Published online Apr 12, 2015. doi: 10.5528/wjtm.v4.i1.1
Peer-review started: July 13, 2014
First decision: September 18, 2014
Revised: January 3, 2015
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: April 12, 2015
Processing time: 275 Days and 14.1 Hours
Translational research is a broad field of medicine with several key phases moving from scientific discovery to bench research and the hospital bedside, followed by evidence-based practice and population-level policy and programming. Understanding these phases is crucial when it comes to preventing and treating illness, especially in global health. Communities around the world struggle with a variety of health problems that are at some times similar and at others quite different. Three major world health issues help to outline the phases of translational research: vaccines, human immunodeficiency virus and acquired immunodeficiency syndrome, and non-communicable diseases. Laboratory research has excelled in many of these areas and is struggling in a few. Where successful therapies have been discovered there are often problems with appropriate use or dissemination to groups in need. Also, many diseases would be better prevented from a population health approach. This review highlights successes and struggles in the arena of global health, from smallpox eradication to the impending epidemic of cardiovascular disease, in an attempt to illustrate of the various phases of translational research.
Core tip: This review summarizes efforts in translational research as applied to the major global health issues of vaccines, human immunodeficiency virus and acquired immunodeficiency syndrome and non-communicable diseases. Historical perspective as well as current efforts are presented in an effort to describe the success and challenges that are concurrent with translational medicine on the international stage.
- Citation: Hoehn RS, Abbott DE. Beyond the bedside: A review of translational medicine in global health. World J Transl Med 2015; 4(1): 1-10
- URL: https://www.wjgnet.com/2220-6132/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i1.1
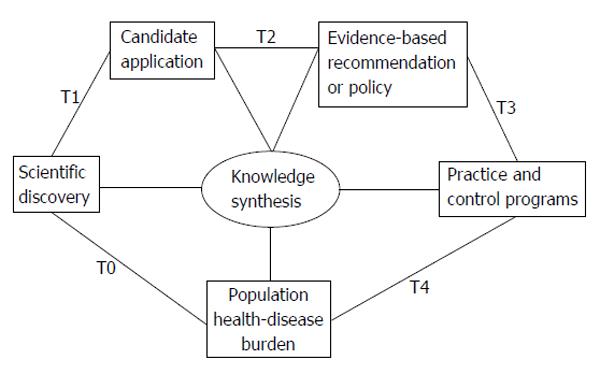
To address the breadth of translational research, the United States National Institutes of Health has recently endorsed a 5-phase model that describes the process of moving from scientific discoveries to population health (Figure 1)[1]. The process starts with scientific discovery of a problem or pathology, termed T0. From there, T1 and T2 encompass the classic “bench to bedside” process of finding a candidate treatment, test, or clinical intervention (T1) and then comparing safety and efficacy of the candidate against a placebo or existing therapy in randomized trials or other study designs (T2). Lastly, T3 research focuses on implementation and dissemination of evidence-based interventions and T4 examines population-level health impact and cost-effectiveness[2].
The purpose of this review is to describe the impact of the phases of translational research on global health. Communities around the world suffer from health issues that are at times very different but can also be quite similar. Some therapies are readily available in resource-rich countries but scarce in less affluent countries. Other therapies simply do not work in certain parts of the world due to disease specificity or cultural issues. Though there are many blights, three major issues affecting the health of the global population are vaccine development, the human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDS), and non-communicable diseases (NCDs). Here we will review the history and current status of research in these areas, highlighting the role of various phases of translational research with respect to their effects on global health.
The history of vaccine development and distribution exemplifies translational research. Edward Jenner discovered the smallpox vaccine in the 1790s by treating patients with “matter” from the sores of cowpox[3]. This technique of sharing the live cowpox virus between patients lasted through the 19th century until the development of a live attenuated vaccine in the early 20th century[4]. In the coming decades the World Health Organization (WHO) would sponsor a smallpox vaccination program, and eradication of the virus was formally declared in 1980 (the only other disease to be declared eradicated is Rinderpest, an RNA virus that affected cattle and water buffalo, mostly in Africa; vaccines for this virus were developed in the early 20th century and two major attempts at mass vaccination led to eradication in 2011).
A second vaccination “victory” resulted from the work of one of the fathers of bacteriology, Robert Koch, who demonstrated that the bacterium Bacillus anthracis was the cause of “woolsorters’ disease” in 1876[5]. Anthrax had plagued livestock for millennia, and humans involved in wool and hide processing were at risk of infection. Following this discovery, Louis Pasteur described a randomized controlled trial in which he treated livestock with an attenuated anthrax vaccine prior to inoculating them with a virulent strain of the bacteria[6]. The results were dramatic; 48 h after inoculation, all vaccinated sheep survived and all un-vaccinated sheep were dead. Virtual eradication was made possible by livestock quarantining and vaccination, but recent terror attacks using the anthrax spore have generated interest in newer vaccines[7-9]. While the vaccine is only available for at-risk patients (veterinarians, researchers, certain military personnel, etc.) due to difficulties with production and storage, research is underway to develop a stable, needleless vaccine for widespread use[10].
Worldwide, diarrheal illness is the second leading cause of death in children under the age of five years (760000 deaths each year)[11]. There are many causes of diarrhea and a significant portion of the disease burden can be prevented through public health efforts to create safe drinking water and adequate sanitation. Infectious causes are well described, and three major sources have been the focus of vaccination efforts in recent decades.
Rotavirus is a leading cause of child mortality worldwide, especially in low-income regions; in children under the age of five in 2008, 5% of all mortality and over one-third of diarrhea-related mortality were attributable to rotavirus infection. Early experiments led to development of monovalent live-oral vaccines with variable success[12]. In 1998 the rhesus rotavirus tetravalent vaccine (RRV-TV) was licensed for administration in children after successful trials. However, after several cases of bowel obstruction and intussusception following vaccine administration, the Centers for Disease Control and Prevention advised against using the vaccine and in 1999 the manufacturer withdrew the vaccine from market[13]. Since that time, three live-attenuated oral vaccines have been approved for use. The monovalent (RV1) and pentavalent (RV5) rotavirus vaccines have been evaluated in several large trials and subsequently approved for use in most countries including the United States and the European Union[14]. A third, the Lanzhou lamb rotavirus vaccine, has been approved for use in China only[15].
Another major cause of diarrheal illness is typhoid fever, caused by Salmonella enterica typhi (S. typhi). Two vaccines, injectable (Vi PS) and oral (Ty21a), have shown efficacy and safety in clinical trials and field settings in Chile, Indonesia, and India, but are not ready for widespread immunization protocols[16]. The Vi PS vaccine is non-immunogenic in children under 2 years, and many S. typhi strains are negative for the Vi polysaccharide. The Ty21a vaccine is not recommended for children under 5 years, and its acid-labile nature creates challenges with oral administration. Several newer typhoid vaccines are currently in phase 1-3 trials worldwide, but not licensed for use[17].
Cholera, caused by Vibrio cholerae, is another area of focus for vaccine manufacturers. Dukoral®, an oral killed whole-cell vaccine, was licensed after a large randomized controlled trial in Bangledash in 1990[18]. The per-dose cost of United States $5.25 was felt to be prohibitive for use in low-income regions, and subsequent development of Shanchol® at United States $1.85 per dose was deemed fiscally feasible; Shanchol® use was then adopted after randomized controlled trials in Vietnam and India confirmed safety and immunogenicity[19,20]. Four single dose, live attenuated oral cholera vaccines are in active clinical programs with hopes to improve efficacy, hasten onset and increase duration of protection[21].
Despite some vaccination success as a result of collaborative translational research implementation, malaria, tuberculosis (TB), and HIV/AIDS are three of the top ten causes of death worldwide[22]. As such, much attention and funding has been directed towards finding vaccines for these diseases. Progress has been made, but there are still significant challenges.
There are approximately 250 million reported cases of malaria every year, including almost one million deaths in Sub-Saharan Africa, mostly in children[23]. Many different vaccines are currently in various trials and they all face a similar challenge; Plasmodium falciparum, the causative agent of malaria, has a complex life cycle, with polymorphic antigens expressed in separate phases of the cycle[24]. The best current vaccine candidate, RTS,S/AS01, is a combination of a portion of the circumsporozoite protein that helps the parasite invade human liver cells and the hepatitis B surface antigen, as well as the liposomal formulation adjuvant AS01. A phase IIb trial of the RTS,S malaria vaccine showed safety and efficacy at 20 mo[25] and phase III trials have demonstrated 31%-56% efficacy for one year, with protection from clinical malaria for at least 3.5 years[26,27]. However, efficacy of the vaccine wanes with time and also varies based on the age of the vaccinated child[28]. Despite mediocre results, RTS,S will likely be the first malaria vaccine to receive regulatory approval[29].
The bacille Calmette-Guérin (BCG) vaccine for Mycobacterium TB is one of the earliest developed vaccines and has been given to over four billion people to date[30,31]. Despite this fact, TB kills 1.4 million people annually and drug-resistant TB is becoming a major problem[32]. BCG protects infants from tuberculous meningitis and miliary TB, but is less effective against pulmonary TB in adolescents and adults. There are currently almost twenty candidate vaccines in various phases of clinical trials, all designed to prevent active TB disease[32,33]. Some of these are live recombinant vaccines that have been genetically engineered for enhanced efficacy and/or safety, meant to replace BCG. Others are proteins or viral vector expressing antigens that are meant to serve as an immune booster following initial treatment with the BCG vaccine[24]. A common challenge among trials evaluating TB vaccines is that the disease has a long latent period; thus, trialing preventative vaccines is slow and expensive[31].
