Copyright
©2013 Baishideng Publishing Group Co.
World J Transl Med. Dec 12, 2013; 2(3): 32-35
Published online Dec 12, 2013. doi: 10.5528/wjtm.v2.i3.32
Published online Dec 12, 2013. doi: 10.5528/wjtm.v2.i3.32
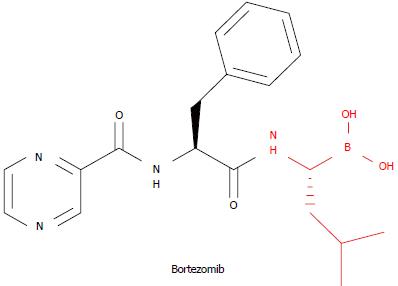
Figure 1 Structure of the proteasome inhibitor bortezomib.
The amino acid residue marked in red at the C-terminus (boroLeu unit) is responsible for its β5-preferring (chymotrypsin-like) activity and represents the electrophilic moiety that covalently traps the catalytic Thr1Oγ with a slow off-rate.
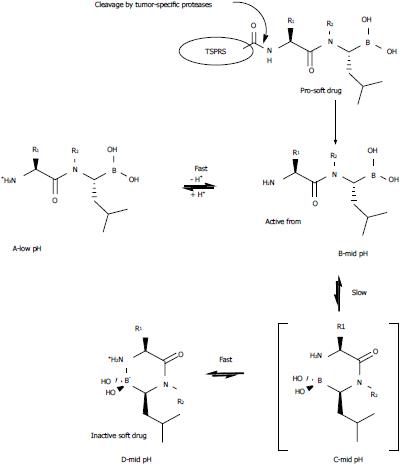
Figure 2 Activation and pH-dependent cyclization of dipeptidyl boroLeu.
A tumor-specific protease recognition sequence (TSPRS) is attached to the N-terminus of the dipeptidyl boroLeu. The dipeptidyl boroLeu is released in its active form by a tumor-specific protease and undergoes a pH-dependent cyclization equilibrium. In the present study, low pH = 2 and mid pH = 7.6.
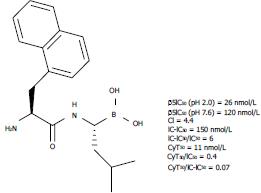
Figure 3 Structure and activity of the most interesting dipeptidyl boroLeu developed by Milo et al[14].
IC-IC50/IC50 ratio → a measure of cell permeability, for which a value of 1.0 corresponds to 100%; CyT50/IC50 ratio → the relationship between cytotoxicity and inhibition, for which a value > 1.0 indicates that more than 50% inhibition is needed to kill 50% of the cells. CI: Cyclization index; IC: Intracellular; CyT50: Cytotoxicity.
- Citation: Micale N. Peptide-based boronates: How to achieve tissue specificity in anticancer therapy. World J Transl Med 2013; 2(3): 32-35
- URL: https://www.wjgnet.com/2220-6132/full/v2/i3/32.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v2.i3.32