Published online Aug 12, 2014. doi: 10.5528/wjtm.v3.i2.58
Revised: June 11, 2014
Accepted: July 17, 2014
Published online: August 12, 2014
Processing time: 136 Days and 19.1 Hours
The scarcity of donor livers has increased the interest in donation after cardiac death (DCD) as an additional pool to expand the availability of organs. However, the initial results of liver transplantation with DCD grafts have been suboptimal due to an increased rate of complications, as well as decreased graft survival. These challenges have led to many developments in DCD donation outcome, as well as basic and translational research. In this article we review the unique characteristics of DCD donors, nuances of DCD organ procurement, the effect of prolonged warm and cold ischemia times, and discuss major studies that compared DCD to donation after brain death liver transplantation, in terms of outcomes and complications. We also review the different methods of donor treatment that has been applied to ameliorate DCD organ outcome, and we discuss the role of machine perfusion techniques in organ reconditioning. We discuss the two major perfusion models, namely, hypothermic machine perfusion and normothermic machine perfusion; we compare both methods, and delineate their major differences.
Core tip: There exists an increased need for liver grafts that currently exceed the availability of organs by a large margin. It is estimated that a third of the patients awaiting for transplantation will perish or become too ill due to the scarcity of grafts. This has led to a renewed interest in marginal organs as a potential pool. Most notably, donation after cardiac death livers has been targeted, and new strategies emerge to ameliorate their quality. Ex-vivo liver perfusion techniques could drastically change the paradigm of organ preservation, conditioning, and amelioration.
- Citation: Bazerbachi F, Selzner N, Seal JB, Selzner M. Liver transplantation with grafts obtained after cardiac death-current advances in mastering the challenge. World J Transl Med 2014; 3(2): 58-68
- URL: https://www.wjgnet.com/2220-6132/full/v3/i2/58.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v3.i2.58
Donation after cardiac death (DCD) was the only mode of organ retrieval in the beginning of organ transplantation era. It was largely abandoned after the establishment of brain death criteria in favour of heart beating organ retrieval to minimize ischemic injury. However, the increasing organ shortage has resulted in a new interest to extend the donor pool for liver transplantation. Excellent outcomes have been reported for kidney transplantation with DCD organs, which triggered new interest for DCD liver transplantation in the 90’s. One study concluded that DCD liver grafts can be used to dramatically reduce wait list time with outcomes comparable to those of standard criteria and donation after brain death (DBD)[1]. Another study reported that a 5% increase in DCD donors will lead to a 27% relative reduction in the wait list volume[2]. The proportion of DCD organs has increased compared with the past decades, and DCD liver transplantation remains at approximately 6% in the United States[3]. However, this group of marginal organs is characterized by increased sensitivity to preservation injury, and the ischemia-reperfusion injury (IRI) pathway is exacerbated by the combination of warm and cold ischemia resulting in cellular injury and energy depletion.
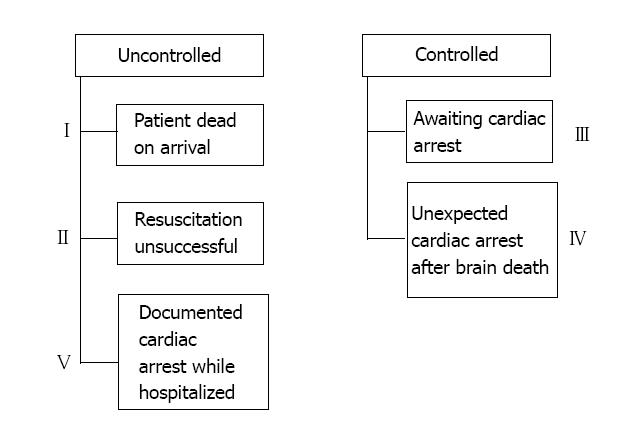
In contrast to the irreversible coma state that defines brain death[4], cardiac death is the irreversible desist of respiratory and circulatory functions. DCD donors [also known as non-heart-beating donors (NHBD)] are divided according to the modified Maastricht classification into 5 categories, which can further be reduced to two main groups (Figure 1): (1) Controlled DCD (categories III and IV), wherein circulatory and respiratory organ support is voluntarily withdrawn by the medical provider, in the setting of a dismal prognosis that renders cardio-respiratory support no longer in the patient’s best interest and survival is deemed futile; and (2) Uncontrolled DCD (categories I, II, and V), in which cardiac death occurs suddenly, and resuscitation is unsuccessful or absent[5].
Debate is ongoing regarding the exact definition of cardiac death, and whether loss of cardiac electricity should be established vs solely relying on the absence of heart sounds, pulse, and blood pressure[5-7]. Today, the irreversible absence of pulse is accepted as the moment of death.
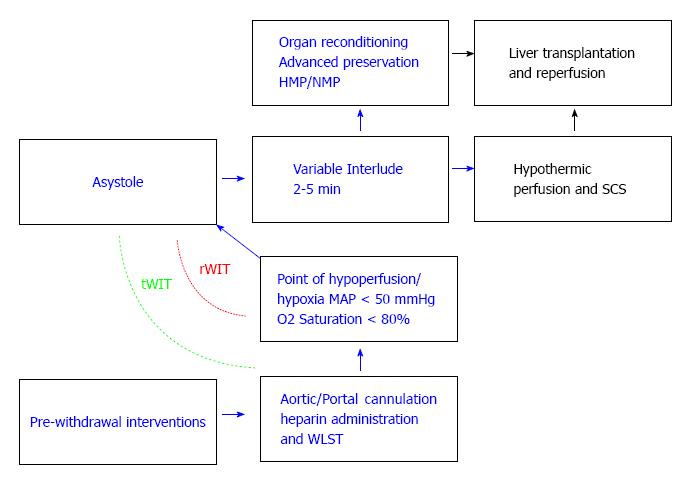
After death is announced, organ procurement starts following a mandatory interlude designated to monitor for spontaneous return of cardiopulmonary function. The American Society of Transplant Surgeons (ASTS) recommends 2 min wait time, and the Society of Critical Care Medicine (SCCM) and Institute of Medicine (IOM) recommend 5 min of sustained death prior to the commencement of procurement[8,9] (Figure 2).