HIV kills two million people annually and infects approximately 7000 people per day, making it one of top causes of death worldwide[34]. Naturally, a significant portion of the world’s research dollars are directed toward treating and preventing this disease. Challenges facing researchers looking for a vaccine against HIV include: global variability of HIV, lack of a validated animal model with appropriate immune response, large variety of infected cells that develop as a result of HIV genome integration into the host’s DNA, and destruction of immune cells by HIV[24]. Phase III trials of most vaccines have failed to show efficacy[35,36] or reduce viral loads[37,38], and some have actually shown increased HIV infection among vaccine recipients[39]. The most exciting results are from a randomized controlled trial comparing placebo to a recombinant canarypox vector vaccine (ALVAC-HIV) and two boosters of a recombinant glycoprotein 120 subunit vaccine (AIDSVAX B/E). In a population of greater than 16000 healthy Thai volunteers, this AIDSVAX B/E showed 31% vaccine efficacy vs placebo by reducing the cumulative probability of infection, but did not reduce viral loads[40]. Nevertheless, finding a safe and effective vaccine against HIV is proving to be one of the most daunting tasks in research today[41].
Even when early-phase translational research has found a safe and effective vaccine for a given disease there are still challenges to widespread availability, and the major barrier to vaccine development for low-income regions is cost[42]. Patients in poor countries cannot afford new, expensive vaccines and pharmaceutical companies are not incentivized to invest capital in developing treatments that will be too expensive for the prospective customers to afford[43]. Not only that, but the opportunity costs of delaying more profitable projects are not appealing to industry. To solve this problem, major institutions in global health came together in 1999 to create the Global Alliance for Vaccines and Immunization (GAVI)[44]. One aim of the alliance is to support new vaccine research, as outlined by their approach to meningitis.
The North African “meningitis belt” is an area that includes countries from Senegal to Ethiopia with a population of around 350 million people[45]. Annual outbreaks in these countries claim hundreds to thousands of lives and are caused mostly by Neisseria meningitidis (N. meningitidis) serogroup A[46]. Controlling these outbreaks requires identifying the culprit strain of N. meningitidis and producing a specific polysaccharide vaccine to treat the population at risk. These polysaccharide vaccines are poorly immunogenic in young children, do not prime immunologic memory, and do not lead to “herd immunity”[47]. To combat this problem, in 2001 the Bill and Melinda Gates Foundation gave United States $70 million to the WHO to establish the Meningitis Vaccine Project[48]. The goal was to eliminate epidemic meningitis in Africa through the development of a serogroup A meningococcal conjugate vaccine that would cost less than United States $0.50 per dose. Vaccine development began in 2003, clinical phase 1-3 trials were completed, and in 2010 MenAfriVac™ was licensed for use in populations aged between one and 29 years; large-scale immunization campaigns began immediately. In 2011, no case of meningococcal A disease occurred in a vaccine recipient in Burkina Faso and the percentage of meningococcal infections in Niger due to serogroup A dropped from 98.6% to less than 2%[49].
The GAVI alliance has also addressed hepatitis B virus (HBV) in China. HBV is a significant problem in low-income countries, and China accounts for up to half of the HBV-related deaths worldwide[50]. Over 260000 people die annually in China from HBV-related liver cancer and cirrhosis. A full 60% have a history of infection, and around 10% are chronic carriers[51]. However, because of high costs, vaccination rates were substantially higher in major cities and wealthy provinces in Eastern China. In 2002, China added HBV vaccination to its National Immunization Programme, and at the same time the China Ministry of Health teamed with GAVI to start the China-GAVI project with a goal of providing free HBV vaccination to people in the poor and western provinces of China. From 1997-2003, overall vaccination coverage increased from 70.7% to 89.8% and timely coverage increased from 29.1% to 75.8%. In the 22 provinces targeted by the China-GAVI Project, timely coverage increased from 64% in 2004 to 81% in 2006, and complete coverage increased from 52% in 2001 to 92% in 2006[52]. National HBV vaccination programs have had similar effects in other countries[53] and have been shown to greatly reduce the incidence of hepatocellular carcinoma in these populations[54].
These examples of meningitis and HBV illustrate the benefit of targeted funding towards developing specific therapies or distributing existing therapies to a population in need. Fifty years after the Sabin and Salk vaccines were developed, polio has been eradicated in much of the world and vaccine campaigns are now addressing the few remaining countries with recently documented cases[55,56]. The Bill and Melinda Gates Foundation has given United States $1.5 billion to the Children’s Vaccine Program for research initiatives in malaria, TB, diarrheal diseases, measles, hookworm, and meningitis[56]. GAVI is targeting the 74 poorest countries in the world with a three-fold approach: improving vaccination infrastructure, purchasing necessary vaccines, and supporting research and development[56].
For many diseases, notably HIV, TB, and malaria, challenges in vaccine development still need to be overcome. For many others, effective vaccines exist and simply need to be distributed effectively. Through this combination of research and distribution it is possible to use discoveries in the lab to prevent and even eliminate the burden caused by these historically tragic diseases.
In 1981 scientists discovered HIV as the causative agent of AIDS, typified by uncommon opportunistic infections in otherwise healthy young men[57]. Since then, highly active antiretroviral therapy (HAART) has been proven to significantly reduce morbidity and mortality by suppressing HIV replication and improving CD4+ T cell counts. Population studies in developed as well as developing countries have shown a significant effect of HAART treatment on reductions in both viral load as well as HIV transmission and new diagnoses[58-63]. The WHO has made evidence-based recommendations for HIV treatment and prevention[64] and the international community has contributed substantially through organizations such as Global Fund to Fight AIDS, TB and Malaria and PEPFAR, the President’s Emergency Plan for AIDS Relief[65]. Despite this progress, over two million people per year become newly infected with HIV worldwide[66].
A major barrier to defeating HIV is the highly mutagenic and drug-resistant nature of the virus[64]. The availability of fixed-dose combination pills and simplified treatment schedules can decrease resistance development by increasing adherence to HAART regimens, but resistance is still developing and can be difficult to monitor[67]. Genotypic testing and viral load monitoring are often not available in resource-limited settings due to cost-constraints and lack of adequate technology. As such, in resource-limited settings the WHO recommends monitoring early warning signs associated with developing drug-resistance: adherence to first-line regimens, changing regimens, inconsistent filling of prescriptions, and missing appointments[67]. Diagnosis of drug resistance in resource-limited settings is a clinical observation and research is now investigating empiric second- and third-line HAART options for patients with suspected resistance[64].
Ultimately, prevention will be the only way to definitively eradicate HIV. Efforts in vaccine development were discussed previously, but another strategy being investigated is prompt treatment of exposed or at-risk individuals with HAART to prevent viral transmission[66]. Treatment of mothers and children in the peri-natal period has been demonstrated to safely and effectively reduce HIV transmission at birth and during breastfeeding[68-70]. HIV post-exposure prophylaxis (PEP) taken within 72 h of an occupational exposure has been shown to prevent transmission in the great majority of cases[71]. Animal trials and observational studies in humans also demonstrate a benefit for non-occupational exposures such as sexual encounters and intravenous drug use[72]. There is promise of using antiretroviral therapy as pre-exposure prophylaxis for certain high-risk groups, but phase I-III trials have shown variable protection from HIV transmission, likely due to poor drug adherence[73]. Potential problems with widespread availability of HIV PEP include increased drug resistance, risky behavior, and decreased cost-effectiveness[66]. To address these issues, dozens of trials in a variety of countries are either planned or ongoing[74].
In the year 1900, the three leading causes of death in the United States were pneumonia, TB, and diarrhea/enteritis. These diseases caused one third of all deaths, of which 40% were among children under five years of age. Over the next century scientists would discover microorganisms and their role in infectious disease as well as determine ways to treat them. Subsequently the burden of disease has shifted; pneumonia, influenza, and HIV were responsible for 4.5% of deaths in the United States in 1997. Conversely, 54.7% of deaths in that year were a result of heart disease and cancer[75].
This shift in disease burden is not unique to the United States or even wealthy, industrialized countries. The incidence of many NCDs such as cardiovascular disease (CVD), cancer, and diabetes is growing so fast in developing countries that many have called it an epidemic[76,77]. From 1909 to 1999, global mortality caused by cancer and CVD increased from 15% to 53%[78]. In China, for example, the percentage of mortality attributable to CVD tripled from 1957-1990[79]. The causes are many and include a worldwide surge in life expectancy, lifestyle changes, urbanization, altered diets, increased tobacco use, poor fetal and childhood nutrition, and diminished physical activity[77].
Epidemiological studies in developing countries have highlighted the substantial presence of risk factors for CVD, many of which are modifiable[80-83]. These risk factors are less prevalent in developing countries than in developed nations and the incidence of NCDs is lower as well. Nonetheless, incidence is increasing and developing nations are also at risk of a NCD epidemic[84]. This provides a unique opportunity to halt disease progression in these regions. Research from developed countries highlights the benefits of preventative medicine in population-based interventions[85], and national public health programs have successfully improved population health in developed as well as developing countries by disseminating information regarding risk factors[86,87]. Social education is especially necessary to confront cultural misconceptions in areas where health professionals are distrusted and obesity is seen as a sign of affluence[88].
When prevention fails, management of affected or high-risk individuals will always be necessary. While there are many known treatments for diabetes, hypertension, hypercholesterolemia, and other chronic diseases, the incidence of these diseases continues to increase both in the United States and worldwide[89-91].
Another challenge to curbing this epidemic is delivery of appropriate therapy. For example, the results of the β-Blocker Heart Attack Trial were published in the United States in 1981, and 15 years later only 62.5% of patients who had had a myocardial infarction were appropriately being prescribed beta-blockers[92]. Despite wide availability of an inexpensive, safe, efficacious intervention, less than two-thirds of patients receive appropriate treatment.
Studies in United States have shown health benefits using one-on-one lifestyle teaching[93] and even text and email reminders[94] to encourage patients to exercise, modify diet, and take medications as instructed. However, these tactics may not apply to resource-limited countries with insufficient supplies of doctors and medicines[95]. As is the case with vaccines, governments in these countries can improve health by investing in cost-effective initiatives to develop and provide medications for their citizens at an affordable price[96-98]. Developing regions have had success improving the management of NCDs by focusing on primary care systems improvements and non-physician-led community initiatives[95,99-102].
Prevention and management of NCDs is a complicated problem, and the challenges faced by developing and developed countries are both similar and different. There is a unique opportunity in the developing world to prevent an epidemic that is currently evolving[84]. Risk factors are increasing, but the prevalence of NCDs in developing countries is still quite low, and research has shown that prevention, risk factor modification, and policy change can prevent the NCD epidemic from equaling others the world is currently battling.
The considerations regarding ethical translational research in global health are diverse. Clearly, the exploitation of economically disadvantaged individuals is egregious, but there are many nuances to consider. When a resource-rich country funds research in a resource-poor country, how do you define the standard of care? Is it wrong to inject live malaria parasites into HIV-positive patients to study the effect CD4+ T cell counts, even in an area where malaria is endemic[103]? Is it fair to randomly assign some malnourished men to receive vitamin-fortified bread and others standard bread when they normally would not have the fortified option anyway[104]? When conducting HIV vaccine trials in high-risk populations, is it necessary to provide condoms or safe-sex counseling[105]? Is it ethical to test a new therapy against subjects who go untreated because they cannot afford medicine which is standard of care[106]? Early-phase translational research has the potential to harm subjects, and that risk increases when crossing international boundaries[107].
Another ethical conundrum is the notion of disproportionate profiting from discoveries made in resource-poor countries host[107]. Not only does it seem wrong to expose patients to a potentially life-altering treatment they could never afford, but such discoveries can further exacerbate international disparities in health, as well as create inequalities in care within the host country[107]. However, international research partnerships can also improve the quality of care in host nations. For over 30 years Cornell has collaborated with the Haitian Ministry of Health on research that started with AIDS and TB but has since expanded to include maternal-child health, family planning, cancer prevention and treatment, immunization, and education. In that time they have successfully reduced rates of HIV and other sexually transmitted infections, increased the number of patients on HAART, and trained thousands of medical personnel, all the while enjoying uninterrupted NIH support since 1983 and generated more than 150 peer-reviewed publications[108]. By funding symbiotic partnerships it is possible not only to generate research data but also to improve population health and reduce the “implementation gap” that plagues global health[109].
The history and current struggles of research in vaccine development, HIV, and NCDs emphasize the importance of the five phases of translational research. Many vaccines have been successfully discovered and their effectiveness proven, some are works in progress, and all must have the potential to be efficiently delivered to populations in need. Treatment for HIV has evolved rapidly and become increasingly effective, but HIV is far from eradicated. Therapies for NCDs are well studied in the developed world, but there is much work to be done in prevention and population health in both developing and developed countries. Moving from clinical observation to bench research to bedside intervention is a hallmark of academic medicine in resource-rich countries. However, stopping at the bedside will not improve population health. Region-specific epidemiologic research can highlight needs, opportunities, and challenges that vary due to economic and cultural differences between communities, and goal-directed funding and research can find solutions to these problems. Research in developing countries can inform policy in developed countries, and vice-versa. As the field of global health grows, research and resources will be shared more efficiently and the successes of smallpox and polio will be translated to HIV and cardiovascular disease.
P- Reviewer: Zhong LP S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 558] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 2. | Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol. 2010;172:517-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Smith KA. Edward jenner and the small pox vaccine. Front Immunol. 2011;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Smith KA. Smallpox: can we still learn from the journey to eradication? Indian J Med Res. 2013;137:895-899. [PubMed] |
| 5. | Chitlaru T, Altboum Z, Reuveny S, Shafferman A. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol Rev. 2011;239:221-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Pasteur L. Summary report of the experiments conducted at Pouilly-le-Fort, near Melun, on the anthrax vaccination, 1881. Yale J Biol Med. 2002;75:59-62. [PubMed] |
| 8. | Keim P, Smith KL, Keys C, Takahashi H, Kurata T, Kaufmann A. Molecular investigation of the Aum Shinrikyo anthrax release in Kameido, Japan. J Clin Microbiol. 2001;39:4566-4567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Blendon RJ, Benson JM, DesRoches CM, Pollard WE, Parvanta C, Herrmann MJ. The impact of anthrax attacks on the American public. MedGenMed. 2002;4:1. [PubMed] |
| 10. | Beierlein JM, Anderson AC. New developments in vaccines, inhibitors of anthrax toxins, and antibiotic therapeutics for Bacillus anthracis. Curr Med Chem. 2011;18:5083-5094. [PubMed] |
| 11. | WHO. Diarrhoeal disease fact sheet. [Cited 2014 May 31]. Available from: http: //www.who.int/mediacentre/factsheets/fs330/en/. |
| 12. | Vesikari T. Rotavirus vaccines against diarrhoeal disease. Lancet. 1997;350:1538-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Centers for Disease Control and Prevention (CDC). Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48:1007. [PubMed] |
| 14. | Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:CD008521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Fu C, Tate JE, Jiang B. Effectiveness of Lanzhou lamb rotavirus vaccine against hospitalized gastroenteritis: further analysis and update. Hum Vaccin. 2010;6:953. [PubMed] |
| 16. | Slayton RB, Date KA, Mintz ED. Vaccination for typhoid fever in sub-Saharan Africa. Hum Vaccin Immunother. 2013;9:903-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res. 2012;135:161-169. [PubMed] |
| 18. | Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270-273. [PubMed] |
| 19. | Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, von Seidlein L, Carbis R, Han SH, Shin SH. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS One. 2008;3:e2323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Anh DD, Canh do G, Lopez AL, Thiem VD, Long PT, Son NH, Deen J, von Seidlein L, Carbis R, Han SH. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine. 2007;25:1149-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Desai SN, Cravioto A, Sur D, Kanungo S. Maximizing protection from use of oral cholera vaccines in developing country settings: an immunological review of oral cholera vaccines. Hum Vaccin Immunother. 2014;10:1457-1465. [PubMed] |
| 22. | WHO. Top 10 causes of death for 2012. [Cited 2014 June 3]. Available from: http: //www.who.int/mediacentre/factsheets/fs310/en/index1.html. |
| 23. | WHO. World Malaria Report 2011. [Cited 2014 June 3]. Available from: http: //www.who.int/malaria/world_malaria_report_2011/en/. |
| 24. | Delany I, Rappuoli R, De Gregorio E. Vaccines for the 21st century. EMBO Mol Med. 2014;6:708-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 25. | Abdulla S, Salim N, Machera F, Kamata R, Juma O, Shomari M, Kubhoja S, Mohammed A, Mwangoka G, Aebi T. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS,S/AS02(D) malaria vaccine in infants living in a malaria-endemic region. Malar J. 2013;12:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Campo JJ, Sacarlal J, Aponte JJ, Aide P, Nhabomba AJ, Dobaño C, Alonso PL. Duration of vaccine efficacy against malaria: 5th year of follow-up in children vaccinated with RTS,S/AS02 in Mozambique. Vaccine. 2014;32:2209-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | RTS , S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmüller B. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 28. | Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 643] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 29. | Bouchie A. GSK plows ahead with EMA malaria vaccine submission. Nat Biotechnol. 2013;31:1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Branch A. The most recent experiments with “bacille calmette guerin”. Can Med Assoc J. 1927;17:720-721. [PubMed] |
| 31. | Nossal GJ. Vaccines of the future. Vaccine. 2011;29 Suppl 4:D111-D115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M. Progress in tuberculosis vaccine development and host-directed therapies--a state of the art review. Lancet Respir Med. 2014;2:301-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Kaufmann SH. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol. 2012;33:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Koff WC, Russell ND, Walport M, Feinberg MB, Shiver JW, Karim SA, Walker BD, McGlynn MG, Nweneka CV, Nabel GJ. Accelerating the development of a safe and effective HIV vaccine: HIV vaccine case study for the Decade of Vaccines. Vaccine. 2013;31 Suppl 2:B204-B208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 632] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 36. | Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 707] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 37. | Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1393] [Cited by in RCA: 1356] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 38. | Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5:357-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Gray GE, Moodie Z, Metch B, Gilbert PB, Bekker LG, Churchyard G, Nchabeleng M, Mlisana K, Laher F, Roux S. Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study. Lancet Infect Dis. 2014;14:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 40. | Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2388] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 41. | Alchin DR. HIV vaccine development: an exploratory review of the trials and tribulations. Immunol Res. 2014;60:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Wenger J. Vaccines for the developing world: current status and future directions. Vaccine. 2001;19:1588-1591. [PubMed] |
| 43. | Mueller-Langer F. Neglected infectious diseases: are push and pull incentive mechanisms suitable for promoting drug development research? Health Econ Policy Law. 2013;8:185-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Wittet S. Introducing GAVI and the Global Fund for Children’s Vaccines. Vaccine. 2000;19:385-386. [PubMed] |
| 45. | Lapeyssonnie L. [Cerebrospinal meningitis in africa]. Bull World Health Organ. 1963;28 Suppl:1-114. [PubMed] |
| 46. | Marc LaForce F, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine. 2009;27 Suppl 2:B13-B19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Greenwood BM, Hassan-King M, Whittle HC. Prevention of secondary cases of meningococcal disease in household contacts by vaccination. Br Med J. 1978;1:1317-1319. [PubMed] |
| 48. | Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac(TM). Hum Vaccin Immunother. 2012;8:715-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 49. | Collard JM, Issaka B, Zaneidou M, Hugonnet S, Nicolas P, Taha MK, Greenwood B, Jusot JF. Epidemiological changes in meningococcal meningitis in Niger from 2008 to 2011 and the impact of vaccination. BMC Infect Dis. 2013;13:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 477] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 51. | Xia G, Liu C, Cao H. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int Hepatol Commun. 1996;10:62-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | Centers for Disease Control and Prevention (CDC). Progress in hepatitis B prevention through universal infant vaccination--China, 1997-2006. MMWR Morb Mortal Wkly Rep. 2007;56:441-445. [PubMed] |
| 53. | Chongsrisawat V, Yoocharoen P, Theamboonlers A, Tharmaphornpilas P, Warinsathien P, Sinlaparatsamee S, Paupunwatana S, Chaiear K, Khwanjaipanich S, Poovorawan Y. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop Med Int Health. 2006;11:1496-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 55. | Minor P. The polio endgame. Hum Vaccin Immunother. 2014;10:2106-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Nossal GJ. Gates, GAVI, the glorious global funds and more: all you ever wanted to know. Immunol Cell Biol. 2003;81:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Broder S. Twenty-five years of translational medicine in antiretroviral therapy: promises to keep. Sci Transl Med. 2010;2:39ps33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, Shannon K, Harrigan PR, Hogg RS, Daly P. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 612] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 59. | Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, Colfax GN. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 567] [Cited by in RCA: 592] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 60. | Fang CT, Hsu HM, Twu SJ, Chen MY, Chang YY, Hwang JS, Wang JD, Chuang CY. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 611] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 62. | Kumarasamy N, Solomon S, Chaguturu SK, Cecelia AJ, Vallabhaneni S, Flanigan TP, Mayer KH. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, Chen RY. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241-251, W-252. [PubMed] |
| 64. | Kumarasamy N, Krishnan S. Beyond first-line HIV treatment regimens: the current state of antiretroviral regimens, viral load monitoring, and resistance testing in resource-limited settings. Curr Opin HIV AIDS. 2013;8:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Piot P, Kazatchkine M, Dybul M, Lob-Levyt J. AIDS: lessons learnt and myths dispelled. Lancet. 2009;374:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Cohen MS, Smith MK, Muessig KE, Hallett TB, Powers KA, Kashuba AD. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013;382:1515-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 67. | Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13 Suppl 2:1-13. [PubMed] |
| 68. | de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 69. | Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 2255] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 70. | Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282-2294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 71. | Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW, Gomaa A, Panlilio AL. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 72. | Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, Struble KA, Cheever L, Johnson M, Paxton LA, Onorato IM. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54:1-20. [PubMed] |
| 73. | Hankins CA, Dybul MR. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: a review. Curr Opin HIV AIDS. 2013;8:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Granich R, Gupta S, Suthar AB, Smyth C, Hoos D, Vitoria M, Simao M, Hankins C, Schwartlander B, Ridzon R. Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res. 2011;9:446-469. [PubMed] |
| 75. | CDC. Achievements in public health 1900-1999: control of infectious diseases. 1999; [Updated 1999; cited 2014 June 6]. Available from: URL: http: //www.cdc.gov/mmwr/preview/mmwrhtml/mm4829a1.htm. |
| 76. | Bonow RO, Smaha LA, Smith SC, Mensah GA, Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation. 2002;106:1602-1605. [PubMed] |
| 77. | Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231-237. [PubMed] |
| 78. | WHO. The World Health Report 1999- making a difference. [Cited 2014 June 9]. Available from: http: //www.who.int/whr/1999/en/. |
| 79. | Yao C, Wu Z, Wu Y. The changing pattern of cardiovascular diseases in China. World Health Stat Q. 1993;46:113-118. [PubMed] |
| 80. | Rahman Al-Nuaim A. High prevalence of metabolic risk factors for cardiovascular diseases among Saudi population, aged 30-64 years. Int J Cardiol. 1997;62:227-235. [PubMed] |
| 81. | Azizi F, Rahmani M, Emami H, Mirmiran P, Hajipour R, Madjid M, Ghanbili J, Ghanbarian A, Mehrabi Y, Saadat N. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1). Soz Praventivmed. 2002;47:408-426. [PubMed] |
| 82. | Yang Z, Xing X, Xiao J, Lu J, Weng J, Jia W, Ji L, Shan Z, Liu J, Tian H. Prevalence of cardiovascular disease and risk factors in the Chinese population with impaired glucose regulation: the 2007-2008 China national diabetes and metabolic disorders study. Exp Clin Endocrinol Diabetes. 2013;121:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Gupta R, Sharma KK, Gupta A, Agrawal A, Mohan I, Gupta VP, Khedar RS, Guptha S. Persistent high prevalence of cardiovascular risk factors in the urban middle class in India: Jaipur Heart Watch-5. J Assoc Physicians India. 2012;60:11-16. [PubMed] |
| 84. | Mensah GA. A heart-healthy and “stroke-free” world through policy development, systems change, and environmental supports: a 2020 vision for sub-Saharan Africa. Ethn Dis. 2003;13:S4-12. [PubMed] |
| 85. | Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427-432; discussion 433-434. [PubMed] |
| 86. | Dowse GK, Gareeboo H, Alberti KG, Zimmet P, Tuomilehto J, Purran A, Fareed D, Chitson P, Collins VR. Changes in population cholesterol concentrations and other cardiovascular risk factor levels after five years of the non-communicable disease intervention programme in Mauritius. Mauritius Non-communicable Disease Study Group. BMJ. 1995;311:1255-1259. [PubMed] |
| 87. | Puska P, Vartiainen E, Laatikainen T, Jousilahti P, Paavola M. The North Karelia Project: From North Karelia to National Action. 2009;. |
| 88. | Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 432] [Article Influence: 28.8] [Reference Citation Analysis (16)] |
| 89. | Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3804] [Cited by in RCA: 3679] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 90. | Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444-2449. [PubMed] |
| 91. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [PubMed] |
| 92. | Lenfant C. Shattuck lecture--clinical research to clinical practice--lost in translation? N Engl J Med. 2003;349:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 526] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 93. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13206] [Cited by in RCA: 12380] [Article Influence: 538.3] [Reference Citation Analysis (1)] |
| 94. | Whalen J. New Ways to Get Children to Take Their Medicine. 2014; [Updated 2014; cited 2014 June 9]. Available from: http: //online.wsj.com/articles/new-ways-to-get-children-to-take-their-medicines-1402064246. |
| 95. | Gill GV, Mbanya JC, Ramaiya KL, Tesfaye S. A sub-Saharan African perspective of diabetes. Diabetologia. 2009;52:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 96. | Soucat A, Levy-Bruhl D, De Bethune X, Gbedonou P, Lamarque JP, Bangoura O, Camara O, Gandaho T, Ortiz C, Kaddar M. Affordability, cost-effectiveness and efficiency of primary health care: the Bamako Initiative experience in Benin and Guinea. Int J Health Plann Manage. 1997;12 Suppl 1:S81-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | Bompart F, Kiechel JR, Sebbag R, Pecoul B. Innovative public-private partnerships to maximize the delivery of anti-malarial medicines: lessons learned from the ASAQ Winthrop experience. Malar J. 2011;10:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Russo G, McPake B. Medicine prices in urban Mozambique: a public health and economic study of pharmaceutical markets and price determinants in low-income settings. Health Policy Plan. 2010;25:70-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Windus DW, Ladenson JH, Merrins CK, Seyoum M, Windus D, Morin S, Tewelde B, Parvin CA, Scott MG, Goldfeder J. Impact of a multidisciplinary intervention for diabetes in Eritrea. Clin Chem. 2007;53:1954-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Coleman R, Gill G, Wilkinson D. Noncommunicable disease management in resource-poor settings: a primary care model from rural South Africa. Bull World Health Organ. 1998;76:633-640. [PubMed] |
| 101. | Mamo Y, Seid E, Adams S, Gardiner A, Parry E. A primary healthcare approach to the management of chronic disease in Ethiopia: an example for other countries. Clin Med. 2007;7:228-231. [PubMed] |
| 102. | Huddle KR, Gill GV. Reducing acute hyperglycaemic mortality in African diabetic patients. Diabet Med. 1989;6:64-66. [PubMed] |
| 103. | Heimlich HJ, Chen XP, Xiao BQ, Liu SG, Lu YH, Spletzer EG, Yao JL. Malariotherapy for HIV patients. Mech Ageing Dev. 1997;93:79-85. [PubMed] |
| 104. | Bishop WB, Laubscher I, Labadarios D, Rehder P, Louw ME, Fellingham SA. Effect of vitamin-enriched bread on the vitamin status of an isolated rural community--a controlled clinical trial. S Afr Med J. 1996;86:458-462. [PubMed] |
| 105. | Lurie P, Wolfe SM. Unethical trials of interventions to reduce perinatal transmission of the human immunodeficiency virus in developing countries. N Engl J Med. 1997;337:853-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 306] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 106. | Kimmelman J. Clinical trials and SCID row: the ethics of phase 1 trials in the developing world. Dev World Bioeth. 2007;7:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 107. | Sofaer N, Eyal N. The diverse ethics of translational research. Am J Bioeth. 2010;10:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Pape JW, Severe PD, Fitzgerald DW, Deschamps MM, Joseph P, Riviere C, Rouzier V, Johnson WD. The Haiti research-based model of international public health collaboration: the GHESKIO Centers. J Acquir Immune Defic Syndr. 2014;65 Suppl 1:S5-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Madon T, Hofman KJ, Kupfer L, Glass RI. Public health. Implementation science. Science. 2007;318:1728-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |