Published online Dec 12, 2014. doi: 10.5528/wjtm.v3.i3.119
Revised: September 23, 2014
Accepted: October 14, 2014
Published online: December 12, 2014
Processing time: 138 Days and 0.2 Hours
Obesity is a multifactorial disease showing a pandemic increase within the last decades in developing, and developed countries. It is associated with several severe comorbidities such as type II diabetes, hypertension, sleep apnea, non-alcoholic steatosis hepatis and cancer. Due to the increasing number of overweight individuals worldwide, research in the field of obesity has become more vital than ever. Currently, great efforts are spend to understand this complex disease from a biological, psychological and sociological angle. Further insights of obesity research come from bariatric surgery that provides new information regarding hormonal changes during weight loss. The initiation of programs for obesity treatment, both interventional and pharmaceutical, are being pursued with the fullest intensity. Currently, bariatric surgery is the most effective therapy for weight loss and resolution of comorbidities in morbid obese patients. Reasons for weight loss and remission of comorbidities following Roux-en-Y-Gastric Bypass, Sleeve Gastrectomy, and other bariatric procedures are therefore under intense investigation. In this review, however, we will focus on obesity treatment, highlighting new insights and future trends of gut hormone research, the relation of obesity and cancer development via the obesity induced chronic state of inflammation, and new potential concepts of interventional and conservative obesity treatment.
Core tip: This review focuses on the latest obesity research breakthroughs, current therapy options, future outlooks, also from a view of a surgeon as well as recently identified molecules that promote obesity and its comorbidities, outlining their great potential as new target molecules in the fight against the global pandemic, called “obesity”.
- Citation: El Gammal AT, Dupree A, Wolter S, Aberle J, Izbicki JR, Güngör C, Mann O. Obesity research: Status quo and future outlooks. World J Transl Med 2014; 3(3): 119-132
- URL: https://www.wjgnet.com/2220-6132/full/v3/i3/119.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v3.i3.119
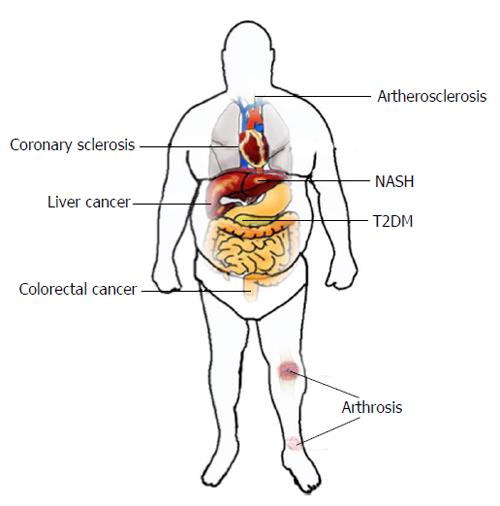
Obesity is a multifactorial disease caused by an energy sparing lifestyle on a predisposed polygenetic background. An obese person is defined as having a body mass index (BMI) greater than 30 kg/m2. Within the last decades, there has been an extraordinary increase in the worldwide prevalence of obesity becoming a major human health threat especially in developing and developed countries with a tendency to rise. Being referred to as a global pandemic[1], the number of overweight or obese individuals increased up to 2.1 billion worldwide. Unfortunately, no single country announced decreasing numbers of obese individuals during the last three decades[2]. Obesity is associated with several severe comorbidities (Figure 1) such as type II diabetes mellitus (T2DM), hypertension, sleep apnea, non-alcoholic steatosis hepatis (NASH) and cancer. Obesity-related diabetes can lead to coronary heart disease, apoplex or kidney failure. Over 80% of all patients with type II diabetes in the United States are overweight and up to 20% of United States health expenditures are estimated to be spent on treating obesity-related diseases[3].
It is expected that NASH will be the leading cause of liver transplantation within the next years[4]. Additionally, obesity is associated with an increased risk of developing various cancer entities such as colorectal-, esophageal-, liver- and breast cancer[5]. Visceral-, orthopedic or cardiac surgical treatment of obese patients is associated with higher complication rates[6-9]. Subsequently, obesity is the origin of a wide spectrum of diseases and a cofounding factor hindering adequate treatment. Due to this reasons, obesity and overweight are associated with an increased risk of death. Thus, therapy for obesity should be individually tailored and various factors such as sex, obesity degree, individual health risks should be taken into account[10,11].
Secondary causes for obesity like endocrine disorders (e.g., hypothyroidism, cushing disease), drug-induced obesity (e.g., glucocorticoids, psychoactive drugs), inherited syndromes (e.g., Prader-Willi syndrome, Bardet-Biedl syndrome) or monogenetic disorders (leptin receptor, melanocortin receptor) play a minor role or are cofactors in causation of obesity in daily practice. Therefore, identifying single reasons for obesity is a complex task. Intervention strategies for weight loss and maintenance at the individual and community level are strongly needed to reduce general health risks as well as health expenditures.
Due to the increasing number of overweight individuals worldwide, research in the field of obesity has become more vital than ever. As a multifactorial disease, research is conducted at a wide variety of areas. Currently, great efforts are spend to understand this complex disease from a biological, psychological and sociological angle. Further insights of obesity research come from bariatric surgery, which display new information regarding the hormonal changes during weight loss. The initiation of programs aiming to treat obesity, both interventional and pharmaceutical, are being pursued with the fullest intensity. There are various scopes of possible research activities. In this review, however, we will focus on obesity treatment, highlighting new insights into gut hormones and the relation of obesity and cancer development.
Among physicians there is consensus, to treat obese patients multidisciplinary. After diagnosis, the patient should undergo a multimodal therapy concept based on individualized dietary education focusing on reducing energy intake, physical exercising, pharmacological therapy and psychological attendance with behavioral therapy. For the latter, many efforts to modify the behavior of obese individuals through encouragement of changes in dietary intake along with physical activity have not declined the obesity epidemic, unfortunately. The primary causes are high rates of therapy abandonment and poor patient compliance.
Patients who completed a comprehensive program including a low-calorie diet are able to lose approximately 15%-25% of their initial body weight during 3 to 6 mo of treatment. After therapy, most patients maintain a weight loss of 8% one year after treatment, 7% three years after treatment, and 5% four years after treatment[12]. These results represent the best-case scenario, excluding patients who dropped out of their programs. It was already shown that patients who have completed structured weight loss programs, maintained their weight loss of less than 3 kg on average after 5 years; patients who accomplished more radical low-calorie diets had significantly higher weight loss of up to 20 kg and maintained more weight loss over time[13]. In a randomized study, Jeffery et al[14] evaluated the efficacy of long-term weight loss comparing one group with behavior therapy and an energy expenditure goal of 1000 kcal per week to a group of patients with high physical activity treatment and an energy expenditure goal of 2500 kcal per week. The high activity group showed significant higher weight loss and long term weight loss maintenance, reflecting that mobility is of high importance[14].
Bariatric surgery is more effective for weight loss and resolution of comorbidities than conventional medical treatment modalities[15,16]. A variety of procedures are described in the literature but only Roux-Y gastric bypass (RYGB) (46.6% of all bariatric procedures worldwide), sleeve gastrectomy (SG) (27.8%), adjustable gastric banding (GB) (17.8%) and in a smaller proportion biliopancreatic diversion with duodenal switch (BPD/DS) (2.2%) are performed in a notable quantity[17].
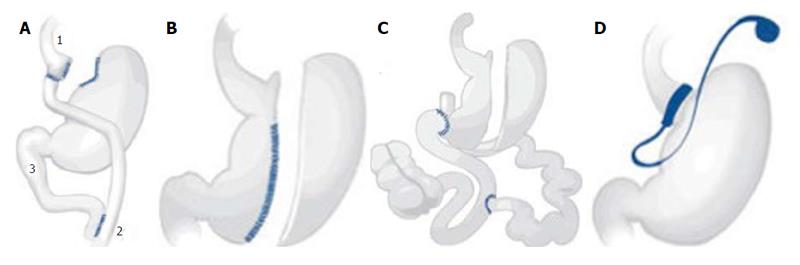
Gastric bypass was first performed in the 1960s by Mason et al[18]. It was modified to a RYGB in the 1970s[19]. It is still the most common bariatric surgical procedure. The combination of food-intake restriction by a small pouch and malabsorption through the smaller common channel (Figure 2A) leads to long-term weight loss[15]. Also, hormonal changes after surgery may have a great impact on weight loss and diabetes remission.
SG (Figure 2B), includes the resection of the greater curve of the stomach. It is the first step of the BPD/DS (Figure 2C). BPD/DS can be performed by a two step procedure; a minority of patients do not need the second surgical step for weight loss[20]. While SG is described as a primary restrictive procedure, including minor hormonal changes, BPD/DS causes malabsorption and leads to a higher rate of deficiencies[21]. GB, which was developed in the 1970s, restricts food-intake by an inflatable, adjustable gastric band resulting in a small gastric pouch (Figure 2D). Since it is based on restriction only, it is the most insufficient bariatric surgical procedure regarding long-term weight loss[22].
Overall, a preoperative multidisciplinary program is recommended. Our own clinical experiences and a review of the literature revealed that bariatric surgery for severe obese patients results in extensive weight loss and long-term comorbidity remission in a very short time frame.
There is no evidence for conventional treatment leading to sufficient excess weight loss in severe obese patients.
Padwal et al[23] performed an observational study of 500 patients with a two years follow-up. Three patient cohorts were included in which 200 patients received medical treatment, 150 patients received bariatric surgical treatment, and 200 patients received no therapy and were grouped as being waitlisted. Medically treated patients received individualized and intensive medical management consisting of a 24-36 wk life style counseling (diet education, physical exercise, and behavioral therapy) and were observed by a multidisciplinary staff which is mandatory before bariatric surgery. Mean weight loss in the waitlisted group was 0.9%, 1.8% in the medically treated group and 22% in the surgery group. The proportion of patients who achieved at least 5% weight loss was 17% in the waitlisted group, 32% in the medically treated group and 75% in the surgery group. The prevalence of hypertension, diabetes and dyslipidemia was reduced in the surgical group, but remained unchanged or increased in the medically treated and waitlisted group[23].
A large meta-analysis included 164 studies (37 randomized controlled trials and 127 observational studies). A total of 161756 patients were analyzed regarding effectiveness and outcome after bariatric surgery.
One year after surgery the patients showed 60% excess weight loss (EWL), and 57% EWL after 3 years. T2DM remission after surgery was 92%, hypertension remission was 75%, dyslipidemia remission was 76%, cardiovascular diseases remission was 58% and remission of sleep apnea was 96%, reflecting that surgical intervention may increase the long-term quality of life[24].
Interestingly, 75.3% of patients that received bariatric surgery showed excess weight loss, whereas patients that had received conventional therapy showed only 11.3% excess weight loss. Moreover, remission of T2DM was reported in 63.5% of cases in surgery group, compared to 15.6% of patients in the conventional therapy group[25]. Subsequently, there is no evidence for conventional treatment leading to sufficient EWL in obese patients with a BMI greater 40 kg/m². In fact, the only efficient treatment showing results in EWL and release of obesity associated diseases results from bariatric surgery. However, there is a strong recommendation to include the patients to a perioperative multidisciplinary medical treatment consisting of dietary changes, exercising and behavioral therapies. There is evidence that preoperative multidisciplinary preparation and education may lead to better long-term effects of bariatric surgery.
In sum, bariatric surgery is currently the only effective treatment for morbid obesity[26]. Reasons for weight loss and remission of comorbidities following RYGB, SG, and other bariatric procedures are therefore in a strong research focus.
Alterations of gut hormone serum levels after RYGB influence appetite, satiety, energy expenditure, and glucose homeostasis[27-29]. Several hormones and peptides are considered to be involved in weight loss in bariatric patients (Table 1).
| Peptide | Production site | Effect | After bariatric surgery | Potential pharmaceutical intervention |
| Ghrelin | Stomach, mainly fundus | Appetite stimulating | ↓ | Receptor antagonists |
| Growth hormone releasing | GOAT inhibition | |||
| Vaccination | ||||
| GLP-1 | L-cells of the distal small bowel | Postprandialinsuline secretion | ↑ | Weight loss in patients with diabetes |
| Suppresses glucagon secretion | Off-label use in obese patients | |||
| Delays gastric emptying | ||||
| Suppresses appetite | ||||
| GIP | Duodenum, jejunum | Postprandial insulin secretion | ↓ | GIP receptor antagonist |
| Energy expenditure | ||||
| CCK | Duodenum, jejunum | Delays gastric emptying | ↑ | CCK analogue substance |
| Suppresses appetite | ||||
| PYY | Distal small bowel | Delays gastric emptying | ↑ | Long-acting analogue substance |
| Suppresses appetite | ||||
| PP | Distal small bowel | Suppresses appetite | ↔ | PP analogue substance |
| OXM | L-cells of the distal small bowel | Delays gastric emptying | ↑ | Receptor agonist |
| Suppresses appetite | ||||
| Increase energy expenditure |
Incretins are gut-derived peptides that increase pancreatic insulin secretion. The Glucagon-like peptide (GLP-1) and Glucose-dependent insulinotropic polypeptide (GIP) are well explored. GLP-1 and its analogues are used to treat diabetes. Beside its stimulating effects on β-cells of pancreatic Langerhans’ islets, GLP-1 also suppresses glucagon secretion, delays gastric emptying and suppresses appetite[30,31]. Therefore, GLP-1 is currently under intense discussion to become a potential therapeutic drug for obesity treatment[32].
Ghrelin is mainly produced in the fundus of the stomach and plays an important role in satiety. When administered to humans, it increases food intake. Several studies showed that postprandial reduction of Ghrelin after bariatric surgery led to weight loss and T2DM remission[33,34]. Therefore, lowering of Ghrelin plasma levels by non-surgical interventions might be a useful approach for obesity treatment. Different approaches already exist in the development of anti-obesity drugs. Pharmacological molecules like Ghrelin antagonists or Ghrelin receptor antagonists showed heterogeneous results in food intake reduction[35]. Other strategies are the inhibition of Ghrelin O-acyltransferase (GOAT) that is required for activation of Ghrelin[36] or lowering body weight by a vaccination targeting Ghrelin[37].
Administration of Oxyntomodulin (OXM) decreases food intake and reduces body weight in rats[38]. Furthermore, OXM has been shown to increase energy expenditure[39]. The combination of decreasing energy intake and increasing energy consumption qualifies OXM to be a potential agent for bariatric treatment. Moreover, a plethora of other gut hormones and peptides are currently under intense investigation regarding weight loss. Interestingly, there is also evidence that various gut hormones are related to cancer growth and cancer development making their physiological understanding even more alluring[40].
Morbid obesity is associated with various types of cancer: Epidemiological studies identified an association of morbid obesity and several types of cancer disease, such as colorectal cancer, endometrium carcinoma, postmenopausal breast cancer, kidney cancer, esophageal cancer, pancreatic cancer, gallbladder cancer, liver cancer, and hematological malignancies[41,42]. Obese patients have a tendency for worse prognosis and outcome after cancer treatment and an increased risk of cancer related morbidity[43]. Calle et al[5] conducted a prospective study to examine the association of obesity and cancer related mortality. They concluded that increased body weight is associated with increased death rates for all cancers combined.
The link between obesity and cancer is still poorly understood. Several adipokines, growth factors, signaling pathways, inflammatory processes as well as the general demodulation of energy-balance and the lack of calorie restriction are being intensively discussed.
Adipokines are involved in cancer development: Traditionally, the adipose tissue was considered to be an energy storage organ. In recent years, however, it became evident that it also functions as an endocrine organ. Besides estrogen, it produces and secretes various adipokines and cytokines. Leptin and adiponectin, two well characterized adipokines, are associated with cancer development[44].
Leptin concentration in serum correlates positively with the patients’ adipose tissue reserves and their nutritional condition. Moreover, leptin has been identified to be a potential mediator of cancer development[45], which is able to activate various key players of different signaling cascades like phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), mitogen-activated proteinkinase (MAPK) and signal transducer and activator of transcription 3 (STAT3). More interestingly, leptin signaling promotes the progression of different cancers[46-48].
Adiponectin is mainly secreted by visceral fat cells and acts adversary to leptin. It is inversely associated with obesity, hyperinsulinemia, and inflammation and may have anti-cancer effects by decreasing insulin-growth factor-1 (IGF-1) and mechanistic target of rapamycin (mTOR) signaling by activation of 5’AMP-activated protein kinase (AMPK). Also anti-inflammatory actions of adiponectin are described through inhibition of nuclear-factor-kappa-light-chain-enhancer activated B cells signaling (NF-κB)[49].
Recent studies suggest a causal link of obesity related diseases (Figure 3) and low-grade/chronic inflammation (Figure 3)[50-52].
In humans, the immune system is of major relevance, which in turn, is able to form a defence shield against bacteria, viruses, or injured cells. A hallmark of the immune system is its most powerful weapon, the “inflammatory response” which was already noticed by a German pathologist called Rudolf Virchow in 1863. Despite the fact that humans without a functional immune system are not able to survive, too much inflammation can have a great impact and may cause serious damage to the healthy individual. Well-known chronic inflammatory diseases occur in patients that suffer from psoriasis or rheumatoid arthritis. A possible link between infections and cancer already exists, since stomach cancer may result from Helicobacter pylori infections or liver cancer from hepatitis (B-, C-) virus infections. A unique feature of these infections is the chronic inflammation response, which is primarily mediated by specific immune cells, such as macrophages and granulocytes that infiltrate the tumor. The latter is known to be recruited by tumor-released attractants. Once leucocytes infiltrate the tumor, they start to secrete chemokines and thereby initiate blood vessel growth/angiogenesis to allocate oxygen and nutrients, which are relevant for tumor growth.
Macrophages: In obese individuals, macrophages infiltrate and expand in adipose tissue. Quantitative and functional changes of these cells affect adipose tissue inflammation. Exposure of macrophages to cytokines promotes two different activation states inducing to divergent polarizations. M1 macrophages are activated by tumor necrosis factor-α (TNF-α), Interferon-γ (IFN-γ), and bacterial endotoxins such as lipopolysaccharides. They are characterized by high levels of interleukin (IL)-12 and IL-23, and low levels of IL-10 as well as inflammatory cytokines[53]. Contrarily, M2 macrophages are attracted by IL-4, IL-13, IL-10, and glucocorticoid hormones. Both types are part of innate immune response. M1 macrophages may induce chronic inflammation, whereas M2 macrophages tend to act anti-inflammatory[54]. It has been suggested, that a phenotypic switch from M2 to M1 occurs in fat tissue[55], however, this model is discussed controversially.
Eosinophiles: Eosinophiles levels are negatively correlated with obesity and adipose tissue in mice. Wu et al[54] could show, that eosinophiles promote an M2-polarization of macrophages by secreting IL-4 and IL-13 and a down regulation of M1 macrophages in adipose tissue.
Mast cells: Mast cell levels in adipose tissue are elevated in obese animals[56]. Mast cell ablation reduces body fat and benefits glucose homeostasis in mice. This effect is induced by IL-6 and IFN-γ. Also, pro-angiogenic factors such as Cathepsins may influence mast cell levels[56].
Myeloid-derived suppressor cells: In adipose tissue, Myeloid-derived suppressor cells (MDSCs) have an inhibitory effect on inflammation by suppressing CD8+-T cells and promoting M1 to M2 macrophage switch in favour for M2 macrophages[57]. The state of chronic inflammation in adipose tissue leads to an accumulation of MDSCs[58]. Being part of immune autoregulation by MDSCs suppress overt inflammatory immune response in chronic inflammation[59].
CD4+-T cells: CD4+-T cell activation is mediated by class II major histocompability complex (MHC II) molecules presented by macrophages and dendritic cells. When activated, CD4+-T cells secrete cytokines, which attract pro-inflammatory cells. Three groups of T cells can be distinguished, namely TH1, TH2, and TH17.
The ratio of TH1/TH2 cells is significantly enhanced in high fat diet induced obesity, since TH2 cells are undermined by IFN-γ producing TH1 cells[60]. CD4+-T cell substitution in immunodeficient mice eventuates in reduction of weight gain, adipocyte cell size, and improvement of glucose homeostasis[60]. The STAT6 pathway is essential for TH2 differentiation, thus STAT6 deficient CD4+-T cells do not show any effect of reconstitution on glucose homeostasis and body weight gain[60].
Regulatory T cells: CD4+-T cells can transdifferentiate into immunosuppressive CD4+CD25+-regulatory T cells (Treg)[61]. Obesity is associated with reduced levels of Treg cells in visceral adipose tissue in mice and humans[62,63]. Treg cell depletion enhances circulating insulin levels and levels of pro-inflammatory cytokines in adipose tissue of lean mice[62]. Up regulation of Treg on the other hand improves insulin sensitivity and enhances anti-inflammatory cytokine IL-10 levels[62]. Also, Treg function to suppress pro-inflammatory immune response and promote macrophage M1 to M2 switch by secreting IL-4, IL-10, and IL-13[64].
CD8+-T cells: CD8+-T cell activation is mediated by MHC I. Activated CD8+-T cells induce lysis of target cells by producing various cytokines and chemokines.
Adipose tissue of obese animals[65] and humans[60] show a significant increase of CD8+-T cell levels. CD8+-T cells lead to elevation of macrophages in adipose tissue and promote polarization into M1 macrophages[63]. CD8+-T cell deficient mice have fewer levels of macrophages in adipose tissue and less levels of TNF-α and IL-6[63].
Natural killer T cells: When activated by lipids, natural killer T (NKT) cells produce a significant amount of TH1- and TH2-responsive cytokines, such as IFN-γ and IL-4[66]. NKT cells can either promote or supress inflammatory response by promoting either TH1 or TH2 cell activation[67,68]. Interestingly, NKT cell levels are reduced in human omental adipose tissue[69]. The role of NKTs in obesity still remains unclear.
B cells: After high fat diet, accumulation of B cells can be detected in adipose tissue of mice. This accumulation is associated with high levels of pro-inflammatory immunoglobulin G2c[70]. B cells promote T cell modulation and macrophage polarization by producing pathogenic Ig-G antibodies. Ig-G, however, increases inflammatory response[70]. The specific role of B-cells and Ig-G in inflammatory response in obesity has yet to be further investigated.
Mediators of inflammatory response: Preadipocytes can transdifferentiate into macrophages[71]. Also they tend to enlarge due to oxygen diffusion resulting in hypoxia, inflammation and increased macrophage infiltration. Enlarged adipocytes produce a variety of inflammatory cytokines and show greater insulin resistance than normal sized ones. Levels of prostaglandin E2, TNF-α, IL-2, IL-8, IL-10, and monocyte chemoattractant protein-1 (MCP-1) are elevated in the microenvironment of enlarged adipocytes. The inflammatory environment attracts macrophages and induces production of additional pro-inflammatory mediators[71].
NF-κB is a central transcription factor that is activated upon bacterial and viral stimuli. It activates gene expression associated with apoptosis, cell proliferation, inflammation, tumorigenesis, metastasis, and angiogenesis[72]. In addition, increased NF-κB expression and activation is associated with insulin resistance.
The frequent up-regulation of NF-κB in many cancers is already known[73]. The increased expres-sion and “uncontrolled” activation of NF-κB may induce cancerogenesis[74,75]. Interestingly, NF-κB gets activated upon leptin stimulation in preneoplastic and neoplastic human colonic epithelial cells in vitro[76,77].
Inflammasomes, by definition cytosolic multiprotein complexes, activate IL-1β and IL-18 during infection or tissue damage[78]. They can be sub-divided into different inflammasome sub-groups such as nucleotide-binding oligomerization domain-like receptors (NLR), NLR pyrin domain-containing 1 and 3 (NLRP1 and NLRP3), absent in melanoma 2, and caspase activation and recruitment domains domain containing 4 (NLRC4/IPAF)[78]. Inflammasomes secrete caspase 1, which cleaves cytokine preforms, such as IL-1β[79,80]. The activity of NLR is associated with autoimmune diseases, malignancies, inflammation, infection, and metabolic disorders[59]. Inflammasome components expression levels are elevated in adipose tissue of obese mice[81-83]. Conversely, NLRP3 and IL-1β are decreased in low calorie dietary restriction[83]. It seems therefore, that NLRP3 integrates multiple signals, causing pathogenic inflammation in obese subjects[84]. Also NLPR6 has a critical role in gut homeostasis[85,86]. Mice with non-functional NLRP6 develop an altered commensal system, preventing normal glycaemic control on a high fat diet and promoting NASH[87].
In summary, there are at least two inflammasome types and substrates that can imbalance metabolism and inflammation in obesity[85].
The role of chronic inflammation as a precursor of tumorigenesis can be observed in various cancers. A gastritis can give rise to gastric cancer, inflammatory bowel disease may promote colorectal cancer and patients suffering from a chronic pancreatitis may have a higher risk to develop pancreatic cancer[88]. The inflammatory effect of adipose fat tissue might therefore be a general precursor of cancerogenesis. Like adipose tissue, tumor microenvironment is composed of multiple cell types like fibroblasts, epithelial cells, mast cells, and cells of innate and adaptive immune system that favor a pro-inflammatory, pro-tumorigenic environment[89-91].
Contribution to the pro-inflammatory environment is the presence of macrophages that are attracted by MCP-1. Tumor tissue classically contains a high amount of M2 polarized macrophages[92]. Macrophages activated by obese states, infiltrate tumors and amplify the inflammatory tumor environment through NF-κB dependent cytokine production and angiogenic factors[88]. Malignancies may be initiated or exacerbate by inflammation, and increased levels of inflammation may be a cause and/or a consequence of malignancy[88,93].
Production of steroid hormones in the adipose tissue are also relevant for various cancers: Steroid hormones such as progesterone, estrogen, androgens and adrenal steroids are associated with energy balance level and obesity associated development of several cancer types[94]. In women, the BMI correlates with the incidence of breast cancer, endometrium cancer and other cancer entities that are associated to sexual hormone levels. The relative contribution of adipose tissue steroid hormone production to the whole steroid metabolism is about 100% in postmenopausal women[44]. The risk of developing breast cancer in post-menopausal women enhances with an increase of circulating levels of steroid hormones such as dehydroepiandrosterone, testosterone, estradiol and estrogen, and low levels of sex hormone binding globuline. There is evidence that estrogens are mitogenic, regulating the expression of insulin, and inducing DNA damage by free radicals, genetic instability and gene mutations in cells[95]. Increased estradiol levels can induce endometrial cell proliferation rates while inhibiting apoptosis and activating the IGF-1 synthesis in endometrial tissue[5].
In men, testosterone has been the focus of most studies on sex hormones, obesity and metabolic complications. Evidence indicates that most tissues, including adipose tissue, express steroid converting enzymes necessary for the local production of androgens and/or estrogens[96]. Up to 40% of the active androgen production (dihydrotestosterone) is accounted for by tissue conversion of adrenal precursors[96]. In men, obesity has generally been associated with reduction of testosterone levels in plasma and elevated estrogen concentrations[97-99]. It has also been reported, that men with visceral adiposity have decreased levels of testosterone[100,101]. A growing body of interest suggests, that obese men are more likely to be diagnosed with aggressive prostate cancer and high tumor volumes[102]. Furthermore, obese patients show a higher risk of cancer recurrence, as well as an increase in disease related deaths compared to lean patients[103,104].
Increased insulin levels and insulin growth factor-1 signaling enhance cancer development[105]. Other observational studies reported an increased mortality of obese cancer patients with T2DM due to hyperinsulinemia and elevated IGF-1 serum levels. In contrast, patients with lower insulin, IGF-1, and IGF-2 levels showed a lower risk to develop cancer[105-107].
Patients treated with insulin or drugs stimulating insulin secretion showed a significantly higher incidence of developing malignancies than those patients treated with anti-diabetic drugs like metformin. Therefore, metformin might be a potential anticancer agent[108].
Caloric restriction, which causes down-regulation of circulating insulin and IGF-1 levels is a potent suppressor in carcinogenesis[74]. Insulin and IGF-1 can trigger cell growth and proliferation, while inhibiting cell survival via proteinkinase B (Akt)/PI3K/mTOR (Akt/PI3K/mTOR) pathway[73]. This signaling pathway is not only the most frequently mutated pathway in human cancers, it is also a signal mediator of leptin, adiponectin and pro-inflammatory cytokines[46,109,110].
Caloric restriction reduces cancer incidence by inhibiting the Akt/PI3K/mTOR pathway via AMPK activation[111-113]. In contrast, Kalaany et al[110] could show that tumors with PI3K activation do not respond to the anti-cancerous effects of caloric restriction.
Interestingly, mTOR activity is increased in obese patients. It plays a central role in obesity related inflammation. Multiple risk factors for cancer development in obesity have been identified, such as the insulin-IGF-1 axis, leptin/adiponectin, and pro-inflammatory cytokines like IL-6, IL-7 and TNF-α. These factors can activate multiple pathways including PI3K/Akt, MAPK and STAT3, resulting in increased mTOR activity. mTOR, however, inhibits the insulin-PI3K pathway by stimulating the STAT3 pathway[46].
IL-6 and TNF-α play a major role in obesity associated hepatocellular carcinoma by activating the STAT3 pathway[114]. The STAT3 pathway is involved in the regulation of various gene expressions including IL-17, IL-23, B-cell lymphoma 2, and vascular epithelial growth factor to promote cell survival, proliferation, invasion, angiogenesis, and metastasis[115]. Consistent activation of STAT3 increases tumor cell proliferation, survival and invasion in suppressing anti-tumor immunity. STAT3 activation also leads to activation of further pro-oncogenic pathways, such as NF-κB and the IL-6/Janus kinase pathways[115].
In 2003, the Human Genome Project was accomplished. After 13 years and estimated costs of 2.7 billion USD, the first human genome was sequenced. In contrast, the human genome of an individual was sequenced over a 5 mo period of time at costs of 1.5 million United States-Dollars in 2008[116].
An overall trend in the public health sector is the tendency towards “individualized therapy” in order to tailor specific therapy options that are currently available for a given patient which is further supported by usage of sophisticated mouse models.
Without doubt, mouse models have helped to understand relevant pathways that are important in the regulation of human body fat on the molecular level[117-123]. Initial insights into molecules that are important in regulating body fat, resulted primarily from genetic mouse screenings[117,124-126]. The identification of specific inactivating gene mutations accompanied by an obese phenotype, have revealed that leptin, leptin receptor and melanocortin-4 receptor play central roles in the regulation of body fat[127-132]. Interestingly, these three obesity phenotypes as a result of inactivating mutations, are also relevant in humans, suggesting that knockout mouse models are a powerful tool to gain new insights into obesity relevant human genes and proteins.
A clinical approach might further support the in vivo findings that resulted from former obesity mouse models. Extensive tissue banking combined with collected clinical data may open up new perspectives in translational medicine as well.
There already exist several methods for screening large patient cohorts such as next generation sequencing. Also, established methods (e.g., Fluorescence in situ hybridization or immunohistochemistry) became powerful tools when featured with high-throughput methods such as tissue microarrays to gain knowledge in the distribution of potential obesity relevant proteins. Tools such as laser mass spectrometry combined with a large tissue database in a microarray format might enable the initiation of virtual protein expression profiling of cells in their natural tissue environment. Further development in this field and others will open up new possibilities to identify causal links between gene expression levels, RNA modification, protein expression levels, post translational modification of proteins, intrinsic enzyme activity, and initiation and progression of diseases on a molecular level.
Automated chip technologies for detection of structural variation discoveries on a DNA- and RNA-level may decrease sequencing time, streamline sample preparations and reduce costs in future studies.
Acquiring great amounts of patient cohorts’ data in large databases combined with blood and tissue sampling will move clinical applicability of new gained knowledge into focus. New potential risk factors and/or therapy targets will be identified by high throughput tissue and blood screenings. Especially the combination of organ-tissue samples with respective blood samples, body fluids, and visceral/subcutaneous fat samples will help to understand complex causal connections between obesity and organ function failure and carcinogenesis on a molecular basis. The novel knowledge will be centralized and digitally organized, accompanied by its’ access that will be provided to health care units and hospitals for data reconciliation.
Preclinical and clinical patient screening will provide the basis for individualized digital patient DNA-, RNA-, protein-, post translational modification-, and enzyme activity profiles that automatically may be compared to already identified risk factors or therapy targets in centralized data bases.
In the present, there already exist research projects that might serve as landmarks for individualized obesity research in the future.
Interestingly, the TG and HDL Working Group were able to identify rare mutations that disrupt apolipoprotein C3 function by sequencing the protein-coding regions of 18666 genes in each of 3734 participants. By correlating loss of function studies with clinical data, carriers of these mutations were found to have a reduced risk of coronary heart disease[133].
In the future, these mutations might serve as clinical risk-markers for coronary heart disease in obese patients. Blood samples of obese patients could be easily tested for gene mutations and the presence of a mutation might then be interpreted as a protective factor in favor of the patients’ health.
Another study conducted an association analysis of single nucleotide polymorphisms, identifying genetic variants that predispose to T2DM[134]. Testing blood or tissue samples right after birth for these genetic variants might probably change the way of clinical diagnostics entirely.
In the future, patients with genetic predisposition for e.g., diabetes might be diagnosed before the onset of disease. This knowledge could then lead to an individualized treatment in terms of dietary intake, physical exercise, or to earlier elective surgical intervention in obese patients.
Also, gut hormone and adipokine serum levels could be screened on regular basis in obese individuals. When out of balance, pharmaceutical intervention with suitable drugs such as GOAT- inhibitors, GIP-Receptor antagonists, inhibitors of the mTOR-, STAT3- and MAPK-, PI3K-pathways or even Metformin might be applicable in the future to prevent relevant comorbidities such as cancer.
Morbid obesity is already a widespread problem not only in first-, but also in second world countries. It causes various major chronic diseases such as coronary heart disease, diabetes, hypertension, and cancer. As living standards in second and third world countries enhance, morbid obesity will proceed to be a huge challenge for health institutions and national health systems. Obesity is a potential human health threat and is likely to become even more present in the future. The relevance and possible long-terms effects of maternal obesity to the health of the offspring are not fully understood. Studies that deal with this issue are of high relevance to precisely understand the long-term adverse health outcomes for the upcoming new generations.
In conclusion, there is an urgent need for obesity research with a straightforward concentration on new studies that aim to identify and interpret the complex, multifactorial variables in order to develop new therapy approaches and prevention programs for patients suffering from this disease.
P- Reviewer: Ji G, Koch TR, Lin GM S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2895] [Cited by in RCA: 2948] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 2. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8003] [Article Influence: 727.5] [Reference Citation Analysis (0)] |
| 3. | Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 796] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 4. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 586] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 5. | Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2509] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 6. | Ditillo M, Pandit V, Rhee P, Aziz H, Hadeed S, Bhattacharya B, Friese RS, Davis K, Joseph B. Morbid obesity predisposes trauma patients to worse outcomes: a National Trauma Data Bank analysis. J Trauma Acute Care Surg. 2014;76:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Wilson MZ, Dillon PW, Hollenbeak CS, Stewart DB. How do risk factors for mortality and overall complication rates following laparoscopic and open colectomy differ between inpatient and post-discharge phases of care? A retrospective cohort study from NSQIP. Surg Endosc. 2014;28:3392-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Wigfield CH, Lindsey JD, Muñoz A, Chopra PS, Edwards NM, Love RB. Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI > or = 40. Eur J Cardiothorac Surg. 2006;29:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Buerba RA, Fu MC, Gruskay JA, Long WD, Grauer JN. Obese Class III patients at significantly greater risk of multiple complications after lumbar surgery: an analysis of 10,387 patients in the ACS NSQIP database. Spine J. 2014;14:2008-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1495] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 11. | Katzmarzyk PT, Janssen I, Ardern CI. Physical inactivity, excess adiposity and premature mortality. Obes Rev. 2003;4:257-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 436] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579-584. [PubMed] |
| 14. | Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684-689. [PubMed] |
| 15. | Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, Jacobson P, Lönroth H, Maglio C, Näslund I. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 541] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 16. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1180] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 17. | Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1004] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 18. | Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47:1345-1351. [PubMed] |
| 19. | Griffen WO, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186:500-509. [PubMed] |
| 20. | Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 334] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | Aasheim ET, Björkman S, Søvik TT, Engström M, Hanvold SE, Mala T, Olbers T, Bøhmer T. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Padwal RS, Rueda-Clausen CF, Sharma AM, Agborsangaya CB, Klarenbach S, Birch DW, Karmali S, McCargar L, Majumdar SR. Weight loss and outcomes in wait-listed, medically managed, and surgically treated patients enrolled in a population-based Bariatric program: prospective cohort study. Med Care. 2014;52:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1385] [Cited by in RCA: 1207] [Article Influence: 109.7] [Reference Citation Analysis (1)] |
| 25. | Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24:437-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1265] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 27. | le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 734] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 28. | Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, Ghatei MA, Bloom SR, le Roux CW, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr. 2011;35:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339-350; discussion 350-352. [PubMed] |
| 30. | Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut. 2006;55:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-López G. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav. 2011;105:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Ng SY, Wilding JP. Liraglutide in the treatment of obesity. Expert Opin Biol Ther. 2014;14:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, Nissen S, Brethauer SA, Schauer PR, Kirwan JP. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: effects of bariatric surgery vs standard medical therapy. Int J Obes (Lond). 2014;38:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Samat A, Malin SK, Huang H, Schauer PR, Kirwan JP, Kashyap SR. Ghrelin suppression is associated with weight loss and insulin action following gastric bypass surgery at 12 months in obese adults with type 2 diabetes. Diabetes Obes Metab. 2013;15:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Schellekens H, Dinan TG, Cryan JF. Lean mean fat reducing ”ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology. 2010;58:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Gualillo O, Lago F, Dieguez C. Introducing GOAT: a target for obesity and anti-diabetic drugs? Trends Pharmacol Sci. 2008;29:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Andrade S, Pinho F, Ribeiro AM, Carreira M, Casanueva FF, Roy P, Monteiro MP. Immunization against active ghrelin using virus-like particles for obesity treatment. Curr Pharm Des. 2013;19:6551-6558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond). 2006;30:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 40. | Ashrafian H, Ahmed K, Rowland SP, Patel VM, Gooderham NJ, Holmes E, Darzi A, Athanasiou T. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117:1788-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 653] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 42. | Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Kaidar-Person O, Bar-Sela G, Person B. The two major epidemics of the twenty-first century: obesity and cancer. Obes Surg. 2011;21:1792-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3338] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 45. | Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc. 2012;71:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H, Zhao L. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009;100:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer. 2008;123:2543-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 474] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 50. | Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2430] [Cited by in RCA: 2481] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 51. | Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91. [PubMed] |
| 52. | Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1684] [Cited by in RCA: 1659] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 53. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3290] [Cited by in RCA: 3116] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 54. | Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1139] [Cited by in RCA: 1064] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 55. | Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3164] [Cited by in RCA: 3575] [Article Influence: 198.6] [Reference Citation Analysis (0)] |
| 56. | Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 573] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 57. | Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591-23599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 58. | Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499-4506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1358] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 59. | Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 60. | Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1155] [Cited by in RCA: 1102] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 61. | Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 62. | Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1750] [Cited by in RCA: 1670] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 63. | Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1719] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 64. | Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446-19451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 671] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 65. | Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond). 2008;32:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 66. | Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845-1847. [PubMed] |
| 67. | Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 1771] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 68. | Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 796] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 69. | Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 70. | Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 71. | Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850-9855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 72. | Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 881] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 73. | Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 74. | Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 76. | Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Rouet-Benzineb P, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, Bado A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J Biol Chem. 2004;279:16495-16502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3880] [Cited by in RCA: 4543] [Article Influence: 302.9] [Reference Citation Analysis (0)] |
| 79. | Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1425] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 80. | Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1286] [Cited by in RCA: 1281] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 81. | Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 82. | Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324-15329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 83. | Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2148] [Cited by in RCA: 2051] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 84. | Masters SL. Specific inflammasomes in complex diseases. Clin Immunol. 2013;147:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc Natl Acad Sci USA. 2011;108:9601-9606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 86. | Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1659] [Cited by in RCA: 1578] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 87. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1880] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 88. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11273] [Article Influence: 490.1] [Reference Citation Analysis (2)] |
| 89. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577-583. [PubMed] |
| 90. | Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, Sirigu P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest. 2003;33:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 342] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 92. | Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 93. | Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb). 2011;21:264-275. [PubMed] |
| 94. | Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Nunez NP, Perkins SN. Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 580] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 96. | Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 356] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 97. | Strain GW, Zumoff B, Kream J, Strain JJ, Deucher R, Rosenfeld RS, Levin J, Fukushima DK. Mild Hypogonadotropic hypogonadism in obese men. Metabolism. 1982;31:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 260] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Tchernof A, Després JP, Bélanger A, Dupont A, Prud’homme D, Moorjani S, Lupien PJ, Labrie F. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism. 1995;44:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 363] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 101. | Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, Capelli M, Bortoluzzi L. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101-104. [PubMed] |
| 102. | Jentzmik F, Schnoeller TJ, Cronauer MV, Steinestel J, Steffens S, Zengerling F, Al Ghazal A, Schrader MG, Steinestel K, Schrader AJ. Corpulence is the crucial factor: association of testosterone and/or obesity with prostate cancer stage. Int J Urol. 2014;21:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011;4:486-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 104. | Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 106. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5282] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 107. | Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 592] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 108. | Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31-R43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 109. | Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2188] [Cited by in RCA: 2155] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 110. | Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 354] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 111. | Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, DiGiovanni J. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila). 2008;1:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 112. | Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 113. | Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492-5499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 114. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1403] [Cited by in RCA: 1369] [Article Influence: 91.3] [Reference Citation Analysis (1)] |
| 115. | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3396] [Article Influence: 212.3] [Reference Citation Analysis (0)] |
| 116. | Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, He W, Chen YJ, Makhijani V, Roth GT. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872-876. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1163] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 117. | Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 579] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 118. | Allan MF, Eisen EJ, Pomp D. The M16 mouse: an outbred animal model of early onset polygenic obesity and diabesity. Obes Res. 2004;12:1397-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 119. | Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127-1128. [PubMed] |
| 120. | Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317-318. [PubMed] |
| 121. | Jürgens HS, Schürmann A, Kluge R, Ortmann S, Klaus S, Joost HG, Tschöp MH. Hyperphagia, lower body temperature, and reduced running wheel activity precede development of morbid obesity in New Zealand obese mice. Physiol Genomics. 2006;25:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 122. | Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia. 1967;3:212-221. [PubMed] |
| 123. | Suzuki W, Iizuka S, Tabuchi M, Funo S, Yanagisawa T, Kimura M, Sato T, Endo T, Kawamura H. A new mouse model of spontaneous diabetes derived from ddY strain. Exp Anim. 1999;48:181-189. [PubMed] |
| 124. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 125. | Duhl DM, Stevens ME, Vrieling H, Saxon PJ, Miller MW, Epstein CJ, Barsh GS. Pleiotropic effects of the mouse lethal yellow (Ay) mutation explained by deletion of a maternally expressed gene and the simultaneous production of agouti fusion RNAs. Development. 1994;120:1695-1708. [PubMed] |
| 126. | Michaud EJ, Bultman SJ, Klebig ML, van Vugt MJ, Stubbs LJ, Russell LB, Woychik RP. A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (Ay) mutation. Proc Natl Acad Sci USA. 1994;91:2562-2566. [PubMed] |
| 127. | Torres-Andrade R, Moldenhauer R, Gutierrez-Bertín N, Soto-Covasich J, Mancilla-Medina C, Ehrenfeld C, Kerr B. The increase in body weight induced by lack of methyl CpG binding protein-2 is associated with altered leptin signalling in the hypothalamus. Exp Physiol. 2014;99:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546. [PubMed] |
| 129. | Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1377] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 130. | van Swieten MM, Pandit R, Adan RA, van der Plasse G. The neuroanatomical function of leptin in the hypothalamus. J Chem Neuroanat. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 131. | Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2235] [Cited by in RCA: 2209] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 132. | Woods SC, Seeley RJ, Porte D, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 763] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 133. | Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 835] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 134. | Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2180] [Cited by in RCA: 2055] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 135. | Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2014;95:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |