Published online Aug 12, 2014. doi: 10.5528/wjtm.v3.i2.37
Revised: March 25, 2014
Accepted: May 28, 2014
Published online: August 12, 2014
Processing time: 211 Days and 5.7 Hours
Metabolic reprogramming and altered energetics have become an emerging hallmark of cancer and an active area of basic, translational, and clinical cancer research in the recent decade. Development of effective anticancer therapeutics may depend on improved understanding of the altered cancer metabolism compared to that of normal cells. Changes in glucose transport and glycolysis, which are drastically upregulated in most cancers and termed the Warburg effect, are one of major focuses of this new research area. By taking advantage of the new knowledge and understanding of cancer’s mechanisms, numerous therapeutic agents have been developed to target proteins and enzymes involved in glucose transport and metabolism, with promising results in cancer cells, animal tumor models and even clinical trials. It has also been hypothesized that targeting a pathway or a process, such as glucose transport or glucose metabolism, rather than a specific protein or enzyme in a signaling pathway may be more effective. This is based on the observation that cancer somehow can always bypass the inhibition of a target drug by switching to a redundant or compensatory pathway. In addition, cancer cells have higher dependence on glucose. This review will provide background information on glucose transport and metabolism in cancer, and summarize new therapeutic developments in basic and translational research in these areas, with a focus on glucose transporter inhibitors and glycolysis inhibitors. The daunting challenges facing both basic and clinical researchers of the field are also presented and discussed.
Core tip: Reprogramming of metabolism has been recognized at the beginning of 21st century as an emerging hallmark of cancer. The Warburg effect is one of the major focuses in the reprogramming. We cannot fully understand or more effectively treat cancer without a better understanding of cancer metabolism. Targeting cancer metabolism, particularly glucose transport and glycolysis, has been shown to be effective in inhibiting cancer growth. This review summarizes recent progresses in developments of therapeutics inhibiting glucose transporters and glycolytic enzymes, provides key information associated with each inhibitor, discusses their promises and problems as well as future challenges and directions of the basic and translational research of the field.
- Citation: Qian Y, Wang X, Chen X. Inhibitors of glucose transport and glycolysis as novel anticancer therapeutics. World J Transl Med 2014; 3(2): 37-57
- URL: https://www.wjgnet.com/2220-6132/full/v3/i2/37.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v3.i2.37
Cancer has long been considered a group of diseases caused by genetic mutations and genetic mutations only. However, in recent decades, extensive biochemical and biological studies have convincingly demonstrated that cancers exhibit significantly reprogrammed metabolism, which plays important roles in tumorigenesis[1-6]. In some cases, altered metabolism may be not only the consequence of genetic mutations, but also a contributing factor or cause of tumorigenesis[7-9]. Cancer metabolic reprogramming and altered energetics have been recognized now as a hallmark of cancer[10].
The importance of metabolism in cancer was actually recognized long time ago. In the 1920s, the German biochemist Otto Warburg, studied glucose metabolism in cancer tissues. He found that, unlike in normal tissue, incubated cancer samples always switched from mitochondrial oxidative phosphorylation (OXPHOS) to cytosolic glycolysis even when oxygen was abundant[11]. This phenomenon of so-called aerobic glycolysis has been known as the Warburg effect[12-15]. Warburg went so far as to claim that the altered glucose metabolism was the cause of cancer. This hypothesis is called the Warburg theory of cancer. He speculated that due to some mitochondrial dysfunctions, mitochondria could not synthesize ATP and thus cells must switch to cytosolic glycolysis, leading to cancer formation[14,16]. Biological studies in recent decades have found that Warburg’s view on the cause of the switch was largely incorrect: many cancers switch to glycolysis even without any mitochondrial defects. New biological and biochemical studies in the past decades revealed that the switch from OXPHOS to glycolysis is not just for ATP synthesis but also for biomass synthesis[15,17], production of NADPH[15,18], a reducing agent needed to remove reactive oxygen species (ROS) generated by cancer cells’ accelerated metabolism, as well as synthesis of amino acids[15,19]. The Warburg effect appears to be a strategic move made by cancer cells to deal with multiple requirements for growth, survival, and proliferation in a microenvironment with numerous constraints.
Altered cancer metabolism has also been recognized as a potential target for cancer therapeutics. Glucose transport and glucose metabolism are significantly upregulated in cancer as revealed by the PET scan and other detection methods[20-24]. The reliance of cancer cells on glucose indicates that they are addicted to the Warburg effect or glucose[25-27]. As a result, cancer cells are more sensitive than normal cells to changes in glucose concentration and will die before normal cells[25-28]. The recognition of this vulnerability in cancer cells has led to targeting glucose transport and metabolism as a new anticancer strategy. Furthermore, although targeted anticancer drugs inhibit one or more proteins or enzymes, cancers demonstrate the ability to escape inhibition using redundant signaling pathway(s). It has been proposed that targeting a signaling pathway or a metabolic process, rather than a protein in a pathway, may be more effective in preventing drug resistance and prolonging treatment effectiveness[29,30]. Potential targets for this proposed new approach include glucose transport and glycolysis, the predominant glucose metabolic changes found in cancer cells.
It should be emphasized that targeting cancer metabolism is not an entirely novel strategy. Some of the earliest chemotherapy drugs, such as methotrexate, also target metabolism and show significant efficacy[31-33]. As we have accumulated more knowledge about cancer metabolism, we should be able to develop more successful anti-cancer-metabolism drugs. In the following sections, recently developed glucose transport and glycolysis inhibitors will be described.
In normal cells under aerobic conditions, OXPHOS is used to make ATP, the universal energy currency in all living organisms[34]. OXPHOS is used because it is the most efficient way for making ATP. For each molecule of glucose, approximately 34 molecules of ATP can be produced by OXPHOS[34]. However, OXPHOS can proceed only when oxygen is present and abundant, a condition called normoxia. When oxygen is lacking, a condition called hypoxia, cells are forced to shift to anaerobic glycolysis to maintain ATP synthesis and energy metabolism[35]. Due to rapid growth and proliferation, a large proportion of the cancer cells in a tumor are in a hypoxic condition and thus use glycolysis to make ATP and other essential biomass molecules such as ribonucleotides. The phenomenon of OXPHOS-to-glycolysis shift in cancer cells is called the Warburg effect[12-16]. Although the Warburg effect was observed more than 80 years ago, its interpretation is still controversial and evolving. Warburg thought that the effect was caused by mitochondrial dysfunctions and the effect is a forced alternative strategy for ATP synthesis. However, research in recent decades largely disagrees with this interpretation. Recently, it has been found that the switch in cancer cells is primarily for the synthesis of biomass (e.g., of RNA precursor and others)[17], the reducing agent NADPH[18], which is needed for clearing ROS, and the amino acid serine[19]. ATP synthesis seems not to be a rate-limiting factor. This conclusion is very different from Warburg’s and is based on the observation that although cancer cells upregulate all glycolytic enzymes, they switch pyruvate kinase (PK), the last enzyme in the glycolytic pathway, from a form with higher higher activity (PKM1) to that with lower activity, PKM2[36-39]. This change suggests that cancer cells do not want all the glucose obtained from the upregulated glucose transport to be converted to pyruvate, but rather diverts some glucose metabolic intermediates to other connected metabolic pathways, such as pentose phosphate pathway (PPP) for synthesis of biomass and reducing agents[17-19,40]. This also suggests that ATP synthesis is not the top priority of the upregulation of glucose transport and metabolism. On the other hand, since glycolysis is about 18 times less efficient compared to OXPHOS, cancer cells must drastically upregulate glycolysis to compensate for the low ATP production.
Currently, the Warburg effect is a very active cancer research area[13]. Targeting glucose metabolism and transport, has been proposed as an effective anticancer strategy[1,3]. Glycolysis, the key process of increased glucose metabolism in cancer cells, has been targeted both in vitro and in vivo[3,41,42]. Glycolysis genes are overexpressed in various cancers[35]. In addition to higher potentials for invasiveness and metastasis[43], the glycolytic switch in cancer also increases cancer’s sensitivity to external interference because of their higher dependence on aerobic glycolysis[25-28].
Glucose deprivation, a method traditionally used to reduce glucose concentration in cultured cells for metabolic studies, has been used frequently in cancer research[44-47]. Glucose deprivation limits glucose supply, forcing cancer cells to slow down proliferation or undergo apoptosis[48-50]. Blocking glucose transport or glycolysis is similar to glucose deprivation, suggesting the possibility of restricting glucose supply with glucose transport or glycolysis inhibitors as an anticancer strategy.
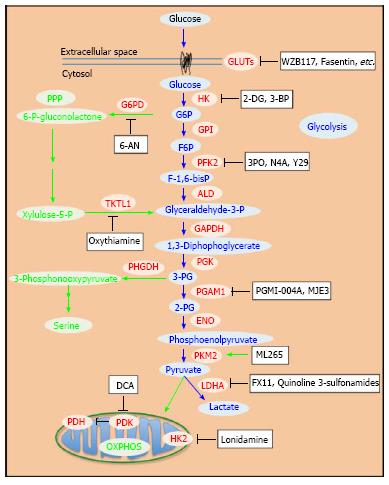
Various inhibitors of glycolytic enzymes have shown significant anticancer efficacy. Most of the reported glycolysis inhibitors are summarized (Table 1 and Figure 1). The enzymes targeted include hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), lactate dehydrogenase (LDH), and pyruvate dehydrogenase kinase (PDK). Related studies revealed that these inhibitors induced apoptosis in cancer cells[51,52]. Moreover, inhibition of glycolysis has been shown to overcome drug resistance in multiple cancer cells associated with mitochondrial respiratory defect and hypoxia[53]. Although numerous attempts to block glycolysis by using various inhibitors in cancer cells and in animal models have been made, developing clinically effective and safe glucose metabolism-targeting therapeutics is still a challenging task.
| Compound name | Target protein | Status | Ref. |
| 2-DG | Inhibits HK | Phase I-completed (Jul 2008) Phase I/II-terminated (Mar 2011) | NCT00096707 NCT00633087 |
| 3-BP | Inhibits HK | Pre-clinical | [66-74] |
| Lonidamine | Inhibits mitochondrial HK2 | Phase II/III-terminated (Aug/Dec 2006) | NCT00237536 NCT00435448 |
| 3PO | Inhibits PFK2 | Pre-clinical | [90] |
| N4A, YZ9 | Inhibits PFK2 | Pre-clinical | [91] |
| PGMI-004A | Inhibits PGAM1 | Pre-clinical | [96] |
| MJE3 | Inhibits PGAM1 | Pre-clinical | [98] |
| TT-232 | Inhibits PKM2 | Phase II-completed (Mar 2008) Phase II-terminated (Oct 2010) | NCT00422786 NCT00735332 |
| Shikonin/alkannin | Inhibits PKM2 | Pre-clinical | [108] |
| ML265 (TEPP-46) | Activates PKM2 | Pre-clinical | [116,117] |
| FX11 | Inhibits LDHA | Pre-clinical | [126] |
| Quinoline 3-sulfonamides | Inhibit LDHA | Pre-clinical | [141] |
| DCA | Inhibits PDK | Phase I-ongoing Phase I-ongoing Phase II-completed (Aug 2009) | NCT00566410 NCT01111097 NCT00540176 |
| 6-AN | Inhibits G6PD | Pre-clinical | [159-161] |
| Oxythiamine | Inhibits TKTL1 | Pre-clinical | [170-173] |
Hexokinase (HK) as the first enzyme in glycolysis phosphorylates glucose to glucose-6-phophate (G6P) irreversibly, which is a rate-limiting step. In cancer cells, type II HK (HK2) is bound to mitochondria, facilitating a high glycolytic flux rate and preventing cancer cell from apoptosis[54]. HK2 is required for cancer initiation and maintenance and the systemic deletion of HK2 is therapeutic in mice bearing tumors[55]. Thus, targeting HK2 may be an effective anti-cancer strategy.
2-deoxy-D-glucose (2-DG) is one of the most widely studied HK inhibitors. 2-DG is a glucose analog with a hydrogen group instead of a hydroxyl group in position 2 of glucose. Due to its structural similarity, 2-DG competes with glucose and inhibits HK with a Ki of 0.25 mmol/L[56]. The product 2-deoxy-D-glucose-6-phosphate made from 2-DG cannot be processed in the following glycolytic steps and therefore blocks glycolysis, leading to ATP depletion, cell cycle arrest and cell death[57,58]. Synergistic studies combining 2-DG and other anti-cancer drugs, such as adriamycin and paclitaxel, indicated that 2-DG is effective in vivo in combination with other drugs[59]. 2-DG sensitizes glioblastoma cells to other anti-cancer treatments and radiation[60-63]. Though effective, 2-DG is relatively toxic with side effects when administered to patients[61,64]. This is at least in part because 2-DG has to be used at high concentrations, around and higher than 5 mmol/L, in order to compete with blood glucose[65].
3-bromopyruvate (3-BP) is another HK inhibitor which has been shown to inhibit the progression of tumors in vivo[66-68]. 3-BP also increases the total ROS in tumor cells[69,70]. A recent study demonstrated that 3-BP inactivates ABC transporters, restoring drug sensitivity in cancer cells[71]. 3-BP has also been studied in combination with various anti-cancer drugs for synergistic effects, and it has been found to be effective in vitro[72] and in vivo[73], although with some hepatotoxicity[74]. However, 3-BP inhibits other enzymes, such as GAPDH, as well[75]. Up to now, no clinical trials have been reported for 3-BP. This may be attributed to its low target specificity and relatively high toxicity.
Lonidamine specifically inhibits mitochondria-bound HK2, which is present mostly in cancer cells but not in normal cells[76]. It effectively inhibits the cell growth, decreasing lactate and ATP generation, in cancer cells[77,78]. Meanwhile, the combination of lonidamine with other anti-cancer agents reverts drug resistance and is effective in the treatment of various cancer cells in both pre-clinical and phase II/III studies[78-80]. However, the combination of lonidamine and epirubicine resulted in no improvement in patients’ survival[81]. Though lonidamine has been widely studied, its hepatotoxicity resulted in the termination of several clinical trials[82,83]. These studies of the HK2 inhibitors suggest that, although HK2 is a potential target, being the first and the rate-limiting step of glycolysis, inhibition of HK2 may result in severe side effects. However, the combination of HK2 inhibitors and other anti-cancer drugs may still be an alternative approach for HK2-overexpressing tumors.
Phosphofructokinase (PFK) has two isoforms. PFK1 promotes the chemical reaction of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F-1,6-bisP), while PFK2 catalyzes the synthesis of fructose-2,6-bisphosphte (F-2,6-biP) and reverses it back to F6P[84]. In tumor cells, PFK2 is ubiquitously and constitutively active to produce F-2,6-biP[85-87]. PFK2 is also inducible by hypoxia in vivo[86,88], which is known as a microenvironment for tumor cell[89]. Thus, targeting PFK may be a good anti-cancer strategy.
3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) is the most specific known PFK2 inhibitor with a Ki of 25 μmol/L[82,90]. 3PO suppresses glucose uptake and glycolytic flux in multiple cancer cell lines, with IC50 values ranging from 1.4 to 24 μmol/L[90]. Animal studies show that 3PO inhibits tumor growth in vivo[90]. In addition, a chromene derivative, N4A, mimics F6P and is a competitive inhibitor of PFK2, with a Ki of 1.29 μmol/L[91]. Its derivative, YZ9, has a Ki as low as 0.094 μmol/L[91]. These inhibitors were shown to inhibit the proliferation of Hela cells (human cervical cancer cells) and T47D cells (human adenocarcinoma cells) in vitro[91]. Using high-throughput screening and structure activity relationship (SAR) studies, Brooke et al[92] identified derivatives of 5-triazolo-2-arylpyridazinone as a novel group of inhibitors of PFK2, with the lowest IC50 of 2.6 μmol/L. Although these inhibitors with extremely low IC50s are potent and promising in vitro, in vivo studies are required to assess their toxicity in animals.
3-phosphoglycerate dehydrogenase (PHGDH) catalyzes the first step of the serine biosynthesis pathway (Figure 1). The increased serine synthesis flux attributed to PHGDH is essential to the viability of a subset of cancer cells in which the enzyme is overexpressed[19,93,94]. Through negative-selection RNAi screening using a human breast cancer xenograft model, Possemato et al[93] showed that PHGDH is required for tumorigenesis in vivo. Meanwhile, using a metabolomics approach with isotope labeling, Locasale et al[19] showed that glycolytic flux is diverted into amino acid (serine and glycine) metabolism in cancer cells. This suggests that cancer cells use this specific pathway to promote oncogenesis. The PHGDH gene was found to be amplified recurrently in both breast cancers and melanoma[19,93,95]. In addition, the protein levels of PHGDH are upregulated in 70% of estrogen receptor (ER)-negative breast cancers[93]. Suppression of PHGDH in cancer cell lines with overexpressed PHGDH, but not in these without, causes a reduction in serine synthesis as well as cell proliferation[19,93]. So far, no PHGDH inhibitors have been reported, although it appears to be a good target.
Phosphoglycerate mutase 1 (PGAM1) catalyzes 3-phosphoglycerate (3-PG) to 2-phosphoglycerate (2-PG). In human cancer cells, loss of TP53 leads to upregulation of PGAM1[96]. In addition, Tyr26 phosphorylation of PGAM1 stabilizes the active conformation of the enzyme[97]. These regulations of PGAM1 contribute to the increased glycolysis and the rapid biosynthesis in cancer cells[96,97].
Inhibition of PGAM1 by shRNA increased 3-PG and decreased 2-PG levels and inhibited the proliferation of cancer cells[96]. Through in situ proteome reactivity profiling, PGAM1 inhibitor MJE3 was identified[98]. MJE3 inhibits PGAM1 activity with an IC50 of 33 μmol/L and reduces the proliferation of breast cancer cells in vitro[98]. PGMI-004A, an alizarin derivative, is another inhibitor of PGAM1 with an IC50 of 13 μmol/L, and it leads to significantly decreased glycolysis, pentose phosphate pathway (PPP) flux and biosynthesis, resulting in attenuated cancer cell proliferation and tumor growth in vivo[96].
Pyruvate kinase (PK) irreversibly catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate coupled with the generation of ATP. PKM2 is the isoform highly expressed in embryonic cells and cancer cells during fast proliferation[99]. The switch of PKM2 to PKM1 was able to inhibit tumor growth in vivo[36]. PKM2 is inactive as a dimer and highly active as a tetramer. Regulation of the transition between the dimer and the tetramer forms depends on the F-1,6 bisP level[100] or the phosphorylation of tyrosine residue 105 of PKM2, which is induced by oncogenic signals in cancer cells[38]. Meanwhile, PKM2 activity is further influenced by serine and succinylaminoimidazolecarboxamide ribose-5’-phosphate (SAICAR), which adds additional complexity to the regulation of PKM2 in cells and suggests that the modulation of PKM2 activity enables cancer cells to adapt their unique metabolic patterns to their specific pathological conditions[38,101].
In tumor cells, the lower activity of PKM2 results in accumulation of upstream glycolytic metabolites for biosynthesis through PPP[37,102]. In addition, the presence of histidine-phosphorylated PGAM1 has been found to correlate with the expression of PKM2 in both cancer cell lines and tumors[103]. In fact, cancer cells with low PKM2 activity allow PEP to transfer its phosphate group to the histidine of PGAM1 and generate pyruvate. This alternate glycolytic pathway bypasses the activity of PKM2 and decouples ATP production from pyruvate generation, facilitating the high rate of glycolysis to support the biosynthesis observed in many proliferating cancer cells[103]. This decoupled ATP production also suggests that ATP may not be the limiting factor for fast proliferation in cancer cells because cancer cells have access to increased interstitial ATP[104-106].
Recently, Israelsen et al[107] demonstrated that PKM2 is not necessary for the proliferation of tumor cells and variable PKM2 expression was found in human tumors. These results suggest that varied PKM2 activity supports the different metabolic requirements of various cancer cells, each with unique metabolic conditions[107]. Though the role of varied expression of the PKM2 isoform in cancer cells is still controversial, ongoing studies focus on both inhibitors and activators of PKM2 to inhibit cancer cell growth both in vitro and in vivo.
Shikonin and alkannin are potent PKM2 inhibitors. Both compounds lower PKM2 activity and decrease glycolysis in MCF-7 human breast cancer cells and A549 human lung cancer cells[108]. TT-232, a synthetic heptapeptide, interferes with the cellular location of PKM2 in tumor cells and induces apoptosis[109]. However, the selectivity of these inhibitors is not very high for PKM2 and side effects were observed[110,111].
In fact, PKM2 was found to be less active than PKM1[36], indicating that cancer cells prefer to use a less active PK to regulate glycolysis and balance their metabolic needs. Thus, in order to inhibit cancer cell growth more effectively, activators, not inhibitors of PKM2, should be used.
Activators of PKM2, such as N, N’-diarylsulfonamides, thieno-pyrrole-pyridazinones and tetrahydroquinoline-6-sulfonamides, have been identified and studied through high throughput screening and SAR exploration[112-114]. These compounds showed potent PKM2 activation activity with a highest AC50 of 38 nmol/L[112]. Kung et al[115] reported a series of quinolone sulfonamides with a unique allosteric binding mode, which activate PKM2 in A549 lung carcinoma cells. The activation of PKM2 reduces carbon flow to serine biosynthesis, which has been known to promote oncogenesis[19,115]. This study suggests that targeting PKM2 confers metabolic stress to cancer cells and attenuates the unique metabolic pattern of cancer cells. Among these compounds, ML265 (or TEPP-46), a potent activator of PKM2 with an AC50 of 92 nmol/L, was found to activate PKM2 by inducing the tetramerization of PKM2[116,117]. ML265 has been shown to reduce tumor size, weight, and occurrence in animal models[116,117]. Recently, Xu et al[118] described a structurally novel series of small molecule 3-(trifluoromethyl)-1H-pyrazole-5-carboxamides as potent PKM2 activators in vitro. Moreover, Guo et al[119] identified 2-((1H-benzo[d]imidazol-1-yl)methyl)-4H-pyrido (1,2-a) pyrimidin-4-ones as novel activators of PKM2 with a unique binding mode. However, their results also suggested that activation of PKM2 alone was insufficient to significantly alter the cancer metabolism[119]. Although the complex roles of PKM2 in tumorigenesis remain to be elucidated, potent and selective activators of PKM2 may be valuable tools for solving the puzzle of PKM2 and combating cancer.
Lactate dehydrogenase (LDH) catalyzes the chemical conversions of pyruvate to lactate and NADH to NAD+ simultaneously. Upregulation of LDHA under c-Myc control promotes aerobic glycolysis and the growth of tumor cells[120]. Increased expression of LDHA was identified in clinical samples of multiple tumor types[121,122]. Inhibition of LDHA expression in fumarate hydratase deficient cells by RNA interference inhibited cell proliferation and tumorigenesis in vivo[42,123]. Thus, LDHA is a potential anti-cancer target with multiple inhibitors already developed[124].
Oxamate competes with pyruvate for LDHA binding with a Ki of 136 μmol/L[125]. However, oxamate also works as an inhibitor of aspartate aminotransferase with an even lower Ki of 28 μmol/L[125]. Thus, oxamate is a non-specific inhibitor of LDHA. FX-11,3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid, competing with NADH as a selective inhibitor of LDHA, inhibited the growth of xenograft tumors[126].
Galloflavin, a new LDHA inhibitor, reduced ATP generation, lactate production, and inhibited growth of human breast cancer cells. However, other mechanisms in addition to inhibition of LDHA were involved in cell death induced by galloflavin[127]. Moorhouse et al[128] used a fragment-based click-chemistry-supported approach to synthesize a series of bifunctional inhibitors of LDHA. In this approach, the structures of both natural substrates pyruvate and NADH were mimicked and linked together in a bifunctional inhibitor. The lead compound has an IC50 of 14.8 μmol/L. ARIAD Pharmaceuticals and Genentech recently have identified numerous LDHA inhibitors[129-132], and Ward et al[133] have identified plant-derived human LDHA inhibitors through high-throughput screening. However, these inhibitors need to be tested in vitro and in vivo in due course. Ward et al[134] used fragment-based lead generation as well as X-ray crystallography to develop very potent inhibitors of LDHA. The lead compound has a remarkable IC50 of 0.27 μmol/L. However, these potent LDHA inhibitors still need to be tested both in vitro and in vivo to demonstrate their potentials as anti-cancer therapeutics.
Granchi et al[135] designed and synthesized a series of N-hydroxyindole (NHI)-based compounds as competitive human LDHA inhibitors. Some representative compounds were tested and shown to possess anti-proliferation activity in multiple human cancer cell lines[136-138]. NHI-1, one of these inhibitors, working with gemcitabine is active against pancreatic cancer cells synergistically[139]. Interestingly, glycosylation of these NHI-based LDHA inhibitors increased potencies and improved cell permeability in cancer cells[140]. Linking the glucose and the LDHA inhibitor facilitates the dual-targeting strategy.
Recently, Billiard et al[141] showed that quinoline 3-sulfonamides inhibit LDHA and reverse the Warburg effect (aerobic glycolysis) in multiple cancer cell lines. Interestingly, compound 1, an LDHA inhibitor in this study, also activates PKM2, if not directly, then at least in part due to the accumulation of F-1,6-bisP caused by LDHA inhibition. Unfortunately, because of low in vivo clearance rates and low oral bioavailability, the quinolone 3-sulfonamides are unsuitable for in vivo use[141]. In sum, though several LDHA inhibitors have been identified, further efforts are needed to test their anti-cancer effects in vivo as well as in clinical trials.
Pyruvate dehydrogenase kinase (PDK) favors glycolysis over mitochondrial oxidative phosphorylation (OXPHOS) by blocking the activity of pyruvate dehydrogenase (PDH) by phosphorylating it[142]. Under normal oxygen pressures, pyruvate goes to mitochondria and is converted to acetyl-CoA in a step catalyzed by PDH. Acetyl-CoA is an important metabolite involved in the citric acid cycle and OXPHOS. In studies in cancer cells, PDK1 expression was induced by HIF-1 in hypoxic conditions and shown to lead to increased glycolysis and suppressed OXPHOS[143,144]. The expression of PDK1 is associated with poor prognosis in head-and-neck squamous cancer[145]. Also, the upregulation of PDK in cancer was associated with a more aggressive phenotype[146]. For these reasons, PDK has been considered an attractive and promising anti-cancer target.
Dichloroacetate (DCA), an analog of pyruvate, has been identified as a PDK inhibitor and widely studied for its ability to inhibit lactate production and cancer growth[147-151]. DCA decreases lactate production by shifting the pyruvate metabolism from glycolytic fermentation towards mitochondrial OXPHOS, and restores mitochondrial function, thus potentially restoring apoptosis-induction, allowing cancer cells to undergo programmed cell death and shrink the tumor[53]. DCA’s research and clinical trials were based on the belief that cancer cells’ mitochondrial function is abnormal and therefore cancer cell growth will be reduced by upregulating and normalizing their OXPHOS. DCA was shown to be effective in suppressing the growth of cancer cells both in vitro and in vivo[152-155]. Several human clinical trials of DCA started after the successful cell and animal studies and still on-going. A phase II clinical trial for malignant glioblastoma has been completed and shows that DCA can be used safely in patients with glioblastoma, suggesting that DCA is a promising anti-cancer agent and inhibiting glycolysis is a potent and effective anti-cancer strategy[156] (Table 1). In addition, several clinical trials combining DCA and other anti-cancer drugs or therapies are in progress. On the other hand, human studies indicate that DCA’s anticancer effects, if any, may be cancer type-related. More basic biomedical studies need to be conducted on the compound before DCA’s anticancer activity can be better evaluated.
Pentose phosphate pathway (PPP), a metabolic pathway branched off from glycolysis, provides metabolic intermediates for biosynthesis and NADPH for clearing ROS in cells. At the first step of PPP, glucose-6-phosphate dehydrogenase (G6PD) catalyzes the conversion of G6P to 6-phophogluconolactone, coupled with generation of NADPH. G6PD has been shown to be overexpressed in cancer cells[157,158]. Therefore, inhibition of G6PH is an attractive strategy to alter cancer metabolism and attenuate cancer growth. 6-aminonicotinamide (6-AN) is an inhibitor of G6PD that induces oxidative stress and sensitizes cancer cells to drugs[159-161]. Recently, Preuss et al[162] used high-throughput screening to identify several hit compounds as novel inhibitors of G6PD with IC50s of < 4 μmol/L. These G6PD inhibitors reduced the viability of MCF10-AT1 mammary carcinoma cells with an IC50 of approximately 25 μmol/L compared to approximately 50 μmol/L for MCF10-A non-carcinoma cells[162]. However, its in vivo efficacy remains to be investigated.
The enzyme transketolase (TKTL) is critical for both PPP and glycolysis[157,163]. Transketolase-like enzyme 1 (TKTL1) has been shown to be increased in tumor cells[164-166]. Down-regulation of TKTL1 inhibited cancer cell proliferation, tumor growth and metastasis[167-169]. Thus, inhibiting TKTL1 is a potential anti-cancer strategy. Oxythiamine inhibits TKTL and the growth of cancer cells both in vitro and in vivo[170,171]. Also, oxythiamine interrupted signaling dynamics in pancreatic cancer cells[172], and attenuated tumor cell metastasis[173]. Further studies on oxythiamine are of interest.
Up to 90% of cancers demonstrate a phenotype of increased glucose uptake, as revealed by PET scan and other detection methods[21,23,174,175]. Cancer cells also show an increased dependence on glucose as a source of energy and biosynthesis precursor for cell growth, while normal cells utilize lipids, amino acids and glucose in a more balanced fashion[25,43]. Increased glucose uptake in cancer is achieved primarily by upregulation of glucose transporters (GLUTs)[176-179] although the recent finding that animal cells transformed with a mutated (oncogenic) KRas gene exhibit macropinocytosis[105] raises the possibility that macropinocytosis and other endocytosis may contribute significantly to glucose uptake in cancer cells. Current research finds that upregulation of GLUTs can be attributed to oncogenic alterations in cancer cells[180].
GLUTs (SLC2A) are plasma membrane-associated transporters that facilitate glucose transport across the cell membrane down the glucose concentration gradients[181]. Up to now, at least 14 different isoforms of GLUTs have been identified in human cells (Table 2)[182]. All GLUTs share a common and highly conserved (97%) transmembrane domain composed of twelve membrane-spanning helices with less conserved and asymmetric extracellular and cytoplasmic domains[183-185]. Different isoforms of GLUTs are structurally and functionally related proteins and divided into 3 classes according to the similarity of their amino acid sequences[182]. They are expressed in various cell types based on cells’ unique physiological requirements for glucose (Table 2)[176]. This differential need and thus transport of glucose is achieved by varied affinities of the GLUTs for glucose[176,186].
| Protein | Class | Expression | Affinity to glucose | Major features | Expression in cancer |
| GLUT1 | I | Ubiquitous (abundant in brain and erythrocytes)[207] | High[201,208,211] | Constitutive basal glucose uptake[207] | Over-expressed[176,203] |
| GLUT2 | I | Liver, retina, pancreatic islet cells[176,198] | Low[201,211] | Glucose sensing, fructose transport[176,200] | Abnormal[176,202-204] |
| GLUT3 | I | Brain[196] | High[201,211] | Supplements GLUT1 in brain[176,196] | Over-expressed[176,205] |
| GLUT4 | I | Muscle, fat, heart[210] | High[208,209,211] | Insulin responsive[210] | Abnormal[188] |
| GLUT5 | II | Intestine, testis, kidney, erythrocytes[213,214] | Very low[212] | Fructose transport[212] | Abnormal[176,203] |
| GLUT6 | III | Spleen, leukocytes, brain[215] | Low[215] | Sub-cellular redistribution[216] | UD[203] |
| GLUT7 | II | Liver, intestine, colon, testis, prostate[216,217] | High[217] | Glucose and fructose transport[217] | ND |
| GLUT8 | III | Testis, brain[219] | High[219] | Sub-cellular redistribution, multisubstrates[216] | Over-expressed[218] |
| GLUT9 | II | Liver, kidney, pancreatic cells[220,222] | High[221] | Multisubstrates[216] | UD[203] |
| GLUT10 | III | Liver, pancreas[223] | High[224] | Glucose transport[224] | ND |
| GLUT11 | II | Heart, muscle[225] | Low[225] | Inhibited by fructose[225] | ND |
| GLUT12 | III | Heart, prostate, muscle, fat, intestine[226] | High[227] | Insulin-reponsive[226] | Abnormal[206] |
| HMIT | III | Brain[228] | No | H+/myo-inositol transport[228] | ND |
| GLUT14 | I | Testis[229] | ND | ND | ND |
GLUTs that are most relevant to cancer are GLUT1 and GLUT3[176,187,188]. GLUT1 is a basal glucose transporter expressed in almost all cell types[189] and is upregulated in almost all cancer types examined[176-179]. PET scans and other analytical methods have revealed membranous overexpression of GLUT1 and increase in glucose uptake by cancer cells[175]. GLUT1 expression level is correlated with the grade, proliferative activity, differentiation, and known prognostic markers in various cancers[175,190-192]. Clinical studies also have shown that high levels of GLUT1 expression correlates with poor prognosis and survival[192-195]. Normally, GLUT3 is expressed primarily in the tissues with high energy demand to supplement GLUT1[176,196]. GLUT3 is over-expressed in various cancers compared with their non-cancerous tissues[176,187,188,197]. GLUT2 is expressed in the liver, pancreatic islet cells, and retina cells[176,198]. GLUT2 has low affinity and high capacity for glucose[199,200]. GLUT2 also has high affinity for fructose[201]. Abnormal levels of GLUT2 expression were detected in gastric, breast, and pancreatic cancers[202-204]. In addition, GLUT4, GLUT5 and GLUT12 have been found to be abnormally expressed in various cancers[187,188,203,205,206].
Transport of glucose from the extracellular space into the cytoplasm is the first rate-limiting step for glycolysis. Glucose metabolism is drastically upregulated in cancer. Thus, inhibition of aerobic glycolysis by blocking glucose uptake may be more efficient than inhibiting glycolytic enzymes in cells. Therefore, GLUTs are potential targets for anti-cancer therapies. All known glucose transporters and their major characteristics are summarized in Table 2.
The rapid growth and proliferation of cancer cells require a large amount of fuel, primarily and preferentially glucose. Numerous clinical and basic science studies have shown that glucose transport is upregulated in various cancers, by overexpressing GLUTs[195,203,230-233]. Studies have identified GLUT1 and GLUT2 as the main glucose transporters in hundreds of tumors[203]. GLUT1 expression was the most widely distributed, while GLUT2 was mainly expressed in breast, colon, and liver carcinomas[203]. Upregulated GLUT3 protein expression was also detected in endometrial, breast and thyroid cancers[233,234]. Recently, constitutive cell membrane localization of GLUT4 was found in myeloma cells[235,236]. Because GLUTs increase glucose transport and enhance cancer cell growth, survival and drug resistance, they are good targets for cancer therapeutic intervention.
GLUT1 is the most widely expressed glucose transporter in different types of cancers[189,194,237,238]. However, GLUT1 was not targeted therapeutically until recently. This is not because GLUT1 is not a good target but because of the lack of specific and potent inhibitors. Anti-GLUT1 antibody was shown to be effective in reducing cancer cell growth in vitro, and the antibody treatment also resulted in cell cycle arrest of the cancer cells[239]. Before and after the report of the GLUT1 antibody, several small molecule GLUT1 inhibitors have been reported. They will be described individually below.
Liu et al[177] recently reported the identification of a group of novel small compounds that inhibit basal glucose transport by cancer. WZB117 is one of the small molecules that best inhibited GLUT1 and cancer cell growth in vitro and in vivo. Its anticancer efficacy and safety was demonstrated in a tumor model of human A549 lung cancer cells in nude mice[28]. Daily intraperitoneal injection of WZB117 at 10 mg/kg reduced tumor size by more than 70%. Mechanism studies showed that WZB117 inhibited glucose transport in human red blood cells (RBC), in which GLUT1 is the only glucose transporter expressed[28]. This conclusively shows that WZB117 inhibits GLUT1. However, it is presently unclear if WZB117 also inhibits other GLUTs. Computer docking studies show that WZB117 binds directly to GLUT1 using three hydrogen bonds with amino acid residues Asn34, Arg126, and Trp412 of the protein[28]. Treatment with WZB117 resulted in changes in levels of GLUT1 protein, intracellular ATP, and related metabolic enzymes such as AMPK in cancer cells, leading to cell-cycle arrest, senescence, and necrosis in red blood cells and tumor cells (IC50 = 10 μmol/L). Synergistic effect with cisplatin and paclitaxel was also demonstrated[28]. A new generation of GLUT1 inhibitors based on the structure of WZB117 but with higher potency and stability are being synthesized and tested.
A small molecule named STF-31 that selectively targets von Hippel-Lindau (VHL) -deficient renal cell carcinoma (RCC) cells was reported by Chan et al[240]. They demonstrated that STF-31 inhibits VHL-deficient cancer cells by inhibiting GLUT1. It was shown that daily intraperitoneal injection of a soluble analogue of STF-31 effectively reduced the growth of tumors of VHL-deficient RCC cells in nude mice[240]. STF-31 specifically targets RCCs because aberrant HIF stabilization regulated by VHL leads to diminished mitochondrial activity in these cells, causing them to become highly dependent on glucose uptake for glycolysis and ATP production. By directly binding GLUT1 and inhibiting glucose uptake, STF-31 targets an RCC-specific vulnerability with limited toxicity to normal kidney cells, which are strictly dependent on neither glycolysis nor GLUT1[240]. Nevertheless, the target spectrum of STF-31 appears to be relatively narrow. The successful animal studies using WZB-117 and STF-31 show in vivo potential of GLUT1-targeting.
Fasentin was first identified as a compound that enhances the death receptor stimuli FAS-mediated cell death in FAS-resistant cancer cells in 2006[241]. Its mechanism of action was further delineated when altered expression of genes associated with nutrient and glucose deprivation were detected[242]. Culturing cells in low-glucose medium led to similar effects of fasentin and sensitized cells to FAS, supporting the conjecture that fasentin inhibits glucose uptake[242]. Computer docking studies suggest fasentin interacts with a unique site on the intracellular domain of GLUT1[242]. The role of fasentin as a chemical sensitizer through glucose transport inhibition was further supported by additional chemical studies[242]. However, no in vivo study has been reported for fasentin.
Apigenin is a natural flavonoid compound existing abundantly in common fruits and vegetables[243]. Previous studies have demonstrated apigenin’s anti-mutagenic, anti-oxidant, anti-cancer, and anti-inflammatory activities[244-247]. In a mechanism study, apigenin was shown to inhibit glucose uptake in a dose-dependent manner (in the 10-100 μmol/L range) in CD18 and S2-013 human pancreatic cancer cell lines[248]. Apigenin was determined to achieve this effect by inhibiting GLUT1 at both mRNA and protein levels[248]. This was further investigated with PI3K inhibitors whose inhibitory effects on GLUT1 mRNA and protein expression are similar to apigenin’s, suggesting that apigenin targets GLUT1 through a PI3K/Akt related pathway[248]. Thus, apigenin inhibits GLUT1 indirectly.
Genistein, an isoflavone, is a natural product present in plants such as soybeans[249,250]. It is a known tyrosine kinase inhibitor and has been shown to exhibit therapeutic effects against a variety of health disorders such as obesity, diabetes and cancer, making it a promising agent for the treatment of metabolic diseases[251,252]. Genistein is also reported to be a potent inhibitor of GLUT1[253,254]. It inhibits the transport of hexose and dehydroascorbic acid through GLUT1 in human HL-60 cells in a dose-dependent fashion[253]. Further investigation demonstrated that genistein binds to the external surface of GLUT1, altering the binding of glucose to the external surface site of GLUT1[254]. However, genistein does not appear to be specific for GLUT1.
Recently, a group of oxime-based GLUT1 inhibitors have been reported[255]. These compounds possess a basic chemical structure different from either phloretin, WZB-117 or other reported GLUT1 inhibitors, and thus represent a novel group of GLUT1 inhibitory compounds. Some of these compounds are as potent as WZB117 in inhibiting glucose transport and cell proliferation in cancer cells[255]. A detailed computer simulation study revealed the potential binding site for these compounds on GLUT1, which appears to be consistent with that reported for 17β-estradiol and genistein[256]. The simulation result and basic structure of these compounds provide bases for designing next generation GLUT1 inhibitors.
Using high-throughput screening coupled with ATP, cell cycle arrest, and lactate assays, two potent GLUT1 inhibitory compounds were identified[257]. These compounds inhibit glucose transport mediated by erythrocyte membrane-derived vesicles with Ki values of 1.2 and 0.8 μmol/L, respectively[257]. These compounds are GLUT1 inhibitors because only GLUT1 is expressed on erythrocytes. However, no in vivo study has been reported for these intriguing compounds.
Phloretin, a natural compound found in fruits such as apples and pears, is reported to be a GLUT2 inhibitor[258-260]. Phloretin has been shown to retard tumor growth both in vitro and in vivo and induce apoptosis in leukemia, melanoma, and colon cancer cells[261-263]. Results from human hepatocellular carcinoma HepG2 cells, which express high levels of GLUT2, suggest that phloretin-induced apoptosis involves inhibition of GLUT2-mediated glucose transport[258]. Additional studies showed that the inhibitory properties of phloretin on GLUT2 sensitize cancer cells to paclitaxel, illustrating the potential use of phloretin in cancer therapy[264].
Quercetin is a flavonoid compound in fruits, vegetables and grains. It was found to be an effective non-competitive GLUT2 inhibitor in Xenopus oocytes with a Ki of 22.8 μmol/L[265]. In rats administered glucose, quercetin inhibits glucose absorption through GLUT2[265]. Quercetin was also suggested to reduce the risk of lung cancer and other types of cancer[266-268]. Quercetin aglycone was shown to affect some receptors associated with cancer development and modulate some signaling pathways involved in inflammation and carcinogenesis[266], although no direct evidence links between inhibition of GLUT2 and cancer prevention. More studies are needed to explore the connection. Quercetin is likely to be a non-specific GLUT2 inhibitor since its anticancer activity cannot be completely explained by its GLUT2 inhibitory activity.
Some DNA-damaging anticancer agents including adriamycin, camptothecin and etoposide were reported to induce cancer cell death by reducing GLUT3 expression in HeLa cells[269]. Real-time PCR results in Hela cells and a tumorigenic HeLa cell hybrid showed that only the expression of GLUT3, rather than GLUT1, was suppressed by these medicines[269]. Mechanism studies suggested that the suppression of GLUT3 expression induced by DNA-damaging agents was through the MEK-ERK pathway in a p53-independent manner[269].
Recently, certain glycogen synthase kinase-3 (GSK-3) inhibitors were identified as inhibitors of GLUT3 expression in GLUT3-overexpressing tumorigenic HeLa hybride cells as compared with non-tumorigenic counterparts that express GLUT1 alone[270]. These inhibitors decreased GLUT3 expression at the transcriptional level through NF-κB signaling in a p53-independent fashion, leading to apoptotic cell death[270]. Thus, GSK-3 inhibitors do not interact with GLUT3 protein directly but reduce GLUT3 expression levels. No small molecule inhibitors of GLUT3 protein have been reported.
Several HIV protease inhibitors were reported to exhibit inhibitory effects on GLUT4: the most potent is ritonavir[235,271,272]. The effects of ritonavir against myeloma cells were investigated in vitro[235]. It was demonstrated that the inhibitory effects of ritonavir were achieved by suppressing the glucose consumption mediated by GLUT4 in myeloma cells, which overexpress GLUT4, as well as localize it to the basal cell surface[235]. The specificity of ritonavir for GLUT4 was confirmed by artificially introducing GLUT1-mediated glucose uptake, which resulted in resistance to prolonged ritonavir treatment[235]. Half of the cell death induced by ritonavir was seen at a concentration of 20 μmol/L[235]. These and other study results highlight the therapeutic potential of ritonavir in mediating GLUT4 inhibition in myeloma treatment[235,272]. Ritonavir has also been investigated for treatment of other types of cancer[273-275] and undergone clinical trials (ClinicalTrials.gov Identifier: NCT01009437, NCT01095094).
Silibinin, also known as silybin, is a natural flavonoid recently shown to be a GLUT4 inhibitor[276,277]. Kinetic analysis revealed that silybin is a competitive inhibitor of GLUT4, modulating glucose transport in CHO cells with a Ki of 60 μmol/L[276]. Inhibitory effects of silibinin on cancer growth have been demonstrated in preclinical models[278,279] and tested in clinical Phase I[280,281] and Phase II trials (ClinicalTrials.gov Identifier: NCT00487721) for prostate cancer, indicating the relative safety of this anticancer agent. Because of its relatively weak GLUT4 inhibitory activity, silibinin’s anticancer effects are likely to be elicited from multiple mechanisms.
From the studies cited above, it can be concluded that GLUTs are rate-limiting for glycolysis in specific tumor contexts. The identification and targeting of upregulated GLUTs in different tumors provide a promising approach to block glucose-regulated cancer metabolism and thus inhibit cancer growth. Key information for all the GLUT inhibitors described above is summarized in Table 3.
| Inhibitor | Target GLUT | Status | Ref. |
| WZB117 | GLUT1 | Animal study | Liu et al[28], 2012 |
| STF-31 | GLUT1 | Animal study | Chan et al[240], 2011 |
| Fasentin | GLUT1 | In vitro | Wood et al[242], 2008 |
| Apigenin | GLUT1 | Phase II | NCT00609310 |
| Genistein | GLUT1 | Phase II/III | NCT00118040; NCT00584532 |
| Oxime-based GLUT1 inhibitors | GLUT1 | Animal study | Tuccinardi et al[255], 2013 |
| Pyrrolidinone derived GLUT1 inhibitors | GLUT1 | In vitro | Ulanovskaya et al[257], 2011 |
| Phloretin | GLUT2 | Animal study | Wu et al[258], 2009 |
| Quercetin | GLUT2 | Phase I | NCT01912820 |
| DNA-damaging anticancer agents | GLUT3 | In vitro | Watanabe et al[269], 2010 |
| GSK-3 inhibitors | GLUT3 | In vitro | Watanabe et al[270], 2012 |
| Ritonavir | GLUT4 | Phase I/II | NCT01009437; NCT01095094 |
| Silibinin | GLUT4 | Phase I/II | Flaig et al[280], 2007; NCT00487721 |
From numerous examples cited in this review, it can be concluded that targeting glucose transport and metabolism offers several advantages: (1) It targets a protein, enzyme or process that is significantly altered or upregulated in cancer compared to those in normal cells. The differences between cancer and normal cells potentially provides a therapeutic window by which cancer cells can be effectively inhibited without harming patients’ normal cells; (2) Targeting GLUTs is equivalent to inhibiting the entire process of glycolysis, leaving cancer cells fewer options for production of sufficient amount of ATP, NADPH, serine, etc. It may also be harder for cancer cells to bypass GLUT inhibition, leading to stronger and longer-lasting inhibition. To compensate for the shortage of glucose, cancer cells will have to use either other glucose transport mechanisms or other energy molecules, such as glutamine for biosynthesis and energy. Although this is possible, it is more difficult than merely bypassing the inhibition of a single enzyme in the middle of a signaling pathway; and (3) Cancer cells are addicted to glucose[25,27], and thus more sensitive to glucose concentration changes triggered by GLUT inhibition than are normal cells. Cancer cells more readily enter cell cycle arrest or apoptose from glucose shortage[28].
However, there are also some weaknesses associated with the strategy of glucose transport inhibition. These include: (1) GLUTs are expressed by both cancer and normal cells. Inhibiting cancer cells’ GLUTs inevitably inhibits normal cells that also use GLUTs for their functions. The identification of a therapeutic window is absolutely essential for the success of this anticancer strategy. Fortunately, key organs in the body such as the brain and heart can use ketone bodies as a substitute for glucose[282,283]. Therefore, GLUT inhibition should not result in significant energy shortage for these vital organs; and (2) Cancer cells’ reliance on glucose is not absolute. Some cancer cells use glutamine[284,285] and others can shift from glucose metabolism to glutamine metabolism[286,287], bypassing glucose transport inhibition. Drugs targeting other metabolic pathways such as glutamine transport/metabolism or targeting cancer cell growth signaling may be used together with GLUT inhibitors to shut down cancer cells’ energy metabolism and cell growth more effectively, leading to cancer cell death. These approaches need to be tested in cancer cells first and then in animal tumor models.
Recently, we have observed that our GLUT1 inhibitor WZB-117[28] more effectively inhibits cancer cell lines that express the wild type KRas gene (KRaswt cells) than KRasmut cancer cell lines (unpublished observations). Although the reason for the difference is unclear, we speculate this may be associated with the “leakiness” of cancer cells to extracellular glucose and ATP. We base this on a recent finding published in a 2013 Nature paper that KRasmut genotype is associated with a phenotype of macropinocytosis[105], a type of endocytosis that non-specifically takes up extracellular molecules as large as proteins[288]. In theory, KRasmut-induced macropinocytosis should be able to take up glucose or ATP as well. Thus, to further enhance cancer treatment efficacy by GLUT inhibitors, it is imperative to ascertain not only which GLUT is upregulated in the targeted cancer, but also the genotype (such as KRas status) of the cancer. We also observed that WZB-117 was less effective in cancer cell lines with higher glycogen content (unpublished observation). It is possible that higher intracellular glycogen content confers some degree of resistance to glucose transport inhibitors. In theory, a longer duration of GLUT inhibition should be able to exhaust intracellular glycogen storage and change GLUT1 inhibitor-insensitive cells into sensitive ones. These new findings may enhance GLUT inhibitors’ success in treating specific cancer types.
In summary, glucose transport and glycolysis inhibitors have been shown to be promising anti-cancer agents that warrant further basic science and clinical investigation. Improvement in inhibitor’s efficacy (IC50), selectivity of the target, and identification of therapeutic windows while taking cancers’ specific genotype and phenotype into account, are needed for such inhibitors to become effective anti-cancer therapeutics.
We thank Dr. Athena Chen for critical review of the manuscript.
P- Reviewer: Kang CM, Ishiguro T S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3702] [Article Influence: 264.4] [Reference Citation Analysis (0)] |
| 2. | Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 960] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 3. | Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1696] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 4. | Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652-3658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 329] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 5. | Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 721] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 6. | Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 7. | Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 728] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 8. | Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog. 2013;12:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Taubes G. Cancer research. Unraveling the obesity-cancer connection. Science. 2012;335:28, 30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 46941] [Article Influence: 3352.9] [Reference Citation Analysis (5)] |
| 11. | Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012;24:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11739] [Article Influence: 733.7] [Reference Citation Analysis (0)] |
| 13. | Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 1826] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 14. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9890] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 15. | Upadhyay M, Samal J, Kandpal M, Singh OV, Vivekanandan P. The Warburg effect: insights from the past decade. Pharmacol Ther. 2013;137:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2439] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 17. | DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3034] [Article Influence: 178.5] [Reference Citation Analysis (0)] |
| 18. | Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 19. | Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 882] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 20. | Burt BM, Humm JL, Kooby DA, Squire OD, Mastorides S, Larson SM, Fong Y. Using positron emission tomography with [(18)F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia. 2001;3:189-195. [PubMed] |
| 21. | Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1161] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 22. | Kurokawa T, Yoshida Y, Kawahara K, Tsuchida T, Okazawa H, Fujibayashi Y, Yonekura Y, Kotsuji F. Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer. 2004;109:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O’shaughnessy J, Guyton KZ, Mankoff DA. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785-2808. [PubMed] |
| 24. | Jadvar H. Molecular imaging of prostate cancer with PET. J Nucl Med. 2013;54:1685-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Bui T, Thompson CB. Cancer’s sweet tooth. Cancer Cell. 2006;9:419-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3472] [Cited by in RCA: 3603] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 27. | Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927-8930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 914] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 29. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3022] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 30. | Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 5579] [Article Influence: 464.9] [Reference Citation Analysis (0)] |
| 31. | Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol. 2013;71:1115-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 32. | Longo-Sorbello GS, Bertino JR. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica. 2001;86:121-127. [PubMed] |
| 33. | Chabner BA, Roberts TG. Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1372] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 34. | Kornberg A. For the love of enzymes: The odyssey of a biochemist. MA: Harvard University Press 1989; 60-83. |
| 35. | Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 36. | Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2001] [Cited by in RCA: 2168] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 37. | Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 792] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 38. | Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 39. | Wong N, De Melo J, Tang D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int J Cell Biol. 2013;2013:242513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 40. | McCarthy N. Metabolism: a TIGAR tale. Nat Rev Cancer. 2013;13:522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633-4646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1080] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 42. | Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1226] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 43. | Garber K. Energy deregulation: licensing tumors to grow. Science. 2006;312:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem. 2008;283:36344-36353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Saito S, Furuno A, Sakurai J, Sakamoto A, Park HR, Shin-Ya K, Tsuruo T, Tomida A. Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Res. 2009;69:4225-4234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe W, Ha J. AMP kinase signaling determines whether c-Jun N-terminal kinase promotes survival or apoptosis during glucose deprivation. Carcinogenesis. 2009;30:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 48. | Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci USA. 1998;95:1511-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 220] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Singh G, Lakkis CL, Laucirica R, Epner DE. Regulation of prostate cancer cell division by glucose. J Cell Physiol. 1999;180:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Krętowski R, Stypułkowska A, Cechowska-Pasko M. Low-glucose medium induces ORP150 expression and exerts inhibitory effect on apoptosis and senescence of human breast MCF7 cells. Acta Biochim Pol. 2013;60:167-173. [PubMed] |
| 51. | Robey RB, Hay N. Akt, hexokinase, mTOR: Targeting cellular energy metabolism for cancer therapy. Drug Discovery Today: Disease Mechanisms. 2005;2:239-246. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. 2013;30:2983-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613-621. [PubMed] |
| 54. | Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta. 2002;1555:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 688] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 57. | Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002;87:805-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 58. | Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P, Schwartz L, Icard P. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26:3561-3566. [PubMed] |
| 59. | Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 358] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 60. | Egler V, Korur S, Failly M, Boulay JL, Imber R, Lino MM, Merlo A. Histone deacetylase inhibition and blockade of the glycolytic pathway synergistically induce glioblastoma cell death. Clin Cancer Res. 2008;14:3132-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Singh D, Banerji AK, Dwarakanath BS, Tripathi RP, Gupta JP, Mathew TL, Ravindranath T, Jain V. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Dearling JL, Qureshi U, Begent RH, Pedley RB. Combining radioimmunotherapy with antihypoxia therapy 2-deoxy-D-glucose results in reduction of therapeutic efficacy. Clin Cancer Res. 2007;13:1903-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Dwarakanath B, Jain V. Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol. 2009;5:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 65. | Strandberg AY, Pienimäki T, Pitkälä KH, Tilvis RS, Salomaa VV, Strandberg TE. Comparison of normal fasting and one-hour glucose levels as predictors of future diabetes during a 34-year follow-up. Ann Med. 2013;45:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 274] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 67. | Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, Hullihen J, Pedersen PL. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, Yang JI, Park SC, Lee HS. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2554-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 69. | Kim JS, Ahn KJ, Kim JA, Kim HM, Lee JD, Lee JM, Kim SJ, Park JH. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells : ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr. 2008;40:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 70. | Ihrlund LS, Hernlund E, Khan O, Shoshan MC. 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol. 2008;2:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 71. | Nakano A, Tsuji D, Miki H, Cui Q, El Sayed SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T. Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS One. 2011;6:e27222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005;19:2153-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Cao X, Bloomston M, Zhang T, Frankel WL, Jia G, Wang B, Hall NC, Koch RM, Cheng H, Knopp MV. Synergistic antipancreatic tumor effect by simultaneously targeting hypoxic cancer cells with HSP90 inhibitor and glycolysis inhibitor. Clin Cancer Res. 2008;14:1831-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Jae HJ, Chung JW, Park HS, Lee MJ, Lee KC, Kim HC, Yoon JH, Chung H, Park JH. The antitumor effect and hepatotoxicity of a hexokinase II inhibitor 3-bromopyruvate: in vivo investigation of intraarterial administration in a rabbit VX2 hepatoma model. Korean J Radiol. 2009;10:596-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF. Anticancer efficacy of the metabolic blocker 3-bromopyruvate: specific molecular targeting. Anticancer Res. 2013;33:13-20. [PubMed] |
| 76. | Floridi A, Paggi MG, Marcante ML, Silvestrini B, Caputo A, De Martino C. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J Natl Cancer Inst. 1981;66:497-499. [PubMed] |
| 77. | Fanciulli M, Valentini A, Bruno T, Citro G, Zupi G, Floridi A. Effect of the antitumor drug lonidamine on glucose metabolism of adriamycin-sensitive and -resistant human breast cancer cells. Oncol Res. 1996;8:111-120. [PubMed] |
| 78. | Floridi A, Bruno T, Miccadei S, Fanciulli M, Federico A, Paggi MG. Enhancement of doxorubicin content by the antitumor drug lonidamine in resistant Ehrlich ascites tumor cells through modulation of energy metabolism. Biochem Pharmacol. 1998;56:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | De Lena M, Lorusso V, Latorre A, Fanizza G, Gargano G, Caporusso L, Guida M, Catino A, Crucitta E, Sambiasi D. Paclitaxel, cisplatin and lonidamine in advanced ovarian cancer. A phase II study. Eur J Cancer. 2001;37:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Di Cosimo S, Ferretti G, Papaldo P, Carlini P, Fabi A, Cognetti F. Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors. Drugs Today (Barc). 2003;39:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Berruti A, Bitossi R, Gorzegno G, Bottini A, Alquati P, De Matteis A, Nuzzo F, Giardina G, Danese S, De Lena M. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of a phase III study with a factorial design. J Clin Oncol. 2002;20:4150-4159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Granchi C, Minutolo F. Anticancer agents that counteract tumor glycolysis. ChemMedChem. 2012;7:1318-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 83. | Price GS, Page RL, Riviere JE, Cline JM, Thrall DE. Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother Pharmacol. 1996;38:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Dunaway GA, Kasten TP, Sebo T, Trapp R. Analysis of the phosphofructokinase subunits and isoenzymes in human tissues. Biochem J. 1988;251:677-683. [PubMed] |
| 85. | Chesney J, Mitchell R, Benigni F, Bacher M, Spiegel L, Al-Abed Y, Han JH, Metz C, Bucala R. An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc Natl Acad Sci USA. 1999;96:3047-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 86. | Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, Mitchell R, Bucala R. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62:5881-5887. [PubMed] |
| 87. | Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, Yoshioka N, Bucala R, Koike T. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res. 2005;11:5784-5792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Minchenko O, Opentanova I, Caro J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1-4) expression in vivo. FEBS Lett. 2003;554:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, Caro J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem. 2002;277:6183-6187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 90. | Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 91. | Seo M, Kim JD, Neau D, Sehgal I, Lee YH. Structure-based development of small molecule PFKFB3 inhibitors: a framework for potential cancer therapeutic agents targeting the Warburg effect. PLoS One. 2011;6:e24179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Brooke DG, van Dam EM, Watts CK, Khoury A, Dziadek MA, Brooks H, Graham LJ, Flanagan JU, Denny WA. Targeting the Warburg Effect in cancer; relationships for 2-arylpyridazinones as inhibitors of the key glycolytic enzyme 6-phosphofructo-2-kinase/2,6-bisphosphatase 3 (PFKFB3). Bioorg Med Chem. 2014;22:1029-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1354] [Cited by in RCA: 1310] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 94. | Liu J, Guo S, Li Q, Yang L, Xia Z, Zhang L, Huang Z, Zhang N. Phosphoglycerate dehydrogenase induces glioma cells proliferation and invasion by stabilizing forkhead box M1. J Neurooncol. 2013;111:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 95. | Mullarky E, Mattaini KR, Vander Heiden MG, Cantley LC, Locasale JW. PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 2011;24:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 96. | Hitosugi T, Zhou L, Elf S, Fan J, Kang HB, Seo JH, Shan C, Dai Q, Zhang L, Xie J. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22:585-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 97. | Hitosugi T, Zhou L, Fan J, Elf S, Zhang L, Xie J, Wang Y, Gu TL, Alečković M, LeRoy G. Tyr26 phosphorylation of PGAM1 provides a metabolic advantage to tumours by stabilizing the active conformation. Nat Commun. 2013;4:1790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 98. | Evans MJ, Saghatelian A, Sorensen EJ, Cravatt BF. Target discovery in small-molecule cell-based screens by in situ proteome reactivity profiling. Nat Biotechnol. 2005;23:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 100. | Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 615] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 101. | Gui DY, Lewis CA, Vander Heiden MG. Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci Signal. 2013;6:pe7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 102. | Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E. Pyruvate kinase type M2: a crossroad in the tumor metabolome. Br J Nutr. 2002;87 Suppl 1:S23-S29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 518] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 103. | Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492-1499. [PubMed] |
| 104. | Qian Y, Wang X, Liu Y, Li Y, Colvin RA, Tong L, Wu S, Chen X. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 2014;351:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 105. | Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1020] [Cited by in RCA: 1282] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 106. | Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 523] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 107. | Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 108. | Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297-4306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 374] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 109. | Steták A, Veress R, Ovádi J, Csermely P, Kéri G, Ullrich A. Nuclear translocation of the tumor marker pyruvate kinase M2 induces programmed cell death. Cancer Res. 2007;67:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 110. | Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed. 1999;38:270-301. [DOI] [Full Text] |
| 111. | Szende B, Kéri G. TT-232: a somatostatin structural derivative as a potent antitumor drug candidate. Anticancer Drugs. 2003;14:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Boxer MB, Jiang JK, Vander Heiden MG, Shen M, Skoumbourdis AP, Southall N, Veith H, Leister W, Austin CP, Park HW. Evaluation of substituted N,N’-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. J Med Chem. 2010;53:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 113. | Jiang JK, Boxer MB, Vander Heiden MG, Shen M, Skoumbourdis AP, Southall N, Veith H, Leister W, Austin CP, Park HW. Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg Med Chem Lett. 2010;20:3387-3393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Walsh MJ, Brimacombe KR, Veith H, Bougie JM, Daniel T, Leister W, Cantley LC, Israelsen WJ, Vander Heiden MG, Shen M. 2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg Med Chem Lett. 2011;21:6322-6327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Kung C, Hixon J, Choe S, Marks K, Gross S, Murphy E, DeLaBarre B, Cianchetta G, Sethumadhavan S, Wang X. Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem Biol. 2012;19:1187-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 116. | Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 605] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 117. | Walsh MJ, Brimacombe KR, Anastasiou D, Yu Y, Israelsen WJ, Hong BS, Tempel W, Dimov S, Veith H, Yang H. ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model. Probe Reports from the NIH Molecular Libraries Program (Internet) 2010-. 2012;. [PubMed] |
| 118. | Xu Y, Liu XH, Saunders M, Pearce S, Foulks JM, Parnell KM, Clifford A, Nix RN, Bullough J, Hendrickson TF. Discovery of 3-(trifluoromethyl)-1H-pyrazole-5-carboxamide activators of the M2 isoform of pyruvate kinase (PKM2). Bioorg Med Chem Lett. 2014;24:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Guo C, Linton A, Jalaie M, Kephart S, Ornelas M, Pairish M, Greasley S, Richardson P, Maegley K, Hickey M. Discovery of 2-((1H-benzo[d]imidazol-1-yl)methyl)-4H-pyrido[1,2-a]pyrimidin-4-ones as novel PKM2 activators. Bioorg Med Chem Lett. 2013;23:3358-3363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658-6663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 856] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 121. | Yao F, Zhao T, Zhong C, Zhu J, Zhao H. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol. 2013;34:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 122. | Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D, Lou W. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 123. | Xie H, Valera VA, Merino MJ, Amato AM, Signoretti S, Linehan WM, Sukhatme VP, Seth P. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 124. | Granchi C, Bertini S, Macchia M, Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem. 2010;17:672-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, Eaton JW, Telang S, Chesney J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10:R84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 126. | Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 1133] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 127. | Farabegoli F, Vettraino M, Manerba M, Fiume L, Roberti M, Di Stefano G. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci. 2012;47:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 128. | Moorhouse AD, Spiteri C, Sharma P, Zloh M, Moses JE. Targeting glycolysis: a fragment based approach towards bifunctional inhibitors of hLDH-5. Chem Commun (Camb). 2011;47:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Kohlmann A, Zech SG, Li F, Zhou T, Squillace RM, Commodore L, Greenfield MT, Lu X, Miller DP, Huang WS. Fragment growing and linking lead to novel nanomolar lactate dehydrogenase inhibitors. J Med Chem. 2013;56:1023-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Dragovich PS, Fauber BP, Corson LB, Ding CZ, Eigenbrot C, Ge H, Giannetti AM, Hunsaker T, Labadie S, Liu Y. Identification of substituted 2-thio-6-oxo-1,6-dihydropyrimidines as inhibitors of human lactate dehydrogenase. Bioorg Med Chem Lett. 2013;23:3186-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 131. | Fauber BP, Dragovich PS, Chen J, Corson LB, Ding CZ, Eigenbrot C, Giannetti AM, Hunsaker T, Labadie S, Liu Y. Identification of 2-amino-5-aryl-pyrazines as inhibitors of human lactate dehydrogenase. Bioorg Med Chem Lett. 2013;23:5533-5539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 132. | Vanderporten E, Frick L, Turincio R, Thana P, Lamarr W, Liu Y. Label-free high-throughput assays to screen and characterize novel lactate dehydrogenase inhibitors. Anal Biochem. 2013;441:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Ward RA, Brassington C, Breeze AL, Caputo A, Critchlow S, Davies G, Goodwin L, Hassall G, Greenwood R, Holdgate GA. High-Throughput Screening to Identify Plant Derived Human LDH-A Inhibitors. European J Med Plants. 2013;3:603-615. [PubMed] |
| 134. | Ward RA, Brassington C, Breeze AL, Caputo A, Critchlow S, Davies G, Goodwin L, Hassall G, Greenwood R, Holdgate GA. Design and synthesis of novel lactate dehydrogenase A inhibitors by fragment-based lead generation. J Med Chem. 2012;55:3285-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 135. | Granchi C, Roy S, Del Fiandra C, Tuccinardi T, Lanza M, Betti L, Giannaccini G, Lucacchini A, Martinelli A, Macchia M. Triazole-substituted N-hydroxyindol-2-carboxylates as inhibitors of isoform 5 of human lactate dehydrogenase (hLDH5). Med Chem Commun. 2011;2:638-643. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Granchi C, Roy S, Giacomelli C, Macchia M, Tuccinardi T, Martinelli A, Lanza M, Betti L, Giannaccini G, Lucacchini A. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem. 2011;54:1599-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 137. | Granchi C, Roy S, De Simone A, Salvetti I, Tuccinardi T, Martinelli A, Macchia M, Lanza M, Betti L, Giannaccini G. N-Hydroxyindole-based inhibitors of lactate dehydrogenase against cancer cell proliferation. Eur J Med Chem. 2011;46:5398-5407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 138. | Granchi C, Roy S, Mottinelli M, Nardini E, Campinoti F, Tuccinardi T, Lanza M, Betti L, Giannaccini G, Lucacchini A. Synthesis of sulfonamide-containing N-hydroxyindole-2-carboxylates as inhibitors of human lactate dehydrogenase-isoform 5. Bioorg Med Chem Lett. 2011;21:7331-7336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 139. | Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 140. | Calvaresi EC, Granchi C, Tuccinardi T, Di Bussolo V, Huigens RW, Lee HY, Palchaudhuri R, Macchia M, Martinelli A, Minutolo F. Dual targeting of the Warburg effect with a glucose-conjugated lactate dehydrogenase inhibitor. Chembiochem. 2013;14:2263-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 141. | Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colón M, Dodson CS, Gilbert SA, Greshock J, Jing J. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 142. | Harris RA, Bowker-Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 143. | Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2879] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 144. | Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1728] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 145. | Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 146. | McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700-22708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 147. | Stacpoole PW, Lorenz AC, Thomas RG, Harman EM. Dichloroacetate in the treatment of lactic acidosis. Ann Intern Med. 1988;108:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 148. | Whitehouse S, Randle PJ. Activation of pyruvate dehydrogenase in perfused rat heart by dichloroacetate (Short Communication). Biochem J. 1973;134:651-653. [PubMed] |
| 149. | Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1170] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 150. | Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 151. | Papandreou I, Goliasova T, Denko NC. Anticancer drugs that target metabolism: Is dichloroacetate the new paradigm? Int J Cancer. 2011;128:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 152. | Sun RC, Fadia M, Dahlstrom JE, Parish CR, Board PG, Blackburn AC. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat. 2010;120:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 153. | Xie J, Wang BS, Yu DH, Lu Q, Ma J, Qi H, Fang C, Chen HZ. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int J Oncol. 2011;38:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 154. | Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol. 2008;109:394-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 155. | Cao W, Yacoub S, Shiverick KT, Namiki K, Sakai Y, Porvasnik S, Urbanek C, Rosser CJ. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate. 2008;68:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 156. | Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 157. | Langbein S, Frederiks WM, zur Hausen A, Popa J, Lehmann J, Weiss C, Alken P, Coy JF. Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer. 2008;122:2422-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 158. | Ramos-Montoya A, Lee WN, Bassilian S, Lim S, Trebukhina RV, Kazhyna MV, Ciudad CJ, Noé V, Centelles JJ, Cascante M. Pentose phosphate cycle oxidative and nonoxidative balance: A new vulnerable target for overcoming drug resistance in cancer. Int J Cancer. 2006;119:2733-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 159. | Budihardjo II, Walker DL, Svingen PA, Buckwalter CA, Desnoyers S, Eckdahl S, Shah GM, Poirier GG, Reid JM, Ames MM. 6-Aminonicotinamide sensitizes human tumor cell lines to cisplatin. Clin Cancer Res. 1998;4:117-130. [PubMed] |
| 160. | Varshney R, Adhikari JS, Dwarakanath BS. Contribution of oxidative stress to radiosensitization by a combination of 2-DG and 6-AN in human cancer cell line. Indian J Exp Biol. 2003;41:1384-1391. [PubMed] |
| 161. | Bhardwaj R, Sharma PK, Jadon SP, Varshney R. A combination of 2-deoxy-D-glucose and 6-aminonicotinamide induces cell cycle arrest and apoptosis selectively in irradiated human malignant cells. Tumour Biol. 2012;33:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 162. | Preuss J, Richardson AD, Pinkerton A, Hedrick M, Sergienko E, Rahlfs S, Becker K, Bode L. Identification and characterization of novel human glucose-6-phosphate dehydrogenase inhibitors. J Biomol Screen. 2013;18:286-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 163. | Langbein S, Zerilli M, Zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94:578-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 164. | Zhang S, Yue JX, Yang JH, Cai PC, Kong WJ. Overexpression of transketolase protein TKTL1 is associated with occurrence and progression in nasopharyngeal carcinoma: a potential therapeutic target in nasopharyngeal carcinoma. Cancer Biol Ther. 2008;7:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 165. | Chen H, Yue JX, Yang SH, Ding H, Zhao RW, Zhang S. Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res. 2009;28:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 166. | Kayser G, Sienel W, Kubitz B, Mattern D, Stickeler E, Passlick B, Werner M, Zur Hausen A. Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology. 2011;43:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 167. | Coy JF, Dressler D, Wilde J, Schubert P. Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin Lab. 2005;51:257-273. [PubMed] |
| 168. | Zhang S, Yang JH, Guo CK, Cai PC. Gene silencing of TKTL1 by RNAi inhibits cell proliferation in human hepatoma cells. Cancer Lett. 2007;253:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 169. | Hu LH, Yang JH, Zhang DT, Zhang S, Wang L, Cai PC, Zheng JF, Huang JS. The TKTL1 gene influences total transketolase activity and cell proliferation in human colon cancer LoVo cells. Anticancer Drugs. 2007;18:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 170. | Boros LG, Puigjaner J, Cascante M, Lee WN, Brandes JL, Bassilian S, Yusuf FI, Williams RD, Muscarella P, Melvin WS. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997;57:4242-4248. [PubMed] |
| 171. | Raïs B, Comin B, Puigjaner J, Brandes JL, Creppy E, Saboureau D, Ennamany R, Lee WN, Boros LG, Cascante M. Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich’s tumor cells through inhibition of the pentose cycle. FEBS Lett. 1999;456:113-118. [PubMed] |
| 172. | Wang J, Zhang X, Ma D, Lee WN, Xiao J, Zhao Y, Go VL, Wang Q, Yen Y, Recker R. Inhibition of transketolase by oxythiamine altered dynamics of protein signals in pancreatic cancer cells. Exp Hematol Oncol. 2013;2:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 173. | Yang CM, Liu YZ, Liao JW, Hu ML. The in vitro and in vivo anti-metastatic efficacy of oxythiamine and the possible mechanisms of action. Clin Exp Metastasis. 2010;27:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 174. | Czernin J, Phelps ME. Positron emission tomography scanning: current and future applications. Annu Rev Med. 2002;53:89-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 175. | Higashi T, Tamaki N, Torizuka T, Nakamoto Y, Sakahara H, Kimura T, Honda T, Inokuma T, Katsushima S, Ohshio G. FDG uptake, GLUT-1 glucose transporter and cellularity in human pancreatic tumors. J Nucl Med. 1998;39:1727-1735. [PubMed] |
| 176. | Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 177. | Liu Y, Zhang W, Cao Y, Liu Y, Bergmeier S, Chen X. Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Lett. 2010;298:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 178. | Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 1996;56:1164-1167. [PubMed] |
| 179. | Cooper R, Sarioğlu S, Sökmen S, Füzün M, Küpelioğlu A, Valentine H, Görken IB, Airley R, West C. Glucose transporter-1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br J Cancer. 2003;89:870-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 180. | Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 757] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 181. | Garvey WT. Mechanisms of insulin signal transduction. DeFronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International textbook of diabetes mellitus. 3rd ed. New Jersey: John Wiley & Sons 2004; 227-252. |
| 182. | Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 905] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 183. | Jung CY. Proteins that interact with facilitative glucose transporters: implication for function. Exp Physiol. 1998;83:267-273. [PubMed] |
| 184. | Zeng H, Parthasarathy R, Rampal AL, Jung CY. Proposed structure of putative glucose channel in GLUT1 facilitative glucose transporter. Biophys J. 1996;70:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 185. | Lachaal M, Rampal AL, Lee W, Shi Y, Jung CY. GLUT1 transmembrane glucose pathway. Affinity labeling with a transportable D-glucose diazirine. J Biol Chem. 1996;271:5225-5230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 186. | Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 578] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 187. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 896] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 188. | Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta. 2013;1835:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 189. | Hruz PW, Mueckler MM. Structural analysis of the GLUT1 facilitative glucose transporter (review). Mol Membr Biol. 2001;18:183-193. [PubMed] |
| 190. | Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895-2898. [PubMed] |
| 191. | Ravazoula P, Batistatou A, Aletra C, Ladopoulos J, Kourounis G, Tzigounis B. Immunohistochemical expression of glucose transporter Glut1 and cyclin D1 in breast carcinomas with negative lymph nodes. Eur J Gynaecol Oncol. 2003;24:544-546. [PubMed] |
| 192. | Cho H, Lee YS, Kim J, Chung JY, Kim JH. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Invest. 2013;31:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 193. | Mori Y, Tsukinoki K, Yasuda M, Miyazawa M, Kaneko A, Watanabe Y. Glucose transporter type 1 expression are associated with poor prognosis in patients with salivary gland tumors. Oral Oncol. 2007;43:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 194. | Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 195. | Kang SS, Chun YK, Hur MH, Lee HK, Kim YJ, Hong SR, Lee JH, Lee SG, Park YK. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn J Cancer Res. 2002;93:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 196. | Kayano T, Fukumoto H, Eddy RL, Fan YS, Byers MG, Shows TB, Bell GI. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988;263:15245-15248. [PubMed] |
| 197. | Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Immunohistochemical detection of Glut3 in human tumors and normal tissues. Anticancer Res. 1997;17:2747-2750. [PubMed] |
| 198. | Watanabe T, Nagamatsu S, Matsushima S, Kondo K, Motobu H, Hirosawa K, Mabuchi K, Kirino T, Uchimura H. Developmental expression of GLUT2 in the rat retina. Cell Tissue Res. 1999;298:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 199. | Eisenberg ML, Maker AV, Slezak LA, Nathan JD, Sritharan KC, Jena BP, Geibel JP, Andersen DK. Insulin receptor (IR) and glucose transporter 2 (GLUT2) proteins form a complex on the rat hepatocyte membrane. Cell Physiol Biochem. 2005;15:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 200. | Efrat S. Making sense of glucose sensing. Nat Genet. 1997;17:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 201. | Gould GW, Thomas HM, Jess TJ, Bell GI. Expression of human glucose transporters in Xenopus oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991;30:5139-5145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 239] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 202. | Noguchi Y, Marat D, Saito A, Yoshikawa T, Doi C, Fukuzawa K, Tsuburaya A, Satoh S, Ito T. Expression of facilitative glucose transporters in gastric tumors. Hepatogastroenterology. 1999;46:2683-2689. [PubMed] |
| 203. | Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García Mde L, Medina RA, Carrasco M, Barberis S, Castro T. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol. 2006;207:614-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 204. | Seino Y, Yamamoto T, Inoue K, Imamura M, Kadowaki S, Kojima H, Fujikawa J, Imura H. Abnormal facilitative glucose transporter gene expression in human islet cell tumors. J Clin Endocrinol Metab. 1993;76:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 205. | Kurata T, Oguri T, Isobe T, Ishioka S, Yamakido M. Differential expression of facilitative glucose transporter (GLUT) genes in primary lung cancers and their liver metastases. Jpn J Cancer Res. 1999;90:1238-1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 206. | Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97:2035-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 207. | Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1152] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 208. | Nishimura H, Pallardo FV, Seidner GA, Vannucci S, Simpson IA, Birnbaum MJ. Kinetics of GLUT1 and GLUT4 glucose transporters expressed in Xenopus oocytes. J Biol Chem. 1993;268:8514-8520. [PubMed] |
| 209. | Keller K, Strube M, Mueckler M. Functional expression of the human HepG2 and rat adipocyte glucose transporters in Xenopus oocytes. Comparison of kinetic parameters. J Biol Chem. 1989;264:18884-18889. [PubMed] |
| 210. | Fukumoto H, Kayano T, Buse JB, Edwards Y, Pilch PF, Bell GI, Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989;264:7776-7779. [PubMed] |
| 211. | Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992;31:10414-10420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 212. | Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523-14526. [PubMed] |
| 213. | Kayano T, Burant CF, Fukumoto H, Gould GW, Fan YS, Eddy RL, Byers MG, Shows TB, Seino S, Bell GI. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990;265:13276-13282. [PubMed] |
| 214. | Concha II, Velásquez FV, Martínez JM, Angulo C, Droppelmann A, Reyes AM, Slebe JC, Vera JC, Golde DW. Human erythrocytes express GLUT5 and transport fructose. Blood. 1997;89:4190-4195. [PubMed] |
| 215. | Doege H, Bocianski A, Joost HG, Schürmann A. Activity and genomic organization of human glucose transporter 9 (GLUT9), a novel member of the family of sugar-transport facilitators predominantly expressed in brain and leucocytes. Biochem J. 2000;350 Pt 3:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 216. | Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol. 2001;18:247-256. [PubMed] |
| 217. | Li Q, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD, Chen XZ, Cheeseman CI. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G236-G242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 218. | Goldman NA, Katz EB, Glenn AS, Weldon RH, Jones JG, Lynch U, Fezzari MJ, Runowicz CD, Goldberg GL, Charron MJ. GLUT1 and GLUT8 in endometrium and endometrial adenocarcinoma. Mod Pathol. 2006;19:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 219. | Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost HG. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem. 2000;275:16275-16280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 220. | Phay JE, Hussain HB, Moley JF. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics. 2000;66:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 221. | Manolescu AR, Augustin R, Moley K, Cheeseman C. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 222. | Evans SA, Doblado M, Chi MM, Corbett JA, Moley KH. Facilitative glucose transporter 9 expression affects glucose sensing in pancreatic beta-cells. Endocrinology. 2009;150:5302-5310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 223. | McVie-Wylie AJ, Lamson DR, Chen YT. Molecular cloning of a novel member of the GLUT family of transporters, SLC2a10 (GLUT10), localized on chromosome 20q13.1: a candidate gene for NIDDM susceptibility. Genomics. 2001;72:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 224. | Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the Type 2 diabetes-linked region of chromosome 20q12-13.1. Mol Genet Metab. 2001;74:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 225. | Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schürmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J. 2001;359:443-449. [PubMed] |
| 226. | Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab. 2002;282:E733-E738. [PubMed] |
| 227. | Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12-functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Commun. 2003;308:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 228. | Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J. 2001;20:4467-4477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 229. | Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002;80:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 230. | Tran A, Pio BS, Khatibi B, Czernin J, Phelps ME, Silverman DH. 18F-FDG PET for staging breast cancer in patients with inner-quadrant versus outer-quadrant tumors: comparison with long-term clinical outcome. J Nucl Med. 2005;46:1455-1459. [PubMed] |
| 231. | Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich J, Oefner PJ. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 232. | Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD. Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett. 2003;193:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 233. | Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 234. | Jóźwiak P, Krześlak A, Pomorski L, Lipińska A. Expression of hypoxia-related glucose transporters GLUT1 and GLUT3 in benign, malignant and non-neoplastic thyroid lesions. Mol Med Rep. 2012;6:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 235. | McBrayer SK, Cheng JC, Singhal S, Krett NL, Rosen ST, Shanmugam M. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: implications for glucose transporter-directed therapy. Blood. 2012;119:4686-4697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 236. | Cheng JC, McBrayer SK, Coarfa C, Dalva-Aydemir S, Gunaratne PH, Carpten JD, Keats JK, Rosen ST, Shanmugam M. Expression and phosphorylation of the AS160_v2 splice variant supports GLUT4 activation and the Warburg effect in multiple myeloma. Cancer Metab. 2013;1:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 237. | Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda). 2007;22:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 238. | Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 384] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 239. | Rastogi S, Banerjee S, Chellappan S, Simon GR. Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett. 2007;257:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 240. | Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 241. | Schimmer AD, Thomas MP, Hurren R, Gronda M, Pellecchia M, Pond GR, Konopleva M, Gurfinkel D, Mawji IA, Brown E. Identification of small molecules that sensitize resistant tumor cells to tumor necrosis factor-family death receptors. Cancer Res. 2006;66:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 242. | Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546-3555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 243. | Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review). Int J Oncol. 2007;30:233-245. [PubMed] |
| 244. | Kuo ML, Lee KC, Lin JK. Genotoxicities of nitropyrenes and their modulation by apigenin, tannic acid, ellagic acid and indole-3-carbinol in the Salmonella and CHO systems. Mutat Res. 1992;270:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 245. | Myhrstad MC, Carlsen H, Nordström O, Blomhoff R, Moskaug JØ. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 246. | Ye Y, Chou GX, Wang H, Chu JH, Yu ZL. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine. 2010;18:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 247. | Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 248. | Melstrom LG, Salabat MR, Ding XZ, Milam BM, Strouch M, Pelling JC, Bentrem DJ. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas. 2008;37:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 249. | Tarkowski M, Kokocińska M, Latocha M. [Genistein in chemoprevention and treatment]. Pol Merkur Lekarski. 2013;34:54-57. [PubMed] |
| 250. | Nagaraju GP, Zafar SF, El-Rayes BF. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr Rev. 2013;71:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 251. | Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 252. | Li QS, Li CY, Li ZL, Zhu HL. Genistein and its synthetic analogs as anticancer agents. Anticancer Agents Med Chem. 2012;12:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 253. | Vera JC, Reyes AM, Cárcamo JG, Velásquez FV, Rivas CI, Zhang RH, Strobel P, Iribarren R, Scher HI, Slebe JC. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J Biol Chem. 1996;271:8719-8724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 254. | Pérez A, Ojeda P, Ojeda L, Salas M, Rivas CI, Vera JC, Reyes AM. Hexose transporter GLUT1 harbors several distinct regulatory binding sites for flavones and tyrphostins. Biochemistry. 2011;50:8834-8845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 255. | Tuccinardi T, Granchi C, Iegre J, Paterni I, Bertini S, Macchia M, Martinelli A, Qian Y, Chen X, Minutolo F. Oxime-based inhibitors of glucose transporter 1 displaying antiproliferative effects in cancer cells. Bioorg Med Chem Lett. 2013;23:6923-6927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 256. | Afzal I, Cunningham P, Naftalin RJ. Interactions of ATP, oestradiol, genistein and the anti-oestrogens, faslodex (ICI 182780) and tamoxifen, with the human erythrocyte glucose transporter, GLUT1. Biochem J. 2002;365:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 257. | Ulanovskaya OA, Cui J, Kron SJ, Kozmin SA. A pairwise chemical genetic screen identifies new inhibitors of glucose transport. Chem Biol. 2011;18:222-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 258. | Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 259. | Zheng Y, Scow JS, Duenes JA, Sarr MG. Mechanisms of glucose uptake in intestinal cell lines: role of GLUT2. Surgery. 2012;151:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 260. | Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 261. | Kobori M, Iwashita K, Shinmoto H, Tsushida T. Phloretin-induced apoptosis in B16 melanoma 4A5 cells and HL60 human leukemia cells. Biosci Biotechnol Biochem. 1999;63:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 262. | Kobori M, Shinmoto H, Tsushida T, Shinohara K. Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett. 1997;119:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 263. | Nelson JA, Falk RE. The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res. 1993;13:2287-2292. [PubMed] |
| 264. | Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH, Chen JH, Wu CH, Ho YS. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol Carcinog. 2009;48:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 265. | Song J, Kwon O, Chen S, Daruwala R, Eck P, Park JB, Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J Biol Chem. 2002;277:15252-15260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 266. | Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 481] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 267. | Nöthlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 268. | Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004;50:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 269. | Watanabe M, Naraba H, Sakyo T, Kitagawa T. DNA damage-induced modulation of GLUT3 expression is mediated through p53-independent extracellular signal-regulated kinase signaling in HeLa cells. Mol Cancer Res. 2010;8:1547-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 270. | Watanabe M, Abe N, Oshikiri Y, Stanbridge EJ, Kitagawa T. Selective growth inhibition by glycogen synthase kinase-3 inhibitors in tumorigenic HeLa hybrid cells is mediated through NF-κB-dependent GLUT3 expression. Oncogenesis. 2012;1:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 271. | Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251-20254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 389] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 272. | Vyas AK, Koster JC, Tzekov A, Hruz PW. Effects of the HIV protease inhibitor ritonavir on GLUT4 knock-out mice. J Biol Chem. 2010;285:36395-36400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 273. | Srirangam A, Milani M, Mitra R, Guo Z, Rodriguez M, Kathuria H, Fukuda S, Rizzardi A, Schmechel S, Skalnik DG. The human immunodeficiency virus protease inhibitor ritonavir inhibits lung cancer cells, in part, by inhibition of survivin. J Thorac Oncol. 2011;6:661-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 274. | Dewan MZ, Tomita M, Katano H, Yamamoto N, Ahmed S, Yamamoto M, Sata T, Mori N, Yamamoto N. An HIV protease inhibitor, ritonavir targets the nuclear factor-kappaB and inhibits the tumor growth and infiltration of EBV-positive lymphoblastoid B cells. Int J Cancer. 2009;124:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 275. | Srirangam A, Mitra R, Wang M, Gorski JC, Badve S, Baldridge L, Hamilton J, Kishimoto H, Hawes J, Li L. Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer. Clin Cancer Res. 2006;12:1883-1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 276. | Zhan T, Digel M, Küch EM, Stremmel W, Füllekrug J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J Cell Biochem. 2011;112:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 277. | Nomura M, Takahashi T, Nagata N, Tsutsumi K, Kobayashi S, Akiba T, Yokogawa K, Moritani S, Miyamoto K. Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biol Pharm Bull. 2008;31:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 278. | Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin--a promising new treatment for cancer. Anticancer Agents Med Chem. 2010;10:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 279. | García-Maceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2009;28:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 280. | Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 281. | Singh RP, Agarwal R. Prostate cancer prevention by silibinin. Curr Cancer Drug Targets. 2004;4:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 282. | Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060-H1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 283. | Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 284. | Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 945] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 285. | Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1227] [Cited by in RCA: 1520] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 286. | Burgess DJ. Metabolism: Glutamine connections. Nat Rev Cancer. 2013;13:293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 287. | DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 1014] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 288. | Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |