Peer-review started: August 27, 2018
First decision: October 4, 2018
Revised: November 2, 2018
Accepted: January 3, 2019
Article in press: January 4, 2019
Published online: January 21, 2019
Processing time: 149 Days and 0.3 Hours
Dehydration and volume depletion describe two distinct body fluid deficit disorders with differing pathophysiology, clinical manifestations and treatment approaches. However, the two are often confused or equated with each other. Here, we address a number of commonly encountered misconceptions about body-fluid deficit disorders, analyse their origins and propose approaches to overcome them.
Core tip: The conceptual error of using the term “dehydration” as a non-specific generic term to represent any type of fluid deficit affecting any fluid compartment, or even worse, to imply extracellular fluid volume depletion remains disturbingly prevalent among medical students and doctors. Careless and casual use of the term “dehydration” for patients who, in fact, have intravascular “volume depletion” contaminates the medical language, creates misleading impressions and unfortunately, in some cases, leads to inappropriate management. We propose a multi-faceted approach that supplements real life clinical scenarios with reflective activities through active participation of students and helps remove these robust misconceptions and instigate conceptual restructuring.
- Citation: Asim M, Alkadi MM, Asim H, Ghaffar A. Dehydration and volume depletion: How to handle the misconceptions. World J Nephrol 2019; 8(1): 23-32
- URL: https://www.wjgnet.com/2220-6124/full/v8/i1/23.htm
- DOI: https://dx.doi.org/10.5527/wjn.v8.i1.23
Students often confuse concepts related to sodium and water balance. One concept that has received considerable attention in recent medical teaching is the notion that disorders of water balance are manifested as hyponatraemia or hypernatraemia, whilst disorders of sodium balance are manifested as disruption of extracellular fluid (ECF) volume. In this review, we focus on another key concept regarding dehydration and volume depletion, and how the two are completely different clinical syndromes with distinct pathophysiological mechanisms, clinical features, biochemical characteristics, and management strategies.
Assessment of body fluid status is an integral component of the physician’s evaluation of most hospitalized patients. A competent fluid assessment requires sound knowledge of the dynamic interaction between body fluid compartments, as well as a skilful examination and careful biochemical analysis of serum and urine. In addition, a command of medical language and terminology is essential to precisely describe and categorize body-fluid status. Mange et al[1] highlighted the importance of recognizing dehydration and volume depletion as two completely different clinical entities. However, the conceptual error of using the term “dehydration” as a non-specific, generic term to represent any type of fluid deficit affecting any fluid compartment, or even worse, to imply ECF volume depletion, remains disturbingly prevalent among medical students and doctors. Careless and casual use of the term “dehydration” for patients who, in fact, have intravascular “volume depletion” contaminates the medical language, creates misleading impressions and unfortunately, in some cases, leads to inappropriate management. Considering the magnitude of the problem, in 2004 the International Classification of Diseases coordination and maintenance Committee made recommendations to modify the coding for body fluid disorders to uniquely identify dehydration and volume depletion[2]. For the sake of this review, we will use the term “students” to refer to medical students as well as to junior physicians who fall prey to the dehydration/volume depletion misconceptions.
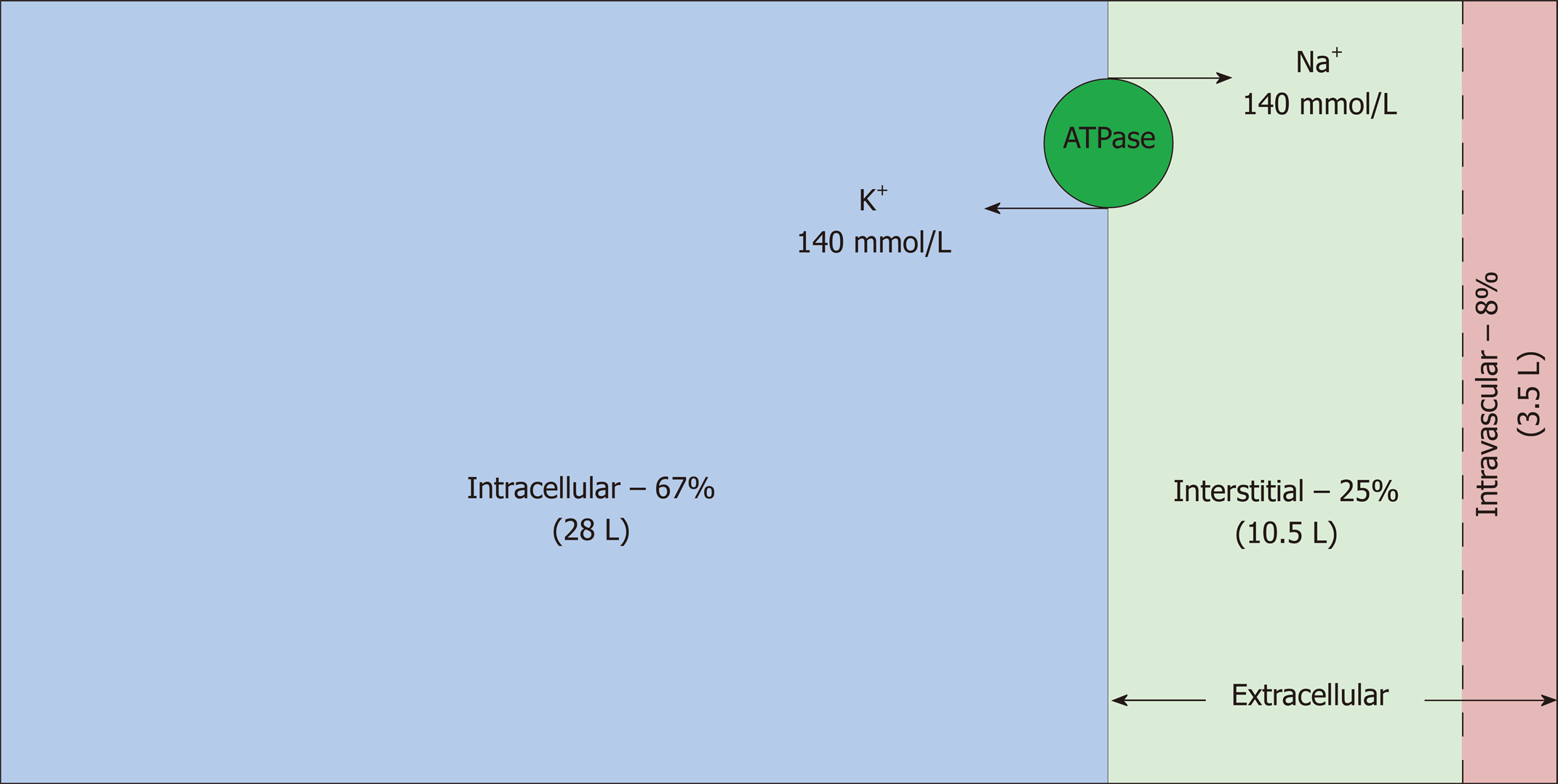
Total body water (TBW) is estimated to be 50%-60% of body weight, varying with age, gender and race, and resides in three main fluid compartments of body (Figure 1)[3,4]. The bulk of the TBW (67%) is confined intracellularly; the remaining 33% is distributed between the two sub-compartments of the extracellular space: interstitial and intravascular (25% and 8% respectively)[5]. Hence, in a 70 kg man, TBW is approximately 42 L, out of which 28 L, 10.5 L and 3.5 L are distributed in the intracellular, interstitial and intravascular compartments, respectively. Another subcategory of ECF, albeit small, is transcellular fluid (not shown in the figure) that resides in pleural, pericardial, peritoneal, synovial, ocular and cerebrospinal spaces, although in some cases, its chemical composition and physical properties may differ from that of intravascular or interstitial fluid[6]. Fluid input and output from the body proceeds via the intravascular compartment.
Intravascular and interstitial compartments are separated solely by highly permeable capillary membranes. Hence, their ionic composition is almost identical; the major cation is sodium (Na+) and the major anions are chloride and bicarbonate. In contrast, the major cation in the intracellular fluid (ICF) is potassium (K+) and the major anions are inorganic phosphates. Sodium chloride is typically confined to the ECF compartment by virtue of the Na-K-ATPase pumps, anchored in the cell membranes, which pump Na+ out and K+ into the cells. This constant active transport of Na+ and K+ across the cell membrane makes the ECF rich in Na+ and the ICF rich in K+. Consequently, the osmolality of the ECF is largely dependent on sodium and chloride whereas the osmolality of the ICF is derived from potassium along with other intracellular osmoles. Water moves freely between all fluid compartments through highly water permeable cell membranes; therefore, the osmolality of the plasma is equal to the osmolality of other compartments.
Of the total plasma volume, 85% is in venous circulation and 15% is in arterial circulation. It is this small arterial volume (approximately 700 mL) that constitutes the effective circulating volume, which is responsible for tissue perfusion and regulation of the body’s salt and water balance[7,8].
Considering the differing permeability of the membranes that separate fluid compartments in the body, administration of different IV fluids will result in differing distribution amongst these compartments. Since water flows freely between all three compartments, infusion of one litre of 5% dextrose water (D5W) will lead to an increase in the volume of the intracellular compartment of approximately 670 mL (67% of one litre), that of the interstitial compartment of 250 mL (25% of one litre) and that of intravascular compartment of 80 mL (8% of one litre). On the other hand, infusion of one litre of normal saline (0.9% NS) will add approximately 750 mL to the interstitial space and 250 mL to the intravascular space due to the inhibition of Na+ entry into the cell by the aforementioned Na/K/ATPase pumps located in the cell membranes. Though the water content of both D5W and 0.9% NS solutions is equal (1000 mL), much more fluid will reside in the intravascular space if given in the form of 0.9% NS (as none enters the intracellular space). Hence, 0.9% NS is preferred over D5W if the aim is to correct intravascular volume depletion. Conversely, if the aim is to correct dehydration (pure water loss) then a fluid that flows to all the compartments, such as D5W is the preferred solution. Giving D5W is equivalent to giving free water because glucose is rapidly metabolized.
TBW and volume of each fluid compartment can be accurately measured by radionuclide and “indicator-dilution” methods or by bioelectrical impedance[9-11].
As indicated by Mange et al[1], two distinct clinical syndromes can develop secondary to excessive body fluid losses: (1) Dehydration, which means pure water loss (“Hydro” originates from the ancient Greek word “hudōr”, meaning “water”; to de-hydrate means removing water). Loss of water reduces the distribution space of Na+, thereby disturbing the Na+ and water ratio, leading to hypernatremia and hypertonicity. Because cell membranes are freely permeable to water, this results in osmotic movement of water from the larger intracellular compartment to the extracellular compartment. There is a contraction of all body water compartments proportional to their share of TBW[12]. Since the intracellular compartment is the largest reservoir of body water, it suffers the largest water deficit. For instance, for each litre of water lost from the body, the intracellular compartment contributes 670 mL. In contrast, the intravascular compartment suffers a loss of only 80 mL; hence pure water loss rarely compromises the effective circulating volume or haemodynamic stability. Pure water loss results in hypernatremia and hypertonicity because Na+ is a membrane-impermeant solute. This induces shrinkage of osmoreceptor cells in the anterior hypothalamus, stimulating the release of antidiuretic hormone (ADH) from the posterior pituitary gland. ADH promotes incorporation of water channels (aquaporin 2) in the distal nephron segments allowing increased water reabsorption. At the same time, the thirst mechanism is triggered leading to increased water ingestion. Renal conservation of water along with increased water intake act to reverse the osmolal changes brought about by the initial water loss by restoring normonatremia (Figure 2); (2) Volume depletion, which implies an ECF volume deficit secondary to the loss of both sodium and water. Sodium is confined into the extracellular compartment by the Na-K-ATPase pumps in the cell membranes, which helps to hold water extracellularly[13]. Sodium and water loss lead to a reduction in the effective circulating volume. The human body orchestrates a number of homeostatic responses to combat hypovolemia that include activation of the renin-angiotensin-aldosterone system (receptors in renal afferent arterioles), stimulation of the sympathetic nervous system (aortic arch and carotid sinus receptors), suppression of ANP (atrial receptors) and stimulation of ADH release. All these lead to renal conservation of both salt and water, thereby restoring normovolemia. It is noteworthy that ADH release is stimulated in both dehydration (due to hypertonicity), and ECF volume depletion (due to decreased effective circulating volume).
Though uncommon, some physicians have insufficient knowledge of body fluids due to a lack of factual information about body fluid compartments and differences in their composition. Most are aware that a patient with haemorrhagic shock has a depleted intravascular compartment, but only a few recognize which compartment suffers the most in a dehydrated patient with a serum sodium of 170 µmol/L. Suppose an elderly patient is admitted with community-acquired pneumonia. He has been rather drowsy for two days before admission with poor oral intake. He is tachypneic and pyrexial, but his blood pressure is normal with no postural change. Initial laboratory tests reveal a serum sodium of 170 µmol/L. He is receiving antibiotics and D5W infusion. When asked “What condition are you treating with D5W infusion?”, most students reply “hypernatremia” rather than “dehydration”, i.e., they mention the biochemical derangement rather than the condition that produced it. Further probing reveals that some students do not recognize that hypernatremia in most instances represents loss of water in relation to Na+ (not an excess of sodium) and is a manifestation of dehydration (hence we calculate the free water deficit to assess the amount of water replacement needed to correct hypernatremia). In other words, it is the water intake/excretion (rather than Na+ handling) that regulates the ECF sodium concentration. It also appears that although some students have knowledge of the different fluid compartments, they fail to apply their knowledge to real life cases.
A number of students have a skewed understanding of body fluid compartments and harbour various misconceptions, the most common of which is erroneously referring to “ECF volume depletion” or “intravascular volume depletion” as “dehydration”. The vast majority of doctors appreciate that patients who present with profuse diarrhoea and vomiting and are consequently hypotensive and tachycardic are intravascularly depleted. They also very appropriately resuscitate these patients with 0.9% NS rather than D5W infusion. However, when presenting such a case during the ward round, they say “this patient was severely dehydrated and resuscitated with 0.9% NS”. So, although they correctly identify and treat the clinical syndrome of intravascular volume depletion, they use imprecise terminology.
Another common misbelief among students is that dehydration can be reliably diagnosed by physical signs such as sunken eyes, decreased skin turgor and dry mucous membranes. Contrarily, the predictive value of these individual clinical signs in diagnosing dehydration is limited in adult populations. Studies endorsing these physical signs were mostly carried out on paediatric and elderly patient populations[14-18]. Many of these patients in fact had ECF volume depletion rather than dehydration, as evidenced by haemodynamic compromise and normal serum sodium levels.
The term “sunken eyes” (enophthalmos) implies posterior displacement of the eyeballs within the orbits due to a decrease in volume of orbital soft tissues. However, exophthalmometry, the standard objective technique for measuring enophthalmos, is not used in general medicine leaving substantial variation in inter-observer agreement for this physical sign. Furthermore, normal anatomical variation amongst individuals and age-related changes (lipodystrophy of orbital fat with increasing age) make “sunken eyes” an unreliable physical sign of dehydration.
Reduced skin turgor means reduced elastic recoil of the skin to its normal contour after being pinched in a fold. As pointed out by Laron et al[19], it reflects contraction of the interstitial and intravascular space (both are subcategories of the extracellular compartment) rather than the loss of intracellular water. Skin turgor also correlates directly with the elastin content of the skin, which decreases significantly with ageing[16,20].
Though it had long been known that primary loss or deprivation of water produces biological disturbances (thirst) dissimilar to those seen in primary loss or deprivation of salt (circulatory instability), both types of deficits were considered to be subcategories of dehydration in the early 20th century[21,22]. These ancient concepts have managed to exercise a strong pull on some modern doctors, who have persevered in using the term “dehydration” to refer to both intracellular water loss and ECF volume loss and to sub-classify dehydration into isonatraemic, hyponatraemic and hypernatraemic forms[23-27].
In fact, it is volume depletion that has isonatraemic, hyponatraemic and hypernatraemic subtypes determined by the tonicity of the fluid lost and the type of fluid ingested[28-31]. If the losses are isotonic, i.e., proportionate quantities of water and sodium are lost (e.g., blood loss), then serum sodium and tonicity will remain unchanged resulting in isonatraemic volume depletion. However, if more sodium relative to water is lost (or the patient takes plenty of salt-free fluids, for example tap water), hyponatraemic volume depletion ensues. Finally, if less sodium is lost relative to water (or if the patient does not drink water, or takes hypertonic soup), hypernatraemic volume depletion follows. In contrast to volume depletion, dehydration is always hypernatraemic (due to loss of pure water); the categories “hyponatraemic” and “isonatraemic” do not apply in dehydration.
Some patients can present with features of both dehydration and intravascular volume depletion. The co-existence of these two different entities is partly responsible for some physicians misjudging them as a single disorder. Indeed, many patients in paediatric clinical studies with diarrhoeal illnesses were both dehydrated and ECF volume depleted[14,32]. This complex pathophysiological state was oversimplified as “dehydration” and the severity of body fluid losses categorized according to percentage of body weight loss. The “dehydration” assessment scales included the physical signs and laboratory parameters of both intracellular water loss and ECF volume depletion[14,32].
Clinically, it is not possible to establish whether hypernatremia in an intravascularly depleted patient is secondary to hypernatraemic intravascular depletion (water loss greater than sodium loss), severe dehydration (profuse water loss alone), or a combination of the two. This differentiation requires marker/tracer studies[9-11]. In clinical situations, there is hardly any need for this differentiation. As a first step, intravascular volume depletion is treated with 0.9% NS to support organ function. Once adequate haemodynamic stability is achieved, hyperosmolality is corrected with D5W.
Usually, dehydration does not lead to intravascular volume depletion as the intravascular space contributes only a small percentage to the TBW loss; the major bulk is lost from the intracellular space, the largest reservoir of body water. As discussed earlier, a loss of 1 L from TBW removes only 80 ml from the intravascular space (2.3% of intravascular volume); consequently, no appreciable deleterious effects on haemodynamics are seen. Development of signs and symptoms of intravascular volume depletion usually require more than 0.5 L of intravascular volume deficit. For this intravascular volume deficit to develop in a 70 kg person with dehydration, a TBW deficit of more than 6 litres (more than 15% of TBW) will be required. By this time severe hypernatremia (serum Na+ > 170 mmol/L) would have developed[12].
Albert Einstein said, “everything should be made as simple as possible, but not simpler”. When discussing a patient’s condition, physicians commonly use shortened forms of legitimate medical words and phrases as a time-saving measure. The drawback of this is that these brief forms can lead to varied interpretations and thus confound medical personnel. The term “volume depletion” is used as a brief (though obscure) form for ECF volume depletion or intravascular volume depletion but might not adequately convey the intended meaning. It neither clarifies whether the loss of fluid is from intracellular or extracellular space, nor indicates the type of fluid lost (hypotonic or isotonic). Hence, for some it may imply depletion of TBW (i.e., dehydration), while for others, depletion of the intravascular fluid alone (i.e., isotonic fluid loss).
An organized approach is imperative in correcting robust misconceptions related to body fluid deficit disorders. First, it is crucial that all faculty members develop a critical understanding of the body-fluids, as misconceptions acquired from faculty members and textbooks are very difficult to eliminate from the minds of young doctors later in their professional lives.
In the following section we present our approach to overcoming misconceptions in a manner that will create a lasting effect on students and prevent them from reverting to their preconceptions.
Although misconceptions about body fluids disorders are widespread, students are generally unaware that the knowledge they possess is faulty. Mere use of medical terminology is not sufficient evidence of students’ knowledge; it needs to be ensured that these terms are used with accurate meaning in the context of body fluid compartments. We actively bring up the subject when encountering patients with body fluid deficits in order to probe students for the presence of misconceptions.
Once identified, we try to make students discontent with their misconceptions. This provides a strong stimulus for refinement or replacement of the flawed concepts with intelligible and plausible ones. Utmost care is given to maintain a favourable learning environment where the students are not ridiculed for holding incorrect preconceptions.
We split teaching into short modules, each with a clear framework and objectives. Most of the modules remain “learner-centred” where students are engaged in meaningful activities which promote thought. Multiple teaching techniques are employed to cater to the diverse learning styles of the students and rekindle students’ interest and participation.
Introductory presentation: We start with a 10-min introductory presentation using visual aids such as a white-board or PowerPoint presentation to orient the students to body fluid compartments. Classifying the body fluid deficit disorder based on the nature of the fluid deficit (water alone vs water with salt) and the main body fluid compartment affected (intracellular vs extracellular) in each disorder generates uneasiness in the minds of those students who misconceive dehydration and volume depletion as one entity. This serves as an important turning point in the students’ learning as they start feeling dissatisfied with their pre-conceptions.
We make a conscious effort to avoid ambiguous linguistic expressions. We use the term “dehydration” to specifically refer to a body-fluid disorder resulting from pure water depletion with consequent hypernatremia, rather than using it as a blanket term for any type of fluid deficit. Furthermore, since the term “volume depletion” gives rise to referential ambiguity (because it does not specify the referent body fluid compartment), we disambiguate this term by adding an adjective, for example, “ECF” or “Intravascular” to indicate the depleted body-fluid compartment. This also encourages students to abandon the habit of using misleading abbreviations.
Clinical encounter: The newly implanted concepts must be supplemented with real life applications within a patient care context ensuring the students do not merely learn the new rote information. Utilizing the “think-pair-share” technique, we invite students to form either pairs or small groups. One group evaluates a pre-selected patient with dehydration while the other group assesses a patient with ECF volume depletion.
Debriefing session 1: After seeing their respective patients, the two groups return to the “classroom”. They are given a 2 min “reflection time” for formulation of ideas after which each group interacts with the other to compare the clinical, laboratory and therapeutic details of their patients. The instructor facilitates the learning process and highlights the contrasting features of the two patients. This session also provides an opportunity for the instructor to point out any common conceptual errors and offer constructive suggestions.
Instead of beginning with the presenting illness, which is the generally considered “norm”, a unique way to provoke curiosity in the minds of the students is to start the discussion with the management of the two patients. This disrupts the students’ expectations, captures their attention and makes them think and reflect retrospectively. An example of this could be asking: “Both the patients are receiving IV fluids because they have body fluid deficits; why is one patient being treated with slow infusion of D5W and the other with rapid administration of 0.9% NS or blood?” or “What factors have influenced the choice of fluid and the rate of infusion?” These queries will encourage the students to talk about the body fluid compartment from which the fluid has been lost in each condition and the concept of replacing “like with like” by choosing appropriate fluids for each condition. These questions also help cement the fact that dehydration and volume depletion are different disorders and hence necessitate different treatments. At this juncture, schematic illustrations of fluid compartments to demonstrate the effect of the addition of different types of fluids (blood, albumin, 0.9% NS, NaHCO3, D5W, etc.) on each body fluid compartment are utilized. Students are interested to note how each type of fluid initially expands the intravascular space but then distributes distinctively through different fluid compartments. This also helps them with the application of theoretical knowledge into direct patient care. The ECF volume maintains blood pressure and perfusion of organs, hence a fluid that is more likely to stay in the ECF compartment (0.9% NS) is administered as a rapid infusion in a volume depleted patient to ensure haemodynamic stability. Conversely, a fluid that predominantly restores the ICF compartment (D5W) is administered to a dehydrated patient in a controlled fashion to prevent the development of cerebral oedema. Again, throughout the session, the terms “ECF volume depletion” and “intravascular volume depletion” are used to dispel referential ambiguity.
Analysis of the laboratory values: After the debriefing session, we focus on the laboratory investigation results of the two patients. This is done either at the computer station on the ward or in the classroom where lab values from electronic medical records are projected on to the screen. It is important to emphasize the dissimilarity between the urinary and serum biochemical values of the two patients. In the ECF volume depleted patient, the urine becomes concentrated and contains very little Na+ consequent to renal conservation of salt and water. In the dehydrated patient, although urine is concentrated (due to water absorption in the distal tubule), urinary Na+ is not decreased. In fact, hypernatremia in a dehydrated patient can augment renal natriuresis by mechanisms that appear to be independent of changes in atrial natriuretic peptide[33]. In ECF volume depletion, BUN/creatinine ratio rises due to renal hypo-perfusion with variable effects on serum sodium (depending upon the type of fluid loss). In contrast, there is no significant increase in serum urea or creatinine in dehydration; the gold standard is hypernatremia and consequent hypertonicity[1,34].
Back to the patients: After going through the laboratory results, students go back to the patients to demonstrate physical signs. If the physical signs have resolved consequent to the treatment, then students review the medical file to note the physical findings at time of presentation. Important findings include orthostatic hypotension, tachycardia, prolonged capillary refill time and decreased skin turgor. These are defined to students as signs of ECF volume depletion, as dehydration cannot be reliably determined by use of clinical examination. Finally, to complete the chain of events in reverse chronological order, we focus on the modes of presentation of the two patients by reviewing their medical records. ECF volume depleted patients usually present with a history of blood-loss or gastrointestinal fluid loss, conditions that cause third-spacing (e.g., ileus or pancreatitis) or sepsis (vasodilatation induced relative hypovolemia). On the other hand, since intracellular hydration influences cellular function, severely dehydrated patients may present with altered cognitive and neuromuscular function. It is noteworthy that altered mentation can be both a cause (by affecting the patient’s ability to access water) and a consequence (due to the resultant deranged neuro-cellular function) of dehydration. Critically ill patients in the ICU are prone to develop dehydration because they are unconscious, sedated or ventilated and are hence unable to voluntarily control their free water intake.
After going through all the aspects of care for their respective patients, the two groups switch and examine the other patient using the same system. This session is usually shorter as it simply affirms the pre-discussed differences between the two clinical conditions, cementing the newly acquired knowledge in the students’ minds. We find that by the end of this session, most students can appreciate the key differences between dehydration and volume depletion and the clinical implications of each.
Home assignment: The above stated clinical and biochemical differences invariably prompt analysis of different homeostatic mechanisms that operate in ECF volume depletion and dehydration. We direct students to carry out further reading to explore pathophysiological differences between the two disorders (Figure 2). We also provide them with additional learning resources that allow them to adequately explore the whole subject at their own comfortable pace to build up their new conceptual frameworks.
Debriefing session 2: On the following day, the pathophysiology of dehydration and volume depletion are addressed in the final session. Representatives from both student groups are invited to draw simple diagrams on a white board illustrating the homeostatic mechanisms. As they do this, it highlights to the audience that the sensors and effectors of both conditions are different (Figure 2); in dehydration, the osmoregulatory mechanisms are activated whereas in volume depletion the ECF volume regulatory pathways are stimulated to restore a normal physiologic environment. Students should then be able to link up the pathophysiological changes to the symptoms, signs and treatment of dehydration and volume depletion. They appreciate that the therapeutic manoeuvres mimic the response of normal kidneys to these conditions – kidneys retain water in dehydration, so we treat dehydration with water administration; kidneys retain salt and water in volume depletion, hence we infuse 0.9% NS to combat volume depletion.
In the second half of this session, students may share their perspectives on the whole topic. They also reflect on how the new concepts will resolve the problems that previously led to dissatisfaction in the management of such patients, and how their new understanding will benefit them in their future clinical experiences.
New concepts that are not reflected upon often fade away rapidly. Therefore, opportunities are tactfully created for the students to continuously apply the new knowledge into clinical practice; acute medical admissions’ wards are good places to see patients with ECF volume depletion whereas ICUs are good avenues to evaluate intubated and ventilated patients who are prone to dehydration. Multiple encounters with volume depleted and dehydrated patients allow the students to revise new concepts and train them to identify and treat these two contrasting conditions appropriately.
Another effective strategy is to have the advanced students in the team (fellows or senior residents who has successfully demonstrated proficiency in the subject) teach the subject to the junior residents or interns. During this process, the senior students invest more personal effort into learning and thus consolidate deeper understandings. The faculty member acts as a facilitator who designs and supervises the learning environment as well as provides on-the-spot advice when required.
Dehydration/volume depletion misconceptions are prone to relapse. We regularly revisit these misconceptions through lectures, case discussions, publications and board review programmes. In our training programme, most residents from general medical teams rotate to nephrology. This opportunity is used to advance-train these residents in the field of fluid balance so that they may act as surrogate teachers to complement professional teaching by faculty. Finally, questions on our in-house exams are crafted to carefully test the conceptual framework of students rather than their ability to memorize facts.
The terms dehydration and volume depletion represent two fundamentally different clinical disorders. Erroneous concepts about these body fluid disorders are worryingly prevalent among medical students and physicians. A multi-pronged approach is required to wipe out these robust misconceptions. We strongly believe that the most effective strategy to instigate conceptual restructuring is by supplementing real life clinical experiences with reflective activities through active participation of students. We recommend that dehydration/volume depletion misconceptions be included in medical school curricula and textbooks so that medical students become aware of the common pitfalls of fluid status evaluation.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: Qatar
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Nechifor G, Stavroulopoulos A S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Mange K, Matsuura D, Cizman B, Soto H, Ziyadeh FN, Goldfarb S, Neilson EG. Language guiding therapy: the case of dehydration versus volume depletion. Ann Intern Med. 1997;127:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | AHIMA. Summary of October 2004 ICD-9-CM Coordination and Maintenance Committee Meeting. Available from: URL: http://library.ahima.org/doc?oid=62385#.W83gHlUzaUl. |
| 3. | Chumlea WC, Guo SS, Zeller CM, Reo NV, Baumgartner RN, Garry PJ, Wang J, Pierson RN Jr, Heymsfield SB, Siervogel RM. Total body water reference values and prediction equations for adults. Kidney Int. 2001;59:2250-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 970] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Edelman IS, Leibman J. Anatomy of body water and electrolytes. Am J Med. 1959;27:256-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 255] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Guyton AC, Hall JE. The Body Fluids and Kidney. In: Textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders, 2006: 292. . |
| 7. | Berl T, Schrier RW. Disorders of water metabolism. In: Schrier RW, editor. Renal and Electrolyte Disorders. 6th ed. Philadelphia: Lippincott Williams Wilkins. 2003;1-63. |
| 8. | Freda BJ, Davidson MB, Hall PM. Evaluation of hyponatremia: a little physiology goes a long way. Cleve Clin J Med. 2004;71:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ritz P; Source Study. Bioelectrical impedance analysis estimation of water compartments in elderly diseased patients: the source study. J Gerontol A Biol Sci Med Sci. 2001;56:M344-M348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | O'Brien C, Baker-Fulco CJ, Young AJ, Sawka MN. Bioimpedance assessment of hypohydration. Med Sci Sports Exerc. 1999;31:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Olde Rikkert MG, Deurenberg P, Jansen RW, van't Hof MA, Hoefnagels WH. Validation of multi-frequency bioelectrical impedance analysis in detecting changes in fluid balance of geriatric patients. J Am Geriatr Soc. 1997;45:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Feig PU, McCurdy DK. The hypertonic state. N Engl J Med. 1977;297:1444-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 144] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Rose BD, Post TW. Introduction to disorders of osmolality. In: Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York: McGraw-Hill 2001; 683. |
| 14. | Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291:2746-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics. 1997;99:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 357] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Gross CR, Lindquist RD, Woolley AC, Granieri R, Allard K, Webster B. Clinical indicators of dehydration severity in elderly patients. J Emerg Med. 1992;10:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Thomas DR, Tariq SH, Makhdomm S, Haddad R, Moinuddin A. Physician misdiagnosis of dehydration in older adults. J Am Med Dir Assoc. 2003;4:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | LARON Z. Skin turgor as a quantitative index of dehydration in children. Pediatrics. 1957;19:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (1)] |
| 20. | Dorrington KL. Skin turgor: do we understand the clinical sign? Lancet. 1981;1:264-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Kerpel-Fronius E. Über die Beziehungen zwischen Salz- und Wasserhaushalt bei experimentellen Wasserverlusten. Ztschr f Kinderh. 1935;57:489. [DOI] [Full Text] |
| 22. | Nadal JW, Pedersen S, Maddock WG. A comparison between dehydration from salt loss and from water deprivation. J Clin Invest. 1941;20:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Ballestero Y, Hernandez MI, Rojo P, Manzanares J, Nebreda V, Carbajosa H, Infante E, Baro M. Hyponatremic dehydration as a presentation of cystic fibrosis. Pediatr Emerg Care. 2006;22:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Finberg L, Bernstein J. Acute hyponatremic dehydration. J Pediatr. 1971;79:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 25. | Caksen H, Odabaş D, Sar S, Celebi V, Arslan S, Kuru M, Abuhandan M. Hyponatremic dehydration: an analysis of 78 cases. Int Urol Nephrol. 2001;33:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Grisanti KA, Jaffe DM. Dehydration syndromes. Oral rehydration and fluid replacement. Emerg Med Clin North Am. 1991;9:565-588. [PubMed] |
| 27. | Moritz ML, Ayus JC. Improving intravenous fluid therapy in children with gastroenteritis. Pediatr Nephrol. 2010;25:1383-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Sarhill N, Walsh D, Nelson K, Davis M. Evaluation and treatment of cancer-related fluid deficits: volume depletion and dehydration. Support Care Cancer. 2001;9:408-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Berk L, Rana S. Hypovolemia and dehydration in the oncology patient. J Support Oncol. 2006;4:447-54; discussion 455-7. [PubMed] |
| 30. | Leaf A. The clinical and physiologic significance of the serum sodium concentration. N Engl J Med. 1962;267:24-30 contd. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Leaf A. The clinical and physiologic significance of the serum sodium concentration. N Engl J Med. 1962;267:77-83 concl. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Duggan C, Refat M, Hashem M, Wolff M, Fayad I, Santosham M. How valid are clinical signs of dehydration in infants? J Pediatr Gastroenterol Nutr. 1996;22:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Andersen LJ, Andersen JL, Pump B, Bie P. Natriuresis induced by mild hypernatremia in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1754-R1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Armstrong LE. Hydration assessment techniques. Nutr Rev. 2005;63:S40-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |