Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.113
Peer-review started: June 29, 2015
First decision: August 25, 2015
Revised: October 16, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: December 12, 2015
Processing time: 175 Days and 0.4 Hours
AIM: To verify in vivo relevance of the categorization of human vascular endothelial cells (VECs) into type-I (pro-proliferative) and type-II (anti-proliferative).
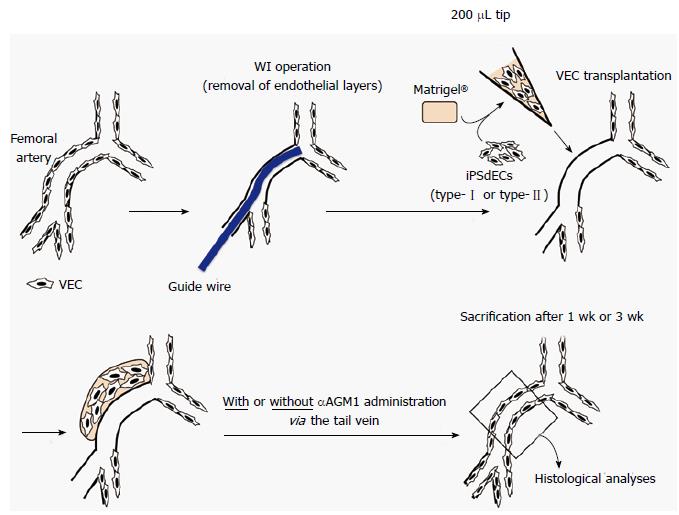
METHODS: Endothelial layers of murine femoral arteries were removed by wire injury (WI) operation, a common technique to induce arteriostenosis. Type-I and type-II VECs produced from human induced pluripotent stem cells (iPSCs), whose characters were previously determined by their effects on the proliferation of vascular smooth muscle cells in in vitro co-culture experiments, were mixed with Matrigel® Matrix. The mixtures were injected into subcutaneous spaces around WI-operated femoral arteries for the transplanted human iPSC-derived VECs (iPSdECs) to take a route to the luminal surface via vasa vasorum, a nutrient microvessel for larger arteries. Histologies of the femoral arteries were examined over time. The presence of human iPSdECs was checked by immunostaining studies using an antibody that specifically recognizes human VECs. Degrees of stenosis of the femoral arteries were calculated after three weeks. To determine the optimal experimental condition, xenotransplantation experiments were performed under various conditions using immunocompromised mice as well as immunocompetent mice with or without administration of immunosuppressants.
RESULTS: Because immunocompromised mice showed unexpected resistance to WI-induced arteriostenosis, we performed xenotransplantation experiments using immunocompetent mice along with immunosuppressant administrations. After one week, luminal surfaces of the WI-operated arteries were completely covered by human iPSdECs, showing the efficacy of our novel transplantation technique. After three weeks, type-I-iPSdECs-transplanted arteries underwent total stenosis, while type-II-iPSdECs-transplanted arteries remained intact. However, untransplanted arteries of immunosuppressant-treated mice also remained intact by unknown reasons. We found that transplanted human VECs had already been replaced by murine endothelial cells by this time, indicating that a transient existence of human type-II-iPSdECs on arterial luminal surfaces can sufficiently prevent the development of stenosis. Thus, we re-performed xenotransplantation experiments using immunocompetent mice without administrating immunosuppressants and found that arteriostenosis was accelerated or prevented by transplantation of type-I or type-II iPSdECs, respectively. Similar results were obtained from the experiments using human embryonic stem cell-derived VECs at early passages (i.e., type-II) and late passages (i.e., type-I).
CONCLUSION: Pro- and anti-stenosis capacities of type-I and type-II human iPSdECs were verified, respectively, promising a therapeutic application of allogenic iPSdECs.
Core tip: We previously reported that human vascular endothelial cells (VECs) were classified into two categories by their in vitro effects on the proliferation of vascular smooth muscle cells: Pro-proliferative VECs (type-I) and anti-proliferative VECs (type-II). Applying our new technique to transplant human VECs onto the luminal surface of endothelial layer-removed murine arteries, the in vivo relevance of the concept for VEC categorization was validated. Transplantation of pro-proliferative VECs (type-I) resulted in total stenosis while that of anti-proliferative VECs (type-II) completely blocked the development of arteriostenosis. Thus, pro-stenosis (type-I) and anti-stenotic (type-II) capacities were verified in vivo.
- Citation: Nishio M, Nakahara M, Saeki K, Fujiu K, Iwata H, Manabe I, Yuo A, Saeki K. Pro- vs anti-stenotic capacities of type-I vs type-II human induced pluripotent-derived endothelial cells. World J Transl Med 2015; 4(3): 113-122
- URL: https://www.wjgnet.com/2220-6132/full/v4/i3/113.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i3.113
In our previous report, we presented a new concept for the categorization of human vascular endothelial cells (VECs) based on their effects on the proliferation of human vascular smooth muscle cells (VSMCs) in in vitro co-culture experiments: Pro-proliferative VECs (type-I) and anti-proliferative VECs (type-II)[1]. We also showed that commercially available primary cultured human VECs were exclusively pro-proliferative VECs (type-I)[1] compatible with an earlier report[2]. Although anti-proliferative (type-II) VECs were producible from commercially available human endothelial progenitor cells depending on donors[1], they were inevitably converted into “type-I” VECs after repetitive subcultures[1]. Human embryonic stem cells (ESCs) stably produced “type-II” VECs; however, ESC-derived type-II VECs were inevitably converted into type-I after repetitive subcultures[1]. Molecular analyses identified that the causative gene for “type-II to type-I” conversion was Regulator of G-protein signaling 5 (RGS5)[1], which is reportedly expressed in the endothelia of pathological vessels but not those of normal vessels[1,3,4]. Besides repetitive subcultures, oxidative stresses also induced “type-II to type-I” conversion[1] Although anti-oxidant treatments lowered the degree of RGS5 induction and delayed the time of “type-II to type-I” conversion, they could not completely block RGS5 induction or “type-II to type-I” conversion[1]. Thus, the problem of obtaining sufficient amounts of type-II VECs remained unsolved.
This problem was resolved by utilization of exogenous gene-free human induced pluripotent stem cells (iPSCs). Although type-II VECs were producible from conventional retrovirus vector-based exogenous gene-containing human iPSCs (Ret-iPSCs) depending on lines, they were inevitably converted into type-I VECs after repetitive subcultures[1]. By contrast, type-II VECs generated from recently established Sendai virus vector-based human iPSCs (SeV-iPSCs)[5] showed particularly high resistance to “type-II to type-I” conversion[1]. This may be explained by an empirical rule that “the older the establishment time of ESC/iPSC is, the more prone to phenotype conversion the ESC/iPSC-derived VECs are” due to larger cumulative stresses. Moreover, SeV-iPSCs get rid of genome stress attributed to vector insertions into chromosomes. Indeed, Ret-iPSCs often expressed RGS5 message in immature states and RGS5 expression levels were further augmented after VEC differentiation. Thus, not only the passage numbers of iPS-derived VECs but also the quality of iPSCs (inc. the time and the method of establishments) are crucial determinants of the stability of type-II characters of iPSC-derived VECs (iPSdECs).
In the current study, we validated in vivo relevance of the new concept for the categorization of human VECs by transplantation experiments using iPSdECs: Ret-iPSCdEC (type-I) and SeV-iPSCs-derived VECs (SeV-iPSCdEC) (type-II). For this aim, we established a new technique to transplant human VECs onto luminal surfaces of murine arteries utilizing a route via vasa vasorum. By applying this unique technique, we verified the pro-stenosis and anti-stenosis capacities of type-I and type-II human VECs, respectively. We also show that xenotransplantation of human VECs to murine arteries was effectively performed even under immunocompetent conditions because transplanted VECs have exerted their full effects within a short period (< 1 wk) before rejected by immune systems. Our studies not only prove the in vivo relevance of our new concept for the categorization of human VECs but also suggest the possible application of allogenic human iPS-derived VECs to therapeutic purposes.
The hESC line (KhES-5) was generously provided by the Institute for Frontier Medical Science, Kyoto University[5]. A SeV-hiPSC line [SeV(BJ)-hiPSC] were established by using iPS-Tune™ (DNAVEC Corp., Ibaraki, Japan) from BJ fibroblast[6]. A Ret-hiPSC line (#25) was established from human fetus lung cells (MRC-5) by infecting recombinant retroviruses expressing the four factors (Oct3/4, Sox2, Klf4 and c-Myc) at Department of Reproductive Biology, Center for Regenerative Medicine, National Research Institute for Child Health and Development as used elsewhere[7,8]. 253G1[9] was provided by CiRA at Kyoto University and used elsewhere[7,8,10]. The type-I and type-II VECs were generated from Ret-hiPSC and SeV-hiPSCs, respectively[1].
The 1st antibody reactions were performed by using a 1:50-diluted rabbit polyclonal anti-human-specific PECAM antibody (sc-8306, Santa Cruz Biotechnology Inc.)[11] or a 1:420-diluted anti-smooth muscle actin antibody (A5228, Santa Cruz Biotechnology Inc.) and the 2nd antibody reactions were performed by using an Alexa Fluor® 488-conjugated goat anti-rabbit IgG (A11008, Life Technologies, Inc.) or an Alexa Fluor® 488-conjugated goat anti-mouse IgG (A11029, Life Technologies, Inc.), respectively. Photomicrographs were taken by either Olympus FluoView™ FV1000 Confocal Microscope (Olympus Optical Co. Ltd., Tokyo, Japan) or Olympus BX51 Fluorescence Phase contrast Microscope (Olympus Optical Co. Ltd.) equipped with DP-2 TWAIN digital camera system (Olympus Optical Co. Ltd.) and cellSens® standard imaging software (Olympus Optical Co. Ltd.).
The operation was performed as described elsewhere[12]. Nine-week-old ICR or NOD/SCID mice were anesthetized by intraperitoneally administrating the mixture of hydrochloric acid medetomidine (0.3 mg/kg), midazolam (4 mg/kg) and butorphanol tartrate (5 mg/kg) using 26 gauge needles. After depilation and disinfection, a 2 cm-long skin incision was made at the inguinal area to expose the femoral artery. After ligating the profunda femoris artery, a small incision was made on its proximal site, from which a 0.014-inch diameter guidewire (Cook Medical Inc., Bloomington, IN, United States) was inserted until it reached the bottom of the aorta descendens. Then, the guidewire was moved back and forth 10 times and rotated 5 times to evenly and completely exfoliate the endothelial layer. Finally, guidewire was removed and the profunda femoris artery was ligated at its most proximal site. After one week to three weeks from the operation, mice were anesthetized by intraperitoneally administrating the mixture of hydrochloric acid medetomidine (0.3 mg/kg), midazolam (4 mg/kg) and butorphanol tartrate (5 mg/kg) using 26 gauge needles and then subjected to perfusion fixation by administrating 50 mL of PBS and subsequently 100 μL of 4% paraformaldehyde solution via the left ventricle. Specimens of femoral artery were prepared and embedded into paraffin blocks for histological analyses.
An anti-asialo-GM1 antibody (300 μg) (WAKO Pure Chemical Industries, Osaka, Japan) was injected into the tail vein of 9-wk-old ICR or NOD/SCID mice, which were subsequently subjected to WI operations. On the other hand, iPSdECs/Matrigel® mixture were prepared just before the end of the WI operation as follows: 1 × 106 iPSdECs were centrifuged and kept in ice-cold sterile 1.5 mL tube, to which 20 μL of ice-cold Matrigel® Matrix was promptly added and mixed. The iPSdECs/Matrigel® mixture was injected into the subcutaneous space around the outer membrane of the femoral artery by using 200 μL pipet tip. An anti-asialo-GM1 antibody (300 μg) was injected into tail vein twice a week to block NK-mediated rejection in some experiments.
All procedures involving animals, including WI operations and pervasa vasorum transplantation (PVVT), were reviewed and approved by the Institutional Animal Care and Use Committee of National Center for Global Health and Medicine, Tokyo, Japan (Authorization No. 15014). The animals were acclimatized to laboratory conditions (23 °C ± 3 °C, 12 h/12 h light/dark, 55% ± 15% humidity, ad libitum access to food and water) prior to experimentation. The animal protocol was designed to minimize pain or discomfort to the animals by using mixed anesthetics as described above.
Experiments were performed independent three experiments using three mice (n = 3) and the data were analyzed using student t test. Results were shown as averages ± standard deviations (AV ± SD).
The luminal surfaces of the arteries are continuously exposed to high-pressured and pulsatile blood flows, and thus, it is difficult for the transplanted human VECs to safely land and stably reside on the luminal surfaces if they are intravenously or intra-arterially injected into the blood stream. Thus, as alternative approach, we applied a strategy to take a rout via vasa vasorum, which is a nutrient vessel that supply or drain the walls of the larger arteries and veins. We hypothesized that the vasa vasorum would effectively guide the transplanted human VECs at least to the middle portion of tunica media and the human VECs would then migrate to the luminal surface, which is the natural site for them to reside.
For an effective replacement of the endothelial cells of the murine femoral artery by the transplanted human VECs, the host endothelial layer was mechanically removed by wire injury (WI) operation, which is a very common technique to induce experimental arteriostenosis. Then, human iPSdECs were transplanted by our unique technique termed “PVVT”, where human iPSdECs were mixed with Matrigel® Matrix and the mixtures were simply injected into subcutaneous spaces around WI-operated femoral arteries (Figure 1). First, we performed experiments using immunodeficient NOD/SCID mice, which lack functional lymphocytes and permit the engraftment of a wide range of human cells. However, these mice did not develop arteriostenosis even after WI operations by unknown reasons (data not shown). Similar results were obtained from nude mice, which suffer from deficient T cell function (data not shown). Therefore, we performed experiments using immunocompetent ICR mice along with an administration of anti-asialo GM1 monoclonal antibody (αAGM1)[13] to prevent immunorejections by natural killer (NK) cells. The reason why we used αAGM1 is as follows. It is known that endothelial cells of the venus graft are replaced by arterial endothelial cells within several days from the coronary artery bypass surgery. Thus, an inhibition of the long-term immunorejections by T/B cells, which are usually induced a couple of weeks later, is not required for our purpose. On the other hand, short-term immnoreactions by NK cells, which are usually induced after several days from transplantation, might possibly affect the efficiency of the transplantations. Therefore, we performed xenotransplantation under the condition where NK cell activities were blocked by an administration of αAGM1.
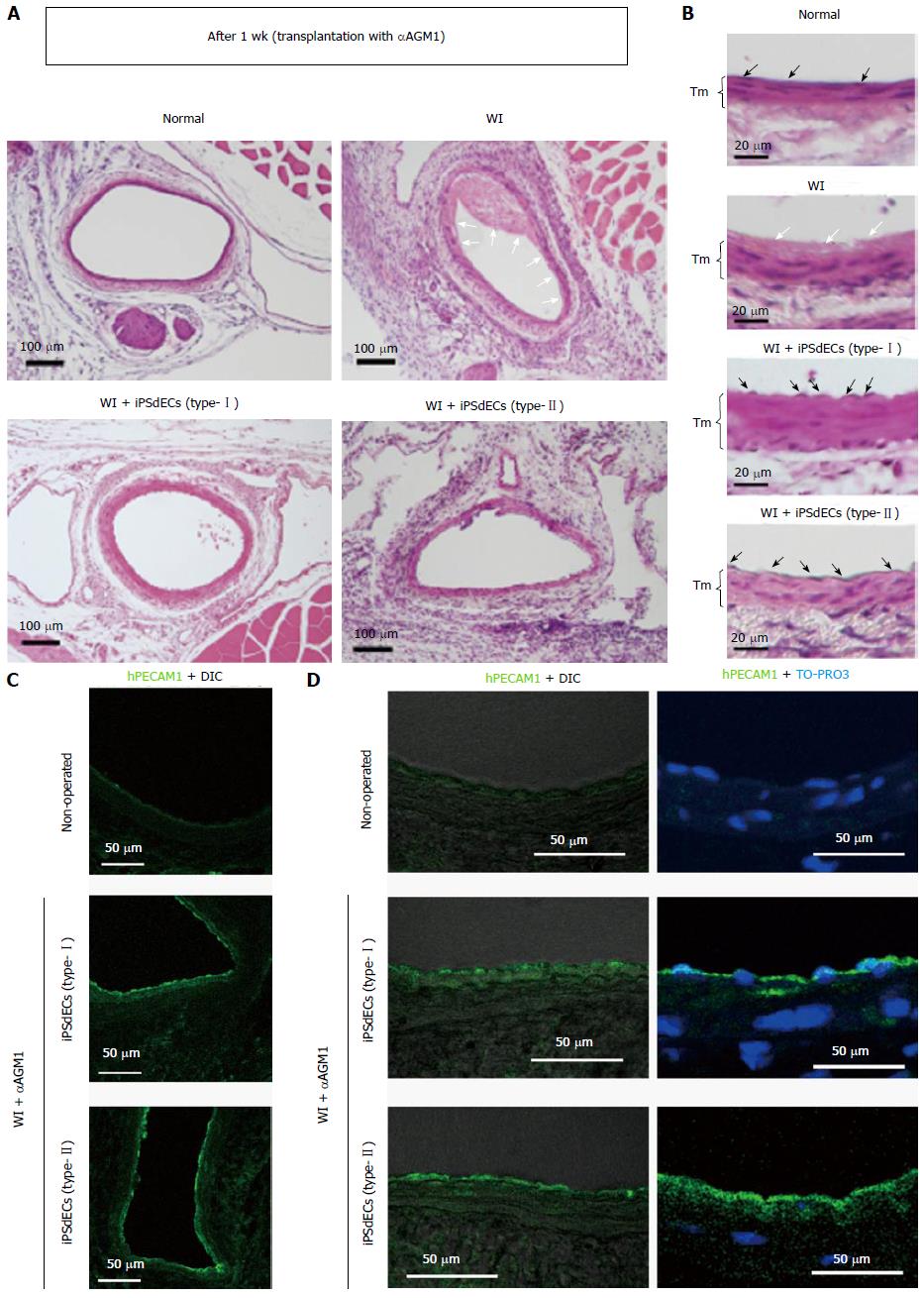
After one week from the transplantation, WI-operated arteries underwent entire loss of endothelial layers with fibrin clot-like substances on the luminal surfaces of the arteries of non-transplanted mice (Figure 2A and B, open arrows). By contrast, the luminal surfaces of the arteries of human iPSdEC-transplanted mice were thoroughly covered by the endothelial cells (Figure 2B, closed arrows). Immunostaining studies using an antibody that specifically recognizes human PECAM1 but not murine PECAM1[11] confirmed that the endothelial cells that covered the luminal surfaces of the arteries were indeed human VECs (Figure 2C and D).
Thus, our new PVVT technique guaranteed the effective transplantation of human VECs onto the luminal surfaces of murine arteries.
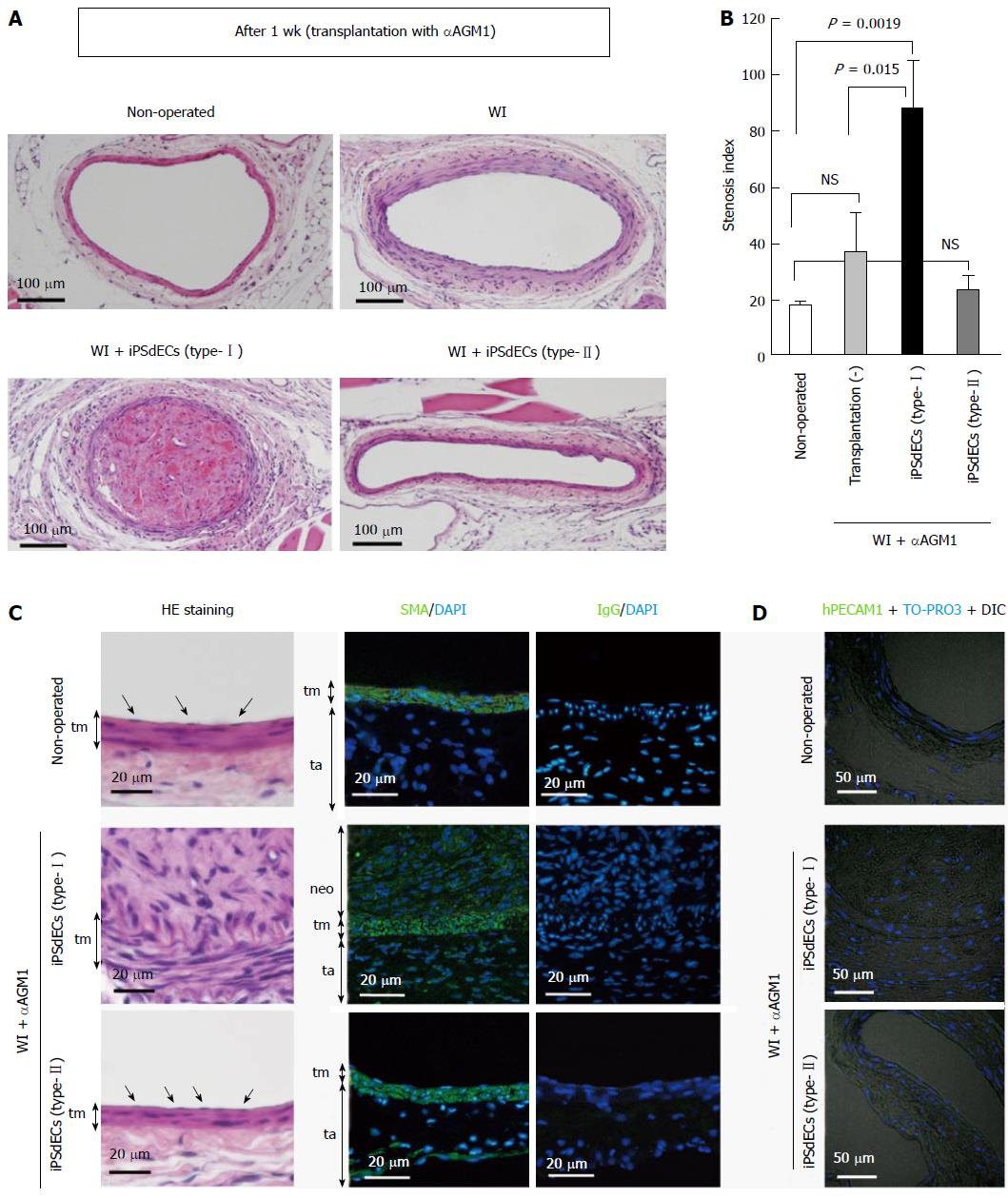
Next, we analyzed the histologies of the arteries after three weeks from the transplantation. We found that type-I iPSdECs-transplanted arteries underwent almost complete stenosis (Figure 3A, lower left). By contrast, “type-II” iPSdEC-transplanted arteries showed intact morphologies (Figure 3A, lower right). For quantitative evaluations, we calculated the ratio of the square measure of the lumen and that of the area surrounded by external elastic membranes in cross sections as an indicative of stenosis. We found that transplantation of “type-I” iPSdECs exacerbated the development of arteriostenosis (Figure 3B). Histological analyses (Figure 3C, left panels) and immunostaining studies using anti-smooth muscle actin (Figure 3C, right panels) confirmed the presence of hyper-proliferated VSMCs within the neointima. Unexpectedly, AGM1 administration significantly inhibited the development of arteriostenosis (Figure 3A, upper right), and thus, we could not detect significant differences between “WI + αAGM1 + transplantation (-)” and “WI + αAGM1 + iPSdEC (type-II)” groups (Figure 3B). Therefore, although we could successfully demonstrate the pro-stenosis capacity of type-II iPSdEC, we failed in showing the anti-stenosis activity of type-II iPSdEC in these experiments. We hypothesized that immunosuppressive states inhibited the development of arteriostenosis as observed in the case of immunodeficient NOD/SCID mice and nude mice (data not shown).
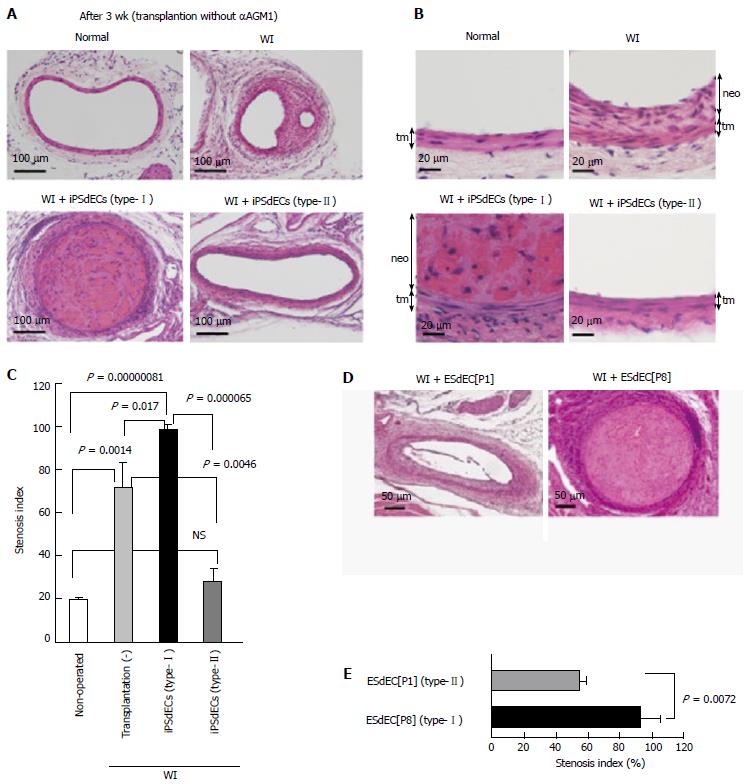
When we examined the presence of human iPSCdECs after 3 wk from transplantation, we noticed that the transplanted human VECs already became undetectable on the arterial luminal surfaces (Figure 3D), indicating that transplanted iPSCdECs had already been replaced by host’s endothelial cells by this time as expected from the finding obtained from bypass surgeries. Our observation at the same time indicates that the time period required for the transplanted iPSdECs to exert their full effects was rather shorts (about one week). Thus, we re-performed the transplantation experiments without administrating αAGM1. We confirmed that arteriostenosis was exacerbated by type-I iPSCdECs transplantation under these conditions (Figure 4A, lower left, and 4B, lower left). By contrast, the development of arteriostenosis was completely prevented in type-II” iPSdECs-transplanted arteries (Figure 4A, lower right, and 4B lower right). Without an administration of αAGM1, WI-operated arteries underwent arteriostenosis (Figure 4A, upper right, and 4B upper right). Thus, anti-stenosis capacities of type-II iPSdECs, as well as the pro-stenosis capacities of type-I iPSdECs, were verified (Figure 4C). We also examined the effects of transplantation with human ESC-derived VECs (ESdECs) at early passages (type-II) and at late passages (type-I)[1]. Although ESdECs at early passages (type-II) underwent “type-II to type-I conversion” after a few rounds of subcultures in vitro and thus their type-II characters were not as solid as type-II SeV-iPSdECs, we could detect clear differences in the results of transplantations between ESdECs at early passages (type-I) and those at early passages (type-II) (Figure 4D and E).
Collectively, the in vivo relevance of the concept for the categorization of human VECs, type-I (pro-proliferative) and type-II (anti-proliferative), were verified in vivo.
In the current study, we verified the in vivo relevance of the new concept for the categorization of human VECs by showing that pro-proliferative VECs (type-I) exacerbated the stenosis of injured arteries whereas anti-proliferative VECs (type-II) prevented the development of arteriostenosis. Our finding highlights an unexpected importance of the endothelial cells for the maintenance of vascular structures, demonstrating that VECs serve as not only the simple cover for the luminal surface but also a crucial regulator of the proliferation of VSMCs. Therefore, an approach to the preservation of type-II VEC phenotypes provides a new strategy for the treatment of arteriostenosis. Although the major cause of arteriostenosis is currently hypercholesterolemia-based atherosclerosis and anticholesteremic agents has been exerting high therapeutic effects, new VEC-targeted drug discoveries may further contribute to the control of ischemic diseases especially in the cases of restenosis after stent therapies or resistance to cholesterol medications.
We found by chance that arteriostenosis is strongly inhibited under immunosuppressive conditions. This finding may explain, at least in part, the high effectiveness of immunosuppressive agents used in the drug-eluting stent. The reason why immunosuppressive states prevent the development of arteriostenosis remains elusive. In WI-injured arteries of immunodeficient NOD/SCID mice, cellular compartments of tunica media were lost by unknown reasons (data not shown). Because a similar phenomenon was observed in nude mice (data not shown), a certain component of T cells might possibly be involved in the regulation of this bizarre phenomenon. We are currently studying which components of T cells are involved in this phenomenon.
In the current study, we also established a new transplantation technique termed PVVT, which guarantees the effective transplantation of human VECs onto the luminal surface of murine arteries. PVVT is a very simple technique, which can be performed by putting human VEC-embedded gels into subcutaneous spaces adjacent to arterial walls through a small skin incision. It can even be carried out as an ambulatory treatment if it is clinically applied in the future. Although we cannot completely exclude the possibility that there are still other paths than vasa vasorum, our new transplantation technique provides the easiest and safest way to transplant VECs on the luminal surfaces of injured arteries. Another merit of our PVVT technique is that it can be performed even under immunocompetent conditions, which indicates that allogenic iPSdECs are used as effectively as autologous iPSdECs when clinically applied. Moreover, a short-term requirement of iPSdECs makes the clinical application of human iPSdECs much safer. In the case of allogenic iPSdECs, the risk of tumor formation following transplantation will be lowered to a minimum because allogenic iPSdECs will be immunologically rejected by adaptive immune systems. Collectively, PVVT-based therapies will widen the applicability of iPSdECs to clinical purposes.
Thus, our finding will shed a new light to an advanced understanding of vascular biology and contribute to the therapeutics development for the control of ischemic diseases.
We thank Mr. Shinnosuke Suzuki and M.S. Yoshinori Yanagi for technical assistance.
Human vascular endothelial cells (VECs) are categorized into two groups by their effects on the proliferation of vascular smooth muscle cells (VSMCs) in in vitro co-culture experiments: Pro-proliferative VECs (type-I) vs anti-proliferative VECs (type-II).
It remains elusive whether pro-proliferative and anti-proliferative human VECs indeed exert pro-stenotic and anti-stenotic potentials in vivo, respectively. To valuate the characters of human VECs in vivo, however, an innovative technique that guarantees high-efficiency transplantation of VECs onto the luminal surface of the artery, which is exposed to high-pressured blood stream, is required.
The problem to effectively transplant human VECs onto the luminal surface of the murine artery has been resolved by the novel transplantation technique, where gel-embedded VECs were injected into the substances space around arteries through a small incision opening on the skin surface. By applying this technique, in vivo relevance the concept for the categorization of human VECs was validated, demonstrating that transplantation of pro-proliferative VECs (type-I) and anti-proliferative VECs (type-II) resulted in deterioration and prevention of stenosis in the injured arteries, respectively.
Towards the control of ischemic diseases, transplantation of human iPSC-derived type-II VECs to the luminal surface of injured arteries via a vaso vasorum route may provide a new adjunct therapy with high efficacy and high safety but low risk of restenosis after revascularization.
Vasa vasorum is a feeding microvessel that supplies the cells in tunica media of larger blood vessels including smooth muscle cells and fibroblasts. Wire injury is a technique to mechanically remove the endothelial cells from arterial lumens by the movement of a metal wire in a longitudinal as well as vertical direction, causing the development of arteriostenosis.
The manuscript is about two types of vascular endothelial cells, which have anti and pro proliferative effect on vascular smooth muscle cells. The study is well-design and written.
P- Reviewer: Tanabe S, Zaminy A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Nishio M, Nakahara M, Sato C, Saeki K, Akutsu H, Umezawa A, Tobe K, Yasuda K, Yuo A, Saeki K. New categorization of human vascular endothelial cells by pro- versus anti-proliferative phenotypes. World J Transl Med. 2015;In press. |
| 2. | Shinoda E, Yui Y, Hattori R, Tanaka M, Inoue R, Aoyama T, Takimoto Y, Mitsui Y, Miyahara K, Shizuta Y. Tissue factor pathway inhibitor-2 is a novel mitogen for vascular smooth muscle cells. J Biol Chem. 1999;274:5379-5384. [PubMed] |
| 3. | Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, Molitor JA, Henstorf G, Lafyatis R, Pritchard DK. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Fleming JN, Nash RA, Mahoney WM, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep. 2009;11:103-110. [PubMed] |
| 5. | Suemori H, Yasuchika K, Hasegawa K, Fujioka T, Tsuneyoshi N, Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926-932. [PubMed] |
| 6. | Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394-406. [PubMed] |
| 7. | Nakamura N, Saeki K, Mitsumoto M, Matsuyama S, Nishio M, Saeki K, Hasegawa M, Miyagawa Y, Ohkita H, Kiyokawa N. Feeder-free and serum-free production of hepatocytes, cholangiocytes, and their proliferating progenitors from human pluripotent stem cells: application to liver-specific functional and cytotoxic assays. Cell Reprogram. 2012;14:171-185. [PubMed] |
| 8. | Gokoh M, Nishio M, Nakamura N, Matsuyama S, Nakahara M, Suzuki S, Mitsumoto M, Akutsu H, Umezawa A, Yasuda K. Early senescence is not an inevitable fate of human-induced pluripotent stem-derived cells. Cell Reprogram. 2011;13:361-370. [PubMed] |
| 9. | Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106. [PubMed] |
| 10. | Hayashi R, Ishikawa Y, Ito M, Kageyama T, Takashiba K, Fujioka T, Tsujikawa M, Miyoshi H, Yamato M, Nakamura Y. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7:e45435. [PubMed] |
| 11. | Nakahara M, Nakamura N, Matsuyama S, Yogiashi Y, Yasuda K, Kondo Y, Yuo A, Saeki K. High-efficiency production of subculturable vascular endothelial cells from feeder-free human embryonic stem cells without cell-sorting technique. Cloning Stem Cells. 2009;11:509-522. [PubMed] |
| 12. | Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |