Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.101
Peer-review started: June 29, 2015
First decision: August 16, 2015
Revised: September 3, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: December 12, 2015
Processing time: 175 Days and 0.7 Hours
AIM: To identify kinases involved in phenotype regulation of vascular endothelial cells (VECs): Pro-proliferative G-protein signaling 5 (RGS5)high (type-I) vs anti-proliferative RGS5low (type-II) VECs.
METHODS: Proteomic kinase assays were performed to identify the crucial kinase involved in the phenotype regulation of human VECs using type-I VECs, which promotes the proliferation of human vascular smooth muscle cells (VSMCs), and type-II VECs, which suppress the proliferation of human VSMCs. The assays were performed using multiple pairs of type-I and type-II VECs to obtain the least number of candidates. The involvement of the candidate kinases was verified by evaluating the effects of their specific inhibitors on the phenotype regulation of human VECs as well as the expression levels of regulator of RGS5, which is the causative gene for the “type-II to type-I” phenotype conversion of human VECs.
RESULTS: p38α mitogen-activated protein kinase (p38α MAPK) was the only kinase that showed distinctive activities between type-I and type-II VECs: p38α MAPK activities were low and high in type-I and type-II VECs, respectively. We found that an enforced expression of RGS5 indeed lowered p38α MAPK activities in type-II VECs. Furthermore, treatments with a p38α MAPK inhibitor nullified the anti-proliferative potential in type-II VECs. Interestingly, MAPK inhibitor treatments enhanced the induction of RGS5 gene. Thus, there is a vicious cycle between “RGS5 induction” and “p38α MAPK inhibition”, which can explain the unidirectional process in the stress-induced “type-II to type-I” conversions of human VECs. To understand the upstream signaling of RGS5, which is known as an inhibitory molecule against the G protein-coupled receptor (GPCR)-mediated signaling, we examined the effects of RGS5 overexpression on the signaling events from sphingosine-1-phosphate (S1P) to N-cadherin, because S1P receptors belong to the GPCR family gene and N-cadherin, one of their downstream effectors, is reportedly involved in the regulation of VEC-VSMC interactions. We found that RGS5 specifically bound with S1P1. Moreover, N-cadherin localization at intercellular junctions in type-II VECs was abolished by “RGS5 overexpression” and “p38α MAPK inhibition”.
CONCLUSION: p38α MAPK plays crucial roles in “type-I vs type-II” phenotype regulations of human VECs at the downstream of RGS5.
Core tip: We previously reported that human vascular endothelial cells (VECs) are categorized into two types by their effects on the proliferation of vascular smooth muscle cells and the expressions of regulator of G-protein signaling 5 (RGS5): Pro-proliferative RGS5high (type-I) and anti-proliferative RGS5low (type-II) VECs. Performing proteomic kinase assays and inhibitor studies, we show here that p38 mitogen-activated protein kinase (p38 MAPK) is the crucial kinase that determines VEC phenotyping at the downstream of RGS5. Not only RGS5 overexpression suppressed p38 MAPK activities but also p38 MAPK inhibitions up-regulated RGS5 expression, indicating that “RGS5 induction” and “p38 MAPK inhibition” creates a vicious cycle in “type-II to type-I” conversions of human VECs.
- Citation: Nakahara M, Nishio M, Saeki K, Yuo A, Saeki K. p38 mitogen-activated protein kinase regulates type-I vs type-II phenotyping of human vascular endothelial cells. World J Transl Med 2015; 4(3): 101-112
- URL: https://www.wjgnet.com/2220-6132/full/v4/i3/101.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i3.101
We previously reported that human vascular endothelial cells (VECs) are classified into two groups by their effects on the proliferation of vascular smooth muscle cells (VSMCs) in in vitro co-culture experiments along with the expression levels of regulator of G-protein signaling 5 (RGS5): Pro-proliferative RGS5high VEC (type-I) vs anti-proliferative RGS5low VECs (type-II)[1]. We also demonstrated that commercially available primary cultured human VECs exclusively belong to pro-proliferative RGS5high VECs (type-I). On the other hand, human bone marrow-derived endothelial progenitor cells (EPCs) and human embryonic stem cells (ESCs) produced anti-proliferative RGS5low VECs (type-II)[1]. Because RGS5 expression was hardly detectable in normal human VECs[1,2] but significantly induced under pathological conditions[1,3,4] and because oxidative stress and subculture-dependent stress induced “type-II to type-I” conversion along with RGS5 gene induction[1], pro-proliferative RGS5high VECs (type-I) should be considered as degenerative VECs. The reason why widely used commercially available primary cultured human VECs belong to type-I degenerative VECs may be attributed to their histories: They have survived the drastic environmental changes from in vivo to in vitro by overcoming multiple stresses during the process of their preparations such as tissue removals, cell dissociations and cell expansions. Therefore, their characters may have changed in such a manner that they lose type-II anti-proliferative capacities. By contrast, EPC/ESC-derived VECs do not have such stressful histories, and thus, it is possible that they preserve type-II anti-proliferative phenotypes. Nevertheless, EPC/ESC-derived type-II RGS5low VECs were inevitably converted into type-I RGS5high VECs after a few rounds of subcultures[1].
In the case of induced pluripotent stem cells (iPSCs), the situations are rather complicated. Regarding VECs produced from retroviral vector-based iPSCs (Ret-iPSCs), type-II anti-proliferative capacities were often deteriorated from early phases and subculture-dependent “type-II to type-I” conversions were often accelerated[1]. By contrast, in the case of Sendai virus vector-based iPSC (SeV-iPSCs), “type-II to type-I” conversion was highly repressed: SeV-iPSC-derived VECs (SeV-iPSdECs) showed high resistance to subculture-dependent and oxidative stress-induced “type-II to type-I” conversions[1]. The phenotype differences between Ret-iPSC-derived VECs (Ret-iPSdECs) and SeV-iPSdECs can be explained, at least in part, by the differences in the degree of stresses. Ret-iPSCs have multiple copies of viral vectors in their chromosomes, and thus, they suffer from genome stresses. On the other hand, SeV-iPSCs completely get rid of such genome stresses because the Sendai virus vector is an RNA virus-based vector and do not integrate into the host chromosomes.
In our previous study, the signaling pathway involved in the “type-II to type-I” conversion at the downstream of RGS5 remained elusive. Because RGS5 was the only gene that showed a discriminative expression pattern between type-I and type-II VECs in microarray analyses[1], we thought that an alternative strategy other than transcriptomic approaches was required to identify the downstream signaling events of RGS5. In the current study, we performed proteomic kinase assays to identify the crucial kinase that regulates the RGS5-mediated “type-I vs type-II” phenotyping of human VECs.
Human umbilical vein endothelial cells (HUVEC), human neonatal dermal microvascular endothelial cells (HMVEC), human adult aortic endothelial cells (HAEC) and human adult coronary arterial endothelial cells (HCAEC) were purchased from Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan). The cells were cultured on 0.1% gelatin-coated plates using EGM®-2 BulletKit (Lonza Group Ltd. Basel, Switzerland). Human aortic smooth muscle cells of different donors were purchased from Lonza Group Ltd. (Basel, Switzerland) and cultured using SmGM™-2 BulletKit™ (Lonza Group Ltd.). Cells were re-seeded at split ratios of 1:3-1:4 twice a week. VECs within 8th passage were used in all experiments. The hESC lines (KhES-1, -3, -5) were established by the Institute for Frontier Medical Science, Kyoto University[2]. SeV-hiPSCs were established from HUVEC[3] and BJ fibroblast[3] by using iPS-Tune™ (ID Pharma Co., Ltd., Ibaraki, Japan). The Ret-hiPSC line of 253G1[4] was provided by CiRA at Kyoto University. Sphingosine-1-phosphate (S1P) (#62570, Cayman Chemical Co., Ann Arbor, MI, United States) was dissolved by 0.3 mol/L NaOH at the concentration of 4 mg/mL as a stock. A p38 MAP Kinase inhibitor (#506126, Calbiochem Co., La Jolla, CA, United States) was dissolved by DMSO at the concentration of 10 mmol/L as a stock. A JNK inhibitor SP600125 (#BML-EI305, Enzo Life Sciences, Inc., Farmingdale, NY, United States) was dissolved by DMSO at the concentration of 10 mmol/L as a stock. An extracellular signal-regulated kinase (ERK) inhibitor PD98059 (#513001, Calbiochem Co.,) was dissolved by DMSO at the concentration of 50 mmol/L stock. FTY720 Phosphate (#10008639, Cayman Chemical Co.,) was dissolved by chloroform at the concentration of 0.5 mg/mL as a stock. All reagents were kept at -20 °C.
VECs were g-irradiated (5 Gy) and stained with carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE) by using CFSE Cell Division Assay Kit (Cayman Chemical Co., Ann Arbor, MI), while VSMCs were stained with PKH26 by using PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich Co. LLC., St. Louis, MO, United States) according to the manufacturer’s guidance. For contact co-culture, irradiated and CFSE-stained VECs were seeded at the density of 2 × 105 cells/well on 0.1% gelatin-coated 24-well culture plates, and on the following day, PKH26-stained VSMCs were seeded at the density of 3.75 × 103 cells/well on VEC layers or gelatin layers as control. After 4 d, total cells were harvested and subjected to flow cytometry analyses by FACSCaliburTM (BD Biosciences, San Jose, CA, United States) and FL1 and FL2 fluorescence intensities were measured by CellQuest™ Pro software (BD Biosciences). FL2 (PKH26) fluorescence intensities were further analyzed mathematically by ModFit LT™ software (Verity Software House Inc., Topsham, ME, United States) to calculate the proliferation index.
Total RNA was extracted from VECs using TRIzol® Reagent (Life Technologies, Inc.). Complementary DNA was prepared from 1 μg of RNA using SuperScriptTM III First-Strand Synthesis System kit (Life Technologies, Inc.), and used in quantitative polymerase chain reaction (PCR) reactions with FAST SYBR® Green Master Mix (Applied Biosystems® from Life Technologies, Inc.). Quantitative real-time PCR was performed using the StepOnePlusTM PCR machine (Applied Biosystems® from Life Technologies, Inc.). Primers used for RGS5 were Forward: 5’-GGAGGCTCCTAAAGAGGTGA-3’ and Reverse: 5’-GGGAAGGTTCCACCAGGTTC-3’, and primers used for GAPDH were Forward: 5’-CCACTCCTCCACCTTTGAC-3’ and Reverse: 5’-ACCCTGTTGCTGTAGCCA-3’.
Human ESC/iPSC-derived cells were fixed by 4% paraformaldehyde for 15 min at room temperature. The 1st antibody reaction was performed by using a 1:100-diluted rabbit polyclonal anti-human N-cadherin antibody (ab12221, Abcam plc, Cambridge, MA, United States). The 2nd antibody reaction was performed by using an Alexa Fluor® 488-conjugated goat anti-rabbit IgG (A11008, Life Technologies, Inc). Photomicrographs were taken by Olympus BX51 Fluorescence Phase contrast Microscope (Olympus Optical Co. Ltd.) equipped with DP-2 TWAIN digital camera system (Olympus Optical Co. Ltd.) and cellSens® standard imaging software (Olympus Optical Co. Ltd.).
The 1 × 105 VECs were lysed by using 20 μL sample buffer solution (2ME+) (× 2) (Cat. 196-11022) (WAKO Pure Chemical Industries, Osaka, Japan). The first antibody reaction was performed by using a 1:1000-diluted anti-human RGS5 antibody (ab83230, Abcam, Cambridge, MA, United States), a rabbit polyclonal anti-p38α antibody (C-20) (sc-535, Santa Cruz Biotechnology Inc., Santa Cruz, CA, United States), a rabbit polyclonal anti-p38 (phospho T180) antibody (ab51050, Abcam plc.), a rabbit monoclonal anti-c-Jun (60A8) (#9165, Cell Signaling Technology, Inc., Beverly, MA, United States), a rabbit monoclonal anti-Phospho-c-Jun (Ser73) (D47G9) (#3270, Cell Signaling Technology, Inc.), a rabbit monoclonal anti-Phospho-c-Jun (Ser63) (54B3) (#2361, Cell Signaling Technology, Inc.), a rabbit polyclonal anti-Phospho-c-Jun (Ser243) Antibody (#2994, Cell Signaling Technology, Inc.), a rabbit polyclonal anti-c-Jun (phospho T93) antibody (ab28854, Abcam plc.) or a 1:1000-diluted anti-human β-tubulin antibody (sc-9104, Santa Cruz Biotechnology Inc., Santa Cruz, CA, United States) and the second antibody reaction was performed by using a 1:2000-diluted anti-rabbit IgG HRP-linked antibody (#7074S) (Cell Signaling Technology, Inc.).
The 3 × 106 HUVECs were lysed by 500 μL of RIPA buffer. Immunoprecipitation was performed using a mouse monoclonal anti-human RGS5 antibody (sc-390245, Santa Cruz Biotechnology Inc.) or a goat polyclonal anti-human S1P1 antibody (sc-16070、Santa Cruz Biotechnology Inc.), anti-human S1P2 antibody (sc-31577, Santa Cruz Biotechnology Inc.), anti-human S1P3 antibody (sc-16076, Santa Cruz Biotechnology Inc.). For control, normal mouse IgG (sc-2025, Santa Cruz Biotechnology Inc.) or normal goat IgG (sc-2028,Santa Cruz Biotechnology Inc.). Precipitated samples were subjected to western blotting using a goat polyclonal anti-human S1P1 antibody (sc-16070, Santa Cruz Biotechnology Inc.) and a rabbit polyclonal anti-human RGS5 antibody (ab-83230, Abcam plc.).
Proteome Profiler Antibody Arrays were performed using Human Phospho-Kinase Antibody Kit (#ARY003B, R&D Systems Inc., Minneapolis, MN, United States) according manufacturer’s guidance. In brief, 5 × 106 cells were lysed by using 500 μL lysis buffer and, after centrifugation, 334 μL of the lysate was diluted by 2 mL blocking buffer and used for the assay. The intensity of each spot was measured by using a free Image J software and its percentage to that of reference spot was plotted in a bar chat.
A Homo sapiens cDNA, FLJ96402, which corresponds to Homo sapiens RGS5, transcript variant 1, mRNA (NM_003617.3), with two nucleotide substitutions, was purchased from National Institute of Technology and Evaluation (Tokyo, Japan), and the two substituted nucleotides were corrected by using KOD-Plus-Mutagenesis Kit (Toyobo Co. Ltd., Osaka, Japan) to become identical to the nucleotide sequences in NM_003617.3. The cDNA was inserted into pmaxCloning™ expression vector (Lonza Group Ltd. Basel, Switzerland). The 2 × 105 human ESC-derived VECs (ESdECs) were transfected with 3 μg vectors using a Nucleofector™ (Lonza Group Ltd.) as reported elsewhere[1] and subjected to Western blotting. Alternatively, RGS5 cDNA was inserted into the simian immunodeficiency virus (SIV) vector (ID Pharma Co., Ltd., Ibaraki, Japan) in either forward (SIV-RGS5) or reverse (SIV-control) direction. The 2 × 105 ESdECs were infected with SIV vectors at MOI = 80. After 6 d, during which the cells were subcultured twice, immunostaining studies were performed.
Experiments were performed independent three or four experiments (n = 3 or 4) and the data were analyzed according Student t-test. Results were shown as averages ± standard deviations (AV ± SD).
Proteomic kinase assays using multiple pairs of type-I and type-II VECs
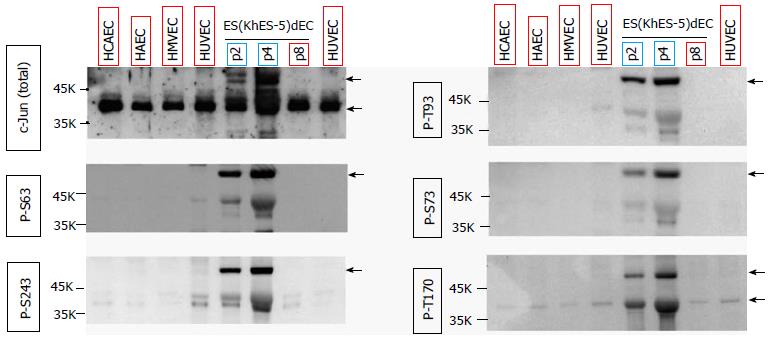
During our trials to identify the downstream signaling target of RGS5, we found by coincidence that there was a clear difference in the phosphorylation state of c-JUN protein between type-I and type-II VECs. c-Jun protein was hyper-phosphorylation at multiples sites such as Ser63, Ser73, Ser243, Thr93 and Thr170 in type-II human VECs including ES(KhES-5)-derived VECs [ESdEC(KhES-5)] at early passages (Figure 1, blue rectangles). By contrast, c-Jun protein was hypo-phosphorylated in type-I VECs including ESdEC (KhES-5) at late passages and commercially available primary cultured human VECs (Figure 1, red rectangles). Therefore, we hypothesized that certain kinases would be working at the downstream of RGS5 for the “type-I vs type-II” phenotyping of human VECs. It is known that c-Jun protein is phosphorylated by multiple kinases including c-Jun N-terminal kinase (JNK), p38α MAPK, ERK, glycogen synthase kinase 3 (GSK) and casein kinase 2. To identify the pivotal kinase involved in the determination of VEC phenotypes, a systemic analysis to quantitatively evaluate the activation states of various kinases using multiple pairs of type-I and type-II VECs is of great use. For this aim, we applied a commercially available proteomic kinase assay, where cell lysates were reacted with an array of site-specific phosphorylation antibodies of 43 kinases on a nitrocellulose membrane to simultaneously detect the activation state of each kinase via an ordinary immunoblotting procedure.
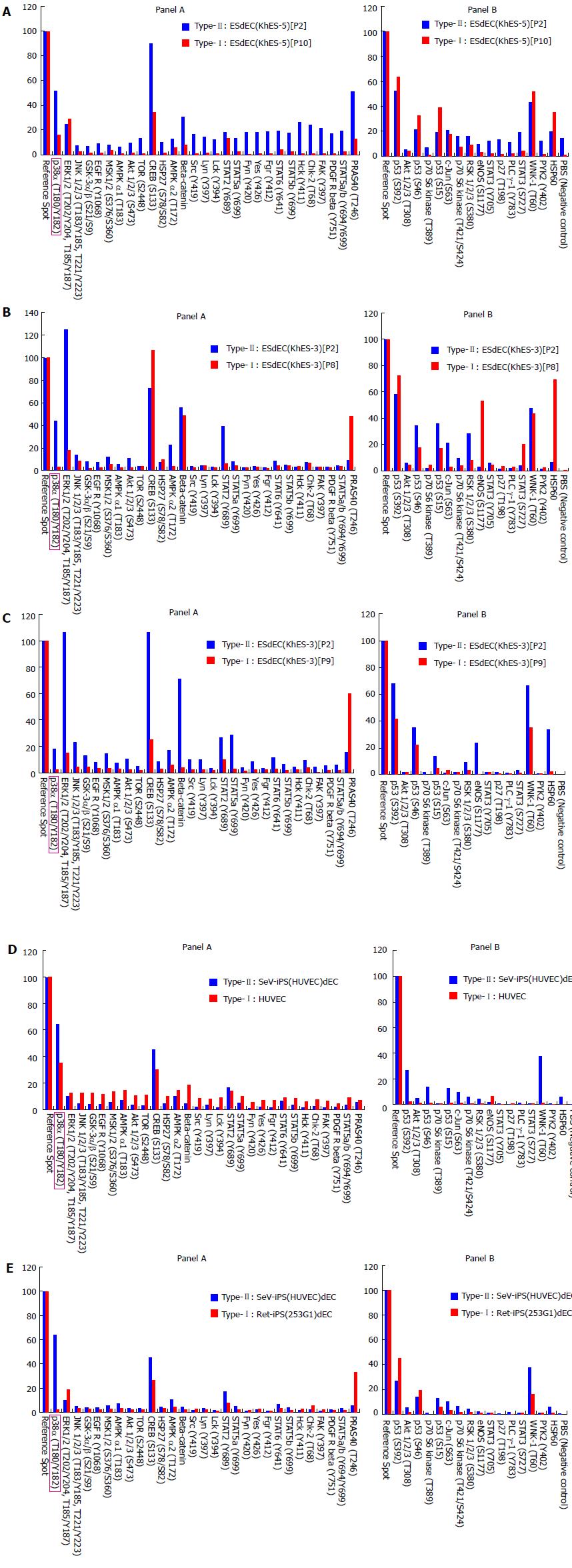
First, we compared the profiles of protein kinase activities between ESdEC(KhES-5) at passage 2 [ESdEC(KhES-5)[P2]], which showed type-II phenotype[1], and those at passages 10 [ESdEC(KhES-5)[P10]], which showed type-I phenotype[1]. As shown in Figure 2A, protein kinase activities were generally higher in ESdEC(KhES-5)[P2] (type-II) than type-I ESdEC(KhES-5)[P10] (type-I) in accordance with the result in Figure 1. To narrow the list of candidate kinases involved in type-I/type-II phenotyping, we performed proteomic kinase assays using VECs generated from KhES-3 line of ESC at passage 2 [ESdEC(KhES-3)[P2]], which showed type-II phenotype[1], and those at passages 8 [ESdEC(KhES-3)[P8]], which showed type-I phenotype[1]. ESdEC(KhES-3) provided similar results to ESdEC(KhES-5) regarding several kinases including p38α MAPK, GSK3, AMPK and AKT (Figure 2B, Panel A) and kinase that phosphorylated p53 and HSP60 (Figure 2B, Panel B). To further narrow the list of candidate kinases, we performed proteomic kinase assays using VECs generated from KhES-1 line of ESC at passage 2 [ESdEC(KhES-1)[P2]], which showed type-II phenotype[1], and those at passages 9 [ESdEC(KhES-1)[P9]], which showed type-I phenotype[1]. Regarding several cases including p38α MAPK, GSK3, AMPK and AKT, consistent results were obtained among the three lines of ESdECs (Figure 2C, Panel A). We still performed proteomic kinase assays using SeV-iPS-derived VECs (SeV-iPS(HUVEC)dEC), which showed type-II phenotype[1], and HUVEC, which showed type-I phenotype[1]. As shown in Figure 2D, only p38α MAPK provided consistent results: Higher p38α MAPK activities in type-II VECs and lower p38α MAPK activities in type-I VECs. To confirm this finding, we further performed proteomic kinase assays using Ret-iPS-derived VECs [Ret-iPS(253G1)dEC] with type-I phenotype[1] and SeV-iPS(HUVEC)dEC with type-II phenotype[1]. Again, p38α MAPK activities were higher in type-II VECs than type-I (Figure 2E). We finally performed proteomic kinase assays using additional commercially available primary cultured human VECs including HAEC, HCAEC and HMVEC, all of which showed type-I phenotype[1], and obtained compatible results (data not shown).
Thus, we obtained p38α MAPK as the strongest candidate for the kinase that is involved in the “type-I vs type-II” phenotyping of human VECs.
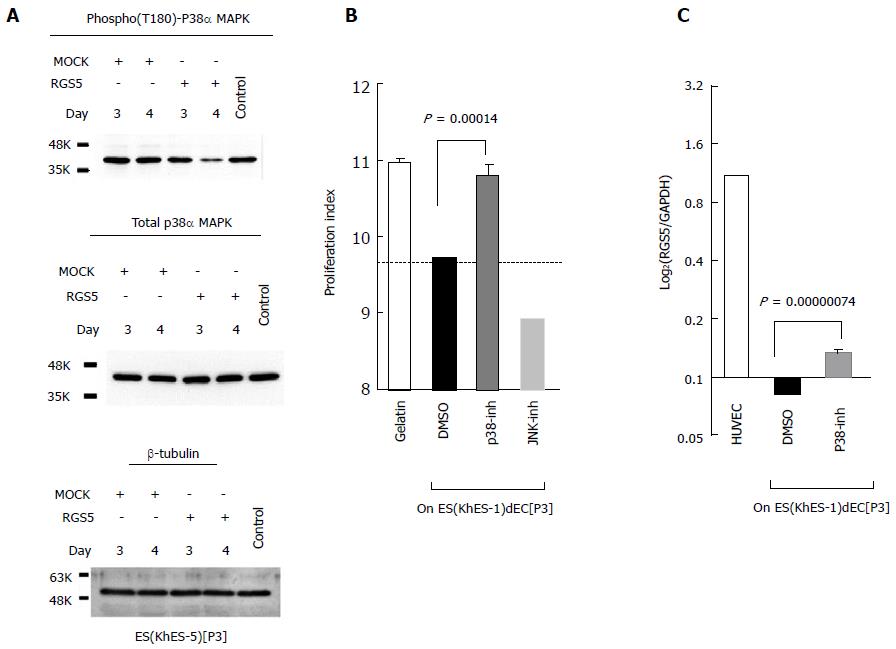
To verify the involvement of p38α MAPK in RGS5-mediated phenotype regulation of human VECs, we first examined whether an enforced expression of RGS5 would affect p38α MAPK activities. As shown in Figure 3A, transfection of the RGS5-expression vector into type-II ESdECs lowered Thr180 phosphorylation of p38α MAPK, and thus, depressing the activity of p38α MAPK. Next, we examined the effects of the p38α MAPK inhibitor on the phenotype regulation of VECs. Treatment with a p38α MAPK inhibitor (Figure 3B, gray column), but not with a JNK inhibitor (Figure 3B, hatched column) or an ERK inhibitor (data not shown), abrogated type-II anti-proliferative capacities of ESdECs (Figure 3B), confirming the involvement of p38α MAPK in RGS5-mediated “type-II vs type-I” phenotyping. Interestingly, we found that p38α MAPK inhibitor treatments up-regulated RGS5 expression (Figure 3C). Thus, there is a positive feedback loop between “RGS5 induction” and “p38α MAPK down-regulation”, running a vicious cycle for the unidirectional conversion from type-II VECs to type-I VECs.
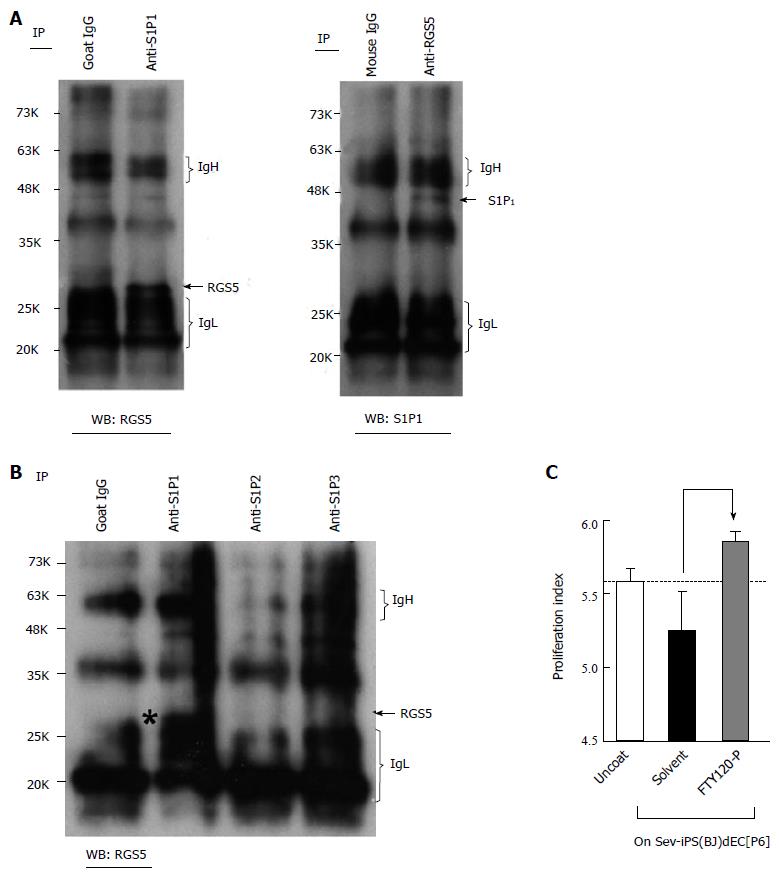
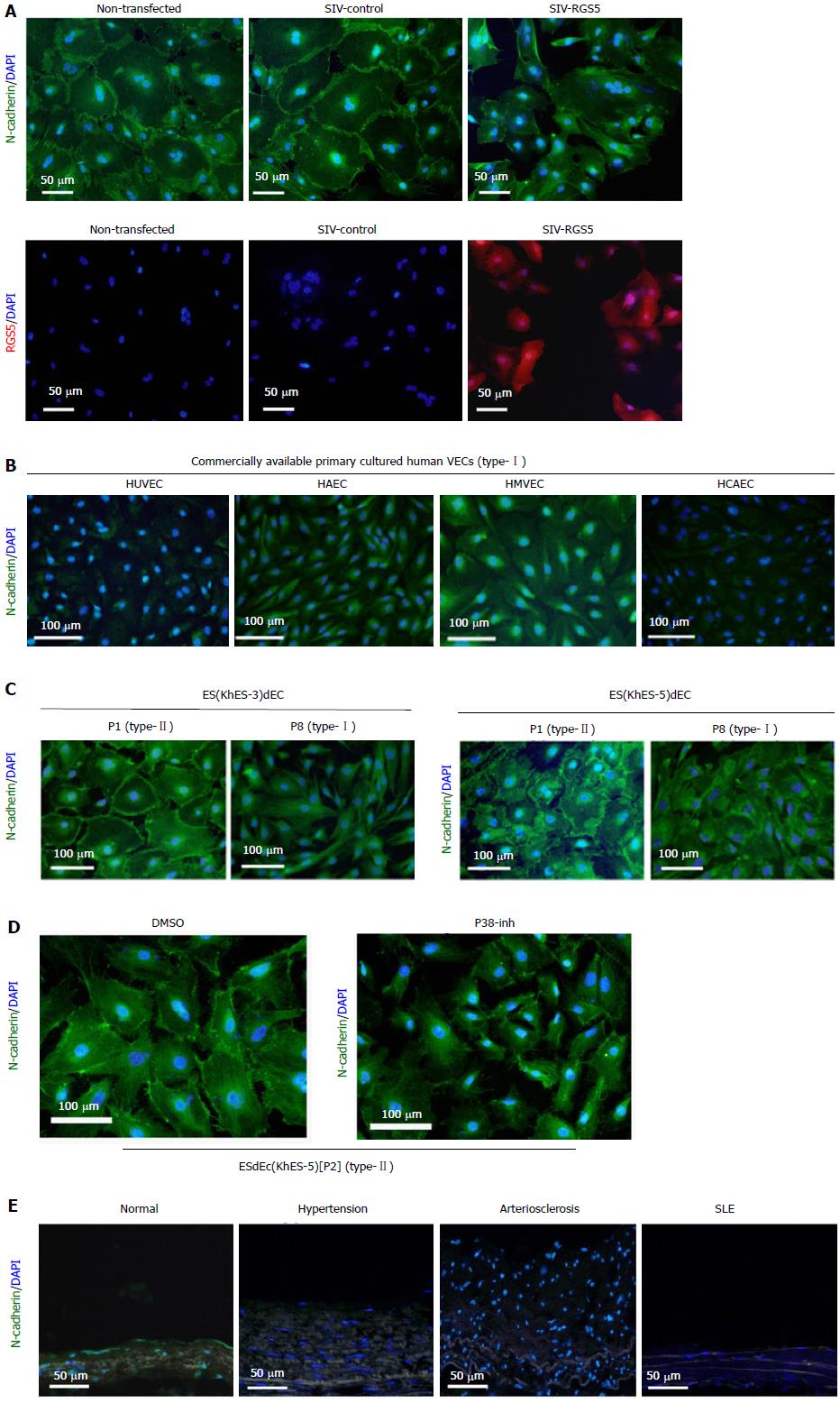
It is known that RGS family proteins function as inhibitory molecules against G protein-coupled receptor (GPCR)-dependent signaling. It is also known that sphingosine-1-phosphate (S1P), whose receptors belong to the GPCR family, activates various kinases including p38α MAPK, ERK and JNK and plays crucial roles in the regulation of VEC-VSMC interactions via N-cadherin[5]. Therefore, we examined the effects of RGS5 expression on the signaling from S1P to N-cadherin. First, we examined the possible interactions between RGS5 and S1P receptors. As shown Figure 4, RGS5 co-precipitated with S1P receptor 1 (S1P1) (Figure 4A), but not with S1P2 or S1P3 (Figure 4B), in type-I RGS5high VECs. In addition, treatments with FTY720-P, an inhibitor of S1P-dependent signaling, induced “type-II to type-I” conversions (Figure 4C). Thus, RGS5 induced “type-II to type-I” conversion, at least in part, by interfering the S1P/S1P1-dependent signaling. To further confirm the disturbance of S1P-dependent signaling by RGS5, we examined the effects of RGS5 on the subcellular localization of N-cadherin because S1P1 receptor activation in VECs is reportedly required for N-cadherin-dependent VEC-VSMC adhesion[5]. We found that the localization of N-cadherin at intercellular junctions was abrogated by an enforced expression of RGS5 in type-II VECs (Figure 5A). We also examined the intracellular localization of N-cadherin in various human VECs. In accordance with a previous report[6], N-cadherin localization at intercellular junctions was hardly detectable in type-I commercially available primary cultured human VECs (Figure 5B). In addition, repetitive subcultures abrogated the localization of N-cadherin at intercellular junctions in type-II ESdECs (Figure 5C). We also examined the effects of an inhibitor of p38α MAPK, which is the pivotal downstream kinase of RGS5 in the phenotype regulation of human VECs (Figures 2 and 3) and found that p38α MAPK inhibition abrogated the localization of N-cadherin at intercellular junctions (Figure 5D). Finally, we examined N-cadherin expressions in clinical specimens of the patients with hypertension, arteriosclerosis and systemic lupus erythematodes (SLE)-associated vasculitis. Our previously report showed that RGS5 expressions in VECs were up-regulated in pathological situations in a severity-dependent manner[1] in accordance with an earlier report on the study of scleroderma patients, which showed endothelial RGS5 overexpression in subcutaneous vessels[3,4]. Compatible with those previous findings, N-cadherin expressions were abrogated in pathological arteries (Figure 5E). Thus, RGS5 overexpression disturbs S1P/S1P1–dependent signaling events in human VECs.
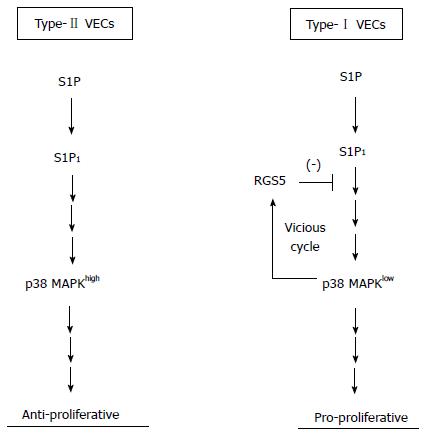
In the current study, we identified p38α MAPK as a pivotal kinase that is involved in phenotype regulations of human VECs at the downstream of RGS5. Therefore, human VECs are finally categorized as follows: Pro-proliferative RGS5high p38α MAPKlow VECs (type-I) and anti-proliferative RGS5low p38α MAPKhigh VECs (type-II) (Figure 6).
It is widely accepted that p38α MAPK plays important roles in the acquisition of stress resistance. It was shown that p38α MAPK mediated cell survival in response to oxidative stress by inducing antioxidant genes[7]. The involvement of p38α MAPK in the acquisition of oncogenic stress resistance was also reported[8,9]. These findings support the idea that the activity of p38α MAPK should be maintained at relatively high levels to prevent the “type-II to type-I” conversions by various kinds of stresses including oxidative stress and aging. It was also reported that macrophage deficiency of p38α MAPK promoted apoptosis and plaque necrosis in advanced atherosclerotic lesions[8]. Thus, p38α MAPK plays indispensable roles in the amelioration of ischemic vascular diseases in both VECs and macrophages.
We also showed that there exists a vicious cycle between “RGS5 inductions” and “p38α MAPK inhibitions”. Because this cycle induces an intensifying expression of RGS5 over time, “type-II to type-I” conversion, which is induced by oxidative stress or aging[1], is generally an irreversible process. Our previous in vitro studies demonstrated that RGS5 expression in type-I VECs cannot be nullified unless they are subjected to iPSC establishment and subsequent VEC differentiation[1]. On the other hand, the cancellation of RGS5 expression can be observed under in vivo conditions. It was reported that, although RGS5 expression was induced in the endothelial cells of subcutaneous vessels in scleroderma patients, it returned to normal after high dose immunosuppressive therapy followed by autologous hematopoietic cell transplant[10,11]. Thus, it seems that there are a sufficient amount of endothelial progenitor cells (EPCs) that can produce healthy RGS5low VECs (type-II) in the bone marrow of scleroderma patients and these EPCs contribute to the regeneration of healthy vessels after an intensive therapy. In this sense, not only autologous iPSC-derived VECs but also autologous hematopoietic stem/progenitor cells may provide a powerful tool for the transplantation therapy for the treatment of vascular diseases.
We also demonstrated that RGS5 disturbed S1P-dependent signaling events. It was reported that the S1P1 activation in VECs was required for N-cadherin-dependent adhesion with mural cells and that knockdown of N-cadherin expression resulted in destabilization of vascular structures[5]. Our immunostaining studies showed that the localization of N-cadherin at intercellular junctions was abrogated in pathological arteries (Figure 5E). Thus, loss of N-cadherin localization at intercellular junctions may possibly promote the arteriostenosis via the destabilization of VEC-VSMC adhesions. Our results (Figure 5B), in accordance with a previous report[6] showed that commercially available primary cultured human VECs lacked the localization of N-cadherin at intercellular junctions. Although the molecular basis for the lack of N-cadherin localization at intercellular junctions has not been elucidated so far, an inappropriately enhanced expression of RGS5 in type-I VECs may possibly be involved in the progression of this process, at least in part.
There may be diverse signals that induce RGS5 expressions. In addition to our previous finding that oxidative stress and subculture stress induced RGS5 expression[1], Jin et al[12] reported that RGS5 worked as a hypoxia-inducible apoptotic stimulator in HUVEC. Therefore, it seems highly reasonable to think that RGS5 is a common downstream effector of various stressors in human VECs. Although the direct downstream signaling event remains elusive, RGS5 may provide a good candidate for drug discovery in various vascular diseases.
Collectively, the preservation of p38α MAPK activity at higher levels in VECs provides a new strategy in the drug discovery for the treatment of ischemic diseases.
We thank Mr. Shinnosuke Suzuki and Mr. Yoshinori Yanagi for technical assistance.
The pathological basis of ischemia is hyper-proliferation of vascular smooth muscle cells (VSMCs). Although there had been a longstanding controversy over the effect of vascular endothelial cells (VECs) on the proliferation of VSMCs, it was recently clarified that human VECs are categorized into two groups by their effects on the proliferation of VSMCs: Pro-proliferative VECs (type-I) vs anti-proliferative VECs (type-II). Various stresses such as oxidative stress and aging induce “type-II to type-I” conversion of human VECs.
Regulator of G-protein signaling 5 (RGS5), which is reportedly induced in endothelial cells of pathological vessels, is identified as a causative gene for “type-II to type-I” conversion.
Signaling events that are working up-stream and down-stream of RGS5 in “type-II to type-I” conversion remain elusive. By applying proteomic kinase assays, we have clarified that RGS5-mediated p38α mitogen-activated protein kinase (p38α MAPK) suppression is the crucial downstream signaling event for "type-II to type-I" conversion.
p38α MAPK activity in type-II VECs even under stressful conditions may provide a useful indicator in drug discovery for ischemic diseases.
p38α MAPK is reportedly required for an acquisition of stress resistance in various cell types.
In this study, the authors have identified that p38α MAPK is a crucial downstream effector of RGS5 in type I-type II VECs conversion. This finding provides a new strategy in the drug discovery for the treatment of ischemic disease. In general, this is a quite interesting and nice study. Experiments were well designed with appropriate controls and executed. Conclusions are significant and justified based on the high quality data. This manuscript certainly deserves to be published.
P- Reviewer: Wang QE, Wang YQ S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Nishio M, Nakahara M, Sato C, Saeki K, Akutsu H, Umezawa A, Tobe K, Yasuda K, Yuo A, Saeki K. New categorization of human vascular endothelial cells by pro- vs anti-proliferative phenotypes. World J Transl Med. 2015;In press. |
| 2. | Suemori H, Yasuchika K, Hasegawa K, Fujioka T, Tsuneyoshi N, Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2072] [Cited by in RCA: 1974] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 5. | Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102:7-17. [PubMed] |
| 7. | Gutiérrez-Uzquiza Á, Arechederra M, Bragado P, Aguirre-Ghiso JA, Porras A. p38α mediates cell survival in response to oxidative stress via induction of antioxidant genes: effect on the p70S6K pathway. J Biol Chem. 2012;287:2632-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Kobayashi Y, Qi X, Chen G. MK2 Regulates Ras Oncogenesis through Stimulating ROS Production. Genes Cancer. 2012;3:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, Molitor JA, Henstorf G, Lafyatis R, Pritchard DK. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Fleming JN, Nash RA, Mahoney WM, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep. 2009;11:103-110. [PubMed] |
| 12. | Jin Y, An X, Ye Z, Cully B, Wu J, Li J. RGS5, a hypoxia-inducible apoptotic stimulator in endothelial cells. J Biol Chem. 2009;284:23436-23443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |