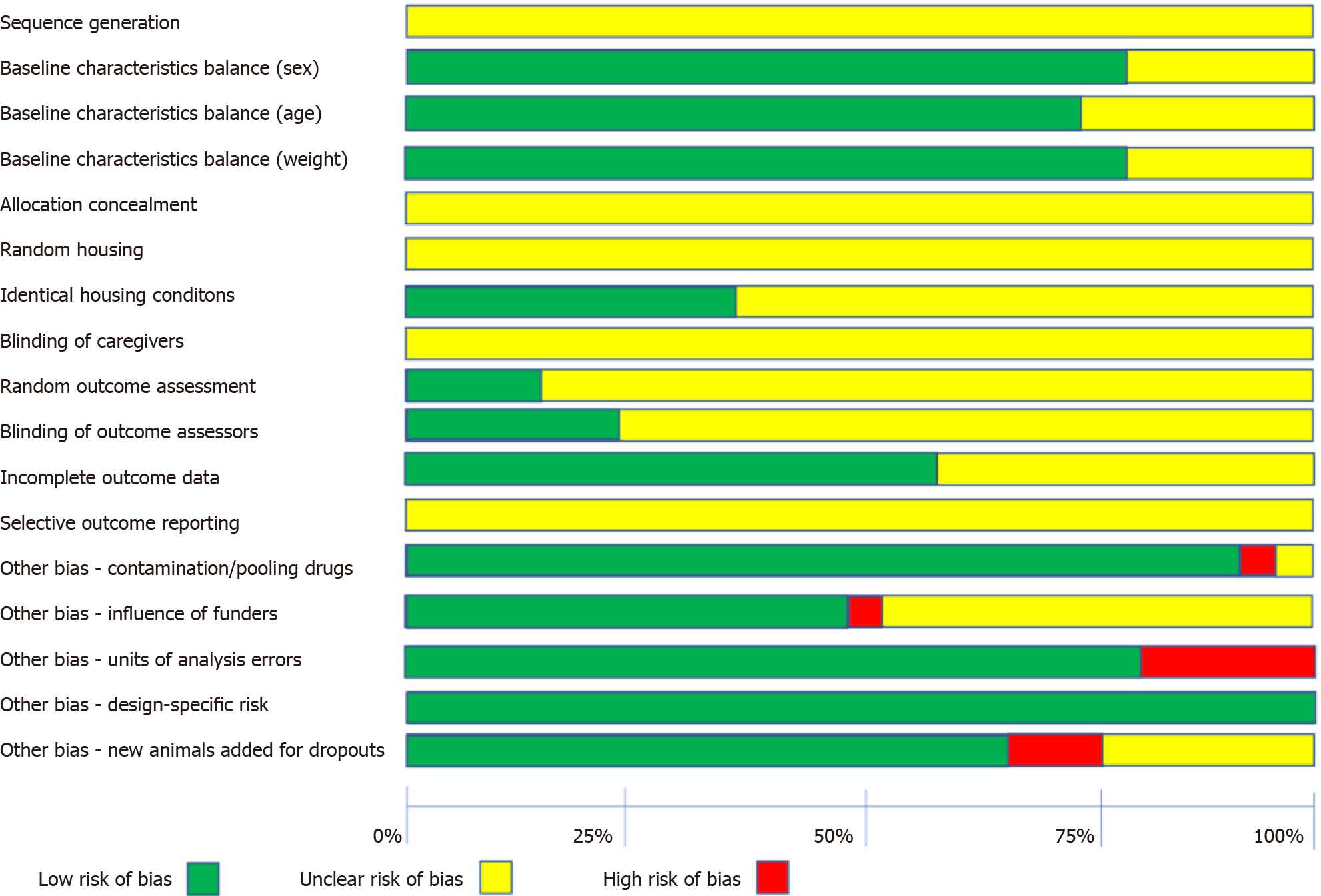
Quality appraisal of individual animal studies performed by means well-established tool such as the SYRCLE’s Risk of Bias tool[9], ensures consistency and prevents discrepancies in assessing risk of bias in systematic reviews of animal intervention studies. SYRCLE’s Risk of Bias tool is an adaptation of the Cochrane Risk of Bias tool which could potentially facilitate transition of animal research into clinical practice. On top of that, due to the relatively standardised use of this instrument in the existing literature, the necessity of improving particular methodological aspects of animal studies can be easily stressed[9]. It should be noted that a graphical quantification of the risk of bias summarising the assessments for each domain could be of essence (Figure 2)[5].
It is an undeniable fact that “negative” laboratory animal results more often than not remain unpublished[10]. Therefore, exploration of selective reporting in animal papers appears to be critical. In other words, merely relying on statistical significance may introduce bias in the results of the statistical analysis and potentially threaten the validity of the meta-analysis findings.
It is highlighted that while a systematic review is generally better than an individual study, a meta-analysis of animal studies should not be placed at the top of the hierarchy in a pyramid that depicts validity[11]. This is because a meta-analysis is as good as the studies identified and included[12]. Nevertheless, in the absence of high-quality evidence, relying on the results of a meta-analysis of animal models is advisable provided that caution is exercised due to potential bias.
It is worthy of note that prior to drawing meta-analysis conclusions, sample size of the included comparison groups, quality rating of the involved studies, effect sizes, and statistical heterogeneity should be taken into account. On top of that, investigating the impact of various sources of clinical heterogeneity by means of a sensitivity analysis (i.e., exclusion of one or more papers from the analysis to assess the impact of a particular confounding factor on the findings of the study) with a view to verify the meta-analysis results is strongly advocated.
Despite the abundance of literature on developing meta-analytic skills relating to human data, methodological papers dealing with animal data synthesis are lacking. In the current article, we focused on the technicalities and implications of pooling animal literature which could be particularly useful when investigating the results of orthopaedic surgical interventions in the absence of human evidence. It is worthy of note that due to the experimental nature of animal papers, a certain amount of uncertainty in the meta-analysis conclusions is anticipated. For that reason, we advise caution when it comes to extrapolating the results of this type of research back to human biology.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pérez-Cabezas V S-Editor: Liu M L-Editor: A P-Editor: Yuan YY
| 1. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17526] [Article Influence: 1095.4] [Reference Citation Analysis (1)] |
| 2. | Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. |
| 3. | Lyman GH, Kuderer NM. The strengths and limitations of meta-analyses based on aggregate data. BMC Med Res Methodol. 2005;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Thorlund K, Mills EJ. Sample size and power considerations in network meta-analysis. Syst Rev. 2012;1:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Tsikopoulos K, Sidiropoulos K, Kitridis D, Hassan A, Drago L, Mavrogenis A, McBride D. Is coating of titanium implants effective at preventing Staphylococcus aureus infections? Int Orthop. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Romanò CL, Scarponi S, Gallazzi E, Romanò D, Drago L. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res. 2015;10:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org. |
| 8. | Giang HTN, Ahmed AM, Fala RY, Khattab MM, Othman MHA, Abdelrahman SAM, Thao LP, Gabl AEAE, Elrashedy SA, Lee PN, Hirayama K, Salem H, Huy NT. Methodological steps used by authors of systematic reviews and meta-analyses of clinical trials: a cross-sectional study. BMC Med Res Methodol. 2019;19:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2511] [Cited by in RCA: 2466] [Article Influence: 224.2] [Reference Citation Analysis (2)] |
| 10. | ter Riet G, Korevaar DA, Leenaars M, Sterk PJ, Van Noorden CJ, Bouter LM, Lutter R, Elferink RP, Hooft L. Publication bias in laboratory animal research: a survey on magnitude, drivers, consequences and potential solutions. PLoS One. 2012;7:e43404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016;21:125-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 729] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 12. | Weir A, Rabia S, Ardern C. Trusting systematic reviews and meta-analyses: all that glitters is not gold! Br J Sports Med. 2016;50:1100-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |