Published online Apr 12, 2014. doi: 10.5528/wjtm.v3.i1.9
Revised: March 6, 2014
Accepted: March 13, 2014
Published online: April 12, 2014
Processing time: 106 Days and 19.1 Hours
Neglected tropical diseases are a group of tropical diseases endemic in poor countries even though medical treatment and cures are available. They are considered a global health problem due to the severity of the physiological changes they induce in their hosts. Malaria is a disease caused by Plasmodium sp. that in its cerebral form may lead to acute or long-term neurological deficits, even with effective antimalarial therapy, causing vascular obstruction, reduced cerebral blood flow and many other changes. However, Plasmodium falciparum infection can also develop into a cerebral malaria (CM) disease that can produce neurological damage. This review will discuss the mechanisms involved in the neuropathology caused by CM, focusing on alterations in cognitive, behavior and neurological functions in human and experimental models.
Core tip: This review attempts to compile the limited current knowledge on the behavioral and cognitive effects of cerebral malaria (CM) and the possible pathological mechanisms related to neurobehavioral manifestations. CM induces acute/chronic neurological damage, affecting several Central Nervous System regions responsible for behavioral, neurological and cognitive functions which may result in motor deficits, epilepsy, blindness, speech/hearing and memory/attention disorders, hyperactivity, anxiety-like behavior, neuropsychiatric manifestations of post malaria neurological syndrome, both in humans and animal models. The action mechanisms involved in the alterations are not yet clearly defined; however proinflammatory mediators have been described with consequent axonal damage and demyelination.
- Citation: Monteiro MC, Oliveira FR, Oliveira GB, Romao PRT, Maia CSF. Neurological and behavioral manifestations of cerebral malaria: An update. World J Transl Med 2014; 3(1): 9-16
- URL: https://www.wjgnet.com/2220-6132/full/v3/i1/9.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v3.i1.9
Malaria, leishmaniasis and tuberculosis together with other neglected tropical diseases (NTDs) cause 32% of the burden of ill health in Africa and seriously impact on health outcomes in many regions of the world. NTDs share common features such as high endemicity in rural and impoverished urban areas of low-income countries. Some NTDs are disfiguring and stigmatizing, being considered poverty-promoting conditions, particularly in Africa, Asia, and the tropical regions of the Americas[1,2].
Among various NTDs, malaria is one of the most life-threatening diseases, provided that the currently recommended interventions are not adequately implemented[2]. In 2011, the World Health Organization (WHO) estimated that 3.3 billion people were at risk of malaria. More than 274 million clinical cases and 1.1 million deaths occurred between 2001 and 2010 worldwide, with approximately 80% of cases and 90% of deaths estimated to occur in the African Region, mostly in children under five years of age and in pregnant women[2,3]. Kiszewski et al[4] estimated that Global resource requirements for malaria control totaling USD 38-45 billion will be spent from 2006 to 2015 for the diagnosis and treatment of malaria, mainly in countries and populations at risk of epidemic, such as sub-Saharan Africa.
Human malaria is caused by five species of obligate intraerythrocytic protozoa of the genus Plasmodium: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi[2], and is transmitted by the bite of an female anopheles mosquito. At least three-dozen different species of Anopheles mosquitoes can transmit malaria worldwide[5]. However, infections can also occur through exposure to infected blood products (transfusion malaria) and via congenital transmission[6].
Of these, P. falciparum is the organism primarily responsible for severe malaria, although P. vivax[3] and P. Knowle[7,8] can also cause severe disease. According to WHO’s criteria[9], severe malaria is defined by clinical or laboratory evidence of vital organ dysfunction and/or high parasite burden; this high parasitemia can be a risk factor for death from P. falciparum malaria[9].
Overall, clinical features of severe malaria include cerebral malaria (CM) with impaired consciousness (including coma), prostration, multiple convulsions, deep breathing and respiratory distress (metabolic acidosis), acute pulmonary edema and acute respiratory distress syndrome, circulatory collapse or shock and acute kidney injury[9,10]. However, severe malaria is a complex multisystem disorder that can mimic many other diseases that are also common in malaria-endemic countries, such as central nervous system (CNS) infections, sepsis, severe pneumonia and typhoid fever[9].
In this review, we described neurocognitive and behavioral outcomes of CM in humans and animals so as to facilitate further understanding of the disease’s pathogenesis in the CNS.
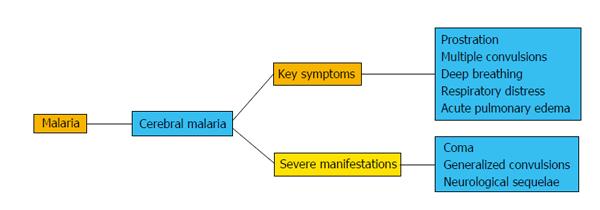
CM is one of the most severe and rapidly fatal neurological complications caused by Plasmodium species, mainly P. falciparum, with around one million deaths per year in children from sub-Saharan Africa[11,12]. The first manifestations of CM are non-specific fever, chills, irritability, agitation or psychotic behavior, vomiting and cough. In adults, complications are severe jaundice, respiratory distress syndrome, and severe intravascular hemolysis leading to hemoglobinuria and anemia, which further contributes to renal failure (Figure 1). The most severe manifestations are impaired consciousness with coma, generalized convulsions and neurological sequelae. Pregnant women are also vulnerable and develop anemia, hypoglycemia, coma and pulmonary edema. In children, the main symptoms are severe anemia, metabolic acidosis, hypoglycemia, coma and gastrointestinal symptoms[13-15], as shown in Figure 1.
CM may result in acute or long-term neurological deficits, even with effective antimalarial therapy[16,17]. CM is a neurological complication that occurs in approximately 1% of infections caused by P. falciparum[18,19]; however, a high mortality rate follows[14,20].
The pathological mechanisms that lead to neurological complications and mortality are not yet clearly defined. It is believed that in infected erythrocytes, platelets, and activated leukocytes inflammatory events occur owing to increased levels of adhesion molecules on the inflamed endothelium, leading to a reduction in microvascular blood flow, decreased delivery of nutrients to affected brain tissue and vessel walls, followed by hemorrhage and neuronal alterations[21-23].
The blood-brain barrier (BBB) acts as a physical barrier that limits the trafficking of substances via trans-cellular transport and is responsible for regulating ion and nutrient transport into the brain, a feature that restricts the free flow of physiological molecules between the bloodstream and brain parenchyma[18,24].
Conversely, perturbations to the BBB can lead to deregulation in any of the neurovascular components, which in turn can alter the brain’s homeostasis leading to a multitude of neural dysfunctions and inappropriate BBB activation as observed in multiple sclerosis, Alzheimer’s disease, stroke, certain depression disorders and parasitic infections, among others[25-29]. Vascular dysfunction with subsequent BBB damage has been observed both in human CM and in animal models[18,19,30].
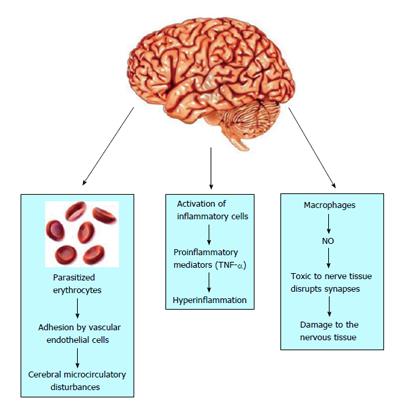
The pathogenesis of CM is associated with cerebral microcirculatory disturbances resulting from the adhesion to and sequestration of parasitized erythrocytes, immune cells and platelets by vascular endothelial cells that line the small blood vessels of the brain, leading to their blockage[30], as shown in Figure 2. In this regard, several studies provide evidence that in the erythrocytic phase the merozoites modify the surface of erythrocytes, inducing the expression of a surface protein-Plasmodium falciparum erythrocyte membrane protein 1-that has a strong affinity for adhesion molecules expressed on the surface of vascular endothelium, such as intracellular adhesion molecule 1, vascular cell adhesion molecule 1, and platelet endothelial cell adhesion molecule 1, among others[31].
In sequestration, P. falciparum-infected erythrocytes adhere to the brain endothelium through binding to PfEMP1[32]. There is no evidence to date of infected erythrocyte entry into brain parenchyma, suggesting that these cells remain in the vascular space where they are sequestered. This sequestration of parasitized erythrocytes leads to multiple vascular effects, including the formation of clusters of agglomerated platelets and leukocytes, increased vasoconstriction, as well as the agglutination of erythrocytes not parasitized by generating so-called rosettes, which significantly reduce cerebral blood flow in the capillaries and cause vascular obstruction, leading to hypoxia, brain parenchymal hemorrhage[22] and disruption of BBB integrity[11,33].
Moreover, hyperinflammation in the brain has also been related to CM and is another mechanism responsible for the vasculopathy observed during infection (Figure 2). Some studies report that during the inflammatory response, activation of inflammatory cells may occur accompanied by an overproduction of type-1 proinflammatory mediators, especially tumor necrosis factor-alpha (TNF-α), which is produced by microglia, astrocytes, monocytes and cerebral vascular endothelium[34]. In humans, this cytokine induces the upregulation of adhesion molecules on endothelial cell surfaces, which contributes to the increased capture of erythrocytes in the cerebral capillaries and other organs[25,35]. Furthermore, inflammation enhances nitric oxide (NO) production by macrophages, which seems linked to the pathogenesis of the disease, considering that it is extremely toxic to nerve tissue and disrupts synapses, contributing to the damage to the nervous tissue[36]. In addition, inflammation can lead to micro- and ring hemorrhages and necrosis of surrounding tissues and cerebral edema, resulting in significant compression of cerebral arteries that can lead to death, as well as the various symptoms of CM, such as confusion or stupor of obtundation, or deep coma with long-term neurological deficits such as cortical blindness[25,37].
Postmortem analyses of children who died with CM revealed that the axonal and myelin damage was associated with ring hemorrhages and vascular thrombosis in the cerebral and cerebellar white matter and brainstem. Disruption of the BBB and accumulation of monocytes with phagocytosed hemozoin within microvessels containing infected erythrocytes was found, suggesting a link between infected erythrocyte sequestration and intravascular/perivascular pathology in fatal pediatric CM[38]. In animal models, the presence of apoptosis was also observed initially in endothelial cells and later in neurons and glia[39], and may be associated with persistent cognitive impairment[17]. These disturbances in the homeostasis of the cerebral microcirculation play an important role in the pathogenesis of CM, generating vascular obstructions, reduced cerebral blood flow and BBB disruption associated with high cerebral vasoconstriction[24,40]. In addition, in the presence of seizures and/or fever, the metabolic demand increases with consequent risk of neural injury[41], as shown in Figure 2.
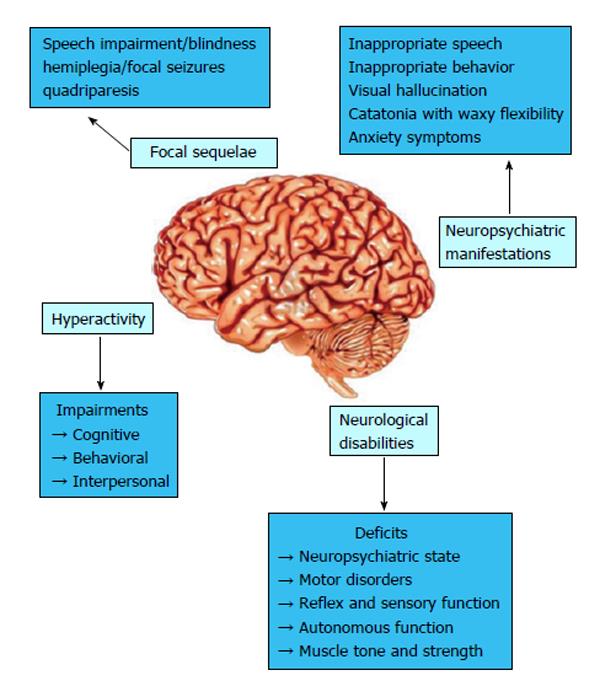
Unfortunately, severe brain injury occurs after CM and 25% of pediatric cases result in epilepsy or long-term neurological and cognitive deficits[42-44]. According to the time of symptom onset, CM may be classified into two patterns of neurological sequelae[45], as shown in Figure 3. The first is immediate and characterized by coma and status epilepticus during the acute illness, resulting in focal sequelae such as hemiplegia and focal seizures, or multifocal sequelae with spastic quadriparesis, motor disorders, cognitive and behavioral impairment, blindness, speech or hearing impairment. The second pattern develops within months or years after CM, and behavioral deficits and/or epilepsy may occur.
Among gross motor deficits, hemiplegia, diplegia, quadriparesis or quadriplegia may be observed after CM[46]. Disorders in movement and gait can be noted, including ataxia, choreoathetosis, dystonia and poor neck control, as well as feeding difficulties[46]. Dai et al[47] demonstrated that motor coordination impairment was associated with dysregulation of Akt and GSK3β signaling in a murine model of CM. The inhibition of the Akt pathway results in modifications in neuronal integrity, since it is a protein kinase playing a key role in the insulin signaling pathway and an important regulator of apoptosis, being consequently important for cell viability, although via an GSK3β-dependent pathway. In addition, the intracranial hypertension may contribute to the motor sequelae, given that it reduces the cerebral perfusion pressure, nutrient and oxygen delivery and, where death does not occur, subsequent global ischemic injury and brainstem compression can lead to cerebral atrophy, which may result in motor and cognitive impairment[48].
Convulsions in CM are common and inflammatory products such as quinolinic acid contribute to the neuropathology, considering that this metabolite from the kynurenine pathway is a N-methyl-D-aspartate agonist that causes neuroinflammation, convulsions, and cell death[49-51]. Dobbie et al[52] demonstrated that quinolinic acid provokes seizures in animals, possibly, altering the neurotransmission excitatory and triggering long-term deleterious effects on cognitive function and/or behavior. Sokol et al[53] demonstrated irreversible neuron damage after long-term seizure activity, followed by gliosis and focal atrophy, resulting in more seizures and brain damage. The epilepsy (recurrence of seizures without apparent cause) occurs in approximately 10% of pediatric cases and may be occasioned by focal or global hypoxia or ischemia[54,55]. The epileptogenesis mechanisms are unclear. Structural brain damage and the presence of Durck’s malarial granuloma may contribute to the epileptogenesis mechanisms[56]; however, other factors should also be considered, like genetic propensity[57].
It has been observed that speech and language were the most common neurocognitive impairments found in Kenyan children who survived severe malaria[58]. The authors suggested that language impairment may be part of a broad impairment that is most noted in the patterns of language, which probably contributes to deficits on verbal components of other cognitive assessments. On the other hand, Dugbartey[59] reported that children affected by CM develop impairments in bimanual tactile discrimination, accuracy of visual scanning, visual memory, perceptual abstraction and rule learning skills, right ear auditory information processing, and dominant-hand motor speed. Other studies revealed deficits in spatial memory, mental processing, sequential processing, and attention tasks[58,60,61]. Indeed, other kinds of memory, such as episodic memory, also seem to be affected by CM[62].
Dai et al[47,63] demonstrated that memory deficits either during or after successful treatment were associated with reduced Akt expression and dysregulation of Akt/GSK3β signaling in a murine CM model. GSK3β plays a key role in the process of neurodevelopment and the transcription of brain derived neurotrophic factor, affecting long-term memory and synaptic plasticity[64]. In this context, it has been associated with hyperphosphorylation of tau protein[65], which is the major component in neurodegenerative disorders like Alzheimer’s disease[66]. Abnormal tau levels in the cerebral spinal fluid in CM survivors[47,67] lead to long-term deficits in cognitive areas like memory, learning, language and psychiatric disorders[17,42,68,69]. Specific damage in neuronal areas such as the hippocampus and sub-cortical white matter may lead to impairments in learning, memory and language function[70-72].
Hyperactivity, impulsiveness and inattentiveness have also been observed in CM survivors[45], similar to what occurs in attention deficit hyperactivity disorder (ADHD), which produces impairments in the cognitive, behavioral, and interpersonal domains[73]. Dysregulated reward processing in the frontostriatal system has been proposed as a central mechanism in prevailing theoretical models of ADHD[74,75], and altered dopamine signaling underlies a number of ADHD symptoms[75]. Several anatomical changes in the brain are related to ADHD, including in the caudate nucleus, prefrontal cortex white matter, corpus callosum, cerebellar vermis[76] and globus pallidus[77], which are all areas that contain high densities of dopamine receptors. Most probably, damage occasioned by CM in the frontostriatal and cerebellar areas by a decrease in local blood flow or neuronal loss may produces impairments in dopamine signaling and consequently ADHD[78].
Animal model parameters may reproduce some symptoms related to ADHD and stroke. In this regard, a murine study demonstrated a lower level of general activity associated with reduced response to touch escape and absent vocalization correlated with large areas of hemorrhage in animals with CM[39]. These findings suggest an important influence of parenchymal hemorrhage distribution on the severity of neurological deficits in the late stage of the illness[46].
An inflammatory cytokine profile has been associated with CNS dysfunction found in human and experimental CM. In the course of experimental CM induced by Plasmodium berghei (strain ANKA), leukocyte migration into the brain, as well as the production of TNF-α and chemokines (CCL2, CCL3, CCL5 and CXCL9) preceded neurological changes including in the neuropsychiatric state, motor behavior, autonomic function, muscle tone and strength, suggesting that the inflammatory changes may be involved in the neurological impairment[79]. In this context, de Miranda et al[80] demonstrated an anxiety-like behavior in C57BL/6 mice infected with P. berghei using the elevated plus maze test. The anxiety symptoms were correlated with histopathological alterations in the brainstem, cerebrum and hippocampus and increased cerebral levels of interleukin-1 beta and TNF-α. In humans, Dugbartey et al[81] described anxiety disorders in a CM patient’s recovery, suggesting that falciparum malaria is associated with enduring, albeit subclinical, anxiety and depressive symptoms.
Some authors have reported correlations between neurological disabilities and glutamate levels and their contribution to the pathogenesis of these deficits, showing increased glutamate levels in cerebral spinal fluid and cerebrocortical synaptosomes from CM animals associated with alterations in neuropsychiatric state, motor behavior, reflex and sensory function, autonomous function, muscle tone and strength[82]. Glutamate is the principal excitatory neurotransmitter in the mammalian CNS, participating in several cognitive and neurological functions under physiological conditions[83]. Therefore, large amounts of glutamate release trigger neurotoxicity and neuronal cell death, being involved in neurodegenerative disorders[84]. Thus, the imbalance in the neurotransmitter glutamate may be important in the establishing the pathogenesis mechanism of CM[82].
The neuropsychiatric manifestations of post malaria neurological syndrome (PMNS) are highly variable and include an acute confusional state or acute psychosis with one or more of the following symptoms: inappropriate speech or behavior, visual hallucination, catatonia with waxy flexibility, generalized convulsion, fine postural tremor, clouding of consciousness and decreased muscle tone. It may occur within 2 mo after acute MC, with either neurologic or psychiatric symptoms[85]. A case report on a Taiwan CM patient reported severe headache, dizziness, delirium and polyneuropathy within 2 mo after recovery[86]; even psychotic symptoms with both visual and auditory hallucinations, aggressiveness, and inability to communicate have been related[87,88] that can last for 12 d. The symptoms observed may not be attributed only to CM, since other factors could be responsible; however, the neurologic and psychiatric presentations were compatible with PMNS and the mechanisms are the same as those related to other neurologic CM deficits, including cerebral hypoperfusion and immunologic mechanism, which prompts psychosis in a small minority[88].
Malaria is a parasitic disease that can affect the CNS, altering cognitive and behavioral functions. Neurological and behavioral changes described in the course of experimental or human CM are mainly a consequence of brain hyperinflammation, vascular obstruction, reduced cerebral blood flow, and disruption of the BBB associated with high levels of cerebral vasoconstriction, thrombus, ring hemorrhage, ruptured capillaries, and cerebral blood vessels filled with infected erythrocytes, with consequent axonal damage and demyelination. Additionally, neurologic alterations have been observed as motor deficits, seizures and epilepsy; neurocognitive impairment in language, speech, learning, and memory; and behavioral damage with hyperactivity, anxiety, PMNS and psychosis.
We are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq), FAPESPA, Federal University of Para and the Federal University of Health Sciences of Porto Alegre for granting financial support for this work. P.R.T. Romao and M.C. Monteiro were recipients of fellowships from CNPq.
P- Reviewer: Zhou M S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | World Health Organization. Neglected tropical diseases. The 17 neglected tropical diseases. access in 2013. Available from: http: //www.who.int/neglected_diseases/diseases/en/. . |
| 2. | World Health Organization. World Malaria Report 2012. Available from: http: //www.who.int/malaria/publications/world_malaria_report_2012/report/en/. . |
| 3. | World Health Organization. Global Health Observatory (GHO). Number of malaria deaths. Access in 2013. Available from: http: //www.who.int/gho/malaria/epidemic/deaths/en/. . |
| 4. | Kiszewski A, Johns B, Schapira A, Delacollette C, Crowell V, Tan-Torres T, Ameneshewa B, Teklehaimanot A, Nafo-Traore F. Estimated global resources needed to attain international malaria control goals. Bull World Health Organ. 2007;85:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med. 2011;8:e1000401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 6. | Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit Care. 2003;7:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Kantele A, Jokiranta TS. Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin Infect Dis. 2011;52:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Management of severe malaria: a practical handbook - 3rd ed. Geneva: World Health Organization 2012; . |
| 10. | Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Desruisseaux MS, Machado FS, Weiss LM, Tanowitz HB, Golightly LM. Cerebral malaria: a vasculopathy. Am J Pathol. 2010;176:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Waknine-Grinberg JH, McQuillan JA, Hunt N, Ginsburg H, Golenser J. Modulation of cerebral malaria by fasudil and other immune-modifying compounds. Exp Parasitol. 2010;125:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Dondorp AM. Pathophysiology, clinical presentation and treatment of cerebral malaria. Neurology Asia. 2005;10:67-77. |
| 14. | Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68:267-274. [PubMed] [DOI] [Full Text] |
| 15. | Jain K, Sood S, Gowthamarajan K. Modulation of cerebral malaria by curcumin as an adjunctive therapy. Braz J Infect Dis. 2013;17:579-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Desruisseaux MS, Gulinello M, Smith DN, Lee SC, Tsuji M, Weiss LM, Spray DC, Tanowitz HB. Cognitive dysfunction in mice infected with Plasmodium berghei strain ANKA. J Infect Dis. 2008;197:1621-1627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Trop Med Int Health. 2006;11:386-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Shikani HJ, Freeman BD, Lisanti MP, Weiss LM, Tanowitz HB, Desruisseaux MS. Cerebral malaria: we have come a long way. Am J Pathol. 2012;181:1484-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog. 2012;8:e1002982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci USA. 2012;109:E1772-E1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Newton CR, Taylor TE, Whitten RO. Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg. 1998;58:673-683. [PubMed] |
| 22. | Faille D, El-Assaad F, Alessi MC, Fusai T, Combes V, Grau GE. Platelet-endothelial cell interactions in cerebral malaria: the end of a cordial understanding. Thromb Haemost. 2009;102:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Cox D, McConkey S. The role of platelets in the pathogenesis of cerebral malaria. Cell Mol Life Sci. 2010;67:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3452] [Cited by in RCA: 3886] [Article Influence: 204.5] [Reference Citation Analysis (0)] |
| 25. | Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier. Int J Parasitol. 2006;36:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 27. | Tung JN, Tsao TY, Chen SL, Tai CJ, Shen SC, Cheng YW, Jiang MC. Presence of secretory cellular apoptosis susceptibility protein in cerebrospinal fluids of patients with intracerebral hemorrhage caused by stroke and neurotrauma. Neuro Endocrinol Lett. 2010;31:390-398. [PubMed] |
| 28. | Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812:220-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2094] [Cited by in RCA: 2409] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 30. | Coltel N, Combes V, Hunt NH, Grau GE. Cerebral malaria -- a neurovascular pathology with many riddles still to be solved. Curr Neurovasc Res. 2004;1:91-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 32. | Milner DA. Rethinking cerebral malaria pathology. Curr Opin Infect Dis. 2010;23:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 34. | Medana IM, Hunt NH, Chaudhri G. Tumor necrosis factor-alpha expression in the brain during fatal murine cerebral malaria: evidence for production by microglia and astrocytes. Am J Pathol. 1997;150:1473-1486. [PubMed] |
| 35. | Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 478] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 36. | Zanini GM, Cabrales P, Barkho W, Frangos JA, Carvalho LJ. Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice. J Neuroinflammation. 2011;8:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol. 2006;36:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, Kamiza S, Molyneux M, Taylor TE. The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol. 2011;178:2146-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 39. | Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbok R, Tannich E, Schmutzhard E. Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol Appl Neurobiol. 2006;32:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Kennan RP, Machado FS, Lee SC, Desruisseaux MS, Wittner M, Tsuji M, Tanowitz HB. Reduced cerebral blood flow and N-acetyl aspartate in a murine model of cerebral malaria. Parasitol Res. 2005;96:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Carter JA, Lees JA, Gona JK, Murira G, Rimba K, Neville BG, Newton CR. Severe falciparum malaria and acquired childhood language disorder. Dev Med Child Neurol. 2006;48:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Ngoungou EB, Dulac O, Poudiougou B, Druet-Cabanac M, Dicko A, Mamadou Traore A, Coulibaly D, Farnarier G, Tuillas M, Keita MM. Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, West Africa. Epilepsia. 2006;47:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, Wu B, Boivin MJ. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92-e99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 45. | Idro R, Kakooza-Mwesige A, Balyejjussa S, Mirembe G, Mugasha C, Tugumisirize J, Byarugaba J. Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes. 2010;3:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Daroff RB. Cerebral malaria. J Neurol Neurosurg Psychiatry. 2001;70:817-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Dai M, Freeman B, Shikani HJ, Bruno FP, Collado JE, Macias R, Reznik SE, Davies P, Spray DC, Tanowitz HB. Altered regulation of Akt signaling with murine cerebral malaria, effects on long-term neuro-cognitive function, restoration with lithium treatment. PLoS One. 2012;7:e44117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Newton CR, Crawley J, Sowumni A, Waruiru C, Mwangi I, English M, Murphy S, Winstanley PA, Marsh K, Kirkham FJ. Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child. 1997;76:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Stone TW, Connick JH, Winn P, Hastings MH, English M. Endogenous excitotoxic agents. Ciba Found Symp. 1987;126:204-220. [PubMed] |
| 50. | Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain. 1993;116:1425-1450. [PubMed] |
| 51. | Nakamura TA, Yamada K, Hasegawa T, Nabeshima T. Possible involvement of nitric oxide in quinolinic acid-induced convulsion in mice. Pharmacol Biochem Behav. 1995;51:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Dobbie M, Crawley J, Waruiru C, Marsh K, Surtees R. Cerebrospinal fluid studies in children with cerebral malaria: an excitotoxic mechanism. Am J Trop Med Hyg. 2000;62:284-290. [PubMed] |
| 53. | Sokol DK, Demyer WE, Edwards-Brown M, Sanders S, Garg B. From swelling to sclerosis: acute change in mesial hippocampus after prolonged febrile seizure. Seizure. 2003;12:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. QJM. 1996;89:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Walker O, Salako LA, Sowunmi A, Thomas JO, Sodeine O, Bondi FS. Prognostic risk factors and post mortem findings in cerebral malaria in children. Trans R Soc Trop Med Hyg. 1992;86:491-493. [PubMed] |
| 56. | Aleem MA. Epilepsy in malaria. Epilepsia. 2005;46:601. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Versteeg AC, Carter JA, Dzombo J, Neville BG, Newton CR. Seizure disorders among relatives of Kenyan children with severe falciparum malaria. Trop Med Int Health. 2003;8:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung’ala-Odera V, Lees JA, Newton CR. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Dugbartey AT. The neuropsychology of cerebral malaria. PhD Dissertation, University of Victoria: Canada 1995; . |
| 60. | Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002;23:353-364. [PubMed] |
| 61. | Carter JA, Mung’ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, Newton CR. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1063] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 63. | Dai M, Reznik SE, Spray DC, Weiss LM, Tanowitz HB, Gulinello M, Desruisseaux MS. Persistent cognitive and motor deficits after successful antimalarial treatment in murine cerebral malaria. Microbes Infect. 2010;12:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Salas TR, Reddy SA, Clifford JL, Davis RJ, Kikuchi A, Lippman SM, Menter DG. Alleviating the suppression of glycogen synthase kinase-3beta by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J Biol Chem. 2003;278:41338-41346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 66. | Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 674] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 67. | Medana IM, Idro R, Newton CR. Axonal and astrocyte injury markers in the cerebrospinal fluid of Kenyan children with severe malaria. J Neurol Sci. 2007;258:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, John CC. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360-e366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 69. | Holding PA, Stevenson J, Peshu N, Marsh K. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93:529-534. [PubMed] |
| 70. | Richardson ED, Spring JA, Varney NR, Struchen MA, Roberts RJ. Dichotic listening in the clinic: new neuropsychological applications. Clinical Neuropsychiatry. 1994;8:416-428. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 71. | Grote CL, Pierre-Louis SJ, Durward WF. Deficits in delayed memory following cerebral malaria: a case study. Cortex. 1997;33:385-388. [PubMed] |
| 72. | Varney NR, Roberts RJ, Springer JA, Connell SK, Wood PS. Neuropsychiatric sequelae of cerebral malaria in Vietnam veterans. J Nerv Ment Dis. 1997;185:695-703. [PubMed] |
| 73. | American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 4th edition. Washington: DSM-IV 1994; . |
| 74. | Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 2008;49:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 75. | Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 76. | Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 573] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 77. | Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 78. | Lou HC, Henriksen L, Bruhn P, Børner H, Nielsen JB. Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol. 1989;46:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 288] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Lacerda-Queiroz N, Rodrigues DH, Vilela MC, Miranda AS, Amaral DC, Camargos ER, Carvalho LJ, Howe CL, Teixeira MM, Teixeira AL. Inflammatory changes in the central nervous system are associated with behavioral impairment in Plasmodium berghei (strain ANKA)-infected mice. Exp Parasitol. 2010;125:271-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | de Miranda AS, Lacerda-Queiroz N, de Carvalho Vilela M, Rodrigues DH, Rachid MA, Quevedo J, Teixeira AL. Anxiety-like behavior and proinflammatory cytokine levels in the brain of C57BL/6 mice infected with Plasmodium berghei (strain ANKA). Neurosci Lett. 2011;491:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Dugbartey AT, Dugbartey MT, Apedo MY. Delayed neuropsychiatric effects of malaria in Ghana. J Nerv Ment Dis. 1998;186:183-186. [PubMed] |
| 82. | Miranda AS, Vieira LB, Lacerda-Queiroz N, Souza AH, Rodrigues DH, Vilela MC, Gomez MV, Machado FS, Rachid MA, Teixeira AL. Increased levels of glutamate in the central nervous system are associated with behavioral symptoms in experimental malaria. Braz J Med Biol Res. 2010;43:1173-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S-1015S. [PubMed] |
| 84. | Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698:6-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 478] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 85. | Nguyen TH, Day NP, Ly VC, Waller D, Mai NT, Bethell DB, Tran TH, White NJ. Post-malaria neurological syndrome. Lancet. 1996;348:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Hsieh CF, Shih PY, Lin RT. Postmalaria neurologic syndrome: a case report. Kaohsiung J Med Sci. 2006;22:630-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Falchook GS, Malone CM, Upton S, Shandera WX. Postmalaria neurological syndrome after treatment of Plasmodium falciparum malaria in the United States. Clin Infect Dis. 2003;37:e22-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 262] [Article Influence: 7.5] [Reference Citation Analysis (0)] |