The first described technique for DCD graft retrieval was the so-called “super-rapid technique” (SRT) presented by Starzl et al[10], and involved en-bloc resection of the abdominal viscera, with subsequent separation of individual organs on the back-table while immersed in ice[10,11]. This technique has been further refined, and the modified technique entails a fast thoraco-laparotomy, hypothermic perfusion of the abdominal aorta, venous exsanguination, cross-clamping of the supradiaphragmatic aorta, and may include portal venous hypothermic perfusion[8]. The rate-limiting step in terminating warm ischemia time (WIT) is the cannulation of the aorta to allow hypothermic perfusion. In experienced centers, this essential step could be done within 1-2 min after declaration of death[12].
In-situ cooling of the liver before and during procurement is imperative. Some have advocated for precannulation of the femoral arteries prior to withdrawing life support to further shorten the time until initiation of the cold flush. A double-balloon, triple-lumen (DBTL) catheter can be used to shunt the cold perfusate solely to abdominal viscera[13]. Although cooling of abdominal organs is facilitated with this technique, ethical concerns have impeded its utilization[14-16].
The role of the initial flushing solution has been controversially debated. DCD rat livers that were flushed with low viscosity solutions showed lower vascular resistance than those flushed with cold Belzer solution [University of Wisconsin solution (UW)] and led to better survival[17]. In contrast, analysis of the UNOS database showed a decrease in graft survival when Histidine–Tryptophan-Ketoglutarate (HTK) vs UW solution was used as a preservative solution in DCD organs[18].
Warm ischemia time (WIT) refers to cellular ischemia under normothermic conditions, and entails two physiological periods[19,20]: (1) Ischemia after withdrawal of life support until cold perfusion is commenced; and (2) Ischemia during implantation, after removal of the organ from ice until reperfusion.
The beginning of asystole is difficult to predict following the withdrawal of life support therapies (WLST). Should asystole not happen within 120 min of WLST, current guidelines recommend ending the attempted DCD organ retrieval and continuing ICU therapy. In about 30% of all attempted DCD organ retrievals death does not occur within the 120 min recommended waiting time. Different time points from the beginning of warm ischemia have been used. Some groups propose the use of total WIT (tWIT): entailing the period from WLST until the start of preservation (whether it is sought through static cold storage (SCS), or through extracorporeal perfusion, organ preservation starts either as a total body cooling approach, or as in-situ cooling approach using the DBTL catheter[21-24]). In contrast, others have proposed to consider the beginning of warm ischemia only if the arterial saturation of oxygen falls under 70%-80% and/or arterial hypotension occurs (MAP < 60-50 mmHg), also dubbed real warm ischemia time (rWIT). It ends once preservation commences[24,25]. According to the ASTS, the maximal acceptable WIT for safe liver transplant is 30 min or less[8,26].
Interestingly, single center studies produced conflicting reports regarding the association between WIT and graft survival[27-29]. Similarly, the association between WIT and the rate of biliary complications has not been consistently established in all available studies[28,30-32]. However, despite the inability to detect these associations, Ho et al[29] have shown that longer rWIT may predict poor survival after liver transplantation. Another recent study by Abt et al[33] demonstrated in a multivariate regression analysis, an association between graft survival and the slope of the systolic blood pressure using values during the first 10 min after donor extubation (SBP10). The authors propose to select donors with a favorable trajectory of blood pressure during the agonal phase[33].
Warm ischemia injury is mediated by several mechanisms, including Na+/K+-ATPase dysfunction, inhibition of nitric oxide synthase (NOS), vascular microthrombosis, changes in bile salts composition, overproduction of hypoxanthine and free radicals, as well as overproduction of vasoconstrictors during reperfusion[34-39].
Reich et al[40] reported the first single-center experience with comparable outcome of DCD vs DBD liver transplantation. However, major studies that followed reported conflicting results (Table 1).
| Ref. | Year | DCD transplants number | Recipient survival rate (%) at 1 yr, 3 yr, and 5 yr post-transplant | Graft survival rate (%) at 1 yr, 3 yr, and 5 yr post-transplant | ITBS rate | Retransplants rate | ||||
| Croome et al[98] | 2013 | HCC DCD = 242 | 76 | 64 | 56 | |||||
| Non-HCC DCD = 2117 | 86 | 77 | 71 | |||||||
| Abt et al[33] | 2013 | 110 | 47 | 14% | ||||||
| Callaghan et al[99] | 2013 | 352 | 81 | 73 | ||||||
| Vanatta et al[100] | 2013 | 38 | 92 | 80 | 92 | 74 | 7% | 2% | ||
| Elaffandi et al[101] | 2012 | 108 | 84 | 2% | ||||||
| Taner et al[28] | 2012 | 200 | 93 | 85 | 81 | 81 | 73 | 69 | 12% | 5% |
| Meurisse et al[52] | 2012 | 30 | 93 | 85 | 85 | 90 | 82 | 82 | 3% | |
| DeOliveira et al[30] | 2011 | 167 | 87 | 85 | 81 | 85 | 83 | 78 | 2% | |
| Hong et al[102] | 2011 | 81 | 78 | 62 | 53 | 10% | 12% | |||
| Mathur et al[53] | 2011 | 1567 | 65 | 13% | ||||||
| Dubbled et al[46] | 2010 | 55 | 85 | 80 | 74 | 68 | 14% | 18% | ||
| Yamamoto et al[45] | 2010 | 24 | 62 | 43 | 43 | 54 | 37 | 38 | ||
| Detry et al[103] | 2010 | 58 | 83 | 67 | 72 | 49 | 38% | |||
| de Vera et al[27] | 2009 | 141 | 79 | 70 | 69 | 56 | 16% | 18% | ||
| Grewal et al[43] | 2009 | 108 | 92 | 88 | 88 | 79 | 74 | 71 | 8% | 15% |
| Jiménez-Galanes et al[104] | 2009 | 20 | 86 | 80 | 5% | |||||
| Pine et al[105] | 2009 | 39 | 82 | 68 | 80 | 64 | 20% | |||
| Nguyen et al[42] | 2009 | 19 | 90 | 90 | 74 | 63 | 10% | 16% | ||
| Fujita et al[106] | 2007 | 24 | 87 | 82 | 69 | 56 | 21% | |||
The initial outcomes of liver transplantation with DCD grafts have been suboptimal due to a high rate (20%-40%) of ischemic-type biliary strictures (ITBS)[36,41-43], higher graft failure rate, as well as increased medical and surgical complications following the procedure.
According to one study by Jay et al[44], post transplantation costs were significantly higher in DCD versus DBD transplant recipients who experienced ITBS or re-transplantation. In their study, DCD costs continued to be higher when the analysis was censored for re-transplanted patients; this may suggest that morbidity is increased and may account for this increase in costs[44]. It follows that an examination of the most common complications of DCD liver transplants may be necessary, if the full scope of economic burden is to be understood[45].
Interestingly, in a study comparing 24 DCD recipients vs 16 DBD recipients, Yamamoto et al[45] showed that, despite an increased rate of hepatic artery thrombosis (HAT) and biliary complications, graft and patient survival did not differ between the groups. Their study suggested that improved surgical and medical management has led to amelioration of transplantation outcomes.
One of the immediate complications is primary graft failure (PGF) following ischemic insult resulting in re-transplantation or patient death. PGF after DCD liver transplantation has decreased in frequency over time, and is reported to be approximately 5% in the most recent studies[31,43,46]. This improvement may be ascribed to improved surgical techniques and amelioration in organ preservation and extraction.
One of the later complications is ITBS [also dubbed as nonanastomotic biliary stricture (NABS), or ischemic cholangiopathy]. ITBS presents as non-anastomotic intrahepatic or extrahepatic biliary strictures (in the absence of arterial thrombosis), which occur within the first 3 mo after transplantation. One hypothesis of ITBS etiology is the arterial supply theory. Since most of the blood that supplies the biliary system emanates from the hepatic arteries, severe decrease in hepatic artery supply may result in biliary necrosis and subsequent stenosis[47,48]. The occurrence of this complication has been estimated to fall between 20% and 40% in DCD recipients, compared to 5% in DBD recipients However, recent studies reported a decreased frequency of this complication, and estimated its incidence to be around 10%[30,31,49].
ITBS appears to be particularly associated with DCD organs, with a 10 fold increase in incidence compared with DBD livers. Other risk factors are increased donor age, increased donor weight, and increased cold ischemia time (CIT) and/or WIT (especially WIT > 30 min)[27,28,32,50-54].
Attempts have been made to reduce ITBS in orthotopic liver transplantation. Moench et al[55] established the utility of arterial back-table pressure perfusion of the hepatic artery prior to transplantation in heart beating donor grafts, and showed an association with decreased ITBS rate in a multivariate analysis. Hashimoto et al[36] investigated the use of tissue plasminogen activator (tPA) administration in 22 patients during DCD liver transplantation. In the implantation phase, tPA was injected in the hepatic artery prior to making the anastomosis. The authors found that this strategy decreased the incidence of ITBS to 9% in DCD liver grafts[36].
As a result of these complications, strict acceptance criteria have been applied for DCD liver transplantation and only a small percentage of DCD livers are currently accepted for transplantation. Harring et al[56] proposed criteria for DCD transplant optimization that focused on strict selection for donors and recipients (donor age < 50 years and WIT < 20 min), however, current ASTS recommendations state that DCD liver grafts should be ideally used in younger recipients with age < 60 years and WIT < 30 min.
Some studies cautioned about using DCD grafts in HCV(+) recipients as they have found that HCV recurrence was more aggressive and advanced more rapidly in this cohort of patients, compared to DBD grafts[57,58], although a recent registry analysis failed to detect this difference[59]. Moreover, a recent match-controlled, retrospective analysis demonstrated that DCD liver grafts did not promote disease progression or negatively affect patient and graft survival in comparison with DBD liver grafts in HCV(+) patients[60].
Animal models were designed using several strategies to optimize DCD grafts, including administration of different pharmacologic agents[61,62]. Administration of heparin and phentolamine prior to asystole resulted in an increase of acinar perfusion and sinusoidal density in rat livers[61]. Experimental data have shown that tacrolimus may incur protection against hepatic IRI when administered intravenously or as a hepatic rinse[63]. Recently, a study protocol has been published for a European randomized multicenter trial comparing ex vivo tacrolimus perfusion of marginal liver grafts vs placebo[64]. Milrinone, a phosphodiesterase 3 inhibitor, exerts positive inotropic and vasodilatory effects, and has been reported to attenuate the graft injury caused by CIT, WIT, and subsequent IRI via an increase in intracellular cAMP levels[65]. Pentoxifylline is a methylxanthine compound and a phosphodiesterase inhibitor with hemorheological, as well as anti-inflammatory properties has also been shown to decrease IRI in animal models[66,67].
As more programmes now accept increasing numbers of DCD livers in which organ function status is uncertain, the need for further evaluation and even reconditioning of the organ is emphasized.
Although the prevailing goal of organ preservation in the past has been to slow the metabolic rate by SCS, this strategy may not be optimal for livers from marginal donors.
Initially, SCS emerged as a method to optimally store organs and thus improve graft survival. However, this simple technique does not allow for adequate evaluation of the organ, as reduction of metabolism to about 5% by cold storage hinders the possibility of meaningful liver evaluation. Moreover, while hypothermia slows down metabolism, it does not prevent continuation of anaerobic glycolysis and does not stop the production of harmful by-products.
Therefore, several groups proposed the use of extracorporeal perfusion systems to reduce IRI, and ameliorate graft outcomes.
Originally suggested by Carrel and Lindbergh in the late 1930s for organs in general, ex vivo liver perfusion emerged as a potential protective strategy[68-72]. The purpose of extracorporeal perfusion is to continuously support the preserved organ with nutrients and oxygen, and to eliminate toxic products from the cellular milieu. Newer studies evaluated these experimental techniques and their effect on late biliary injury[73].
Ex vivo perfusion systems could be classified according to the perfusate temperature, and it includes: normothermia (35 °C-37 °C), mild hypothermia/sub-normothermia (32 °C-35 °C), moderate hypothermia (28 °C-32 °C), severe hypothermia (20 °C-28 °C), and profound hypothermia (< 20 °C)[74] (Table 2).
| Hypothermic machine perfusion HMP | Normothermic machine perfusion NMP |
| Temperature 0 °C-4 °C | Temperature 37 °C |
| Logistically easier | Logistically demanding |
| Modest resumption of energy production with low perfusion rate | |
| Improves the state of mitochondria during preservation | Recreates the physiological milieu by maintenance of normal temperature |
| Performed at sub-physiologic pressures[107] | Performed at physiological pressures[70,82] |
| Requires low perfusion rates[108] | Requires high perfusion rates[108] |
| No requirement for a specific oxygen carrier in the perfusate as demand for O2 is low[108] | Oxygen is provided by using blood, modified hemoglobin, or using a high oxygen tension in special preservation solutions[70,82,84,88,109] |
| Less occurrence of graft infection considering the hypothermic state More tendency for endothelial cell, kupffer cell, and macrophage cell damage due to shear stress and hypothermic activation[110-113] | Reduces IRI |
| When compared to SCS it decreases inflammatory cytokines but no difference in graft or patient survival was found[77,114] | Provides nutrients (glucose, amino acids, etc.), medications to prevent micro-circulatory failure (e.g., prostacyclin, heparin, antibiotics), and oxygen |
| May help protect marginal livers by converting PNF into allograft dysfunction[71] | Allows the assessment of organ viability (e.g., Galactose elimination, factor V production, bile flow) |
| May allow the use of gene therapy prior to transplantation, to reduce the risk of rejection, or decrease the ischemia-reperfusion injury[115-117] |
Henry et al[75] and Guarrera et al[76-78] performed hypothermic (4 °C) machine perfusion (HMP) of the hepatic artery and portal vein without oxygenation. The authors used sub-physiologic perfusion pressures, and no benefits of hypoxic HMP were observed in an animal model. However, in a case control study with 20 human liver transplants using low risk donors, the same group observed a decrease of serum AST/ALT after transplantation when HMP was compared with SCS.
In another porcine DCD model, de Rougemont et al[71] studied the effects of oxygenated HMP prior to transplantation. Livers were exposed to 1 h WIT followed by 7 h of SCS preservation or 1 h of WIT plus 6 h of SCS and 1 h of oxygenated HMP. After liver transplantation, AST levels were similar in both groups. Median recipient survival after transplant was slightly increased by oxygenated HMP from 5 to 8 h.
Despite these results, early experiments that examined machine perfusion of animal liver grafts showed that a hypothermic perfusate is a risk factor for post-transplant HAT. Ikeda and colleagues demonstrated that, compared to normothermic perfusion (NMP), HMP was associated with increased hepatic artery resistance and decreased bile flow[79]. More recently, Tolboom et al[80] showed that bile production increased concordantly with increased perfusate temperature, and was the highest at a degree of 37 °C[80]. Consequently, there existed an increased interest in normothermic techniques[81].
In 2001, Schön et al[82] were the first to successfully describe NMP in porcine livers. The Oxford group headed by Dr. Peter Friend showed conserved hepatic function with NMP up to 72 h[83-85]. SCS cannot be completely avoided, even in NMP, due to the complexity of the procurement process, as well as the logistics of the apparatus. Although NMP could not salvage porcine livers that received 4 h of SCS prior to perfusion, it was able to assess liver function, and maintain cellular replenishment when used throughout the preservation period[83,85-87]. Brockmann and colleagues showed that NMP was advantageous to DBD and DCD livers that endured a prolonged period of preservation (approximately 20 h)[72].
Our group in Toronto[70] was the first to examine bile duct injury using NMP in a DCD porcine model while simulating transplantation. Our study was designed to simulate a clinical scenario in which organs are retrieved at a remote donor hospital and transported with SCS to the transplant center to commence NMP, and our machine perfusion model utilized Steen solution[88] for preservation rather than cellular products. Livers managed with SCS alone had significantly higher ALT levels, decreased oxygen extraction, and increased hepatic necrosis. Levels of bilirubin, phospholipids and bile salts in the bile fluid were fivefold decreased, while LDH was sixfold higher in the SCS vs NMP group. Hepatic artery perfusion was decreased and bile duct necrosis was increased as well, favoring NMP. The protective mechanisms of machine perfusion remain under investigation[89].
Despite these advances, the majority of the studies that examined machine perfusion, focused on early liver graft injury and acute survival. However, in humans, the majority of biliary lesions occur within the first year after transplantation[90].
Regional perfusion (RP) of the liver is used in-vivo, prior to organ retrieval, and act as a bridge between asystole and retrieval, thus limiting WIT, and mitigating ischemia. Moreover, it prevents the depletion of mitochonodrial ATP stores, favoring aerobic metabolism, and acting as an ischemia pre-conditioning period[91-93].
Future research is needed to focus on synergistic liver perfusion modalities such as RP extracorporeal oxygenation, followed by NMP.
The NMP system could also benefit from optimization in terms of portability. In its current form, NMP-dependent techniques cannot avoid a period of SCS prior to perfusion. Experimental data suggest that prolonged cold ischemia of the organ before attachment to the ex-vivo perfusion system could impair the protective effects[86,94].
Another area to explore is liver assessment methods during ex-vivo perfusion to predict function and viability of DCD liver grafts. If clinical validation of such parameters could be established, the procurement team would be able to determine suboptimal grafts without putting the recipient at risk with the liver transplant procedure. Alternatively, optimal DCD grafts would avoid unjustified rejection, therefore adding more livers to the donor pool[87].
NMP may also provide ground for pre-transplantation gene therapy of donor grafts. Cypel et al[95] investigated this method with an adenoviral vector encoding human interleukin-10 (AdhIL-10) to repair injured donor lungs ex-vivo (through NMP) before transplantation. In their study, AdhIL-10-treated lungs showed significant improvement in function when compared to controls, a favorable shift from proinflammatory to anti-inflammatory cytokine expression, and recovery of alveolar-blood barrier integrity[95]. The range of potential targets for gene therapy prior to transplantation includes recruitment of heat shock proteins (some of which have been shown to protect against IRI[96]), modulation of co-stimulatory and apoptosis pathways, amelioration of immunologic profile to prevent rejection, and manipulation of leukocyte recruitment[87,97].
The scarcity of donor livers has resulted in an increased interest in extended criteria donors (ECD), and more specifically DCD grafts, as a potential source to significantly expand the donor pool. Initially, this pool provided disappointing results as it was associated with high incidence of ITBS, HAT, and PGF, however, outcome has improved with better donor selection and pre-transplant treatment. Future application of machine perfusion modalities might allow graft assessment and repair, resulting in more extensive use of DCD liver grafts and provide ground for pre-transplantation conditioning of organs.
P- Reviewer: Boucek C, Kubota K, Tsuchida A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Tector AJ, Mangus RS, Chestovich P, Vianna R, Fridell JA, Milgrom ML, Sanders C, Kwo PY. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Chaib E, Massad E. The potential impact of using donations after cardiac death on the liver transplantation program and waiting list in the state of Sao Paulo, Brazil. Liver Transpl. 2008;14:1732-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Available from: http: //srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_ liver_12.pdf. Accessed: August 18, 2013. |
| 4. | A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205:337-340. [PubMed] |
| 5. | Browne A. The Institute of Medicine on non-heart-beating organ transplantation. Camb Q Healthc Ethics. 2008;17:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sánchez-Fructuoso AI, Prats D, Torrente J, Pérez-Contín MJ, Fernández C, Alvarez J, Barrientos A. Renal transplantation from non-heart beating donors: a promising alternative to enlarge the donor pool. J Am Soc Nephrol. 2000;11:350-358. [PubMed] |
| 7. | Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893-2894. [PubMed] |
| 8. | Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D’Alessandro A, Pomfret EA, Freeman RB, Markmann JF, Hanto DW. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Ethics Committee ACoCCM, Society of Critical Care M. Recommendations for nonheartbeating organ donation. A position paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 2001;29:1826-1831. [PubMed] |
| 10. | Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343-348. [PubMed] |
| 11. | Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Fung JJ, Starzl TE. Experience with liver and kidney allografts from non-heart-beating donors. Transplant Proc. 1995;27:2898. [PubMed] |
| 12. | Perera MT. The super-rapid technique in Maastricht category III donors: has it developed enough for marginal liver grafts from donors after cardiac death? Curr Opin Organ Transplant. 2012;17:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Fondevila C, Hessheimer AJ, Ruiz A, Calatayud D, Ferrer J, Charco R, Fuster J, Navasa M, Rimola A, Taurá P. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant. 2007;7:1849-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Moriyama Y, Morishita Y, Shimokawa S, Taira A. Multiple organ procurement for transplantation: the effect of peritoneal cooling. Transplant Proc. 1991;23:2324-2325. [PubMed] |
| 15. | Kato M, Mizutani K, Hattori R, Kinukawa T, Uchida K, Hoshinaga K, Ono Y, Ohshima S. In situ renal cooling for kidney transplantation from non-heart-beating donors. Transplant Proc. 2000;32:1608-1610. [PubMed] |
| 16. | Robertson JA. The dead donor rule. Hastings Cent Rep. 1999;29:6-14. [PubMed] |
| 17. | Tojimbara T, Wicomb WN, Garcia-Kennedy R, Burns W, Hayashi M, Collins G, Esquivel CO. Liver transplantation from non-heart beating donors in rats: influence of viscosity and temperature of initial flushing solutions on graft function. Liver Transpl Surg. 1997;3:39-45. [PubMed] |
| 18. | Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N. Warm ischemia in transplantation: search for a consensus definition. Transplant Proc. 2007;39:1329-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Shemie SD, Baker AJ, Knoll G, Wall W, Rocker G, Howes D, Davidson J, Pagliarello J, Chambers-Evans J, Cockfield S. National recommendations for donation after cardiocirculatory death in Canada: Donation after cardiocirculatory death in Canada. CMAJ. 2006;175:S1. [PubMed] |
| 21. | Gómez M, Alvarez J, Arias J, Barrio R, Mugüerza J, Balibrea JL, Martín F. Cardiopulmonary bypass and profound hypothermia as a means for obtaining kidney grafts from irreversible cardiac arrest donors: cooling technique. Transplant Proc. 1993;25:1501-1502. [PubMed] |
| 22. | Barber SD. The tell-tale heart: ethical and legal implications of in situ organ preservation in the non-heart-beating cadaver donor. Health Matrix Clevel. 1996;6:471-502. [PubMed] |
| 23. | Garcia-Rinaldi R, Lefrak EA, Defore WW, Feldman L, Noon GP, Jachimczyk JA, DeBakey ME. In situ preservation of cadaver kidneys for transplantation: laboratory observations and clinical application. Ann Surg. 1975;182:576-584. [PubMed] |
| 24. | Abradelo De Usera M, Jiménez Romero C, Loinaz Segurola C, Moreno González E. [Liver transplant with donated graft after controlled cardiac death. Current situation]. Cir Esp. 2013;91:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Manara AR, Murphy PG, O’Callaghan G. Donation after circulatory death. Br J Anaesth. 2012;108 Suppl 1:i108-i121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 26. | Tariciotti L, Rocha C, Perera MT, Gunson BK, Bramhall SR, Isaac J, Buckels JA, Mayer AD, Muiesan P, Mirza DF. Is it time to extend liver acceptance criteria for controlled donors after cardiac death? Transplantation. 2011;92:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, Fontes P, Marsh JW. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Taner CB, Bulatao IG, Willingham DL, Perry DK, Sibulesky L, Pungpapong S, Aranda-Michel J, Keaveny AP, Kramer DJ, Nguyen JH. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Ho KJ, Owens CD, Johnson SR, Khwaja K, Curry MP, Pavlakis M, Mandelbrot D, Pomposelli JJ, Shah SA, Saidi RF. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation. 2008;85:1588-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | DeOliveira ML, Jassem W, Valente R, Khorsandi SE, Santori G, Prachalias A, Srinivasan P, Rela M, Heaton N. Biliary complications after liver transplantation using grafts from donors after cardiac death: results from a matched control study in a single large volume center. Ann Surg. 2011;254:716-722; discussion 722-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D’Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 32. | Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, Reyes JD, Larson AM, Levy AE. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Abt PL, Praestgaard J, West S, Hasz R. Donor hemodynamic profile presages graft survival in donation after cardiac death liver transplantation. Liver Transpl. 2014;20:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Hansen TN, Haworth RA, Southard JH. Warm and cold ischemia result in different mechanisms of injury to the coronary vasculature during reperfusion of rat hearts. Transplant Proc. 2000;32:15-18. [PubMed] |
| 35. | Selzner M, Selzner N, Jochum W, Graf R, Clavien PA. Increased ischemic injury in old mouse liver: an ATP-dependent mechanism. Liver Transpl. 2007;13:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Hashimoto K, Eghtesad B, Gunasekaran G, Fujiki M, Uso TD, Quintini C, Aucejo FN, Kelly DM, Winans CG, Vogt DP. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant. 2010;10:2665-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Yska MJ, Buis CI, Monbaliu D, Schuurs TA, Gouw AS, Kahmann ON, Visser DS, Pirenne J, Porte RJ. The role of bile salt toxicity in the pathogenesis of bile duct injury after non-heart-beating porcine liver transplantation. Transplantation. 2008;85:1625-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Nagayama M, Katsuramaki T, Kimura H, Isobe M, Meguro M, Matsuno T, Nui A, Hirata K. Prediction of graft viability from non-heart-beating donor pigs using hepatic microdialysate hypoxanthine levels. J Surg Res. 2002;107:210-218. [PubMed] |
| 39. | Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant. 2012;2012:164329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Reich DJ, Munoz SJ, Rothstein KD, Nathan HM, Edwards JM, Hasz RD, Manzarbeitia CY. Controlled non-heart-beating donor liver transplantation: a successful single center experience, with topic update. Transplantation. 2000;70:1159-1166. [PubMed] |
| 41. | Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Nguyen JH, Bonatti H, Dickson RC, Hewitt WR, Grewal HP, Willingham DL, Harnois DM, Schmitt TM, Machicao VI, Ghabril MS. Long-term outcomes of donation after cardiac death liver allografts from a single center. Clin Transplant. 2009;23:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Grewal HP, Willingham DL, Nguyen J, Hewitt WR, Taner BC, Cornell D, Rosser BG, Keaveny AP, Aranda-Michel J, Satyanarayana R. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009;15:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Jay CL, Lyuksemburg V, Kang R, Preczewski L, Stroupe K, Holl JL, Abecassis MM, Skaro AI. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Yamamoto S, Wilczek HE, Duraj FF, Groth CG, Ericzon BG. Liver transplantation with grafts from controlled donors after cardiac death: a 20-year follow-up at a single center. Am J Transplant. 2010;10:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Dubbeld J, Hoekstra H, Farid W, Ringers J, Porte RJ, Metselaar HJ, Baranski AG, Kazemier G, van den Berg AP, van Hoek B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 47. | Deltenre P, Valla DC. Ischemic cholangiopathy. Semin Liver Dis. 2008;28:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006;44:806-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 51. | Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, Lyuksemburg V, Martin JA, Feinglass JM, Preczewski LB, Abecassis MM. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543-552; discussion 552-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 52. | Meurisse N, Vanden Bussche S, Jochmans I, Francois J, Desschans B, Laleman W, Van der Merwe S, Van Steenbergen W, Cassiman D, Verslype C. Outcomes of liver transplantations using donations after circulatory death: a single-center experience. Transplant Proc. 2012;44:2868-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Monbaliu D, Crabbé T, Roskams T, Fevery J, Verwaest C, Pirenne J. Livers from non-heart-beating donors tolerate short periods of warm ischemia. Transplantation. 2005;79:1226-1230. [PubMed] |
| 55. | Moench C, Moench K, Lohse AW, Thies J, Otto G. Prevention of ischemic-type biliary lesions by arterial back-table pressure perfusion. Liver Transpl. 2003;9:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Harring TR, Nguyen NT, Cotton RT, Guiteau JJ, Salas de Armas IA, Liu H, Goss JA, O’Mahony CA. Liver transplantation with donation after cardiac death donors: a comprehensive update. J Surg Res. 2012;178:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Hernandez-Alejandro R, Croome KP, Quan D, Mawardi M, Chandok N, Dale C, McAlister V, Levstik MA, Wall W, Marotta P. Increased risk of severe recurrence of hepatitis C virus in liver transplant recipients of donation after cardiac death allografts. Transplantation. 2011;92:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Jay C, Ladner D, Wang E, Lyuksemburg V, Kang R, Chang Y, Feinglass J, Holl JL, Abecassis M, Skaro AI. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant - an analysis of the national registry. J Hepatol. 2011;55:808-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 59. | Uemura T, Ramprasad V, Hollenbeak CS, Bezinover D, Kadry Z. Liver transplantation for hepatitis C from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2012;12:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Taner CB, Bulatao IG, Keaveny AP, Willingham DL, Pungpapong S, Perry DK, Rosser BG, Harnois DM, Aranda-Michel J, Nguyen JH. Use of liver grafts from donation after cardiac death donors for recipients with hepatitis C virus. Liver Transpl. 2011;17:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Richter S, Yamauchi J, Minor T, Menger MD, Vollmar B. Heparin and phentolamine combined, rather than heparin alone, improves hepatic microvascular procurement in a non-heart-beating donor rat-model. Transpl Int. 2000;13:225-229. [PubMed] |
| 62. | Le Dinh H, de Roover A, Kaba A, Lauwick S, Joris J, Delwaide J, Honoré P, Meurisse M, Detry O. Donation after cardio-circulatory death liver transplantation. World J Gastroenterol. 2012;18:4491-4506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | St Peter SD, Post DJ, Rodriguez-Davalos MI, Douglas DD, Moss AA, Mulligan DC. Tacrolimus as a liver flush solution to ameliorate the effects of ischemia/reperfusion injury following liver transplantation. Liver Transpl. 2003;9:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Pratschke S, Eder M, Heise M, Nadalin S, Pascher A, Schemmer P, Scherer MN, Ulrich F, Wolters H, Jauch KW. Protocol TOP-Study (tacrolimus organ perfusion): a prospective randomized multicenter trial to reduce ischemia reperfusion injury in transplantation of marginal liver grafts with an ex vivo tacrolimus perfusion. Transplant Res. 2013;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Ikegami T, Nishizaki T, Hiroshige S, Ohta R, Yanaga K, Sugimachi K. Experimental study of a type 3 phosphodiesterase inhibitor on liver graft function. Br J Surg. 2001;88:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Genovés P, García D, Cejalvo D, Martin A, Zaragoza C, Toledo AH, Toledo-Pereyra LH, Lloris-Carsi JM. Pentoxifylline in liver ischemia and reperfusion. J Invest Surg. 2014;27:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Ribeiro EA, Poli-de-Figueiredo LF, Vincenzi R, Galvao FH, Margarido N, Rocha-E-Silva M, Cruz RJ. Intraportal versus Systemic Pentoxifylline Infusion after Normothermic Liver Ischemia: Effects on Regional Blood Flow Redistribution and Hepatic Ischemia-Reperfusion Injury. HPB Surg. 2013;2013:689835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Carrel A, Lindbergh CA. The culture of whole organs. Science. 1935;81:621-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 69. | Dutkowski P, Furrer K, Tian Y, Graf R, Clavien PA. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006;244:968-976; discussion 976-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Boehnert MU, Yeung JC, Bazerbachi F, Knaak JM, Selzner N, McGilvray ID, Rotstein OD, Adeyi OA, Kandel SM, Rogalla P. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant. 2013;13:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | de Rougemont O, Breitenstein S, Leskosek B, Weber A, Graf R, Clavien PA, Dutkowski P. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009;250:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, Morovat A, Roy D, Winter L, Friend PJ. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 73. | Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 2013;59:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 74. | Roberts M. Rosen’s Emergency Medicine: Concepts and Clinical Practice, 5th ed. Emergency Medicine. 2003;15:196-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 75. | Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando). 2012;26:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Guarrera JV, Henry SD, Chen SW, Brown T, Nachber E, Arrington B, Boykin J, Samstein B, Brown RS, Emond JC. Hypothermic machine preservation attenuates ischemia/reperfusion markers after liver transplantation: preliminary results. J Surg Res. 2011;167:e365-e373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 78. | Guarrera JV, Polyak M, O’Mar Arrington B, Kapur S, Stubenbord WT, Kinkhabwala M. Pulsatile machine perfusion with Vasosol solution improves early graft function after cadaveric renal transplantation. Transplantation. 2004;77:1264-1268. [PubMed] |
| 79. | Ikeda T, Yanaga K, Lebeau G, Higashi H, Kakizoe S, Starzl TE. Hemodynamic and biochemical changes during normothermic and hypothermic sanguinous perfusion of the porcine hepatic graft. Transplantation. 1990;50:564-567. [PubMed] |
| 80. | Tolboom H, Izamis ML, Sharma N, Milwid JM, Uygun B, Berthiaume F, Uygun K, Yarmush ML. Subnormothermic machine perfusion at both 20°C and 30°C recovers ischemic rat livers for successful transplantation. J Surg Res. 2012;175:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 81. | Hessheimer AJ, Fondevila C, García-Valdecasas JC. Extracorporeal machine liver perfusion: are we warming up? Curr Opin Organ Transplant. 2012;17:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Schön MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, Schnoy NC, Neuhaus P. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114-123. [PubMed] |
| 83. | St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Butler AJ, Rees MA, Wight DG, Casey ND, Alexander G, White DJ, Friend PJ. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73:1212-1218. [PubMed] |
| 85. | Reddy SP, Brockmann J, Friend PJ. Normothermic perfusion: a mini-review. Transplantation. 2009;87:631-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Reddy S, Greenwood J, Maniakin N, Bhattacharjya S, Zilvetti M, Brockmann J, James T, Pigott D, Friend P. Non-heart-beating donor porcine livers: the adverse effect of cooling. Liver Transpl. 2005;11:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Vogel T, Brockmann JG, Coussios C, Friend PJ. The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplant Rev (Orlando). 2012;26:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Steen S, Sjöberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 462] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 89. | Izamis ML, Tolboom H, Uygun B, Berthiaume F, Yarmush ML, Uygun K. Resuscitation of ischemic donor livers with normothermic machine perfusion: a metabolic flux analysis of treatment in rats. PLoS One. 2013;8:e69758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 90. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 91. | Amador A, Grande L, Martí J, Deulofeu R, Miquel R, Solá A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | García-Valdecasas JC, Tabet J, Valero R, Taurá P, Rull R, García F, Montserrat E, González FX, Ordi J, Beltran J. Liver conditioning after cardiac arrest: the use of normothermic recirculation in an experimental animal model. Transpl Int. 1998;11:424-432. [PubMed] |
| 93. | Net M, Valero R, Almenara R, Barros P, Capdevila L, López-Boado MA, Ruiz A, Sánchez-Crivaro F, Miquel R, Deulofeu R. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am J Transplant. 2005;5:2385-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 94. | Reddy SP, Bhattacharjya S, Maniakin N, Greenwood J, Guerreiro D, Hughes D, Imber CJ, Pigott DW, Fuggle S, Taylor R. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77:1328-1332. [PubMed] |
| 95. | Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, Sato M, Medin J, Davidson BL, de Perrot M. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1:4ra9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 96. | Balogun E, Foresti R, Green CJ, Motterlini R. Changes in temperature modulate heme oxygenase-1 induction by curcumin in renal epithelial cells. Biochem Biophys Res Commun. 2003;308:950-955. [PubMed] |
| 97. | Laurence JM, Allen RD, McCaughan GW, Logan GJ, Alexander IE, Bishop GA, Sharland AF. Gene therapy in transplantation. Transplant Rev (Orlando). 2009;23:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Croome KP, Wall W, Chandok N, Beck G, Marotta P, Hernandez-Alejandro R. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver Transpl. 2013;19:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Callaghan CJ, Charman SC, Muiesan P, Powell JJ, Gimson AE, van der Meulen JH. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: a cohort study. BMJ Open. 2013;3:e003287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 100. | Vanatta JM, Dean AG, Hathaway DK, Nair S, Modanlou KA, Campos L, Nezakatgoo N, Satapathy SK, Eason JD. Liver transplant using donors after cardiac death: a single-center approach providing outcomes comparable to donation after brain death. Exp Clin Transplant. 2013;11:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Elaffandi AH, Bonney GK, Gunson B, Scalera I, Mergental H, Isaac JR, Bramhall SR, Mirza DF, Perera MT, Muiesan P. Increasing the donor pool: consideration of prehospital cardiac arrest in controlled donation after circulatory death for liver transplantation. Liver Transpl. 2014;20:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Hong JC, Yersiz H, Kositamongkol P, Xia VW, Kaldas FM, Petrowsky H, Farmer DG, Lipshutz G, Markovic D, Hiatt JR. Liver transplantation using organ donation after cardiac death: a clinical predictive index for graft failure-free survival. Arch Surg. 2011;146:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 103. | Detry O, Donckier V, Lucidi V, Ysebaert D, Chapelle T, Lerut J, Ciccarelli O, Pirenne J, Monbaliu D, De Roover A. Liver transplantation from donation after cardiac death donors: initial Belgian experience 2003-2007. Transpl Int. 2010;23:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | Jiménez-Galanes S, Meneu-Diaz MJ, Elola-Olaso AM, Pérez-Saborido B, Yiliam FS, Calvo AG, Usera MA, González MC, González JC, González EM. Liver transplantation using uncontrolled non-heart-beating donors under normothermic extracorporeal membrane oxygenation. Liver Transpl. 2009;15:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Pine JK, Aldouri A, Young AL, Davies MH, Attia M, Toogood GJ, Pollard SG, Lodge JP, Prasad KR. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. 2009;15:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Fujita S, Mizuno S, Fujikawa T, Reed AI, Kim RD, Howard RJ, Hemming AW. Liver transplantation from donation after cardiac death: a single center experience. Transplantation. 2007;84:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | ‘t Hart NA, der van Plaats A, Leuvenink HG, van Goor H, Wiersema-Buist J, Verkerke GJ, Rakhorst G, Ploeg RJ. Determination of an adequate perfusion pressure for continuous dual vessel hypothermic machine perfusion of the rat liver. Transpl Int. 2007;20:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Fuller BJ, Lee CY. Hypothermic perfusion preservation: the future of organ preservation revisited? Cryobiology. 2007;54:129-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Olschewski P, Gass P, Ariyakhagorn V, Jasse K, Hunold G, Menzel M, Schöning W, Schmitz V, Neuhaus P, Puhl G. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology. 2010;60:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Xu H, Lee CY, Clemens MG, Zhang JX. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation. 2004;77:1676-1682. [PubMed] |
| 111. | Jain S, Xu H, Duncan H, Jones JW, Zhang JX, Clemens MG, Lee CY. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology. 2004;48:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 112. | Carles J, Fawaz R, Hamoudi NE, Neaud V, Balabaud C, Bioulac-Sage P. Preservation of human liver grafts in UW solution. Ultrastructural evidence for endothelial and Kupffer cell activation during cold ischemia and after ischemia-reperfusion. Liver. 1994;14:50-56. [PubMed] |
| 113. | Manekeller S, Schuppius A, Stegemann J, Hirner A, Minor T. Role of perfusion medium, oxygen and rheology for endoplasmic reticulum stress-induced cell death after hypothermic machine preservation of the liver. Transpl Int. 2008;21:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Henry SD, Nachber E, Tulipan J, Stone J, Bae C, Reznik L, Kato T, Samstein B, Emond JC, Guarrera JV. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am J Transplant. 2012;12:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 115. | Yeung JC, Wagnetz D, Cypel M, Rubacha M, Koike T, Chun YM, Hu J, Waddell TK, Hwang DM, Liu M. Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol Ther. 2012;20:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 116. | Lu S, Yu Y, Gao Y, Li GQ, Wang XH. Immunological inhibition of transplanted liver allografts by adeno-associated virus vector encoding CTLA4Ig in rats. Hepatobiliary Pancreat Dis Int. 2008;7:258-263. [PubMed] |
| 117. | Sandovici M, Henning RH, van Goor H, Helfrich W, de Zeeuw D, Deelman LE. Systemic gene therapy with interleukin-13 attenuates renal ischemia-reperfusion injury. Kidney Int. 2008;73:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |