Published online May 20, 2021. doi: 10.5662/wjm.v11.i3.23
Peer-review started: January 13, 2021
First decision: March 1, 2021
Revised: March 2, 2021
Accepted: March 19, 2021
Article in press: March 19, 2021
Published online: May 20, 2021
Processing time: 119 Days and 0.9 Hours
There exists a complex interaction between obesity, type 2 diabetes mellitus (T2DM) and cancer, and an increase in the incidence of cancer is expected with the growing obesity-diabetes pandemic. The association of cancer with diabetes mellitus and obesity appears to be site-specific, the highest risk being for post-menopausal breast cancer, endometrial cancer, and colorectal cancer. Moreover, there is worsening of hyperglycaemia with the onset of cancer, evidencing a bi-directional link between cancer and diabetes mellitus and the need for monitoring for diabetes in cancer survivors. In this review, we look at the epidemiological evidence from observational studies and Mendelian randomization studies linking obesity, diabetes, and cancer, as well as the complex pathophysiological mechanisms involved, including insulin resistance with associated hyperinsu
Core Tip: Cancer is the second most common cause of death globally, and the complex pathogenic mechanisms in the development of cancer are not yet fully elucidated. The interplay between obesity, type 2 diabetes and some forms of cancer are well known for the past few years. With a steady increase in the obesity and diabetes pandemics, the incidence of cancer is expected to increase exponentially in the coming years. This review discusses the complex pathophysiological mechanisms linking these three major disease entities, to enhance clinician awareness across the globe, and proposes emerging potential therapeutic strategies.
- Citation: Fernandez CJ, George AS, Subrahmanyan NA, Pappachan JM. Epidemiological link between obesity, type 2 diabetes mellitus and cancer. World J Methodol 2021; 11(3): 23-45
- URL: https://www.wjgnet.com/2222-0682/full/v11/i3/23.htm
- DOI: https://dx.doi.org/10.5662/wjm.v11.i3.23
Cancer is the second most common cause of mortality from non-communicable diseases in the world, accounting for nearly 9.6 million deaths in the year 2017[1]. Apart from the excess mortality, cancer also contributed to 233.5 million disability-adjusted life-years in 2017. The incidence of cancer has been rising, due to a rise in the associated risk factors like aging population, obesity, diabetes mellitus, and lifestyle-related factors. Globally, the incidence of obesity has reached pandemic proportions, irrespective of the socioeconomic status and the age group[2]. Rising proportion of individuals with obesity has been the driving force for the diabetes pandemic[3], the incidence/prevalence of which is increasing at a faster rate in low-income and middle-income countries than in high-income countries[4].
There is plenty of evidence supporting the association between cancer and either obesity or diabetes mellitus on an individual basis. A recent study evaluated the impact of combined obesity and diabetes mellitus on cancer risk, by calculating the population attributable fraction (PAF) of incident cancers attributable to obesity and diabetes mellitus[5]. They observed that 5.7% of all incident cancers in 2012 were related to the combined effects of diabetes mellitus and obesity. When they limited their calculation to include only twelve adiposity-related cancers (colorectal cancer, postmenopausal breast cancer, endometrial cancer, gallbladder cancer, pancreatic cancer, liver cancer, kidney cancer, ovarian cancer, gastric-cardia cancer, thyroid cancer, multiple myeloma, and oesophageal adenocarcinoma), and six diabetes-related cancers (colorectal cancer, endometrial cancer, breast cancer, gallbladder cancer, pancreatic cancer, and liver cancer), they observed that 13.5%-15.3% of the cancers were attributable to the combined effects of diabetes mellitus and obesity. The study also observed that nearly one-fourth of the diabetes-related cancers and one-third of the adiposity-related cancers, happened due to a rise in prevalence of these risk factors[5].
As the global burden of obesity and diabetes mellitus is going to rise further, the burden of cancer will continue to increase. Therefore, interventions should be done at multiple levels including individual, community, health-care system, and policy making to prevent the development of cancer from these non-communicable diseases. This review will discuss the epidemiological studies linking obesity and type 2 diabetes mellitus (T2DM) to cancer and will explore the potential pathophysiological mechanisms linking obesity, and T2DM to cancer.
The obese population shows an increase in relative risk (RR) for developing various cancers, compared to the non-obese population. A recently published systematic review[6] using the data collected from a meta-analysis of epidemiological studies observed that the RR was highest for endometrial cancer (2.54; 95%CI: 2.11-3.06)[7], followed by renal cancer (1.77; 95%CI: 1.68-1.87)[8]. This was followed by pancreatic cancer (1.48; 95%CI: 1.15-1.92)[9], breast cancer (1.42; 95%CI: 1.30-1.45)[10], liver cancer (1.35; 95%CI: 1.24-1.47)[11], colorectal cancer (1.32; 95%CI: 1.18-1.48)[12], melanoma (1.31; 95%CI: 1.19-1.44)[13], ovarian cancer (1.30; 95%CI: 1.10-1.50)[14], thyroid cancer (1.29; 95%CI: 1.18-1.41)[15], leukaemia (1.26; 95%CI: 1.17-1.37)[13], prostate cancer (1.16; 95%CI: 1.08-1.24)[16], gastric cancer (1.13; 95%CI: 1.03-1.24)[17], and bladder cancer (1.10; 95%CI: 1.06-1.42)[18]. However, a previous study also noted that the obese population has a low RR of getting lung cancer (0.79; 95%CI: 0.73-0.85) compared to the non-obese population, indicating an inverse association[19]. The RR for squamous cell carcinoma, adenocarcinoma, and small cell carcinoma of the lung were 0.68 (95%CI: 0.58-0.80), 0.79 (95%CI: 0.65-0.96), and 0.99 (95%CI: 0.66-1.48) respectively indicating that obesity is protective against all types of lung cancer among both current and former smokers.
Obesity is associated with an increased risk of some cancers and decreased risk of other cancers, suggesting that the association between obesity and cancer clearly depends on the site of the cancer (site-specific association). This suggests that if the epidemiological studies analysing the relationship between obesity and cancer are not adequately stratified for the site of cancer, the associations with less common cancers can be masked. Nearly 4% of all new cancers can be attributed to overweight and obesity (adiposity-related cancers), in which endometrial, postmenopausal breast, and colorectal cancers account for more than 60%[20]. Worldwide, the population attributable fraction (PAF) of cancer related to high body mass index (BMI) was greater among women compared to men (5.4% vs 1.9%). Moreover, the countries with very high and high human development index (HDI) had higher PAF (5.3% and 4.8%, respectively), compared to countries with moderate and low HDI (1.6% and 1.0%, respectively)[20]. With increasing rates of obesity at younger age, the adiposity-related cancers are detected at a much younger age.
A dose-response meta-analysis of prospective observational studies reported that each 5 kg weight gain is associated with an increase in the RR for postmenopausal endometrial cancer by 39% among hormone replacement therapy (HRT) non-users (RR 1.39; 95%CI: 1.29-1.49) and by 9% among HRT users (RR 1.09; 95%CI: 1.02-1.16)[21]. Similar weight gain is associated with an increase in RR for postmenopausal ovarian cancer by 13% among HRT non-users (RR 1.13; 95%CI: 1.03-1.23), postmenopausal breast cancer by 11% among HRT non-users (RR 1.11; 95%CI: 1.08-1.13), and colorectal cancer by 9% in men (RR 1.09; 95%CI: 1.04-1.13). Weight gain is also associated with a 42% increase in the RR for renal cancer when the highest and lowest level of adult weight gain are compared (RR 1.42; 95%CI: 1.11-1.81)[21]. However, weight gain is not associated with a rise in colorectal cancer in women, premenopausal breast cancer, postmenopausal breast cancer among HRT users, prostate cancer, and thyroid cancer.
A meta-analysis of one hundred and twenty-six observational cohort studies among breast cancer patients reported that each 5 kg of adult weight gain is associated with a 7% increase in postmenopausal breast cancer (RR 1.07; 95%CI: 1.05-1.09), and each 5 kg/m2 of gain in BMI is associated with a 17% increase in postmenopausal breast cancer (RR 1.17; 95%CI: 1.11-1.23)[22]. Moreover, each 10 cm increase in waist circumference (WC) and hip circumference (HC), are associated with 11% (RR 1.11; 95%CI: 1.08-1.14) and 6% (RR 1.06; 95%CI: 1.04-1.09) increase in postmenopausal breast cancer, respectively. Furthermore, each 0.1 unit increase in waist-hip ratio is associated with a 10% increase in postmenopausal breast cancer (RR 1.10; 95%CI: 1.05-1.16). The increased risk was noted among hormone receptor positive breast cancers compared to receptor negative breast cancers, and among HRT non-users compared to HRT users. Adult weight gain and BMI gain are not consistently associated with premenopausal breast cancer. Each 5 kg of adult weight loss is associated with a 4% decrease in postmenopausal breast cancer (RR 0.96; 95%CI: 0.88-1.04). The study reported that BMI gain in early adult life (between 18-30 years) is inversely associated with postmenopausal (RR 0.81; 95%CI: 0.75-0.87), and premenopausal (RR 0.86; 95%CI: 0.78-0.96) breast cancer[22].
Another meta-analysis of seven prospective observational studies comprising of 18668 men and 24751 women with a mean age of 62 and 63 years (respectively), with a median follow-up period of 12 years reported 1656 first-incident adiposity-related cancers including postmenopausal breast, colorectum, lower oesophagus, gastric, liver, gallbladder, pancreas, endometrium, ovary, and kidney cancers[23]. The hazard ratios (HR) for first-incident cancers, per standard deviation increment in various adiposity indicators including BMI, WC, HC, and waist-hip ratio (WHR) were calculated. The results were 1.11 (95%CI: 1.02-1.21) for BMI, 1.13 (95%CI: 1.04-1.23) for WC, 1.09 (95%CI: 0.98-1.21) for HC, and 1.15 (95%CI: 1.00-1.32) for WHR. For example, the HR for colorectal cancer for each standard deviation increment in BMI, WC, HC, and WHR are 16%, 21%, 15%, and 20%, respectively. These values are not surprising as WC and WHR are better surrogate markers of visceral fat, than BMI. Moreover, HRT non-users have 20% increased risk per standard deviation of BMI, WC, and HC for getting postmenopausal breast cancer, compared to HRT users[23].
A recent prospective study evaluated the effect of weight gain during adult years with or without metabolic dysfunction on the risk of getting adiposity-related cancers[24]. The study reported that, compared to people maintaining a stable weight, those with weight gain of greater than 0.45 kg or 1 pound/year was associated with 38% increase in overall cancer risk (HR 1.38; 95%CI: 1.09-1.76), with women (HR 1.39; 95%CI: 1.03-1.87) having higher risk compared to men (HR 1.32; 95%CI: 0.88-2.00). Compared to weight gain without metabolic dysfunction [metabolically healthy obesity; (MHO)], weight gain with metabolic dysfunction increases the overall risk of cancer risk by 77% (HR 1.77; 95%CI: 1.21-2.59), with men (HR 1.85: 95%CI: 1.00-3.44) having higher risk compared to women (HR 1.74; 95%CI: 1.07-2.82). The study also observed that men and women who gained weight during adult life from non-overweight status at baseline, were associated with 2.18-fold and 1.60-fold overall cancer risk, whereas those who were overweight throughout the study period (from baseline) were associated with statistically non-significant increased cancer risks of 28% (HR 1.28; 95%CI: 0.76-2.14) and 33% (HR 1.33; 95%CI: 0.94-1.88), in men and women, respectively[24].
Nearly 10%-30% of obese individuals are metabolically healthy with lesser visceral and hepatic fat, greater leg fat, expandable subcutaneous fat, preserved cardiores
A meta-analysis of 230 cohort studies including over 30 million individuals observed that, though overweight and obesity were associated with an increased risk of all-cause mortality, there was a U-shaped association[27]. The concept that cancer patients with elevated BMI might have improved survival compared to cancer patients with normal BMI is known as ‘obesity paradox in cancer’. According to many, the term ‘obesity paradox’ is misleading as the paradox is due to the limitations of BMI, which relies on height and weight without delineating the distribution of adipocytes or distinguishing between adipose tissue and skeletal muscle. According to them, cancer patients with higher BMI might be having higher levels of protective skeletal muscle mass[28]. Others consider that, the paradox is due to methodological flaws including reverse causation, selection bias, and confounding[29]. However, a recent meta-analysis of 203 observational studies including 6320365 participants observed that even though obesity is associated with increased overall mortality, cancer specific mortality, and relapse rate in various cancers, it (obesity) is associated with an apparent protective effect in patients with lung cancer and melanoma[30].
Another meta-analysis of eight population-based cohort studies including 635642 participants who underwent bariatric surgery observed that, bariatric surgery is associated with a significantly reduced incidence of cancer (OR 0.72; 95%CI: 0.59-0.87) overall, and obesity-related cancer in particular (OR 0.55; 95%CI: 0.31-0.96)[31]. However, the reduction in incidence of breast cancer reached statistical significance (OR 0.50; 95%CI: 0.25-0.99), whereas reduction in other cancers did not reach statistical significance. A recent meta-analysis of 21 cohort studies comprising of 304516 participants who underwent bariatric surgery, revealed that bariatric surgery was not only associated with decreased cancer incidence (OR 0.56; 95%CI: 0.46-0.68), but also with decreased cancer mortality (OR 0.56; 95%CI: 0.41-0.75)[32]. The study also observed a significant reduction in breast and endometrial cancers in post-bariatric surgery participants.
Few observational studies reported a controversial observation about an increased incidence of colorectal cancer, in the post-bariatric surgery participants[33]. However, even in these trials, the absolute incidence of colorectal cancer was lower in the bariatric surgery group compared to the obese patients who did not undergo bariatric surgery. The cessation of statin therapy, avoidance of high fibre diet, and changes in colonic microbiome after bariatric surgery could explain a possible increase in the incidence of colorectal cancer in post-bariatric surgery cases.
A large study including 22198 participants who underwent bariatric surgery from the Kaiser Permanente Integrated health data reported a 33% reduction in any cancer incidence (HR 0.67; 95%CI: 0.60-0.74), and 41% reduction in adiposity-related cancer incidence (HR 0.59; 95%CI: 0.51-0.69)[34]. Among the adiposity related cancers, surgery is associated with a statistically significant reduction in postmenopausal breast (HR 0.58; 95%CI: 0.44-0.77), colon (HR 0.59; 95%CI: 0.36-0.97), endometrial (HR 0.50; 95%CI: 0.37-0.67), and pancreatic cancers (HR 0.46; 95%CI: 0.22-0.97), compared to obese patients who did not undergo bariatric surgery. Furthermore, a recent meta-analysis of seven studies including 1213727 participants observed that bariatric surgery reduces colorectal cancer by 36% (RR 0·64, 95%CI: 0.42-0.98)[35].
Observational studies have consistently reported that people with T2DM have an increased risk for several types of cancers including liver, pancreas, endometrium, colorectal, breast, and bladder, and a decreased risk for prostate cancer. The observed association between T2DM and cancer could either be a causal (caused by hyperin
An umbrella review of ‘meta-analyses of observational studies that examined the association between T2DM and cancer’ carefully assessed the robustness of the reported associations, considering the quality of the studies and their substantial heterogeneity[36]. The review observed that only a minority of these reported associations have evidence-base without hints of bias. These observed summary associations in the descending order of random effects include endometrial cancer (1.97; 95%CI: 1.71-2.27), intrahepatic cholangiocarcinoma (1.97; 95%CI: 1.57-2.46), colorectal cancer (1.27; 95%CI: 1.21-1.34), and breast cancer (1.20; 95%CI: 1.12-1.28).
A meta-analysis of forty-five observational studies comprising more than eight million participants and 132331 prostate cancer patients revealed a statistically significant inverse association between T2DM and carcinoma of prostate (RR 0.86; 95%CI: 0.80-0.92)[37]. One point supporting the lower incidence of cancer prostate in T2DM is the fact that some men with T2DM with/without obesity have lower androgen levels that results in reduced stimulation of androgen sensitive prostate cancer cells[38]. Another point supporting the lower incidence of cancer prostate is a lower circulating prostate-specific antigen levels seen in men with T2DM with high hemoglobin A1c and fasting blood glucose in the obese, and men with raised alanine transaminase levels which would delay the diagnosis of cancer prostate[39].
Among cancer patients with pre-existing diabetes mellitus, there is a 41% increase in all-cause mortality (HR 1.41; 95%CI: 1.28-1.55)[40]. A subgroup analysis showed increased all-cause mortality with cancers of endometrium (HR 1.76; 95%CI: 1.34-2.31), breast (HR 1.61; 95%CI: 1.46-1.78), and colorectum (HR 1.32; 95%CI: 1.24-1.41). Another meta-analysis on colorectal cancer patients with pre-existing diabetes mellitus observed that the all-cause mortality was increased by 17% (RR 1.17; 95%CI: 1.09-1.25), and cancer specific mortality by 12% (RR 1.12; 95%CI: 1.01-1.24), compared to colorectal cancer patients without diabetes mellitus[41]. Moreover, presence of pre-existing diabetes mellitus was associated with a 51% higher post-operative mortality (OR 1.51; 95%CI: 1.13-2.02) among cancer patients[42]. Cancer patients with pre-existing diabetes mellitus exhibited advanced stage of the disease at the time of diagnosis[43], increased risk of cancer recurrence[44], and decreased disease-free survival (RR 1.27; 95%CI: 1.06-1.52)[41].
However, we ought to bear in mind that observational epidemiological studies are susceptible to certain biases including reverse causality bias, detection bias, and depletion of the susceptible[45]. Mendelian randomization (MR) studies are analytic methods (genetic epidemiological studies) that are used to strengthen the evidence for a causal relationship between an exposure and an outcome. MR studies utilize germline variants obtained from large-scale genome-wide association studies. As these germline variants are determined at the time of birth and remain constant throughout life[46], studies utilizing them will minimize the effects of bias and residual confounding that are observed in observational studies. However, the genetic observational or MR studies have their own strengths and weaknesses. Once the observational and genetic epidemiological studies agree between each other, the results are likely to be more robust.
MR studies have shown that adiposity has a very strong causal association with renal, endometrial, ovarian, oesophageal, pancreatic, and colorectal cancer[46]. Hyperinsulinaemia has a strong association with endometrial, breast, pancreatic and renal cancer risk. Raised circulating insulin-like growth factor-1 (IGF-1) levels have a moderate association with breast and prostate cancer risk. Sex hormone dysregulation and puberty timing have a moderate association with breast and endometrial cancer risk; puberty timing has a moderate association with prostate cancer risk. There is only a weak association between hyperglycaemia and various cancers including those of lung, pancreas, endometrium, kidney, and breast. Finally, no association is observed between T2DM and cancers including pancreatic, endometrial, renal cell, and ovarian cancers[46].
The increased incidence of various cancers including breast, endometrial, and colorectal cancers is observed within few months after the diagnosis of T2DM, or even in the prediabetic phase, indicating that in patients with T2DM, it is the endogenous hyperinsulinaemia, rather than hyperglycaemia, that is associated with an increased risk of cancer[47-50]. Moreover, in breast, colorectal and endometrial cancer patients, the endogenous hyperinsulinaemia is associated with cancer progression, recurrence, and excess mortality[51-54]. Compared to normal cells which preferentially rely on mitochondrial oxidative phosphorylation, the cancer cells rely on glycolysis, even in the presence of oxygen (aerobic glycolysis), as a source of energy, possibly due to damaged mitochondria in cancer cells and also as a measure to maximize the available energy sources to support the rapid proliferation. This observation, known as the Warburg effect, suggests an increased glucose uptake and increased reliance on glucose metabolism by the cancer cells[55].
Studies have shown that hyperglycaemia alone may not cause development of cancer in the absence of hyperinsulinaemia, indicating that the key driver of cancer initiation and progression in patients with diabetes, and obesity is hyperin
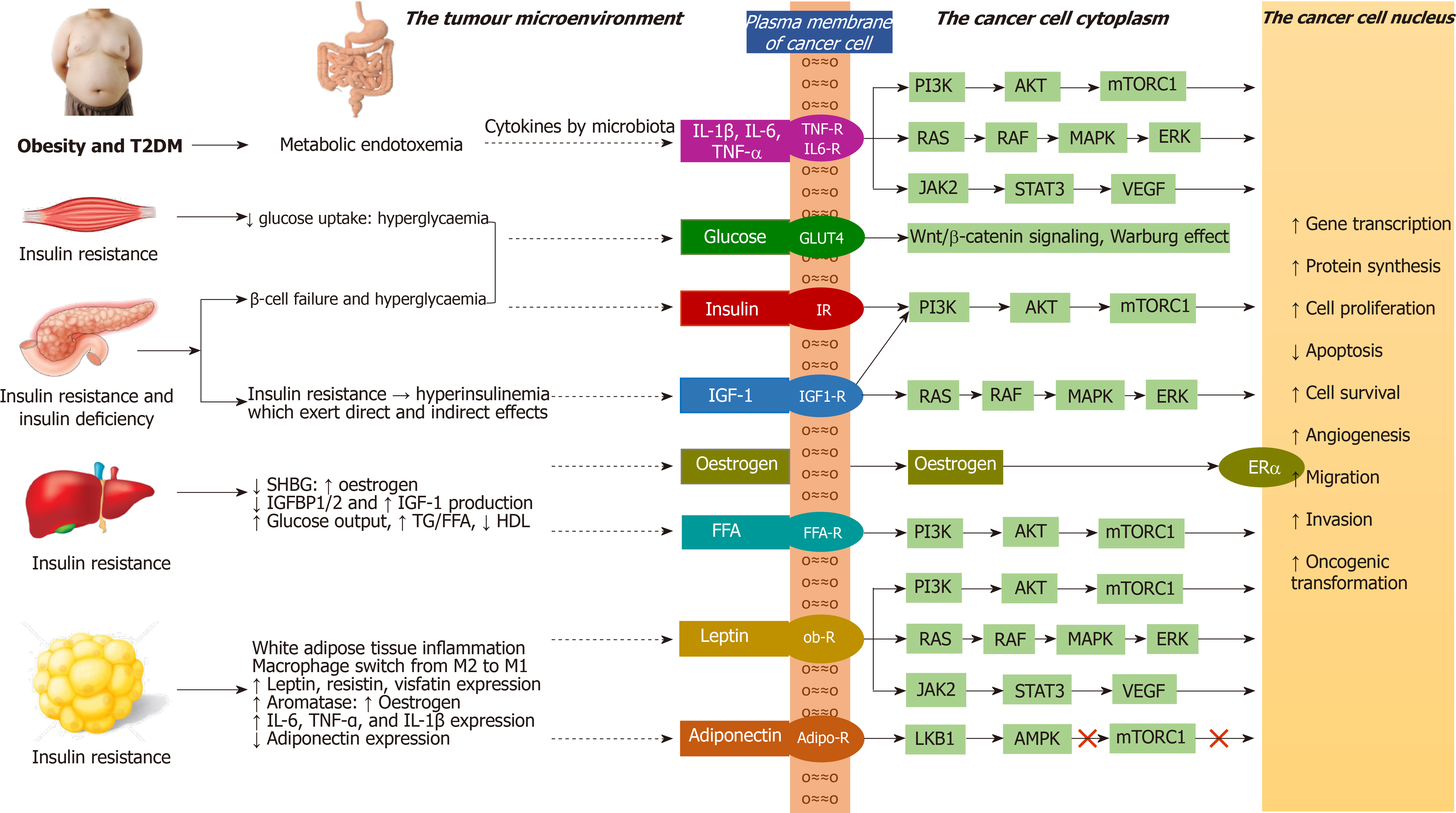
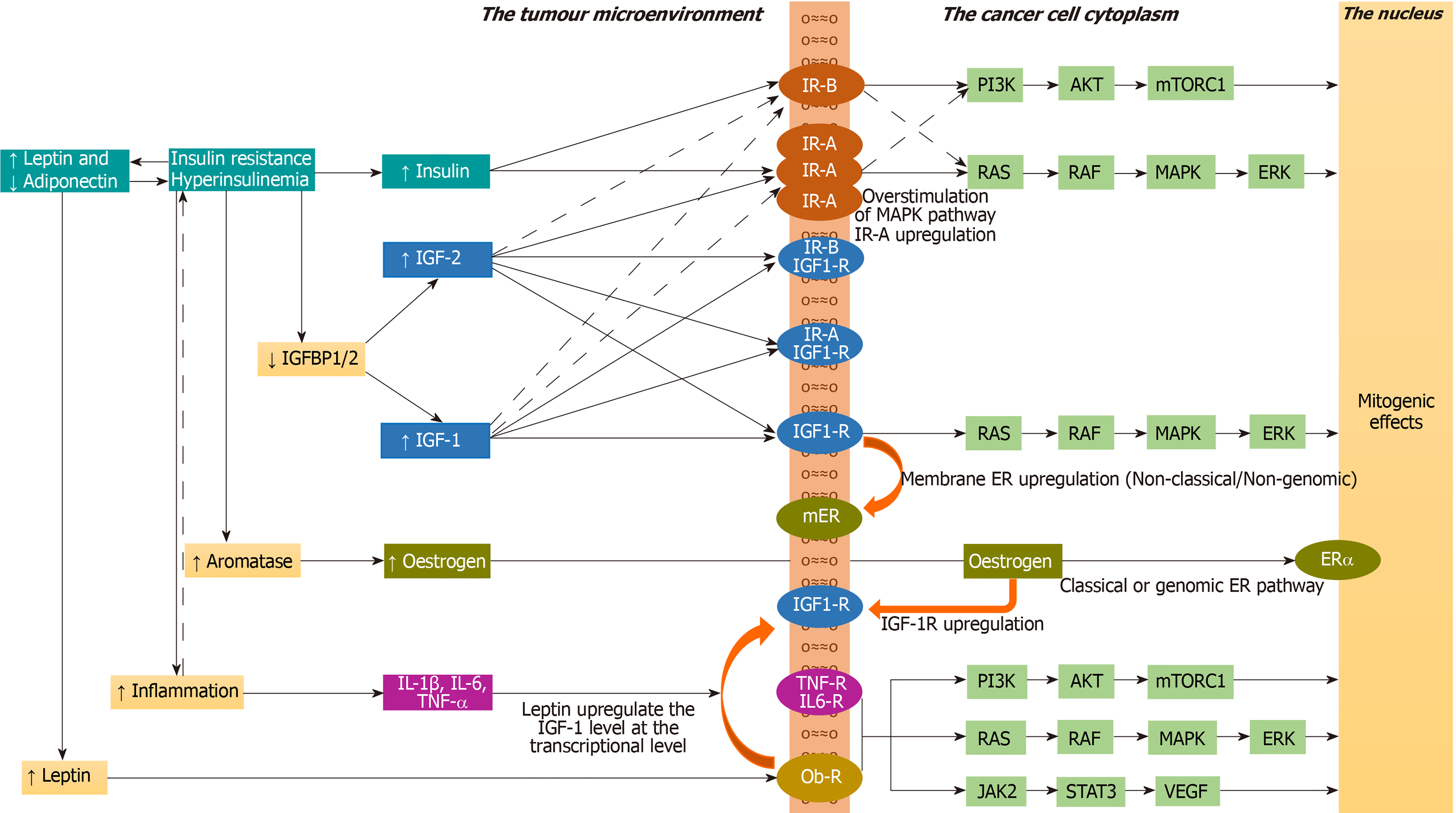
The insulin/IGF family consists of ligands including insulin, IGF-1, and IGF-2; their tyrosine kinase receptors including insulin receptor-A (IR-A), insulin receptor-B (IR-B), IGF-1 receptor (IGF-1R), IR-A/IGF-1R hybrid, and IR-B/IGF-1R hybrid; and six IGF-binding proteins (IGFBPs) that bind to IGF-1 and IGF-2, but not to insulin. Only the free IGFs, unbound to IGFBPs, are biologically available for binding to their receptors. As the IGFs bound to IGFBPs are protected from degradation, the IGFBPs maintain a stable serum IGF levels. Hyperinsulinaemia decreases IGFBP-1 and IGFBP-2 levels, thus increasing the levels of bioavailable IGF-1 and IGF-2[57]. Moreover, hyperin
The IR signalling exerts both metabolic and mitogenic effects. Among the two isoforms that are formed by differential splicing of the insulin receptor gene (splice variants), the IR-B is predominantly expressed in the metabolic tissues including liver, skeletal muscle, adipose tissue, and kidney, whereas IR-A is mainly expressed in the foetal and cancer tissues[60]. IR-B predominantly exerts metabolic effects, whereas IR-A predominantly exerts mitogenic effects. The ratio of IR-A to IR-B in the cell is determined by the expression of certain splicing factors in cells. Insulin, IGF-1, and IGF-2 bind to IR-A, and IR-B with different affinities. Insulin binds to IR-A with a 1.7-fold greater affinity compared to IR-B (only a modest difference in affinity). IGF-2 binds to IR-A with a 40-fold greater affinity compared to IR-B, whereas IGF-1 binds to IR-A with a 10-fold greater affinity compared to IR-B. Insulin binds only to IR-B or IR-A, not to IGF-1R or hybrid receptors. Both IGF-1 and IGF-2 bind to IGF-1R, hybrid receptors, and to IR-A or IR-B. IR-A has 100-fold higher affinity for IGF-2 compared to IGF-1[60]. Thus, IR-A has high affinity for IGF-2 and low affinity for IGF-1, whereas IR-B has a low affinity for IGF-2 and a very low affinity for IGF-1. High IR-A expression, resulting from altered expression of splicing factors in the cell is detrimental in adult life as it is associated with insulin resistance, dysregulated cell proliferation and cancer[61].
While normal cells downregulate the IRs in presence of hyperinsulinaemia, many cancer cells upregulate the IRs and IGF-1Rs in presence of hyperinsulinaemia and associated high IGF-1 levels, leading to mitogenic effects, increased cancer growth and metastasis[62,63]. Cancers that overexpress IR-A include breast, endometrial, lung, colorectal, hepatocellular, prostate, ovary, thyroid, and renal cancers[64-66]. Similarly, the cancers that overexpress IGF-1R include colorectal, breast, hepatocellular, and prostate cancers[67]. The loss of function mutations of tumour suppressor genes including BRCA1, p53, and PTEN lead to high IGF-1R expression[68]. Cancers that overexpress IGF-2 include mesenchymal tumours, breast, oesophageal, ovarian, and hepatocellular; tenosynovial giant cell tumours, Wilms' tumour, and Ewing's sarcoma[69].
Under physiological conditions (in people without hyperinsulinaemia), interaction of insulin and IR-B with subsequent stimulation of phosphatidyl-inositol-3-kinase/protein kinase B/mechanistic target of rapamycin complex 1 (PI3K /AKT/mTORC1) cascade mediate the anabolic effects of insulin including glucose uptake, glycogen synthesis, protein synthesis, and lipid synthesis. In people with hyperinsulinaemia (associated with high IR-A expression) and in cancer cells (associated with high IR-A expression and raised IGF-2), the interaction of insulin and/or IGF-2 with IR-A and the subsequent activation of rat sarcoma/rapidly accelerated fibrosarcoma/mitogen activated protein kinase/extracellular-regulated kinase cascade (RAS/RAF/MAPK/ERK) mediate the mitogenic effects of insulin including cell proliferation, survival, and migration[61]. An imbalance between MAPK and PI3K cascades results in impaired glucose/lipid metabolism in target tissues such as liver, muscle, and adipose tissue with cell proliferation in other tissues[70]. Under physiological conditions the interaction with IR-B is phasic (occurs only in postprandial state) resulting in metabolic effects, whereas under hyperinsulinaemic conditions or in cancer cells the interaction with IR-A is steady or continuous resulting in mitogenic effects[61].
Hyperinsulinaemia is associated with increased expression of aromatase enzyme in the TME resulting in increased oestrogen levels. Furthermore, hyperinsulinaemia is associated with decreased sex hormone-binding globulin levels that will increase the levels of bioavailable oestrogens that act on the tumour cells through oestrogen receptor-α, increasing the risk of oestrogen dependent cancers like breast and endometrial cancers[71]. The oestrogen receptor activation augments the insulin/IGF-mediated mitogenic effects in several cancers including that of breast, prostate, neuroblastoma, and pituitary adenoma[72].
Moreover, in carcinoma of prostate, activation of oestrogen receptors and of androgen receptors located at cell membrane induces IGF-1R upregulation to enhance IGF-1 mediated biological effects[73]. Similarly, in breast cancer, activation of IGF-1R and IR upregulate the non-classical or non-genomic membrane oestrogen receptors to potentiate the mitogenic effects[74,75]. Hyperinsulinaemia is also associated with inflammation in the TME leading to cytokine production and activation of the Janus Kinase-2 and Signal Transducer and Activator of Transcription-3 (JAK2-STAT3) and MAPK cascade inside the tumour cells[71]. Insulin upregulates the cellular metabolic activity leading to generation of reactive oxidative species (ROS) and resultant DNA damage, thereby promoting oncogenesis[76].
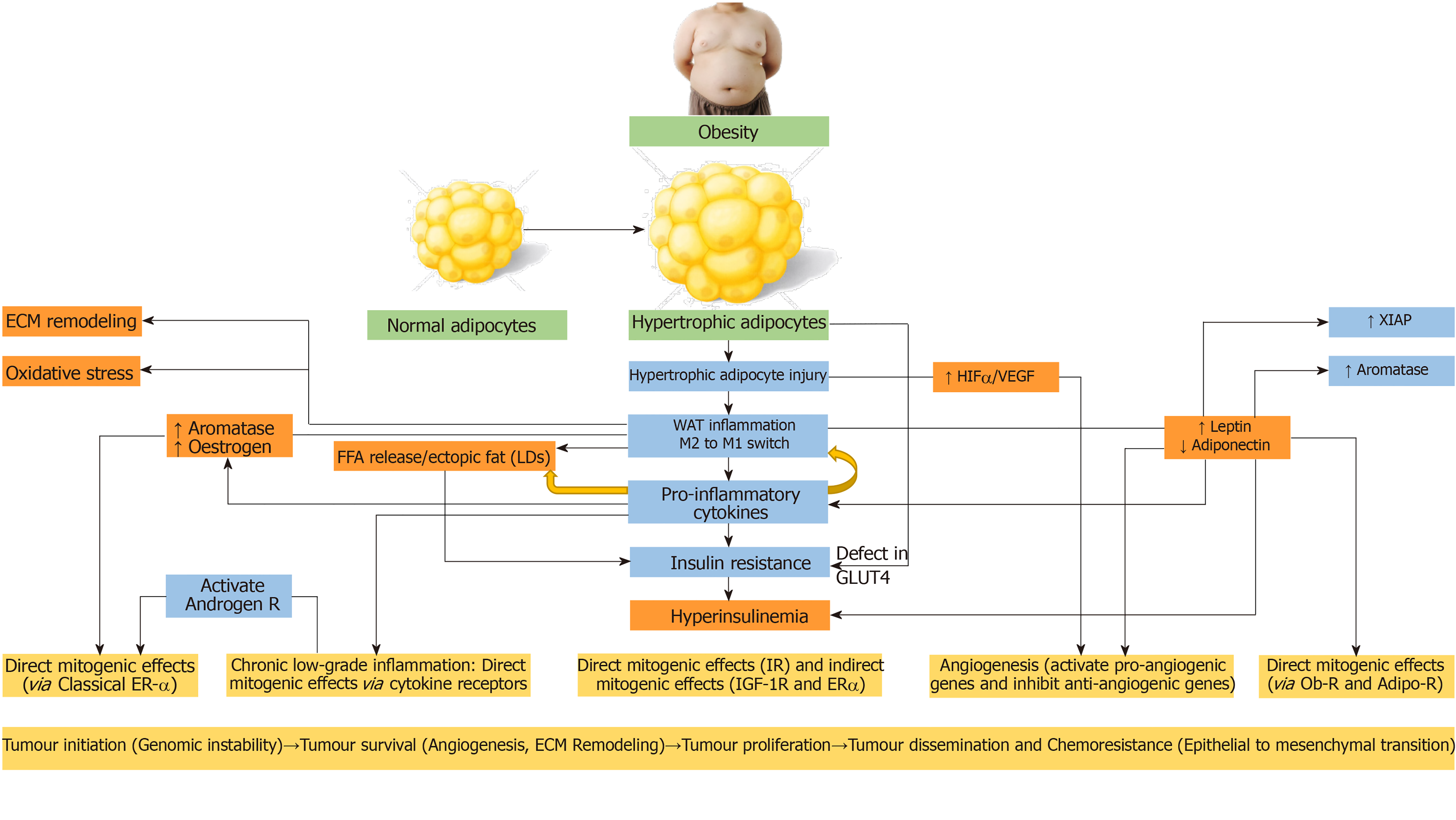
The white adipose tissue (WAT) comprising of subcutaneous and visceral adipose tissues, act as an energy reservoir for other organs. In response to over-nutrition and obesity, the adipose tissue undergoes dynamic remodelling characterized by alterations in the adipocyte number (adipocyte hyperplasia) in cases of childhood obesity or size (adipocyte hypertrophy) in cases of adult obesity[77]. The potential pathophysiological mechanisms linking obesity to cancer with special emphasis to WAT remodelling is outlined in the Figure 3. The hypertrophic adipose tissue outgrows its blood supply, leading to hypoxia, adipocyte injury/death, adipose tissue macrophage recruitment and a switch from anti-inflammatory to pro-inflammatory macrophages (M2 to M1 switch)[78]. This leads to increased expression of pro-inflammatory cytokines including tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β and monocyte chemoattractant protein-1 (MCP-1), and insulin resistance[79].
Pro-inflammatory cytokines propagate the adipose tissue inflammation by recruiting more macrophages (MCP-1)[80]. The macrophages envelope the dead or dying hypertrophic adipocytes to form crown-like structures and these macrophages later become lipid-loaded foam cells[81]. There is increased release of free fatty acids (FFA) from the entrapped adipocytes with subsequent ectopic fat deposition in the liver and skeletal muscle leading to worsening insulin resistance and lipotoxicity[82]. Lipolysis and FFA release from WAT are also stimulated by pro-inflammatory cytokines[83]. The hypertrophic adipocytes exhibit impaired insulin-dependent glucose uptake due to a defect in glucose transporter 4 trafficking, indicating another mechanism for insulin resistance in obese patients with adipocyte hypertrophy, apart from the effect of pro-inflammatory cytokines and ectopic lipid deposition[84].
Pro-inflammatory cytokines generated by chronic low-grade inflammation of WAT can exert direct mitogenic effects via cytokine receptors or indirect mitogenic effects via increased insulin resistance and resultant hyperinsulinaemia. Moreover, the cytokines can activate androgen receptors to promote survival and proliferation of prostate cancer cells in men[85], and can induce the aromatase enzyme to increase the incidence of oestrogen-dependent tumours in the postmenopausal women[86]. The hyperleptinaemia that accompanies WAT inflammation is another inducer of aromatase enzyme[87].
Obesity and hyperinsulinaemia are associated with raised leptin and reduced adiponectin levels. Similarly, hyperleptinaemia and hypoadiponectinaemia are associated with the development of insulin resistance and hyperinsulinaemia[88,89]. The elevated leptin levels activate various cascades like PI3K, MAPK, and predominantly JAK2/STAT3[90]. Leptin induces IL-6 and TNF-α production, thereby sustaining a chronic inflammatory state[91]. It increases the expression of anti-apoptotic proteins (X-linked inhibitor of apoptosis protein), and pro-angiogenic factors including vascular endothelial growth factor (VEGF), and hypoxia-inducible factor-1α (HIF-1α)[92]. On the other hand, adiponectin, acting via liver kinase B1 (LKB1), induces the adenosine monophosphate-activated protein kinase (AMPK) involved in the induction of cell cycle arrest and inhibition of mTOR activity. Elevated leptin and decreased adiponectin levels are known to be associated with proliferation, survival and migration of cancers including that of breast, colon, endometrium, and prostate[92].
The hypertrophic adipose tissue outgrows its blood supply and develops hypoxia. HIF expressed in the hypoxic TME is a dimeric transcription factor having inducible subunits (HIF-1α, HIF-2α, or HIF-3α), and a constitutive subunit (HIF-1β)[93]. Hypoxia stabilizes HIF-1α and promotes its association with HIF-1β. The HIFα-HIFβ dimer enters the nucleus leading to activation of the downstream targets. Under hypoxic conditions, HIF-1α promotes tumour angiogenesis by activating the pro-angiogenic genes [(VEGFA, VEGF receptor-1 (VEGFR1), Angiopoietin (ANGPT), and Ephrin type-A receptor 1 (EphA1)], and inhibiting anti-angiogenic genes (VEGFA, VEGFR1, ANGPT, EphA1)[93]. Tumour angiogenesis is essential for the survival, growth, invasion, and metastasis of malignant lesions.
The metabolically active adipose tissue is a source of ROS/reactive nitrogen species. The adipose tissue from lean individuals expresses antioxidant enzymes including glutathione peroxidase, catalase, and superoxide dismutase 1, whereas these antioxidant enzymes are downregulated in the adipose tissue from obese individuals[83]. The oxidative stress is known to cause DNA double strand breaks and other complex DNA alterations[94]. Low or intermediate levels of oxidative stress result in genomic instability associated with the activation of oncogenes, inactivation of tumour suppressor genes, angiogenesis, and mitochondrial dysfunction[95]. Obesity per se is associated with increased DNA damage and decreased DNA repair. Oxidative stress can be a consequence of obesity. Moreover, oxidative stress can be the trigger for obesity by altering the food intake and stimulating WAT deposition[96-98].
In obesity associated WAT inflammation, the inflammatory environment increases the oxidative stress to a level that it results in DNA damage, genomic instability, augmented cell survival, and cell proliferation resulting in the development of cancer[99]. Increased ROS production has been observed in various cancers. Tumour cells express high levels of antioxidants to detoxify ROS, to establish a redox balance while maintaining a resistance to apoptosis. Though ROS can be pro-tumourigenic in most, they can also be anti-tumourigenic, initiating tumour cell death, especially when the ROS levels exceed the antioxidant threshold of cancer cells[100].
The TME comprises of a cellular and a non-cellular component. The cellular component includes immune cells, fibroblasts, adipocytes, and endothelial cells, whereas the non-cellular structural component, known as the extracellular matrix (ECM) include a meshwork of polymeric proteins like collagen, elastin, and fibronectin. The ECM provides the biochemical and biomechanical environment within which the cancer cells exist[101]. WAT inflammation induces mechanical changes in the ECM, including myofibroblast enrichment with associated increased stiffness that promote tumourigenesis[102]. Moreover, crosstalk between cancer cells and the microenvironment is an important aspect of tumour progression, as this determines the ability of cancer cells to cross the ECM barrier, access the circulation, and establish metastases[103]. The biochemical and biomechanical properties of the ECM influence the ability of the cancer cells to modify physiological features (plasticity) to survive in the hostile microenvironment, and to resist therapy through acquisition of stemness characteristics and epithelial to mesenchymal or mesenchymal to epithelial transitions[103,104].
The obesity associated chronic low-grade inflammatory state in the adipose tissue, results in genomic instability contributing to tumourigenesis. Moreover, obesity is associated with aggressive cancers, due to the crosstalk between adipose tissue and tumours during cancer progression[105]. The mature adipocytes supply adipokines and lipids to the proliferating cancer cells, whereas the adipose stromal cells, and the immune cells infiltrate the tumour tissue to secrete various paracrine factors within the TME to aid tumour progression. Presence of high levels of leptin and/or leptin-receptor is associated with poor prognosis in several cancers as evidenced by the presence of invasive tumours, lymph node involvement, distant metastasis, and chemoresistance[106]. Elevated leptin levels can upregulate the IGF-1 level acting at the stage of transcription[107]. Resistin and visfatin acts through their receptors to promote tumour cell proliferation, angiogenesis, metastasis, and chemoresistan
Obesity is associated with ectopic fat deposition containing FFAs, triglycerides and cholesterol esters in non-adipose tissues. These lipid bodies, known as lipid droplets (LDs), are seen in many cancers, where they are thought to modulate the crosstalk between tumour cells and the cellular component of the TME. LDs are associated with tumour proliferation, chemoresistance, and aggressiveness[110]. Recently, fatty acid receptors with selectivity towards medium-long chain fatty acids (FFAR4 and FFAR1), and towards short chain fatty acids (FFAR2 and FFAR3) are discovered. FFAR4 is associated with proliferation, survival and migration of various cancers including colorectal, pancreatic and bone cancers[111]. The FFAs mediate the proliferation and metastasis of the tumour cells by activating the PI3K-AKT-mTORC1 pathway[112].
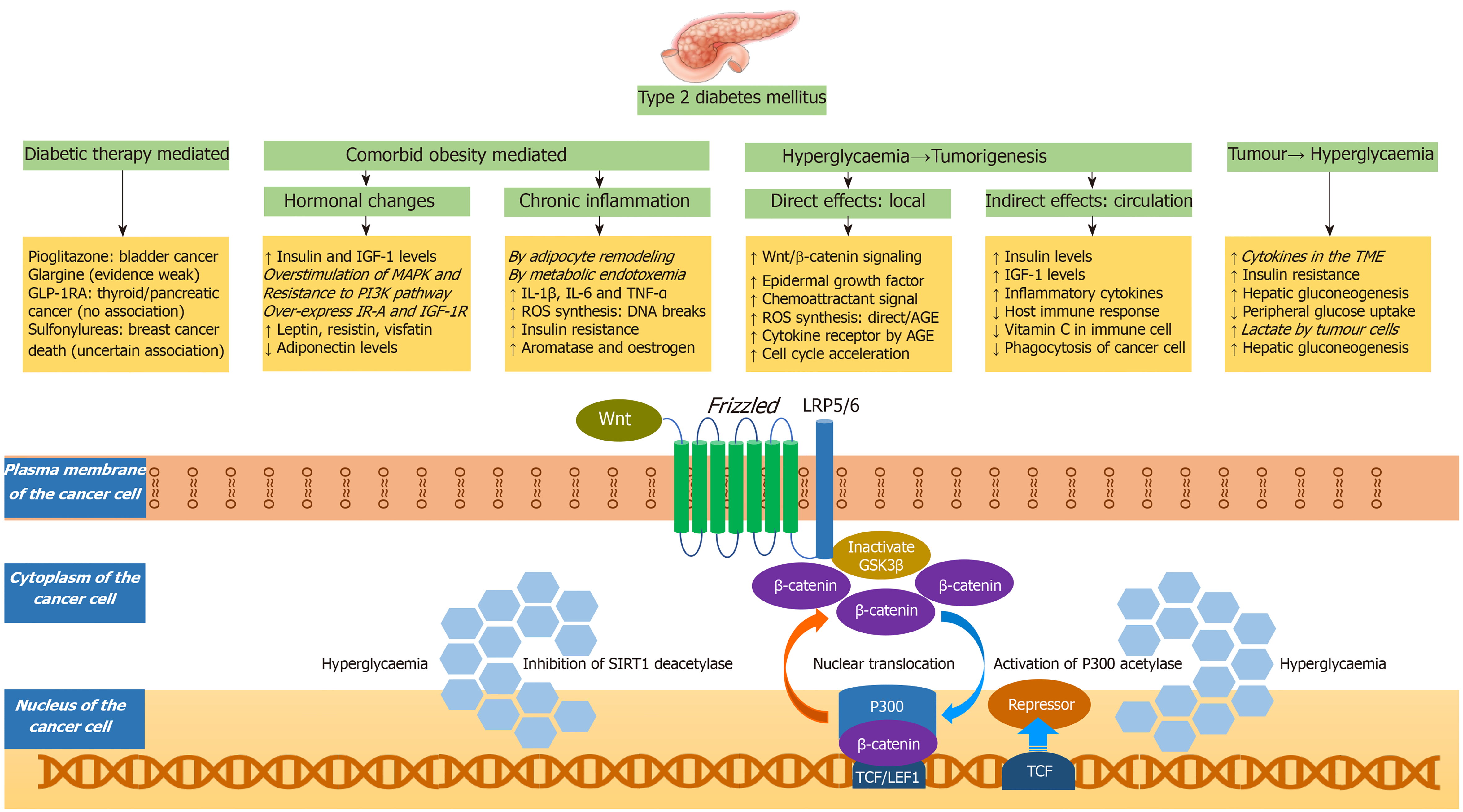
There are many mechanisms that can contribute to high cancer risk in patients with diabetes. The potential mechanisms, with a special emphasis to the Wnt/β-catenin signalling pathway, are portrayed in the Figure 4. These mechanisms can be related to antidiabetic medications[113], hormonal changes (exogenous or endogenous hyperinsulinaemia, raised IGF-1, hyperleptinaemia, and hypoadiponectinaemia), chronic inflammatory state associated with diabetes, oxidative stress associated with diabetes, decreased immunological response to cancer cells arising from competitive impairment of ascorbic acid transport into the immune cells by hyperglycaemia[114], enhanced signalling of epidermal growth factor receptor[115], accelerated cell cycle[116], chemoattractant upregulation, such as glial cell line-derived neurotrophic factor that is involved in the cancer invasiveness and migration[117], cytokine receptor upregulation and ROS generation by the advanced glycation end products (AGEs)[118], and most importantly enhanced Wnt/β-catenin signalling pathway resulting in increased proliferation, survival, invasion, and migration[119]. The raised insulin and IGF-1 levels are associated with overstimulation of MAPK pathway, resistance to PI3K pathway, over-expression of IR-A and activation of IGF-1R[120]. The oxidative stress associated with diabetes can occur through multiple mechanisms: direct effect of hyperglycaemia through glucose metabolism and auto-oxidation, or indirect effect from AGEs, or inflammatory cytokines[120].
Wnt is a family of secreted cysteine-rich glycoprotein ligands that bind to their membrane receptors to activate pathways including non-canonical Wnt-Ca2+ pathway, non-canonical planar cell polarity pathway, and canonical Wnt/β-catenin signalling pathway[121]. The classification of Wnt family into canonical or non-canonical is based on the presence or absence of β-catenin. In the canonical Wnt/β-catenin pathway, Wnt binds to its membrane co-receptor having Frizzled and lipoprotein receptor-related protein. This inactivates the Glycogen Synthase Kinase-3β (GSK3β), resulting in β-catenin accumulation in the cytoplasm. GSK3β is an enzyme that phosphorylates the cytosolic β-catenin to trigger degradation of β-catenin by the destruction complex. GSK3β is thereby considered as a tumour suppressor, due to its ability to inhibit the Wnt/β-catenin signalling pathway[122].
In the absence of hyperglycaemia, β-catenin accumulated in the cytoplasm cannot be translocated to the nucleus, to induce the expression of Wnt target genes. However, hyperglycaemia induces p300 acetyl transferase to achieve β-catenin acetylation. Moreover, hyperglycaemia inhibits Sirtuin 1 deacetylase activity. These favour formation of lymphoid enhancer factor 1 (LEF1)/β-catenin/p300 complex and its accumulation inside the nucleus, where it displaces the transcriptional repressor known as T-cell factor (TCF)7L2-corepressor complex, and induce the expression of Wnt target genes (LEF, TCF)[118,123]. These Wnt target genes are involved in initiation, proliferation, senescence bypass, epithelial to mesenchymal transition, and metastasis of tumours[124-127].
In patients with cancer, the circulating cytokines increases the insulin resistance, decreases the peripheral glucose uptake, increases the hepatic gluconeogenesis, thereby worsens hyperglycaemia. Increased inflammatory cytokines in the TME worsens this hyperglycaemia. Moreover, the product of glycolysis by tumour cells (lactate) stimulates the hepatic gluconeogenesis, further worsening the hyperglycaemia[119]. A recently published study from Korea has shown that cancer can increase the risk of getting subsequent diabetes mellitus in cancer survivors independent of traditional risk factors for diabetes mellitus (HR 1.35; 95%CI: 1.26-1.45)[128]. Though the risk was highest in the first 2 years, it remained high for 10 years following cancer diagnosis (HR 1.19; 95%CI: 1.00-1.43). Though the risk was highest for cancer survivors of pancreatic, kidney, and liver cancers, the risk remained significantly high even for gallbladder, lung, blood, breast, stomach, and thyroid cancers.
The therapeutic agents based on Wnt/β-catenin signalling include those that act by inhibiting Wnt ligands, inhibiting Wnt receptors/co-receptors, stabilizing the destruction complex, and inhibiting β-catenin-dependent transcriptional pathway[129]. Moreover, GSK3β inhibitors are being developed and entering clinical trials as novel cancer treatments due to their ability to inhibit the Wnt/β-catenin signalling pathway[130]. mTOR participates in multiple signalling pathways to regulate proliferation, autophagy, and apoptosis. Various newly developed mTOR inhibitors are entering clinical studies[112]. The free fatty acid receptors agonists are potential therapeutic agents in the management of cancers of colorectum, and ovary[131,132].
Various inhibitors of MAPK signalling pathway including RAS inhibitors, RAF inhibitors, MAPK inhibitors, and ERK inhibitors have also been recently developed[133]. Three RAF inhibitors and three MAPK inhibitors have received approval for the treatment of late-stage B-RAF harbouring cancers, either alone or in combination with other agents. However, these drugs are associated with intrinsic drug resistance in patients with RAS mutations or acquired drug resistance in patients with B-RAF mutations (after 6-10 mo of treatment). Targeting MAPK and AMPK signalling pathways together represents a promising therapeutic intervention in patients with RAS or RAF mutations[134]. Another promising intervention in patients with B-RAF mutation-associated cancers, is dual inhibition of the MAPK and JAK2/STAT3 pathways using a combination of three MAPK pathway inhibitor types including BRAF inhibitor, MAPK inhibitor, and ERK inhibitor along with either of JAK2 or STAT3 inhibitor[135].
Though PI3K signalling pathway is important in cell proliferation, and survival, the drugs acting on this pathway, including pan-PI3K inhibitors or dual PI3K/mTOR inhibitors are only modestly effective as monotherapy, with a relatively high incidence of side effects. However, isoform selective PI3K inhibitors are undergoing clinical trials with improved specificity and reduced toxicity[136]. Similarly, several AKT inhibitors are currently in various stages of clinical trials for diverse types of malignancies[137]. AMPK acts as tumour suppressor, as it mediates the effects of the LKB1 tumour suppressor by inhibiting mTORC1 production. Though metformin, and fluoxetine can activate AMPK, several small molecular AMPK agonists are under various stages of development and few of them are expected to enter clinical trials within next few years[138].
As hyperinsulinaemia is the key driver of cancer initiation and progression in patients with diabetes and obesity, drugs that could reduce hyperinsulinaemia could potentially prevent development of cancer. At supra-physiological concentrations, metformin can exert direct anti-proliferative effects. However, at physiological concentrations the anti-proliferative effects are due to its indirect effects including reduction in hyperglycaemia, insulin, IGF-1, and leptin[139]. Clinical trials with the use of metformin in cancer therapy and prevention are ongoing. Peroxisome proliferator-activated receptor gamma (PPARγ) is expressed in cancers including breast, prostate, colon, bladder, and thyroid cancers. Preclinical trials have shown that the PPARγ agonists have tumour suppressor effect as they are pro-apoptotic, induce autophagy, decrease cancer cell invasion and metastatic potential. However, the results of these clinical trials are disappointing due to their side effect profiles[140].
Obesity and T2DM are associated with high risk of cancer, and the strongest associations are for postmenopausal breast and endometrial cancers, and colorectal carcinomas. Mendelian randomization studies have shown that obesity and hyperin
Manuscript source: Invited manuscript
Specialty type: Medical laboratory technology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao ZF, Ghobadloo SM S-Editor: FanJR L-Editor: A P-Editor: Xing YX
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1749] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4666] [Article Influence: 583.3] [Reference Citation Analysis (2)] |
| 3. | NCD Risk Factor Collaboration (NCD-RisC). Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 4. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2527] [Article Influence: 280.8] [Reference Citation Analysis (0)] |
| 5. | Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6-e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 6. | Gutiérrez-Salmerón M, Chocarro-Calvo A, García-Martínez JM, de la Vieja A, García-Jiménez C. Epidemiological bases and molecular mechanisms linking obesity, diabetes, and cancer. Endocrinol Diabetes Nutr. 2017;64:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Liu H, Yang S, Zhang J, Qian L, Chen X. Overweight, obesity and endometrial cancer risk: results from a systematic review and meta-analysis. Int J Biol Markers. 2014;29:e21-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135:1673-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2014; 12: 1635-44. quiz e103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Rui R, Lou J, Zou L, Zhong R, Wang J, Xia D, Wang Q, Li H, Wu J, Lu X, Li C, Liu L, Xia J, Xu H. Excess body mass index and risk of liver cancer: a nonlinear dose-response meta-analysis of prospective studies. PLoS One. 2012;7:e44522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Okabayashi K, Ashrafian H, Hasegawa H, Yoo JH, Patel VM, Harling L, Rowland SP, Ali M, Kitagawa Y, Darzi A, Athanasiou T. Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1175-85; quiz 1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | García-Jiménez C, Gutiérrez-Salmerón M, Chocarro-Calvo A, García-Martinez JM, Castaño A, De la Vieja A. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer. 2016;114:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43:690-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Zhao ZG, Guo XG, Ba CX, Wang W, Yang YY, Wang J, Cao HY. Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J Int Med Res. 2012;40:2041-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Hu MB, Xu H, Bai PD, Jiang HW, Ding Q. Obesity has multifaceted impact on biochemical recurrence of prostate cancer: a dose-response meta-analysis of 36,927 patients. Med Oncol. 2014;31:829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Lin XJ, Wang CP, Liu XD, Yan KK, Li S, Bao HH, Zhao LY, Liu X. Body mass index and risk of gastric cancer: a meta-analysis. Jpn J Clin Oncol. 2014;44:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Qin Q, Xu X, Wang X, Zheng XY. Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:3117-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Yang Y, Dong J, Sun K, Zhao L, Zhao F, Wang L, Jiao Y. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 672] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 21. | Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 22. | Chan DSM, Abar L, Cariolou M, Nanu N, Greenwood DC, Bandera EV, McTiernan A, Norat T. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30:1183-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Freisling H, Arnold M, Soerjomataram I, O'Doherty MG, Ordóñez-Mena JM, Bamia C, Kampman E, Leitzmann M, Romieu I, Kee F, Tsilidis K, Tjønneland A, Trichopoulou A, Boffetta P, Benetou V, Bueno-de-Mesquita HBA, Huerta JM, Brenner H, Wilsgaard T, Jenab M. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116:1486-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Chadid S, Singer MR, Kreger BE, Bradlee ML, Moore LL. Midlife weight gain is a risk factor for obesity-related cancer. Br J Cancer. 2018;118:1665-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Blüher M. Metabolically Healthy Obesity. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 571] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 26. | Lin CJ, Chang YC, Cheng TY, Lo K, Liu SJ, Yeh TL. The association between metabolically healthy obesity and risk of cancer: A systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2020;21:e13049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romundstad P, Vatten LJ. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 562] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 28. | Cespedes Feliciano EM, Kroenke CH, Caan BJ. The Obesity Paradox in Cancer: How Important Is Muscle? Annu Rev Nutr. 2018;38:357-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Lee DH, Giovannucci EL. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr Nutr Rep. 2019;8:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 30. | Petrelli F, Cortellini A, Indini A, Tomasello G, Zaniboni A. Obesity paradox in patients with cancer: A systematic review and meta-analysis of 6,320,365 patients. medRxiv 2020; 04.28. 20082800;. [DOI] [Full Text] |
| 31. | Wiggins T, Antonowicz SS, Markar SR. Cancer Risk Following Bariatric Surgery-Systematic Review and Meta-analysis of National Population-Based Cohort Studies. Obes Surg. 2019;29:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 32. | Zhang K, Luo Y, Dai H, Deng Z. Effects of Bariatric Surgery on Cancer Risk: Evidence from Meta-analysis. Obes Surg. 2020;30:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Bruno DS, Berger NA. Impact of bariatric surgery on cancer risk reduction. Ann Transl Med. 2020;8:S13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, Powers JD, Yenumula PR, Arterburn DE. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann Surg. 2019;269:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 35. | Almazeedi S, El-Abd R, Al-Khamis A, Albatineh AN, Al-Sabah S. Role of bariatric surgery in reducing the risk of colorectal cancer: a meta-analysis. Br J Surg. 2020;107:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 564] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 37. | Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16:151-158, S1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 38. | Fernandez CJ, Chacko EC, Pappachan JM. Male Obesity-related Secondary Hypogonadism - Pathophysiology, Clinical Implications and Management. Eur Endocrinol. 2019;15:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 39. | Kobayashi M, Mizuno T, Yuki H, Kambara T, Betsunoh H, Nukui A, Abe H, Fukabori Y, Yashi M, Kamai T. Association between serum prostate-specific antigen level and diabetes, obesity, hypertension, and the laboratory parameters related to glucose tolerance, hepatic function, and lipid profile: implications for modification of prostate-specific antigen threshold. Int J Clin Oncol. 2020;25:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754-2764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 695] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 41. | Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56:1304-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29:40-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 339] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 44. | Stein KB, Snyder CF, Barone BB, Yeh HC, Peairs KS, Derr RL, Wolff AC, Brancati FL. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:1839-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Lega IC, Lipscombe LL. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 46. | Vincent EE, Yaghootkar H. Using genetics to decipher the link between type 2 diabetes and cancer: shared aetiology or downstream consequence? Diabetologia. 2020;63:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Increased prevalence of prior breast cancer in women with newly diagnosed diabetes. Breast Cancer Res Treat. 2006;98:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Onitilo AA, Stankowski RV, Berg RL, Engel JM, Glurich I, Williams GM, Doi SA. Breast cancer incidence before and after diagnosis of type 2 diabetes mellitus in women: increased risk in the prediabetes phase. Eur J Cancer Prev. 2014;23:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Onitilo AA, Berg RL, Engel JM, Glurich I, Stankowski RV, Williams G, Doi SA. Increased risk of colon cancer in men in the pre-diabetes phase. PLoS One. 2013;8:e70426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Salinas-Martínez AM, Flores-Cortés LI, Cardona-Chavarría JM, Hernández-Gutiérrez B, Abundis A, Vázquez-Lara J, González-Guajardo EE. Prediabetes, diabetes, and risk of breast cancer: a case-control study. Arch Med Res. 2014;45:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Ahern TP, Hankinson SE, Willett WC, Pollak MN, Eliassen AH, Tamimi RM. Plasma C-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1786-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Cui P, Chen Y, Waili N, Li Y, Ma C. Associations of serum C-peptide and insulin-like growth factor binding proteins-3 with breast cancer deaths. PLoS One. 2020;15:e0242310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Jenab M, Riboli E, Cleveland RJ, Norat T, Rinaldi S, Nieters A, Biessy C, Tjønneland A, Olsen A, Overvad K, Grønbaek H, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Boeing H, Pischon T, Trichopoulos D, Oikonomou E, Trichopoulou A, Panico S, Vineis P, Berrino F, Tumino R, Masala G, Peters PH, van Gils CH, Bueno-de-Mesquita HB, Ocké MC, Lund E, Mendez MA, Tormo MJ, Barricarte A, Martínez-García C, Dorronsoro M, Quirós JR, Hallmans G, Palmqvist R, Berglund G, Manjer J, Key T, Allen NE, Bingham S, Khaw KT, Cust A, Kaaks R. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 54. | Cust AE, Allen NE, Rinaldi S, Dossus L, Friedenreich C, Olsen A, Tjønneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Chang-Claude J, Boeing H, Schulz M, Benetou V, Trichopoulou A, Trichopoulos D, Palli D, Berrino F, Tumino R, Mattiello A, Vineis P, Quirós JR, Agudo A, Sánchez MJ, Larrañaga N, Navarro C, Ardanaz E, Bueno-de-Mesquita HB, Peeters PH, van Gils CH, Bingham S, Khaw KT, Key T, Slimani N, Riboli E, Kaaks R. Serum levels of C-peptide, IGFBP-1 and IGFBP-2 and endometrial cancer risk; results from the European prospective investigation into cancer and nutrition. Int J Cancer. 2007;120:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1910] [Cited by in RCA: 3257] [Article Influence: 361.9] [Reference Citation Analysis (1)] |
| 56. | Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 685] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 57. | Li JB, Wang CY, Chen JW, Feng ZQ, Ma HT. Expression of liver insulin-like growth factor 1 gene and its serum level in patients with diabetes. World J Gastroenterol. 2004;10:255-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Crowe FL, Key TJ, Allen NE, Appleby PN, Overvad K, Grønbæk H, Tjønneland A, Halkjær J, Dossus L, Boeing H, Kröger J, Trichopoulou A, Zylis D, Trichopoulos D, Boutron-Ruault MC, de Lauzon-Guillain B, Clavel-Chapelon F, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, van Gils CH, Peeters PH, Gram IT, Rodríguez L, Jakszyn P, Molina-Montes E, Navarro C, Barricarte A, Larrañaga N, Khaw KT, Rodwell S, Rinaldi S, Slimani N, Norat T, Gallo V, Riboli E, Kaaks R. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Hum Biol. 2011;38:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Vella V, Pandini G, Sciacca L, Mineo R, Vigneri R, Pezzino V, Belfiore A. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J Clin Endocrinol Metab. 2002;87:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 753] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 61. | Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, Morrione A, Vigneri R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr Rev. 2017;38:379-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 62. | Mulligan AM, O'Malley FP, Ennis M, Fantus IG, Goodwin PJ. Insulin receptor is an independent predictor of a favorable outcome in early stage breast cancer. Breast Cancer Res Treat. 2007;106:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Harrington SC, Weroha SJ, Reynolds C, Suman VJ, Lingle WL, Haluska P. Quantifying insulin receptor isoform expression in FFPE breast tumors. Growth Horm IGF Res. 2012;22:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Flannery CA, Saleh FL, Choe GH, Selen DJ, Kodaman PH, Kliman HJ, Wood TL, Taylor HS. Differential Expression of IR-A, IR-B and IGF-1R in Endometrial Physiology and Distinct Signature in Adenocarcinoma. J Clin Endocrinol Metab. 2016;101:2883-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Huang J, Morehouse C, Streicher K, Higgs BW, Gao J, Czapiga M, Boutrin A, Zhu W, Brohawn P, Chang Y, Viner J, LaVallee T, Richman L, Jallal B, Yao Y. Altered expression of insulin receptor isoforms in breast cancer. PLoS One. 2011;6:e26177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Jiang L, Zhu W, Streicher K, Morehouse C, Brohawn P, Ge X, Dong Z, Yin X, Zhu G, Gu Y, Ranade K, Higgs BW, Yao Y, Huang J. Increased IR-A/IR-B ratio in non-small cell lung cancers associates with lower epithelial-mesenchymal transition signature and longer survival in squamous cell lung carcinoma. BMC Cancer. 2014;14:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 975] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 68. | Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20:R321-R339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 70. | Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 765] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 71. | Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J Clin Oncol. 2016;34:4261-4269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 72. | Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Andò S, Sisci D. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Genua M, Pandini G, Sisci D, Castoria G, Maggiolini M, Vigneri R, Belfiore A. Role of cyclic AMP response element-binding protein in insulin-like growth factor-i receptor up-regulation by sex steroids in prostate cancer cells. Cancer Res. 2009;69:7270-7277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | De Marco P, Cirillo F, Vivacqua A, Malaguarnera R, Belfiore A, Maggiolini M. Novel Aspects Concerning the Functional Cross-Talk between the Insulin/IGF-I System and Estrogen Signaling in Cancer Cells. Front Endocrinol (Lausanne). 2015;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Othman EM, Leyh A, Stopper H. Insulin mediated DNA damage in mammalian colon cells and human lymphocytes in vitro. Mutat Res. 2013;745-746:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne). 2016;7:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 769] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 78. | Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118-E1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 639] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 79. | Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208:501-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 393] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 80. | Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 81. | Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 1769] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 82. | Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683-16689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 951] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 83. | Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016;34:4270-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 659] [Article Influence: 73.2] [Reference Citation Analysis (1)] |
| 84. | Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35:1686-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 85. | Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog. 2011;10:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 349] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 86. | Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. Int J Cancer. 2006;118:1915-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, Morrow M, Wang H, Pollak M, Jones LW, Hudis CA, Dannenberg AJ. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin Cancer Res. 2016;22:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 88. | Pérez-Hernández AI, Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol (Lausanne). 2014;5:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Leon-Cabrera S, Solís-Lozano L, Suárez-Álvarez K, González-Chávez A, Béjar YL, Robles-Díaz G, Escobedo G. Hyperleptinemia is associated with parameters of low-grade systemic inflammation and metabolic dysfunction in obese human beings. Front Integr Neurosci. 2013;7:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667-2676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 91. | Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 92. | Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. J Clin Oncol. 2016;34:4277-4283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 93. | Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y, Liao Q, Xiang B, Zhou M, Guo C, Zeng Z, Li G, Xiong W. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 94. | Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 668] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 95. | Crujeiras AB, Díaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res. 2013;47:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 96. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3370] [Cited by in RCA: 3836] [Article Influence: 191.8] [Reference Citation Analysis (0)] |
| 97. | Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab. 2009;20:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 98. | Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC, Liu GS. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 99. | Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 100. | Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1313] [Article Influence: 164.1] [Reference Citation Analysis (0)] |
| 101. | Poltavets V, Kochetkova M, Pitson SM, Samuel MS. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front Oncol. 2018;8:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 102. | Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ, Fischbach C. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 103. | Nallanthighal S, Heiserman JP, Cheon DJ. The Role of the Extracellular Matrix in Cancer Stemness. Front Cell Dev Biol. 2019;7:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 104. | Scott LE, Weinberg SH, Lemmon CA. Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition. Front Cell Dev Biol. 2019;7:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 105. | Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer. 2018;4:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 106. | Ray A, Cleary MP. The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev. 2017;38:80-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 107. | Min DY, Jung E, Kim J, Lee YH, Shin SY. Leptin stimulates IGF-1 transcription by activating AP-1 in human breast cancer cells. BMB Rep. 2019;52:385-390. [PubMed] |
| 108. | Sudan SK, Deshmukh SK, Poosarla T, Holliday NP, Dyess DL, Singh AP, Singh S. Resistin: An inflammatory cytokine with multi-faceted roles in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 109. | Lin TC. The role of visfatin in cancer proliferation, angiogenesis, metastasis, drug resistance and clinical prognosis. Cancer Manag Res. 2019;11:3481-3491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 110. | Cruz ALS, Barreto EA, Fazolini NPB, Viola JPB, Bozza PT. Lipid droplets: platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020;11:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 111. | Senatorov IS, Moniri NH. The role of free-fatty acid receptor-4 (FFA4) in human cancers and cancer cell lines. Biochem Pharmacol. 2018;150:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 620] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 113. | Suh S, Kim KW. Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment. Diabetes Metab J. 2019;43:733-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 114. | Krone CA, Ely JT. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther. 2005;4:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Li W, Ma Q, Li J, Guo K, Liu H, Han L, Ma G. Hyperglycemia enhances the invasive and migratory activity of pancreatic cancer cells via hydrogen peroxide. Oncol Rep. 2011;25:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 116. | Masur K, Vetter C, Hinz A, Tomas N, Henrich H, Niggemann B, Zänker KS. Diabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferation. Br J Cancer. 2011;104:345-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 117. | Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol Cell Biochem. 2011;347:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | Leclerc E, Vetter SW. The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochim Biophys Acta. 2015;1852:2706-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 119. | García-Jiménez C, García-Martínez JM, Chocarro-Calvo A, De la Vieja A. A new link between diabetes and cancer: enhanced WNT/β-catenin signaling by high glucose. J Mol Endocrinol. 2014;52:R51-R66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 120. | Ferroni P, Riondino S, Buonomo O, Palmirotta R, Guadagni F, Roselli M. Type 2 Diabetes and Breast Cancer: The Interplay between Impaired Glucose Metabolism and Oxidant Stress. Oxid Med Cell Longev. 2015;2015:183928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget. 2018;9:5492-5508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 122. | Vijay GV, Zhao N, Den Hollander P, Toneff MJ, Joseph R, Pietila M, Taube JH, Sarkar TR, Ramirez-Pena E, Werden SJ, Shariati M, Gao R, Sobieski M, Stephan CC, Sphyris N, Miura N, Davies P, Chang JT, Soundararajan R, Rosen JM, Mani SA. GSK3β regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019;21:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 123. | Chocarro-Calvo A, García-Martínez JM, Ardila-González S, De la Vieja A, García-Jiménez C. Glucose-induced β-catenin acetylation enhances Wnt signaling in cancer. Mol Cell. 2013;49:474-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 124. | Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1384] [Cited by in RCA: 1527] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 125. | Larue L, Luciani F, Kumasaka M, Champeval D, Demirkan N, Bonaventure J, Delmas V. Bypassing melanocyte senescence by beta-catenin: a novel way to promote melanoma. Pathol Biol (Paris). 2009;57:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Kovacs D, Migliano E, Muscardin L, Silipo V, Catricalà C, Picardo M, Bellei B. The role of Wnt/β-catenin signaling pathway in melanoma epithelial-to-mesenchymal-like switching: evidences from patients-derived cell lines. Oncotarget. 2016;7:43295-43314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 127. | Brown K, Yang P, Salvador D, Kulikauskas R, Ruohola-Baker H, Robitaille AM, Chien AJ, Moon RT, Sherwood V. WNT/β-catenin signaling regulates mitochondrial activity to alter the oncogenic potential of melanoma in a PTEN-dependent manner. Oncogene. 2017;36:3119-3136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 128. | Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, Chang YJ, Choi KS, Jung SY, Woo SM, Ahn JS, Sim SH, Hong YS, Pastor-Barriuso R, Guallar E, Lee ES, Kong SY, Cho J. Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol. 2018;4:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 129. | Kim MJ, Huang Y, Park JI. Targeting Wnt Signaling for Gastrointestinal Cancer Therapy: Present and Evolving Views. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 130. | Sahin I, Eturi A, De Souza A, Pamarthy S, Tavora F, Giles FJ, Carneiro BA. Glycogen synthase kinase-3 beta inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol Ther. 2019;20:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 131. | Bartoszek A, Fichna J, Tarasiuk A, Binienda A, Fabisiak A, Krajewska JB, Mosińska P, Niewinna K, Salaga M. Free Fatty Acid Receptors as New Potential Targets in Colorectal Cancer. Curr Drug Targets. 2020;21:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Hopkins MM, Meier KE. Free fatty acid receptor (FFAR) agonists inhibit proliferation of human ovarian cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2017;122:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Najafi M, Ahmadi A, Mortezaee K. Extracellular-signal-regulated kinase/mitogen-activated protein kinase signaling as a target for cancer therapy: an updated review. Cell Biol Int. 2019;43:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 134. | Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 135. | Zhao K, Lu Y, Chen Y, Cheng J, Zhang W. Dual Inhibition of MAPK and JAK2/STAT3 Pathways Is Critical for the Treatment of BRAF Mutant Melanoma. Mol Ther Oncolytics. 2020;18:100-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1065] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 137. | Kang BW, Chau I. Molecular target: pan-AKT in gastric cancer. ESMO Open. 2020;5:e000728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 138. | Cao W, Li J, Hao Q, Vadgama JV, Wu Y. AMP-activated protein kinase: a potential therapeutic target for triple-negative breast cancer. Breast Cancer Res. 2019;21:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 139. | Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: a preoperative prospective trial. Cancer. 2014;120:2986-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 140. | Augimeri G, Giordano C, Gelsomino L, Plastina P, Barone I, Catalano S, Andò S, Bonofiglio D. The Role of PPARγ Ligands in Breast Cancer: From Basic Research to Clinical Studies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |